Abstract
Background
Metabolic syndrome (MetS) is relatively common worldwide and an important risk factor for cardiovascular diseases. It is closely linked to arterial stiffness of the carotid artery. However, the association of MetS with the safety of carotid revascularization has been rarely studied. The aim of this study was to observe the current status of MetS and its components in Chinese carotid revascularized patients, and investigate the impact on major adverse clinical events (MACEs) after carotid endarterectomy (CEA) or carotid artery stenting (CAS).
Methods
From January 2013 to December 2017, patients undergoing CEA or CAS in the Neurosurgery Department of Xuanwu Hospital were retrospectively recruited. The changes in prevalence of MetS and each component with time were investigated. The primary outcome was 30-day post-operative MACEs. Univariable and multivariable analyses were performed to identify the impact of MetS on CEA or CAS.
Results
A total of 2068 patients who underwent CEA (766 cases) or CAS (1302 cases) were included. The rate of MetS was 17.9%; the prevalence rate of MetS increased with time. The occurrence rate of MACEs in CEA was 3.4% (26 cases) and in CAS, 3.1% (40 cases). There was no statistical difference between the two groups (3.4% vs. 3.1%, P = 0.600). For CEA patients, univariate analysis showed that the MACE (+) group had increased diabetes history (53.8% vs. 30.9%, P = 0.014) and MetS (34.6% vs. 15.8%, P = 0.023). For CAS patients, univariate analysis showed that the MACE (+) group had increased coronary artery disease history (40.0% vs. 21.6%, P = 0.006) and internal carotid artery tortuosity (67.5%% vs. 37.6%, P < 0.001). Furthermore, the MACE (+) group had higher systolic blood pressure (143.38 ± 22.74 vs. 135.42 ± 17.17 mmHg, P = 0.004). Multivariable analysis showed that the influencing factors for MACEs in CEA included history of diabetes (odds ratio [OR] = 2.345; 95% confidence interval [CI] = 1.057–5.205; P = 0.036) and MetS (OR = 2.476; 95% CI = 1.065–5.757; P = 0.035). The influencing factors for MACEs in CAS included systolic blood pressure (OR = 1.023; 95% CI = 1.005–1.040; P = 0.010), coronary artery disease (OR = 2.382; 95% CI = 1.237–4.587; P = 0.009) and internal carotid artery tortuosity (OR = 3.221; 95% CI = 1.637–6.337; P = 0.001).
Conclusions
The prevalence rate of MetS increased with time in carotid revascularized patients. MetS is a risk for short-term MACEs after CEA, but not CAS.
Keywords: Metabolic syndrome, Carotid endarterectomy, Carotid artery stenting, Major adverse clinical events, Influencing factors
Introduction
Stroke is a leading cause of death worldwide and approximately 15% to 20% of ischemic strokes are caused by carotid artery stenosis.[1,2] Carotid endarterectomy (CEA) is the golden standard surgical therapy for atherosclerotic carotid artery stenosis.[1] On the contrary, carotid artery stenting (CAS), a less invasive intervention, has been shown by many clinical studies to be an effective alternative.[3,4]
Metabolic syndrome (MetS) is a combination of related cardiovascular risk factors including obesity, hypertension, high fasting blood glucose (FBG, a pre-diabetic state), high triglycerides, and low levels of high-density lipoprotein (HDL).[5,6] MetS is relatively common worldwide and may be increasing as a result of social, economic, and lifestyle factors.[7–9] MetS has been recognized as an important risk factor for cardiovascular diseases, and it is closely linked to arterial stiffness, especially of the carotid artery.[10] Moreover, MetS can increase plaque insatiability and reduce cerebrovascular conductance, thus leading to a higher risk of cerebrovascular events.[11] In addition, cardiovascular morbidity and mortality was higher in patients with MetS than without MetS.[12]
However, the association of MetS with the safety of carotid revascularization has been rarely studied, with conflicting results.[5,6,13–15] Furthermore, MetS may have ethnic disparity,[9] and no previous studies have investigated MetS in the Chinese population, with the exception of one small study that demonstrated that MetS could influence the safety of CAS.[13] Therefore, this retrospective study, based on a large sample size, aimed to clarify the association of MetS and carotid revascularization.
Methods
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki. Considering its retrospective nature, Institutional Review Board approval was waived for this study and de-identification of all patients in this series. Informed written consent was obtained from all patients prior to their enrollment in this study.
Study Design and Patients
In this single-center and retrospective study, data were extracted from the medical records of patients with carotid artery stenosis who received carotid revascularization of CAS or CEA in the Neurosurgery Department of Xuanwu Hospital from January 2013 to December 2017. Surgery or intervention indication for patients was recent symptomatic carotid stenosis of 50% to 99% or asymptomatic carotid stenosis of 70% to 99%, according to the method of carotid stenosis measurement by the North American Symptomatic Carotid Endarterectomy Trial (NASCET).[16] A symptomatic lesion was defined as transient ischemic attack, retinal ischemic event, or ischemic stroke within the narrowed carotid artery within the previous 6 months[17]; otherwise, it was defined as asymptomatic. The degree of stenosis was evaluated with initial duplex ultrasound, magnetic resonance angiography or computed tomographic angiography and then confirmed by digital subtraction angiography (DSA).
Variables and data measurement
The analyzed variables included patients’ baseline demographic characteristics (eg, age, sex), comorbidities, physical, and laboratory examinations on admission (eg, systolic blood pressure [SBP], diastolic blood pressure [DBP], low HDL, MetS[5]), and vascular anatomy from DSA (eg, arch type, common carotid artery tortuosity, internal carotid artery [ICA] tortuosity). Blood pressure was measured and the highest values for SBP and DBP were used for influencing factor analysis. Baseline blood pressure was measured at the admission day by the nurses. Overnight fasting blood samples were collected on the second day of admission from 6:00 am to 7:00 am for routine biochemical examination, including complete blood cell count, and lipid panel. Plaque ulceration and calcification were determined by ultrasound, while plaque length and stenosis were assessed by DSA. Arch type, common carotid artery tortuosity, and ICA tortuosity distal to the lesion were in accordance with previously published studies.[18] According to previously published researches,[5,14] MetS was defined as the presence of three or more of the following criteria: hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg), low HDL (≤40 mg/dL or 1.03 mmol/L for men; ≤50 mg/dL or 1.29 mmol/L for women), high triglycerides (TG, ≥150 mg/dL or 1.7 mmol/L), high FBG (≥110 mg/dL or 5.6 mmol/L), and body mass index (BMI) ≥30.0 kg/m2. The changes in the prevalence changes of MetS and each component according to year were observed.
Surgery or Intervention
All CEAs were performed under general anesthesia by neurosurgeons, with transcranial Doppler used throughout to monitor the procedure. The surgical techniques, standard CEA or eversion CEA, with or without shunt, were determined by surgeons. All stenting procedures were performed by surgeons with a vast experience in angiographic procedures. Embolic protection devices (EPDs) were routinely used, and the choice of catheters, guidewires, balloons, and stents was based on surgeons’ experience. Neurologic examinations were conducted in each patient before and after CAS by experienced neurologists who did not perform the CAS procedure and were blinded to the study. Both experiences of CEA and CAS procedures in our center were published previously.[4,19]
Outcome Assessment
The 30-day post-operative incidence of major adverse clinical events (MACEs),[5] which was defined as death, stroke, and myocardial infarction (MI) was evaluated. Stroke was defined as focal neurologic function acute disturbance that lasted over 24 h and resulted from intracranial vascular disturbance. The definition of minor strokes was neurologic deficits that resolved completely within 30 days or led to no functional impairment in daily activities. All other strokes were considered major strokes. MI was defined as the appearance of new pathologic Q waves on a standard electrocardiogram in two or more contiguous leads and/or total creatinine kinase rise greater than twice the upper limit of normal with an elevated creatinine kinase myocardial band fraction. The short-term follow-up data were got through clinical visit or telephone. Patients were divided into the MACE (+) group and the MACE (–) group.
Statistically Analysis
Continuous variables with normal distribution are demonstrated as mean ± standard deviation, while categorical variables are demonstrated as number and percentage. The differences in categorical variables between the MACEs (+) group and MACEs (–) group were analyzed separately for CEA and CAS by Chi-squared test or Fisher exact test. The differences in continuous variables were analyzed by t test. After variables with significant differences were identified by above tests, multivariate logistic regression was further conducted to assess risk factors for post-operative MACEs in CEA and CAS. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for 30-day post-operative MACEs. SPSS version 19.0 (IBM, Armonk, NY, USA) was used for the data analysis. A P < 0.05 was considered statistically significant. If the data for any necessary item is missed, the patient will be excluded for the final analysis.
Results
From January 2013 to December 2017, 1049 cases of CEA and 1586 cases of CAS performed in Neurosurgery Department of Xuanwu Hospital were identified. After excluding patients with incomplete pre-operative or intra-operative data and loss of follow-up, a total of 2068 patients who underwent CEA (766 cases) or CAS (1302 cases) were included in this study [Figure 1].
Figure 1.
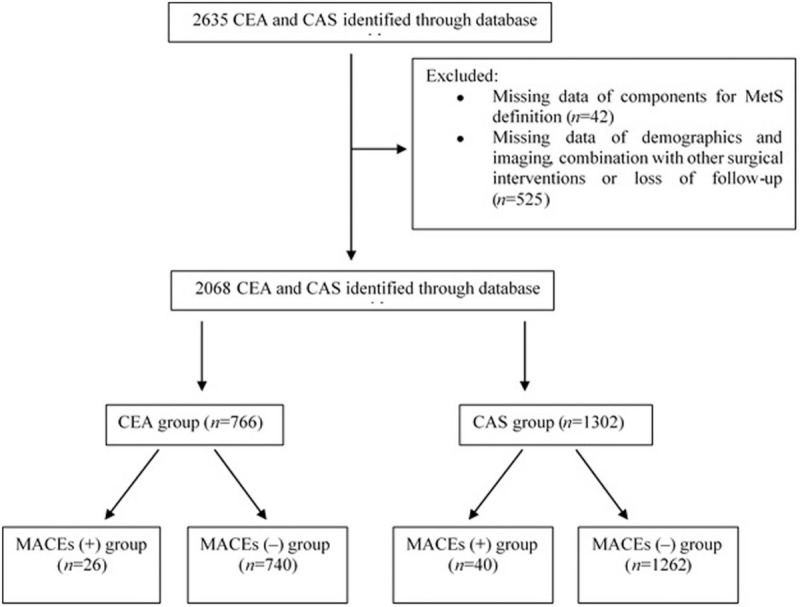
Flow diagram of the study. CEA: Carotid endarterectomy; CAS: Carotid artery stenting; MetS: Metabolic syndrome; MACEs: Major adverse clinical events major adverse; MI: Myocardial infarction.
Baseline information and MACEs results
The rate of MetS was 17.9% (370 patients with MetS and 1698 without). The mean age of patients was 64.7 ± 8.3 years (range: 22–88 years). In total, 61.2% (1266 cases) were symptomatic and 83.7% (1731 cases) were men. The occurrence rate of MACEs within 30 days was 3.4% (26 cases) in CEA and 3.1% (40 cases) in CAS, without significant difference (P = 0.687). The post-operative stroke rate was 2.5% (19 cases) and there were 2 deaths due to hemorrhagic stroke in the CEA group. The post-operative stroke rate was 3.0% (38 cases) and there was one death because of hemorrhagic stroke in the CAS group. The stroke rate was not significantly different between CEA and CAS (2.5% vs. 3.0%, P = 0.557). However, major stroke was more common in CEA group (2.0% vs. 0.9%, P = 0.045) and minor stroke was more common in CAS group (2.0% vs. 0.5%, P = 0.007). Moreover, CEA had a higher rate of MI than CAS (0.9% vs. 0.2%, P = 0.028) [Table 1].
Table 1.
Short-term MACEs in 2068 patients with carotid artery stenosis receiving CEA and CAS, n (%).
| MACEs | CEA (n = 766) | CAS (n = 1302) | Chi-squared values | P |
| Total | 26 (3.4) | 40 (3.1) | 0.162 | 0.687 |
| Death | 2 (0.3) | 1 (0.1) | 0.216 | 0.642 |
| Stroke | 19 (2.5) | 38 (3.0) | 0.345 | 0.557 |
| Major stroke | 15 (2.0) | 12 (0.9) | 4.021 | 0.045 |
| Minor stroke | 4 (0.5) | 26 (2.0) | 7.337 | 0.007 |
| MI | 7 (0.9) | 2 (0.2) | 4.798 | 0.028 |
MACEs: Major adverse clinical events; CEA: Carotid endarterectomy; CAS: Carotid artery stenting; MI: Myocardial infarction.
Changes in Prevalence of MetS and components
MetS was common in carotid stenosis patients who required surgery, and the prevalence rate increased continually with year [Figure 2]. In addition, an increasing trend of low HDL was apparent. An increased incidence of abnormalities in HDL and FBG were observed according to year, and abnormal HDL and FBG had a greater incidence than other components. Indeed, no obvious changes in TG, HTN, and BMI were observed according to year [Figure 3].
Figure 2.
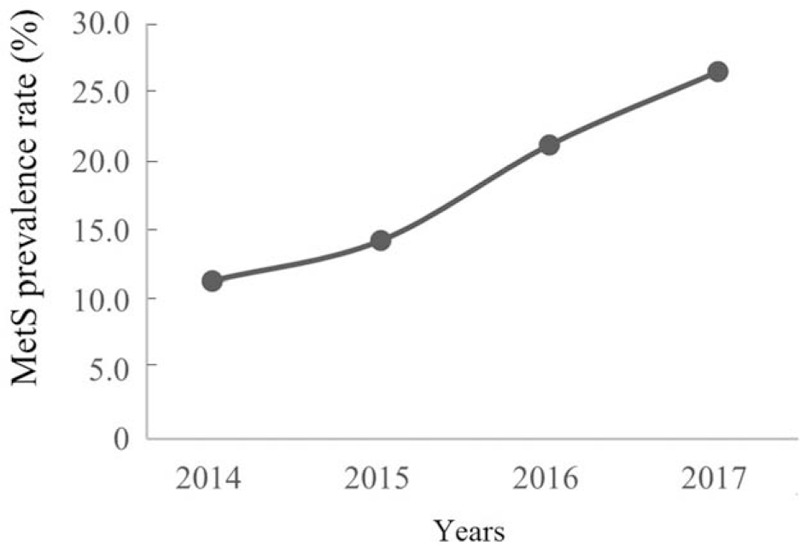
MetS prevalence rates increased continually with year. MetS: Metabolic syndrome.
Figure 3.
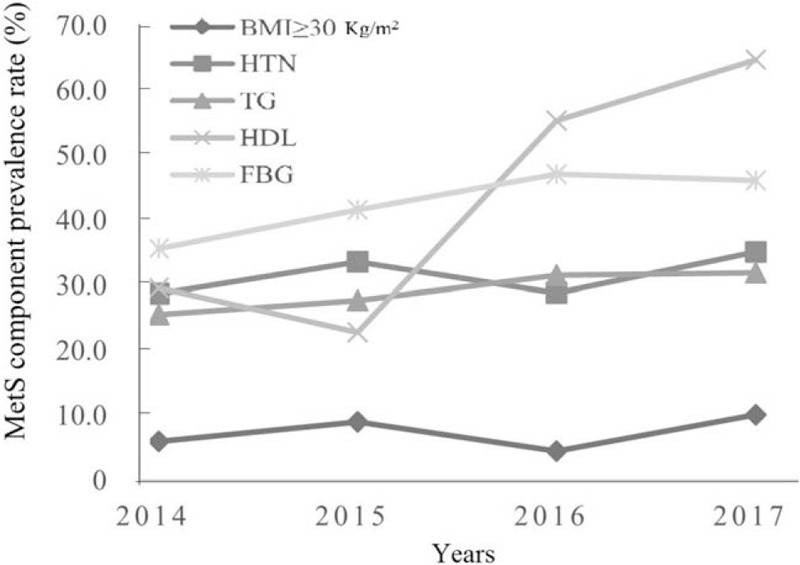
Changes of MetS component prevalence rate according to year. MetS: Metabolic syndrome; BMI: Body mass index; HTN: Hypertension; TG: Triglyceride; HDL: High-density lipoprotein; FBG: Fasting blood glucose.
MetS and MACEs
For patients receiving CEA, compared to the MACE (–) group, the MACE (+) group had increased diabetes history (30.9% vs. 53.8%, P = 0.014) and MetS (15.8% vs. 34.6%, P = 0.023). Other variables showed no significant differences between two groups, including age, gender, symptomatic lesions, hypertension, hyperlipidemia, coronary artery disease (CAD), smoking, drinking, stroke history, BMI, SBP, DBP, creatinine, low HDL, TG, TC, LDL, FBG, lesion side, plaque calcification, plaque ulceration, degree of stenosis, and contralateral carotid stenosis ≥70% [Table 2].
Table 2.
Comparison between the MACE (+) group and MACE (–) group for patients receiving CEA.
| Items | MACE (+) (n = 26) | MACE (–) (n = 740) | Statistical values | P |
| Demographics | ||||
| Age (years) | 64.0 ± 7.3 | 62.7 ± 7.9 | –0.082∗ | 0.408 |
| Male | 21 (80.8) | 629 (85.0) | 0.098† | 0.754 |
| Symptomatic | 20 (76.9) | 466 (63.1) | 2.084† | 0.149 |
| Hypertension | 16 (61.5) | 280 (37.8) | 1.038† | 0.308 |
| Diabetes | 14 (53.8) | 229 (30.9) | 6.081† | 0.014 |
| Hyperlipidemia | 19 (73.1) | 475 (64.2) | 0.866† | 0.352 |
| CAD | 7 (26.9) | 134 (18.1) | 0.779† | 0.377 |
| Smoking | 12 (46.2) | 395 (53.4) | 0.526† | 0.468 |
| Drinking | 8 (30.8) | 264 (35.7) | 0.264† | 0.607 |
| Stroke history | 10 (38.5) | 243 (32.8) | 0.359† | 0.549 |
| Laboratory | ||||
| BMI (kg/m2) | 24.48 ± 2.39 | 25.03 ± 3.37 | 0.825∗ | 0.410 |
| SBP (mmHg) | 136.00 ± 16.59 | 134.29 ± 17.59 | –0.516∗ | 0.610 |
| DBP (mmHg) | 76.92 ± 9.12 | 77.21 ± 10.31 | 0.142∗ | 0.887 |
| Creatinine (μmol/L) | 72.15 ± 19.13 | 69.06 ± 16.97 | –0.909∗ | 0.363 |
| Low HDL | 9 (34.6) | 306 (41.4) | 0.471† | 0.493 |
| LDL (mmol/L) | 1.91 ± 0.70 | 1.99 ± 0.71 | 0.580∗ | 0.567 |
| TC (mmol/L) | 3.45 ± 1.00 | 3.48 ± 0.87 | 0.148∗ | 0.883 |
| TG (mmol/L) | 1.66 ± 1.00 | 1.52 ± 0.88 | –0.827∗ | 0.408 |
| FBG (mmol/L) | 6.22 ± 2.12 | 5.75 ± 1.65 | –1.399∗ | 0.162 |
| MetS | 9 (34.6) | 117 (15.8) | 5.167† | 0.023 |
| Imaging | ||||
| Right side operation | 15 (57.7) | 387 (52.3) | 0.293† | 0.588 |
| Calcified plaque | 7 (26.9) | 239 (32.3) | 0.333† | 0.564 |
| Ulcerative plaque | 9 (34.6) | 159 (21.5) | 2.529† | 0.112 |
| Stenosis degree | 85.04 ± 9.64 | 84.83 ± 10.13 | –0.106∗ | 0.916 |
| Contralateral stenosis ≥70% | 5 (19.2) | 93 (12.6) | 0.492† | 0.483 |
t values.
Chi-squared values.
Data are shown as mean ± standard deviation or n (%). CEA: Carotid endarterectomy; MACEs: Major adverse clinical events; BMI: Body mass index; CAD: Coronary artery disease; SBP: Systolic blood pressure; DBP: Diastolic blood pressure, LDL: Low-density lipoprotein; HDL: High-density lipoprotein; TC: Total cholesterol; TG: Triglyceride; FBG: Fasting blood glucose; MetS: Metabolic syndrome.
For patients receiving CAS, the MACE (+) group had greater CAD history (40.0% vs. 21.6%, P = 0.006) and ICA tortuosity (67.5%% vs. 37.6%, P < 0.001) compared to the MACE (–) group. In addition, the MACE (+) group had higher SBP (143.38 ± 22.74 mmHg vs. 135.42 ± 17.17 mmHg, P = 0.004). Other variables were not significantly different between two groups, including age, gender, symptomatic lesion, hypertension, hyperlipidemia, diabetes history, smoking, drinking, stroke history, BMI, DBP, creatinine, low HDL, TG, TC, LDL, MetS, lesion side, plaque calcification, plaque ulceration, degree of stenosis, contralateral carotid stenosis ≥70%, and other vascular anatomy characteristics and intervention features [Table 3].
Table 3.
Comparison between the MACE (+) group and MACE (–) group for patients receiving CAS.
| Items | MACE (+) (n = 40) | MACE (–) (n = 1262) | Statistical values | P |
| Demographics | ||||
| Age (years) | 67.4 ± 7.2 | 65.9 ± 8.4 | –1.161∗ | 0.246 |
| Male | 34 (85.0) | 1043 (82.6) | 0.150† | 0.698 |
| Symptomatic | 27 (67.5) | 742 (58.8) | 1.215† | 0.270 |
| Hypertension | 10 (25.0) | 468 (37.1) | 2.437† | 0.119 |
| Diabetes | 21 (52.5) | 703 (55.7) | 0.161† | 0.688 |
| Hyperlipidemia | 31 (77.5) | 842 (66.7) | 2.040† | 0.153 |
| CAD | 16 (40.0) | 272 (21.6) | 7.659† | 0.006 |
| Smoking | 25 (62.5) | 643 (51.0) | 2.070† | 0.150 |
| Drinking | 14 (35.0) | 415 (32.9) | 0.079† | 0.779 |
| Stroke history | 18 (45.0) | 406 (32.2) | 2.906† | 0.088 |
| Laboratory | ||||
| BMI (kg/m2) | 25.50 ± 2.83 | 25.23 ± 3.61 | –0.483∗ | 0.629 |
| SBP (mmHg) | 143.38 ± 22.74 | 135.42 ± 17.17 | –2.852∗ | 0.004 |
| DBP (mmHg) | 77.83 ± 11.03 | 76.86 ± 10.09 | –0.594∗ | 0.553 |
| Creatinine (μmol/L) | 76.45 ± 22.84 | 71.31 ± 17.62 | –1.795∗ | 0.073 |
| Low HDL | 20 (50.0) | 526 (41.7) | 1.102† | 0.294 |
| LDL (mmol/L) | 1.95 ± 0.61 | 2.09 ± 0.74 | 1.137∗ | 0.256 |
| TC (mmol/L) | 3.43 ± 0.76 | 3.60 ± 0.98 | 1.080∗ | 0.280 |
| TG (mmol/L) | 1.49 ± 0.63 | 1.50 ± 0.81 | 0.071∗ | 0.943 |
| FBG (mmol/L) | 6.94 ± 3.92 | 5.95 ± 1.88 | –1.589∗ | 0.120 |
| MetS | 7 (17.5) | 237 (18.8) | 0.042† | 0.838 |
| Imaging | ||||
| Right side intervention | 19 (47.5) | 650 (51.5) | 0.249† | 0.618 |
| Calcified plaque | 13 (32.5) | 388 (30.7) | 0.056† | 0.813 |
| Ulcerative plaque | 8 (20.0) | 261 (20.7) | 0.011† | 0.917 |
| Stenosis degree | 78.72 ± 12.71 | 77.96 ± 11.83 | –0.402∗ | 0.687 |
| Contralateral stenosis ≥70% | 9 (22.5) | 156 (12.4) | 3.601† | 0.058 |
| Vascular anatomy | ||||
| Arch type II/III | 19 (47.5) | 565 (44.8) | 0.117† | 0.733 |
| CCA tortuosity | 5 (12.5) | 166 (13.2) | 0.015† | 0.904 |
| ICA tortuosity | 27 (67.5) | 475 (37.6) | 14.593† | <0.001 |
| ECA stenosis ≥50% | 3 (7.5) | 70 (5.5) | 0.032† | 0.857 |
| Intervention | ||||
| Pre-dilation | 30 (75.0) | 1006 (79.7) | 0.530† | 0.467 |
| Post-dilation | 6 (15.0) | 307 (24.3) | 1.847† | 0.174 |
| Open-cell stent | 28 (70.0) | 931 (73.8) | 0.284† | 0.594 |
t values.
Chi-squared values.
Data are shown as mean ± standard deviation or n (%). CAS: Carotid artery stenting; MACEs: Major adverse clinical events; BMI: Body mass index; CAD: Coronary artery disease; SBP: Systolic blood pressure; DBP: Diastolic blood pressure, LDL: Low-density lipoprotein; HDL: High-density lipoprotein; TC: Total cholesterol; TG: Triglyceride; FBG: Fasting blood glucose; MetS: Metabolic syndrome; CCA: Common carotid artery; ICA: Internal carotid artery; ECA: External carotid artery.
The results of multivariate logistic regression analysis are shown in Table 4. For patients receiving CEA, the factors influencing MACEs included diabetes history (OR = 2.345, 95% CI = 1.057–5.205, P = 0.036) and MetS (OR = 2.476, 95% CI = 1.065–5.757, P = 0.035). For patients receiving CAS, the factors influencing MACEs included SBP (OR = 1.023, 95% CI = 1.005–1.040, P = 0.010), CAD (OR = 2.382, 95% CI = 1.237–4.587, P = 0.009) and ICA tortuosity (OR = 3.221, 95% CI = 1.637–6.337, P = 0.001) [Table 4].
Table 4.
Multivariate analysis for short-term MACEs in CEA and CAS.
| CEA (n = 766) | CAS (n = 1302) | |||||
| Characteristics | OR | 95% CI | P | OR | 95% CI | P |
| Diabetes | 2.345 | 1.057–5.205 | 0.036 | |||
| MetS | 2.476 | 1.065–5.757 | 0.035 | |||
| SBP | 1.023 | 1.005–1.040 | 0.010 | |||
| CAD | 2.382 | 1.237–4.587 | 0.009 | |||
| ICA tortuosity | 3.221 | 1.637–6.337 | 0.001 | |||
CEA: Carotid endarterectomy; CAS: Carotid artery stenting; MACEs: Major adverse clinical events; OR: Odds ratio; CI: Confidence interval; MetS: Metabolic syndrome; SBP: Systolic blood pressure; CAD: Coronary artery disease; ICA: Internal carotid artery.
Discussion
MetS has been recognized as an important risk factor for cardiovascular diseases in recent years.[12] It is closely associated with carotid artery stiffness, plaque formation, cerebrovascular events, and cardiovascular morbidity and mortality.[10,11,20] However, MetS may have both time and ethnic differences.[9] In this study, the prevalence of MetS was observed to increase with time, and the rate was similar to that reported by other studies,[5,6,14,15,21,22] especially those comprising a Chinese study population.[13,23] Furthermore, obvious increasing trends were observed for abnormal HDL and FBG. Indeed, dyslipidemia and diabetes are well-known predictors for endothelial dysfunction and cardiovascular events,[24,25] and these observed trends may be related to changes in eating habits and lifestyle with socioeconomic development.
Whether MetS could impact the outcomes of carotid revascularization has rarely been investigated. To date, there are only five studies relating to this issue and they report inconsistent results[5,6,13–15] Only one study investigated CAS[13] and two studies investigated only CEA.[5,6] The remaining two studies analyzed the influence of MetS on CEA and CAS.[5,6] Studies of Williams et al[15] and Visser et al[14] showed no relationship between MetS and CEA outcome. Furthermore, the study of Protack et al[6] showed that MetS did not increase the 30-day complication rate in patients after CAS. On the contrary, Dong et al[13] found that MetS is closely associated with CAS complications. In addition, Casana et al[5] suggested that MetS could increase the risk for both CEA and CAS. In the current study, MetS was a risk factor for MACEs in CEA group but not CAS, which was similar to the results reported by Protack et al.[6]
History of diabetes is an influencing factor for CEA, and it is especially important in the context of ischemic cerebral vascular diseases as diabetes was found to increase the risk of carotid revascularization in many studies. In the study by Visser et al,[14] DM but not MetS increased ipsilateral transient ischemic attack or cerebrovascular accident. Furthermore, the presence of both diabetes and MetS was found to be associated with peri-operative risk for carotid revascularization in the study of Protack et al.[6] Hyperglycemia may worsen cortical intra-cellular brain acidosis and mitochondrial function in the ischemic penumbra,[26] reducing penumbra salvage in stroke patients. At the same time, hyperglycemia may indicate a more procoagulant state.[27] Furthermore, hyperglycemia mainly impacts on cerebral micro-perfusion locally or globally, which decreases the micro-thrombi clearance capacity of the brain.
High blood pressure, another component of MetS, could impact CAS outcomes. Higher SBP could increase the risk of CAS, which is consistent with previous results.[28–30] A previous systematic review showed that baseline blood pressure could affect the outcome of surgery,[31] and blood pressure control could lowering the risk of carotid revascularization.[32] Indeed, blood pressure control to lower the risk of recurrent of stroke has gained increasing attention in recent years.[32] Both hyperglycemia and hypertension are important components of MetS, and could impact micro-perfusion of the brain. These findings may indirectly indicate that MetS should still be given due consideration in the context of CAS, despite the lack of statistical significance observed in the current study.
In addition, CAD was found to be related to MACEs after CAS. Carotid artery stenosis and CAD may frequently coexist and may be manifestations of the same atherosclerotic process that reflects generalized systemic inflammation.[33,34] Moreover, the study of Chung et al[33] showed that prior MI could lead to greater number of peri-CAS embolic particles with a smaller minimum size and higher chance of passing filter pores of the EPDs. At same time, MetS was reported to be a risk factor for both CAD and stroke.[35] Again, the impact of MetS or its components on CAS safety is difficult to ignore.
This study found that MetS is related with higher risk of complication after CEA, but not CAS. The reasons for these results remain unclear. Previous researches reported that MetS could contribute smooth muscle cell dysregulation and increase surgical risk.[36–38] As CEA is performed under general anesthesia, patients with MetS may be more likely to have systemic vascular dysregulation including that of the cerebrovascular system,[36,37] thus may be at a higher risk than CAS under local anesthesia. But the mechanism should be further explored in the future studies. The results of this study may provide new evidence for strategy selection between CEA and CAS for carotid artery stenosis patients.
This study has some limitations. It was a retrospective study, with no long-term follow-up, and only peri-operative MACEs were studied. However, to our knowledge, this was the largest sample size study to investigate the association of MetS with the safety of carotid revascularization worldwide. CEA and CAS were analyzed separately to provide more valuable clinical evidence. At the same time, the changes in prevalence of MetS and its components according to time were observed. Current MetS criteria included only increased TG and decreased HDL, but not abnormal LDL or TC. Therefore, the current MetS criteria involving dyslipidemia is probably not appropriate or comprehensive for researches related to carotid revascularization.[23]
In conclusion, the prevalence rate of MetS increased with time in carotid revascularized patients, and MetS was a risk for short-term MACEs after CEA, but not CAS.
Acknowledgements
The authors thank Yang Kun (Department of Evidence-Base Medicine, Xuanwu Hospital, Capital Medical University, E-mail: yang.kun@wxhosp.org) for statistical revision.
Funding
The study was funded by grants from the National Key Research and Development Project (No. 2016YFC1301703) and the Beijing Scientific and Technologic Project (No. D161100003816002).
Conflicts of interest
None.
Footnotes
How to cite this article: Bai XS, Feng Y, Wang T, Zhang X, Yang CL, Wang YB, Hua Y, Lu J, Zhu FS, Chen YF, Gao P, Yang RJ, Ma Y, Jiao LQ. Impact of metabolic syndrome on short-term outcome of carotid revascularization: a large sample size study in Chinese population. Chin Med J 2020;133:2688–2695. doi: 10.1097/CM9.0000000000001038
References
- 1.Fanous AA, Jowdy PK, Morr S, Vakharia K, Shallwani H, Lorincz K, et al. Vascular anatomy and not age is responsible for increased risk of complications in symptomatic elderly patients undergoing carotid artery stenting. World Neurosurg 2019; 128:e513–e521. doi: 10.1016/j.wneu.2019.04.187. [DOI] [PubMed] [Google Scholar]
- 2.Chen WH, Jin W, Lyu PY, Liu Y, Li R, Hu M, et al. Carotid atherosclerosis and cognitive impairment in nonstroke patients. Chin Med J 2017; 130:2375–2379. doi: 10.4103/0366-6999.215331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonati LH, Gregson J, Dobson J, McCabe DJH, Nederkoorn PJ, van der Worp HB, et al. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): secondary analysis of a randomised trial. Lancet Neurol 2018; 17:587–596. doi: 10.1016/s1474-4422(18)30195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao LQ, Song G, Li SM, Miao ZR, Zhu FS, Ji XM, et al. Thirty-day outcome of carotid artery stenting in Chinese patients: a single-center experience. Chin Med J 2013; 126:3915–3920. doi: 10.3760/cma.j.issn.0366-6999.20131870. [PubMed] [Google Scholar]
- 5.Casana R, Malloggi C, Tolva VS, Odero A, Jr, Bulbulia R, Halliday A, et al. Does metabolic syndrome influence short and long term durability of carotid endarterectomy and stenting? Diabetes Metab Res Rev 2019; 35:e3084.doi: 10.1002/dmrr.3084. [DOI] [PubMed] [Google Scholar]
- 6.Protack CD, Bakken AM, Xu J, Saad WA, Lumsden AB, Davies MG. Metabolic syndrome: a predictor of adverse outcomes after carotid revascularization. J Vasc Surg 2009; 49:1172–1180.e1. doi: 10.1016/j.jvs.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Liu CY, Chen CQ. Intra- and extracranial atherosclerotic stenosis in China: epidemiology, diagnosis, treatment and risk factors. Eur Rev Med Pharmacol Sci 2014; 18:3368–3379. [PubMed] [Google Scholar]
- 8.Maksimovic MZ, Vlajinac HD, Radak DJ, Marinkovic JM, Jorga JB. Prevalence of the metabolic syndrome in patients with carotid disease according to NHLBI/AHA and IDF criteria: a cross-sectional study. BMC Cardiovasc Disord 2012; 12:2.doi: 10.1186/1471-2261-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report 2009; 13:1–7. [PubMed] [Google Scholar]
- 10.Nilsson PM. Arterial stiffness, the metabolic syndrome, and the brain. Am J Hypertens 2017; 31:24–26. doi: 10.1093/ajh/hpx152. [DOI] [PubMed] [Google Scholar]
- 11.Pasha EP, Birdsill AC, Oleson S, Haley AP, Tanaka H. Impacts of metabolic syndrome scores on cerebrovascular conductance are mediated by arterial stiffening. Am J Hypertens 2017; 31:72–79. doi: 10.1093/ajh/hpx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001; 24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 13.Dong S, Peng Z, Tao Y, Huo Y, Zhou H. Metabolic syndrome is associated with increased risk of short-term post-procedural complications after carotid artery stenting. Neurol Sci 2017; 38:1933–1939. doi: 10.1007/s10072-017-3085-4. [DOI] [PubMed] [Google Scholar]
- 14.Visser L, Wallis de Vries BM, Mulder DJ, Uyttenboogaart M, Veen SV, Zeebregts CJ, et al. The influence of the metabolic syndrome on the short- and long-term outcome after carotid endarterectomy. Angiology 2017; 68:306–314. doi: 10.1177/0003319716664283. [DOI] [PubMed] [Google Scholar]
- 15.Williams WT, Assi R, Hall MR, Protack CD, Lu DY, Wong DJ, et al. Metabolic syndrome predicts restenosis after carotid endarterectomy. J Am Coll Surg 2014; 219:771–777. doi: 10.1016/j.jamcollsurg.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325:445–453. doi: 10.1056/nejm199108153250701. [DOI] [PubMed] [Google Scholar]
- 17.Russjan A, Goebell E, Havemeister S, Thomalla G, Cheng B, Beck C, et al. Predictors of periprocedural brain lesions associated with carotid stenting. Cerebrovasc Dis 2012; 33:30–36. doi: 10.1159/000332088. [DOI] [PubMed] [Google Scholar]
- 18.Müller MD, Ahlhelm FJ, Von Hessling A, Doig D, Nederkoorn PJ, MacDonald S, et al. Vascular anatomy predicts the risk of cerebral ischemia in patients randomized to carotid stenting versus endarterectomy. Stroke 2017; 48:1285–1292. doi: 10.1161/STROKEAHA.116.014612. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Song G, Jiao L, Wang Y, Ma Y, Ling F. A study of carotid endarterectomy in a Chinese population: initial experience at a single center. Clin Neurol Neurosurg 2014; 126:88–92. doi: 10.1016/j.clineuro.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Leng XY, Chen XY, Chook P, Xiong L, Lin WH, Liu JY, et al. Association between metabolic syndrome and carotid atherosclerosis: a community-based study in Hong Kong. Metab Syndr Relat Disord 2013; 11:109–114. doi: 10.1089/met.2012.0099. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Zhou Y, Guo YC, Chen Q, Lei XY, Hu HP. Association between elevated resting heart rate and metabolic syndrome in a community-based population. Chin Med J 2018; 131:1003–1004. doi: 10.4103/0366-6999.229885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li NY, Yu H, Li XL, Wang QY, Zhang XW, Ma RX, et al. Astragalus membranaceus improving asymptomatic left ventricular diastolic dysfunction in postmenopausal hypertensive women with metabolic syndrome: a prospective, open-labeled, randomized controlled trial. Chin Med J 2018; 131:516–526. doi: 10.4103/0366-6999.226077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamin Z, Xuesong B, Zhen Z, Yue H, Liwei L, Fei L. Correlation of dyslipidemias and gallbladder polyps-A large retrospective study among Chinese population. Asian J Surg 2020; 43:181–185. doi: 10.1016/j.asjsur.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Ertek S. High-density lipoprotein (HDL) dysfunction and the future of HDL. Curr Vasc Pharmacol 2018; 16:490–498. doi: 10.2174/1570161115666171116164612. [DOI] [PubMed] [Google Scholar]
- 25.Long CA, Fang ZB, Hu FY, Arya S, Brewster LP, Duggan E, et al. Poor glycemic control is a strong predictor of postoperative morbidity and mortality in patients undergoing vascular surgery. J Vasc Surg 2019; 69:1219–1226. doi: 10.1016/j.jvs.2018.06.212. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RE, Tan WK, Martin HS, Meyer FB. Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke 1999; 30:160–170. doi: 10.1161/01.str.30.1.160. [DOI] [PubMed] [Google Scholar]
- 27.Gentile NT, Vaidyula VR, Kanamalla U, DeAngelis M, Gaughan J, Rao AK. Factor VIIa and tissue factor procoagulant activity in diabetes mellitus after acute ischemic stroke: impact of hyperglycemia. Thromb Haemost 2007; 98:1007–1013. doi: 10.1160/th06-12-0719. [DOI] [PubMed] [Google Scholar]
- 28.Ueki K, Sasako T, Okazaki Y, Kato M, Okahata S, Katsuyama H, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5:951–964. doi: 10.1016/s2213-8587(17)30327-3. [DOI] [PubMed] [Google Scholar]
- 29.Huibers A, Calvet D, Kennedy F, Czuriga-Kovacs KR, Featherstone RL, Moll FL, et al. Mechanism of procedural stroke following carotid endarterectomy or carotid artery stenting within the International Carotid Stenting Study (ICSS) randomised trial. Eur J Vasc Endovasc Surg 2015; 50:281–288. doi: 10.1016/j.ejvs.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doig D, Turner EL, Dobson J, Featherstone RL, de Borst GJ, Stansby G, et al. Risk factors for stroke, myocardial infarction, or death following carotid endarterectomy: results from the international carotid stenting study. Eur J Vasc Endovasc Surg 2015; 50:688–694. doi: 10.1016/j.ejvs.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ 1997; 315:1571–1577. doi: 10.1136/bmj.315.7122.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa K, Yamamoto Y, Arima H, Maeda T, Sunami N, Kanzawa T, et al. Effect of standard vs intensive blood pressure control on the risk of recurrent stroke: a randomized clinical trial and meta-analysis. JAMA Neurol 2019; 76:1309–1318. doi: 10.1001/jamaneurol.2019.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung C, Shah TR, Shin H, Han D, Marin ML, Faries PL. Determinants of embolic risk during angioplasty and stenting: neurologic symptoms and coronary artery disease increase embolic risk. Ann Surg 2010; 252:618–624. doi: 10.1097/SLA.0b013e3181f57ad2. [DOI] [PubMed] [Google Scholar]
- 34.Huang KL, Chang YJ, Chang CH, Chang TY, Liu CH, Hsieh I, et al. Impact of coexisting coronary artery disease on the occurrence of cerebral ischemic lesions after carotid stenting. PLoS One 2014; 9: doi: 10.1371/journal.pone.0094280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pigna G, Napoli A, Zaccagna F, Marincola BC, Monticolo R, Catalano C, et al. The relationship between metabolic syndrome, its components, and the whole-body atherosclerotic disease burden as measured by computed tomography angiography. Atherosclerosis 2011; 215:417–420. doi: 10.1016/j.atherosclerosis.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 36.Tung A. Anaesthetic considerations with the metabolic syndrome. Br J Anaesth 2010; 105:i24–i33. doi: 10.1093/bja/aeq293. [DOI] [PubMed] [Google Scholar]
- 37.Tzimas P, Petrou A, Laou E, Milionis H, Mikhailidis DP, Papadopoulos G. Impact of metabolic syndrome in surgical patients: should we bother? Br J Anaesth 2015; 115:194–202. doi: 10.1093/bja/aev199. [DOI] [PubMed] [Google Scholar]
- 38.Lawandy I, Liu Y, Shi G, Zhang Z, Scrimgeour LA, Pavlov V, et al. Increased coronary arteriolar contraction to serotonin in juvenile pigs with metabolic syndrome. Mol Cell Biochem 2019; 461:57–64. doi: 10.1007/s11010-019-03589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


