Abstract
The purpose is to determine if the preoperative central endothelial cell density (ECD) in triple (phacoemulsification plus intraocular lens implantation plus DSAEK) and non-triple Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) procedures have a relationship with the 5-year postoperative ECD or percent Endothelial Cell Loss (ECL).
Out of 986 consecutive DSAEK surgeries for Fuchs dystrophy, 241 eyes had 5-year ECD measurements available. Endothelial cell densities were then evaluated against preoperative ECDs to obtain measures of ECL. Triple and non-triple procedures were isolated and compared independently.
One hundred eighty two eyes had undergone a triple procedure and 59 had not. The mean ECD at 5 years was 1560 ± 648 cells/mm2 for triples and 1483 ± 621 cells/mm2 for non-triples (P = .42). Endothelial Cell loss was 44.4% ± 21.7% and 44.4% ± 22.0%, respectively for eyes that underwent a triple or non-triple (P = .99). There was a moderate, but significant correlation between preoperative ECD and the ECD at 5 years after DSAEK for both triples (r = 0.39, P < .001), and non-triples (r = 0.32, P = .01), respectively.
In Descemets stripping automated endothelial keratoplasty grafts, higher preoperative donor ECD was correlated with higher ECD at 5 years postoperatively but was unaffected by a concurrent cataract surgery in the triple procedure.
Keywords: DSAEK, ECD at 5 years, triple procedure
1. Introduction
Endothelial Keratoplasty (EK) has been rapidly evolving in the past 2 decades. The first modern EK procedure was performed in 1999 and called Posterior Lamellar Keratoplasty (PLK).[1,2] Deep Lamellar Endothelial Keratoplasty (DLEK) was introduced in the following year.[3] In 2004, descemetorhexis technique was introduced and the new procedure was named Descemet Stripping Endothelial Keratoplasty (DSEK), which was further improved through the introduction of automated microkeratome, a procedure named Descemet Stripping Automated Endothelial Keratoplasty (DSAEK).[4] Descemet Membrane Endothelial Keratoplasty (DMEK) was introduced in 2006,[5] which was later modified by DMAEK (Descemet Membrane Automated Endothelial Keratoplasty).[6,7] Although DMEK is becoming more widely adapted, DSEK/DSAEK is still the most commonly performed procedure for EK.[8]
DSAEK is frequently performed as part of a triple procedure (phacoemulsification plus intraocular lens implantation plus DSAEK). Removing the lens in the triple procedure obviates the risk of intraoperative crystalline lens damage, as well as eliminates the risk of accelerated cataract formation after DSAEK.[9,10] However, there is a theoretical risk that the greater instability of the newly placed intraocular lens (IOL) in a triple procedure and the added postoperative inflammation from the concomitant cataract surgery may increase the endothelial cell loss rate in a triple procedure graft v a DSAEK without other simultaneous surgery.[11] While the Cornea Preservation Time Study[12] (CPTS) and other single center studies[13,14] did not show a significant difference in endothelial cell loss of Triples v DSAEK alone, the study presented here has a longer postoperative time frame than the CPTS and more patients to analyze for the 5 year postoperative visit than any prior single center study.
Another major issue in DSAEK is the relevance of the preoperative endothelial cell density to the long-term health of the graft. The CPTS found that a higher preoperative cell density was not correlated with a higher graft survival rate at 3 years,[15] but did find that there was a significant positive correlation with the postoperative endothelial cell density at 3 years.[12] In this single center study, we evaluated the correlation of preoperative cell densities to postoperative cell densities out to a longer time frame of 5 years.
This study is the largest single center, uniform surgical technique study of DSAEK to look at the influence of triple procedures and preoperative cell density on endothelial cell loss and endothelial cell density at 5 years after surgery.
2. Materials and methods
2.1. Protocol
This is part of the EK patient registry at Devers Eye Institute, and a continuation of our prospective data collection on patients receiving EK. A Legacy Institutional Review Board approved and Health Insurance Portability and Accountability Act compliant clinical protocol and surgical consent form was developed and enrollment was initiated for patients with endothelial dysfunction.
No specific requests were made of the eye bank to provide tissue for EK with any different characteristics than what is normally requested for full thickness PK tissues. Donor tissues with an ECD more than 2000 cells/mm2, any age between 4 and 75 years old, and death to transplantation time of up to 12 days were accepted.
Patients receiving triple procedure (DSAEK combined with cataract extraction and posterior chamber intraocular lens (PCIOL)) for Fuchs endothelial dystrophy and cataract, and patients receiving non-triple (DSAEK only) procedure for Fuchs endothelial dystrophy were considered eligible for the study.
All DSAEK procedures were performed between 12/2005 and 10/2013 and only those eyes that had 5 year specular imaging were included in the analysis. Eyes that were not included in the final analysis were either lost to follow-up, experienced graft failure, had unanalyzable specular images, or missed the 5-year visit.
Eyes with a history of age-related macular degeneration, cystoid macular edema, controlled glaucoma and other comorbidities were not excluded as visual acuity was not an outcome of the study. Eyes with uncontrolled glaucoma or eyes with prior or subsequent glaucoma surgery were excluded.
In all cases, the phacoemulsification and intraocular lens implantation were done before DSAEK through the same 5 mm temporal scleral tunnel, but with only a 2.8 mm keratome opening into the AC. This opening was subsequently enlarged to the full 5 mm for the insertion of the donor tissue. The exact, standardized, forcep-insertion DSAEK procedure was used by attending and corneal fellows for all surgeries.
2.2. Specular microscopy data
The vast majority of donor tissue came from Lions VisionGift in Portland, Oregon. Specular images were obtained with an EB-3000 XYZ Eyebank specular microscope (HAI Laboratories, Inc., Lexington, MA). The preoperative cell counts were obtained using an apices digitized method and the manufacturers calibrations for magnification. The apices of at least 100 cells from the endothelial images of each cornea were counted. Preoperative donor tissue specular microscopy was performed by 3 trained eye bank technicians, all of whom had at least 1 year of experience with this technology. The preoperative endothelial cell density measurement was taken before the donor tissue was cut for a DSAEK graft.
Postoperative specular microscopy measurements of ECD were acquired at Devers Eye Institute using the Konan SP4000 noncontact specular microscope (Konan Medical Corp., Fairlawn, NJ). A certified ophthalmic technician (COT) performed all postoperative testing of patients using the same specular microscope each time.
These postoperative cell counts were obtained using the manufacturers calibrations for magnification and were counted with a fixed-frame method with the protocol requiring the marking of at least 50 to 100 cells for each image. Specular microscopy measurements with insufficient quality of the image were not included in the analysis. Insufficient quality of the image was determined subjectively by the examining physician based on the edge clarity of the individual cells. Approximately 8% of specular images are rejected in the authors clinical program, and approximately 10% of the time central specular images could not be obtained despite a crystal-clear graft. Clarity of the graft was not an issue with any rejected specular image. Analysis of endothelial pleomorphism and polymegathism was not performed in this study.
2.3. Surgical procedure
Phacoemulsification cataract extraction and intraocular lens implantation were done before endothelial replacement through the same 5 mm temporal scleral tunnel, but utilizing only a 2.8 mm keratome opening into the anterior chamber (AC). The scleral tunnel wound was then enlarged to 5.0 mm at the completion of the cataract surgery.
Descemets stripping automated endothelial keratoplasty was performed with a standard technique as previously published.[13,16] In most cases, the tissue was precut by an eye bank technician and then provided to the surgeon, usually within 26 hours of precutting.[16–18] DSAEK grafts were cut by the surgeon in the operating room for surgeries conducted prior to 11/2006. A video of the DSAEK technique using precut donor tissue is available online (https://www.youtube.com/watch?v=mtu8dxZUCx0) from a previous report on the use of precut tissue in DSAEK.
In brief, the standard DSAEK procedure involves placement of 2 limbal 1-mm wide paracentesis incisions on either side of a 5-mm temporal scleral access wound. This wound was placed approximately 0.5 mm peripheral to the temporal limbus with a scleral–corneal tunnel of approximately 2 mm until entry into the anterior chamber. After filling the anterior chamber with cohesive viscoelastic, a circular template mark was placed on the surface of the cornea to delineate the area of stripping, and this determines the same size for the donor tissue. The donor diameter chosen was individualized for each patient and was based on the largest diameter circle that can be fit on the individual cornea without overlap of the edge of the tissue over the 2 paracentesis sites or the corneal portion of the main insertion wound. After stripping of the recipient Descemets membrane and scraping of the recipient bed with a Terry Scraper (Bausch & Lomb Surgical, St. Louis, MO), the Healon (Abbott Medical Optics, Santa Ana, CA) viscoelastic was removed from the eye with standard automated irrigation-and aspiration technique. The pupil was constricted with Miochol-E (Novartis Pharmaceuticals Corporation, Basel, Switzerland), the microscope was moved to the donor table, and the donor tissue was trephined with the same size diameter as the recipient template mark. After preparation, the donor tissue was grasped and then inserted with noncoapting Charlie II insertion forceps (Bausch &Lomb Surgical) in a taco configuration with the 60% edge placed anteriorly in the chamber. The tissue was unfolded with deepening of the anterior chamber with balanced salt solution and injection of air to complete unfolding of the tissue into position. After surface sweeping with the Cindy Sweeper (Bausch & Lomb Surgical) to remove interface fluid while the anterior chamber was filled with air, the procedure was completed with complete removal of the air from the chamber and then reinjection of a specified amount of air to yield only a 5 or 6 mm residual bubble for minimal graft support. This small air bubble is preferred to avoid the event of pupillary block that can occur with larger air-bubble retention at the conclusion of surgery.
Surgery was performed by 11 corneal surgeons, 3 senior surgeons and 8 surgeons-in-training, at Devers Eye Institute. Senior surgeons performed 62% (149 eyes) and surgeons-in-training performed 38% (92 eyes) of the cases included in the study, with senior surgeon MAT performing 53% (127 eyes) of the cases. All surgeons used the exact same surgical technique for every case.
2.4. Statistical analysis
Postoperative ECD and percentage ECL was recorded at 5 years and compared to the preoperative ECD. Mean ECD and percentage ECL were compared for eyes with DSAEK only procedures and eyes with the triple procedure using a two-sided Students t test. Correlation analysis was performed where appropriate using a Pearson correlation test. A P value of ≤.05 was determined to be significant.
3. Results
The 241 eyes in this study were from 163 patients, with a mean age at surgery of 68 years (range 47–88 years old). One hundred seventy nine (74%) eyes were from females. All surgeries were performed for endothelial dysfunction due to Fuchs endothelial dystrophy. One hundred eighty two eyes had undergone a triple procedure and 59 had not. Of the non-triple eyes, 6 eyes received a phakic DSAEK, the rest of the non-triple surgeries were psuedophakic. Four of the phakic eyes did not have any follow-up, 1 went on to have cataract surgery and IOL implantation 1 year after DSAEK surgery, and 1 eye remained phakic through the 5 year postoperative period.
Overall mean DSAEK graft thickness was 164.4 ± 31.7 μm (range: 87–265 μm). Mean triple and non-triple DSAEK graft thicknesses were not statistically different (162.6 ± 31.5 μm vs 169.6 ± 32.1 μm, P = .17). There were 42 out of the 241 surgeries that did not have a reported graft thickness (36 eyes from the triple group 6 eyes from the non-triple group) because the tissue was cut by the surgeon in the OR and did not have post-cut OCT measurements.
Of the original 986 eyes, graft failures in the first 5 years postoperative (primary graft failure or late endothelial failure) were found in 7 eyes in the triple group and 4 eyes in the non-triple group.
Postoperative graft dislocations requiring a re-bubble procedure was found in 3 triple eyes and in 10 non-triple eyes. Only 1 re-bubbled eye had 5 year ECD data available.
Patient demographics did not differ between groups with respect to gender or surgeon experience.
The mean ECD and percentage ECL at 5 years postoperative for all 241 eyes was 1541 ± 641 cells/mm2 (44.4% ± 21.7% ECL) (Table 1). There was a significant correlation between preoperative ECD and the postoperative ECD at 5 years for all 241 eyes. (r = 0.39, P < .001).
Table 1.
Summary of mean preoperative ECD and 5-year postoperative ECD, for DSAEK triple, DSAEK non-triple, and combined 241 eyes.
| Preop ECD (cells/mm2) | 5-Year Postop ECD (cells/mm2) | Pearson r | P value | |
| Triples (n = 182) | 2792 ± 354 | 1560 ± 648 | 0.39 | <.001 |
| Non-Triples (n = 59) | 2653 ± 280 | 1483 ± 621 | 0.32 | .01 |
| All Eyes (n = 241) | 2758 ± 342 | 1541 ± 641 | 0.39 | <.001 |
No significant difference between the type of procedure (triple vs non-triple procedure) and the percentage ECL was found. The mean ECD at 5 years was 1560 ± 648 cells/mm2 for triple procedures and 1483 ± 621 cells/mm2 for DSAEK alone procedures (P = .42, Fig. 1 and Table 1). Endothelial cell loss at 5 years was 44.4% ± 21.7% and 44.4% ± 22.0%, respectively for eyes that underwent a triple or non-triple procedure (P = .99). There was a moderate, but significant correlation between preoperative ECD and the postoperative ECD at 5 years after DSAEK for both triple (r = 0.39, P < .001), and non-triple (r = 0.32, P = .01), respectively (Fig. 2A, B).
Figure 1.

5-year postoperative ECD results in DSAEK triple vs non-triple surgeries t test comparing ECD for triple vs non-triple was not statistically significant, P = .42.
Figure 2.
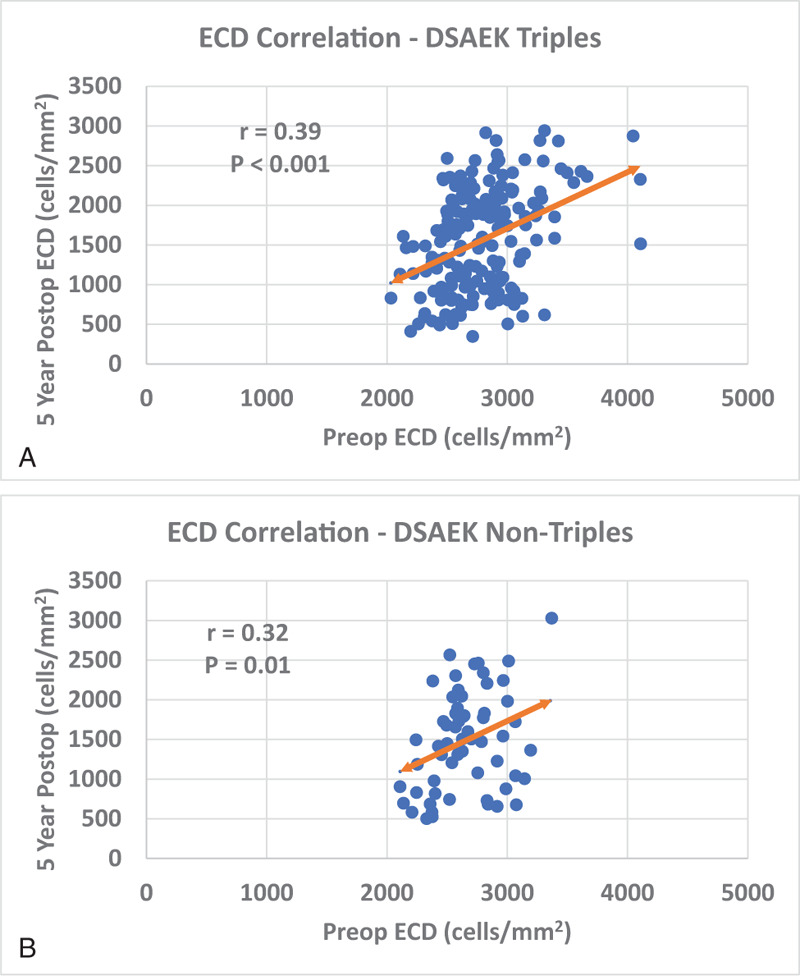
A, B: Preoperative ECD vs 5-year postoperative ECD Pearson correlation for DSAEK triple and non-triple procedures. Preoperative and 5-year postoperative ECD had a statistically significant correlation in both DSAEK triple and DSAEK non-triple groups (P < .001, P = .01, respectively).
4. Discussion
This study has shown that higher preoperative ECD in DSAEK was significantly correlated to higher ECD at 5 years postoperatively. It has also shown that the ECD was not significantly affected by a concurrent cataract surgery in triple procedures.
Cataract and Fuchs dystrophy are 2 eye pathologies that are frequently encountered concomitant in the elderly, and it has been well established that corneal grafting (Penetrating Keratoplasty (PK) or EK) accelerates cataract formation.[9,10,19,20] This is related to the intraoperative manipulation and the steroid use postoperatively.[18] This rate has been shown to be maximum after the age of 50.[9] On the other hand, cataract extraction is a major risk factor for corneal decompensation in eyes with Fuchs dystrophy.[21] For these reasons, triple procedures (phacoemulsification+ IOL+ EK) have often been recommended for the management of patients with both mild cataract and corneal endothelial dysfunction. The complication rate and postoperative ECD for triple procedures compared to the DSAEK alone cases have not been shown to be different for short-term periods (6 months and 12 months) or longer-term follow-up of 3 to 5 years.[12–14] The 5 year ECL in our study (44.4%) was comparable to the 3 year ECL in the CPTS for the Fuchs dystrophy subgroup (44%).[12] The Price et al study reported a higher 5 year ECL at 53% compared to our study.[22] In our study here with a longer follow-up time than the CPTS and over 200% more analyzed eyes than prior single center studies,[22] we have confirmed that the triple procedure is comparable to DSAEK alone surgery at 5 years postoperatively. Therefore, it is recommended that surgeons not perform sequential surgery of phacoemulsification followed weeks later by endothelial keratoplasty in the setting of significant cataract and endothelial disease. These dual pathologies should be remedied in a single setting operation.
Corneal transplant surgeons often have a bias toward wanting donor tissue with a high preoperative endothelial cell density. The CPTS study analyzed 913 clear grafts at 3 years postoperatively and found that a lower preoperative ECD was significantly associated with a lower ECD at 3 years.[12] Our study confirms that finding with follow-up at a longer time of 5 years postoperatively in 241 eyes of strictly Fuchs dystrophy eyes and with a uniform surgical technique at a single center. Interestingly, a higher postoperative ECD did not influence graft survival in the CPTS study and eyes with a higher preoperative ECD did not have an advantage for a greater 3 year graft survival rate.[15] The relationship between ECD and graft survival over an extended postoperative time warrants further study. Our report also confirms findings from a Price group study in which they found a weak but still significant correlation between the preoperative ECD and the 5 years postoperative ECD.[22] We believe that having higher number of eyes showed more significance to the correlation. Although some previous reports have shown no significant correlation between preoperative and postoperative ECD at the short-term postoperative time points,[23,24] more reports have shown that preoperative ECD is significantly correlated with the medium-long term postoperative ECD.[15,22,24] This could be related to the fact that endothelial cells tend to expand and migrate to cover areas of endothelial loss and damage in a slow process that may show its effect over a longer period of time.
The strengths of this study are that it provides a longer period of follow-up (5 years) than the CPTS study (3 years). This study is also the largest single-center study of DSAEK at 5 years with 241 eyes analyzed vs 95 eyes in a prior study.[22] Another strength of the study is that the exact same surgical technique was used for all of the surgeries as opposed to the CPTS where multiple surgical techniques by over 60 surgeons were used. The weakness of the study is that, although the data was collected prospectively, it is an unmasked, retrospective study at a single center and may not be applicable to surgeons using a different surgical technique. Also, a large portion of the 986 consecutive surgeries did not have 5-year postoperative ECD measurements due to being lost to follow-up. We are aware that only 24% of the original consecutive surgeries had 5 years ECD data available and how those missing data points could affect the mean ECD and ECL values. In order to determine that the 5 year ECD values in our sample size of 241 eyes was representative of the entire series of 986 consecutive surgeries, we recorded the number of rejection episodes, 6 month ECL, and 6 month ECD in eyes that had 5 year ECD data and in eyes that did not have 5 year ECD data. Rejection episodes can negatively influence the long-term ECD in patients receiving DSAEK for Fuchs dystrophy. A previous study from our group has shown a statistically significant increase in 2 and 3 year postoperative ECL in eyes experiencing a rejection episode compared to eyes that did not have a rejection.[25] Of the 986 consecutive DSAEK surgeries in this study, 66 eyes experienced a rejection episode within 5 years of surgery. 35 of those 66 eyes had 5 year ECD data.
The CPTS has reported that ECD and ECL at 6 months is associated with late endothelial graft failure (LEGF) up to 5 years postoperative in DSAEK (P < .001).[26] The authors reported a LEGF rate of 6.5% for grafts with 6 month ECD less than 1200 cells/mm2. In our study we found comparable mean 6 month ECL (P = .062) and 6 month ECD (P = .59) in eyes with vs without 5 year ECD, 25.7% ± 16.3% (2050 ± 427 cells/mm2, n = 213) and 23.2% ± 16.1% (2031 ± 433 cells/mm2, n = 484), respectively.
Without having a graft survival analysis of all 986 consecutive surgeries, we believe comparing the 6 month ECL, 6 month ECD, and the number of eyes having a rejection episode is a surrogate way to assess the reliability of our ECL data when only 24% had 5 year data available.
The data from this study indicate that a triple procedure of DSAEK with cataract extraction and intraocular lens insertion should be performed in cases of clinically significant Fuchs corneal dystrophy and cataract. Cataract surgery and DSAEK should not be done as separate procedures sequentially. Furthermore, the preoperative ECD does influence the postoperative ECD after DSAEK at 5 years, but this does not indicate at this time that DSAEK graft survival is dependent upon preoperative ECD.
Author contributions
Conceptualization: Asem A Alqudah.
Data curation: Asem A Alqudah.
Formal analysis: Asem A Alqudah.
Investigation: Asem A Alqudah.
Methodology: Asem A Alqudah.
Project administration: Asem A Alqudah.
Software: Asem A Alqudah.
Supervision: Asem A Alqudah.
Validation: Asem A Alqudah.
Visualization: Asem A Alqudah.
Writing – original draft: Asem A Alqudah.
Writing – review & editing: Asem A Alqudah.
Footnotes
Abbreviations: ECD = endothelial cell density, DSAEK = Descemet Stripping Automated Endothelial Keratoplasty, ECL = Endothelial Cell Loss, EK = Endothelial Keratoplasty, PLK = Posterior Lamellar Keratoplasty, DLEK = Deep Lamellar Endothelial Keratoplasty, DSEK = Descemet Stripping Endothelial Keratoplasty, DMEK = Descemet Membrane Endothelial Keratoplasty, DMAEK = Descemet Membrane Automated Endothelial Keratoplasty, IOL = intraocular lens, CPTS = Cornea Preservation Time Study, PCIOL = posterior chamber intraocular lens, COT = certified ophthalmic technician, AC = anterior chamber, PK = Penetrating Keratoplasty, LEGF = late endothelial graft failure.
How to cite this article: Alqudah AA, Bauer AJ, Straiko MD, Sanchez PJ, Terry MA. Descemet Stripping Automated Endothelial Keratoplasty: the influence of preoperative endothelial cell density and triple procedures on grafts at 5 years postoperative. Medicine. 2020;99:45(e23139).
General research support is provided by Lions VisionGift, Portland, OR. But no specific funding support was obtained for this article.
Mark A. Terry has received royalties from Bausch and Lomb Surgical on the DSAEK instruments which he designed. Terry has also received unrestricted educational grants from both Bausch and Lomb Surgical and Moria for a surgeons breakfast educational program annually at the AAO meeting.
None of the other authors have relevant financial acknowledgements regarding this manuscript content.
General research support provided by Lions VisionGift, Portland, OR.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The Pearson correlation r comparing preoperative ECD and 5-year postoperative ECD for triple (r = 0.39), non-triple (r = 0.32), and combined (r = 0.39) groups is statistically significant (P < .001, P = .01, and P < .001).
ECD = Endothelial Cell Density, DSAEK = Descemet Stripping Automated Endothelial Keratoplasty.
References
- [1].Melles GR, Eggink FA, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea 1998;17:618–26. [DOI] [PubMed] [Google Scholar]
- [2].Melles GR, Lander F, Beekhuis WH, et al. Posterior lamellar keratoplasty for a case of pseudophakic bullous keratopathy. Am J Ophthalmol 1999;127:340–1. [DOI] [PubMed] [Google Scholar]
- [3].Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty in the first United States patients: early clinical results. Cornea 2001;20:239–43. [DOI] [PubMed] [Google Scholar]
- [4].Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea 2006;25:886–9. [DOI] [PubMed] [Google Scholar]
- [5].Melles GR, Ong TS, Ververs B, et al. Descemet membrane endothelial keratoplasty (DMEK). Cornea 2006;25:987–90. [DOI] [PubMed] [Google Scholar]
- [6].McCauley MB, Price FW, Jr, Price MO. Descemet membrane automated endothelial keratoplasty: hybrid technique combining DSAEK stability with DMEK visual results. J Cataract Refract Surg 2009;35:1659–64. [DOI] [PubMed] [Google Scholar]
- [7].Pereira Cda R, Guerra FP, Price FW, Jr, et al. Descemet's membrane automated endothelial keratoplasty (DMAEK): visual outcomes and visual quality. Br J Ophthalmol 2011;95:951–4. [DOI] [PubMed] [Google Scholar]
- [8].Eye Bank Association of America. 2016 Eye Banking Statistical Report. Washington D.C: Eye Bank Association of America; 2017. [Google Scholar]
- [9].Price MO, Price DA, Fairchild KM, et al. Rate and risk factors for cataract formation and extraction after Descemet stripping endothelial keratoplasty. Br J Ophthalmol 2010;94:1468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sati A, Ramappa M, Chaurasia S. Cataract following endothelial keratoplasty (EK) in a child. Med J Armed Forces India 2013;69:398–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sharma N, Singhal D, Nair SP, et al. Corneal edema after phacoemulsification. Indian J Ophthalmol 2017;65:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lass JH, Benetz BA, Patel SV, et al. Cornea Preservation Time Study Group. Donor, Recipient, and Operative Factors Associated With Increased Endothelial Cell Loss in the Cornea Preservation Time Study. JAMA Ophthalmol. 2019 Feb 1;137(2):185-193. doi: 10.1001/jamaophthalmol.2018.5669. Erratum in: JAMA Ophthalmol. 2019 Feb 1;137(2):233. PMID: 30422157; PMCID: PMC6439830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty for Fuchs’ dystrophy with cataract: complications and clinical results with the new triple procedure. Ophthalmology 2009;116:631–9. [DOI] [PubMed] [Google Scholar]
- [14].Jones SM, Fajgenbaum MA, Hollick EJ. Endothelial cell loss and complication rates with combined Descemets stripping endothelial keratoplasty and cataract surgery in a UK centre. Eye (Lond) 2015;29:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Terry MA, Aldave AJ, Szczotka-Flynn LB, et al. Donor, recipient, and operative factors associated with graft success in the cornea preservation time study. Ophthalmology 2018;125:1700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology 2008;115:1179–86. [DOI] [PubMed] [Google Scholar]
- [17].Chen ES, Terry MA, Shamie N, et al. Precut tissue in Descemet's stripping automated endothelial keratoplasty donor characteristics and early postoperative complications. Ophthalmology 2008;115:497–502. [DOI] [PubMed] [Google Scholar]
- [18].Terry MA, Shamie N, Chen ES, et al. Precut tissue for Descemet's stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survival. Ophthalmology 2009;116:248–56. [DOI] [PubMed] [Google Scholar]
- [19].Rathi VM, Krishnamachary M, Gupta S. Cataract formation after penetrating keratoplasty. J Cataract Refract Surg 1997;23:562–4. [DOI] [PubMed] [Google Scholar]
- [20].Tsui JY, Goins KM, Sutphin JE, et al. Phakic descemet stripping automated endothelial keratoplasty: prevalence and prognostic impact of postoperative cataracts. Cornea 2011;30:291–5. [DOI] [PubMed] [Google Scholar]
- [21].Seitzman GD. Cataract surgery in Fuchs’ dystrophy. Curr Opin Ophthalmol 2005;16:241–5. [DOI] [PubMed] [Google Scholar]
- [22].Price MO, Fairchild KM, Price DA, et al. Descemet's stripping endothelial keratoplasty five-year graft survival and endothelial cell loss. Ophthalmology 2011;118:725–9. [DOI] [PubMed] [Google Scholar]
- [23].Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty: the influence of preoperative donor endothelial cell densities on dislocation, primary graft failure, and 1-year cell counts. Cornea 2008;27:1131–7. [DOI] [PubMed] [Google Scholar]
- [24].Lekhanont K, Vanikieti K, Nimvorapun N, et al. Outcomes of Descemet stripping automated endothelial keratoplasty using imported donor corneas. BMC Ophthalmol 2017;17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li JY, Terry MA, Goshe J, et al. Graft rejection after Descemet's stripping automated endothelial keratoplasty: graft survival and endothelial cell loss. Ophthalmology 2012;119:90–4. [DOI] [PubMed] [Google Scholar]
- [26].Patel SV, Lass JH, Benetz BA, et al. Postoperative endothelial cell density is associated with late endothelial graft failure after Descemet stripping automated endothelial keratoplasty. Ophthalmology 2019;126:1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


