Abstract
Dental general anesthesia (DGA) is a safe and high-quality restorative and preventive treatment option for children with severe early childhood caries (S-ECC), who require extensive dental treatment and exhibit anxiety and emotional or cognitive immaturity or are medically compromised. However, several postoperative complications have been reported in children under DGA. This study aimed to evaluate and analyze the prevalence of the relevant factors of postoperative complications in healthy Chinese children following DGA to provide a foundation for pre-, intra-, and postoperative overall health management for healthy and disabled children after DGA.
A total of 369 systematically healthy Chinese children (36–71 months old) undergoing a DGA were studied. Data were collected on patients’ histories, characteristics, anesthesia, and dental procedures. Parents or caregivers were interviewed before and 72 hours after the procedure. Data were analyzed using logistic regression.
Approximately 94.86% of the enrolled children reported one or more complications. The most prevalent complication was postoperative pain (62.70%), followed by weariness, agitation, masticatory problems, drowsiness, oral bleeding, coughing, fever, sore throat, nausea, constipation, epistaxis, vomiting, excitement, and diarrhea. The long duration of the operation was a risk factor for postoperative pain and weariness. A high nutritional status could be a protective factor for postoperative fever.
Prolonged operation means complex treatment, such as pulp therapy or extraction. We speculate that the longer the duration is, the more difficult the dental procedures are. The accumulation of discomfort leads to pain. We suspect that children in lower nutritional levels are more likely to suffer from bacteremia or dehydration, resulting in fever.
Postoperative pain was the most prevalent complication after the DGA. A decrease in dental procedure duration might reduce the odds of postoperative pain and weariness. A high nutritional status could be a protective factor for postoperative fever. Children with low nutritional status could be more susceptible to postoperative fever.
Keywords: dental general anesthesia, fever, postoperative pain, severe early childhood caries, weariness
1. Introduction
Nowadays, early childhood caries (ECC) remains a significant challenge of public health in Chinese children. According to the results of the 4th National Oral Health Epidemiological Survey in mainland China, the ECC prevalence rates in children aged 3, 4, and 5 years were 50.8%, 63.6%, and 71.9%, respectively. However, the constituent ratios of filled teeth were only 1.5%, 2.9%, and 4.1%, respectively.[1] In most cases, children with ECC could accept dental treatment under non-pharmacological behavior management or sedation. However, some very young children or those suffering severe anxiety, mental or physical disabilities, could only be treated under dental general anesthesia (DGA).
DGA is a day-stay, general anesthesia procedure and was first brought to mainland China in 1999, becoming widely accepted in recent years.[2] Compared to outpatient treatments in pediatric dentistry, DGA for children is the most effective option to provide complicated and high-quality dental treatments in one appointment, such as treating dental caries and its complications, and extraction of supernumerary teeth, frenotomy, and the like. It can relieve the distress of patients, parents, and dentists. It is cost-effective for dental institutions and minimizes the economic burden for children's families. After the DGA, the morphology and masticatory function recover in most carious teeth. The oral micro-ecological environment is balanced, and children's caries risk and dental anxiety decrease.[3]
However, children could experience several postoperative complications following a DGA procedure, as reported in previous studies.[4–7] For a day-stay, general anesthesia procedure to be an acceptable option, care during and after surgery must be of the highest quality, and postoperative morbidity must be minimized. Postoperative instructions and predictive maintenance should be communicated with parents in detail. For the sake of predicting, controlling, and reducing postoperative complications, decreasing parents’ anxiety, and improving the quality of medical care, it is essential to analyze and find the relevant factors for postoperative complications and provide a foundation for peri- and intraoperative health management of healthy and disabled children under DGA.
2. Materials and methods
2.1. Subject selection
This prospective study was approved by the West China Hospital of Stomatology Ethics Committee (WCHSIRB-D-2019-085). Chinese children scheduled for DGA in the Department of Pediatric Dentistry, West China Hospital of Stomatology, Sichuan University, from July 2017 to October 2019, were enrolled in this study. Children's demographic characteristics, including birth date, gender, height, and weight, were collected. The history of systemic diseases and allergies and oral history were obtained. Oral conditions, teeth, and dentitions had been checked by pediatric dentists thoroughly before planning the DGA.
The pediatric dentist and dental assistants would communicate with parents about the status of their children's oral health, the individualized treatment plan, the procedures of DGA, and the possible prognosis preoperatively. The legal guardians of children signed all the written informed consent forms. Children were included if they:
-
1)
were not younger than 36 months and older than 72 months;
-
2)
suffered from severe early childhood caries (S-ECC);
-
3)
were classified as American Society of Anesthesiologists Physical Status Class I.
However, the children whose guardians or caregivers had a problem in communicating with the medical staff, or refused postoperative follow-up, or did not complete the follow-up questionnaire, were excluded.
2.2. DGA procedure
The general anesthesia procedure was standardized to avoid confounding variables. Preoperative fasting and water-deprivation were 6 hours. Upon entering the operative room, the patients were provided with standard monitors by the anesthesiologist, who had at least five years of experiences in children's general anesthesia until the general anesthesia ended; the heart rate, breathing rate, non-invasive blood pressure, electrocardiogram, oxygen saturation, end-tidal carbon dioxide, and temperature were monitored. Sevoflurane inhalation (3%–8% in 2 L/min oxygen) was used to induce anesthesia. Once the eyelash reflex disappeared, intravenous access was established, and cisatracurium, propofol, and sulfentanyl were injected. Nasotracheal intubation was performed on all the patients. The combination of sevoflurane and propofol was used to maintain anesthesia. A throat gauze pack was applied to prevent the aspiration of the secretions and dental materials in every patient before surgery. Dental treatment was provided by one certified pediatric dentist and three dental assistants under the Guidelines of the American Academy of Pediatric Dentistry (AAPD).[8,9] During the dental treatment, a rubber dam was applied, if possible. Local anesthesia was applied before extractions and pulp therapy. After the surgery, the tracheal cannula was extubated when patients’ consciousness, respiration, and swallowing and cough reflexes were re-established. The patients were then transferred to the post-anesthesia care unit. Monitoring and oxygen supplementation continued until the patients reached the discharge standard. Before discharge, the anesthetists provided the guardians or caregivers with standardized post-anesthesia advice. Pediatric dentists informed them of the dental procedures performed and provided oral hygiene instructions. Anesthetists and pediatric dentists completed medical records, including anesthesia data (starting and ending time, dose and time of anesthetics used, and vital signs). Table 1 lists the dental treatments provided (restorations, pulp therapies, extractions, and the like).
Table 1.
Dental procedures.
| Procedures | Proportion of Children Received this Treatment | Average Treated Teeth |
| Pulp therapy-primary teeth | 98.92% | 6.05 ± 3.27 |
| Pulpectomy or root canal therapy-primary anterior teeth | 83.20% | 3.20 ± 2.30 |
| Pulpectomy or root canal therapy-primary molars | 81.84% | 2.85 ± 2.11 |
| Pulpotomy-primary molars | 63.41% | 1.49 ± 1.56 |
| Sum of extracted teeth | 58.92% | 1.63 ± 1.98 |
| Extraction- primary anterior teeth | 43.90% | 1.05 ± 1.51 |
| Extraction- primary molars | 31.98% | 0.53 ± 0.93 |
| Extraction-supernumerary teeth | 3.52% | 0.04 ± 0.26 |
| Stainless steel crown--primary molars | 99.7% | 7.20 ± 1.26 |
2.3. Preoperative and postoperative survey
Before the dental treatment, the investigator explained the postoperative complications questionnaire and Face Pain Scale-Revised (FPS-R) tool (Fig. 1) to the guardians or caregivers.[10] The questionnaire, consisting of postoperative complications shown in Table 2, was surveyed via SO JUMP on the third day after discharge. The patients were guided by their guardians or caregivers to complete the FPS-R 2, 12, 24, 48, and 72 hours after discharge if awake, and if not, complete it until they were awake. Children scoring >4 were advised to take ibuprofen dosed according to the manufacturer's guidelines.
Figure 1.
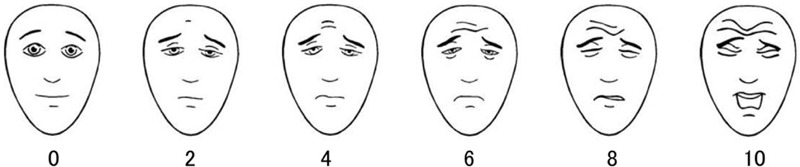
Face Pain Scale-Revised (These faces show how much something can hurt. The face [point to left-most face] shows no pain. The faces show more and more pain [point to each from left to right] up to this one [point to right-most face]. It shows very much pain. Point to the face that shows how much you hurt).
Table 2.
Incidence of the post-operative complications.
| Post-operative complications | Incidence |
| Post-operative pain | 62.7% |
| Weariness | 47.57% |
| Agitation | 45.14% |
| Mastication problem | 42.43% |
| Drowsiness | 36.49% |
| Oral bleeding | 30.27% |
| Fever | 27.03% |
| Cough | 27.57% |
| Sore throat | 7.9% |
| Nausea | 9.46% |
| Constipation | 9.19% |
| Epistaxis | 7.84% |
| Vomiting | 7.57% |
| Excitement | 5.68% |
| Diarrhea | 2.7% |
Pilot testing was conducted in 10 patients and their guardians to assure it is understandable and acceptable to the participants. Neither the questions nor the answers were modified after the pilot study.
2.4. Statistical analysis
Data on demographic variables, oral treatments, the prevalence of postoperative complications, and the peak score of FPS-R were analyzed with SPSS 21.0 (IBM, USA). Univariate analysis of the potential risk factors for postoperative complications was made with chi-squared test and independent-sample t-test. The significant variables (P < .1) were then entered into a multivariable logistic regression model with a .05 two-sided significance level.
3. Results
3.1. General information
Finally, 369 healthy Chinese children who met the inclusion criteria and finished the survey were recruited for this study, including 214 boys and 155 girls. The average age was 4.38 ± 0.77 years; 56.22% of the patients experienced gingival abscesses, sinus tracts, or fistulae before DGA.
3.2. Dental treatments under GA
The average duration of the procedure was 124.84 ± 28.09 minutes. The average number of treated teeth was 16.46 ± 3.26. All the patients underwent restorative procedures, 98.92% received pulp therapy, and 58.92% had one or more of their teeth extracted (Table 1).
3.3. Analysis of postoperative complications
Overall, 94.86% of the patients reported at least one type of postoperative discomfort in the first 72 hours after surgery. Three postoperative complications with the highest prevalence rates were postoperative pain, weariness, and agitation, and three with the lowest rates were diarrhea, excitement, and vomiting (Table 2). The median peak score of FPS-R was 4 (0, 6); 36.7% of children scored >4.
Univariate analysis was performed to evaluate the relevant factors of the most common postoperative complications (Table 3). The variables of P < .1 were included in multivariable logistic regression analysis. The results showed that operation duration was a risk factor for postoperative pain (OR = 1.010, 95% CI: 1.002–1.019) and weariness (OR = 1.011, 95% CI: 1.003–1.018). A high nutrition level is a protective factor for postoperative fever (OR = 0.449, 95% CI: 0.281–0.718) (Tables 4–6).
Table 3.
Univariate analysis of related factors of post-operative pain, weariness and fever.
| Post-operative complications | ||||||
| Variables | Pain | No pain | Weariness | No weariness | Fever | No fever |
| Male | 87 (23.58%)∗ | 127 (34.42%)∗ | 100 (27.10%) | 114 (30.89%) | 57 (15.45%) | 157 (42.55%) |
| High nutrition level | 78 (21.14%) | 119 (32.25%) | 98 (26.56%) | 99 (26.83%) | 39 (10.57%)∗ | 158 (42.82%)∗ |
| Gingival abscess, sinus or fistula before therapy | 78 (21.14%) | 130 (35.23%) | 98 (26.56%) | 110 (29.81%) | 52 (14.09%) | 156 (42.28%) |
| Age | 52.31 ± 9.04 | 52.28 ± 9.63 | 52.86 ± 9.21 | 52.49 ± 9.32 | 51.77 ± 9.60 | 53.00 ± 9.13 |
| Duration of fasting | 759.66 ± 92.22 | 746.75 ± 85.32 | 755.63 ± 88.81 | 754.17 ± 90.96 | 750.60 ± 91.73 | 756.45 ± 89.22 |
| Duration of procedure | 128.49 ± 27.00∗ | 118.65 ± 28.91∗ | 129.06 ± 26.78∗ | 120.98 ± 28.77∗ | 125.75 ± 30.05 | 124.50 ± 27.38 |
| Sum of treated teeth | 16.82 ± 3.04∗ | 15.85 ± 3.52∗ | 16.88 ± 3.24∗ | 16.08 ± 3.23∗ | 16.55 ± 3.18 | 16.42 ± 3.29 |
| Sum of extracted teeth | 1.62 ± 1.96 | 1.64 ± 1.95 | 1.73 ± 2.04 | 1.53 ± 1.88 | 1.42 ± 1.64 | 1.70 ± 2.06 |
| Pulpectomy or root canal therapy-primary teeth | 6.35 ± 3.19∗ | 5.58 ± 3.33∗ | 6.49 ± 3.32∗ | 5.64 ± 3.17∗ | 6.04 ± 3.50 | 6.05 ± 3.18 |
| Pulpotomy-primary molars | 1.56 ± 1.64 | 1.38 ± 1.41 | 1.61 ± 1.55 | 1.38 ± 1.57 | 1.65 ± 1.56 | 1.44 ± 1.56 |
| Stainless steel crown-primary molars | 7.2 ± 1.28 | 7.20 ± 1.22 | 7.32 ± 1.08∗ | 7.09 ± 1.39∗ | 7.30 ± 1.19 | 7.16 ± 1.28 |
| Dose of propofol (mg/kg) | 1.51 ± 0.76 | 1.51 ± 0.93 | 1.43 ± 0.83∗ | 1.57 ± 0.82∗ | 1.58 ± 0.80 | 1.48 ± 0.84 |
| Dose of cisatracurium (mg/kg) | 0.13 ± 0.04 | 0.14 ± 0.18 | 0.13 ± 0.04 | 0.14 ± 0.15 | 0.13 ± 0.03 | 0.14 ± 0.13 |
| Dose of sufentanil (ug/kg) | 0.26 ± 0.13 | 0.25 ± 0.12 | 0.26 ± 0.12 | 0.25 ± 0.13 | 0.25 ± 0.13 | 0.26 ± 0.13 |
Table 4.
Multiple logistic regression analysis of related factors of post-operative pain.
| Variables | β | SD (β) | WALD | P value | Odds Ratio | 95% confidence interval |
| Male | −0.308 | 0.226 | 1.863 | .172 | 0.735 | 0.472∼1.144 |
| Duration of procedure∗ | 0.010 | 0.004 | 5.946 | .015 | 1.010 | 1.002∼1.019 |
| Sum of treated teeth | 0.062 | 0.036 | 2.983 | .084 | 1.063 | 0.992∼1.140 |
| Pulpectomy or root canal therapy-primary teeth | 0.001 | 0.043 | 0.001 | .977 | 1.001 | 0.920∼1.090 |
Table 6.
Multiple logistic regression analysis of related factors of post-operative fever.
| Variables | β | SD (β) | WALD | P value | Odds Ratio | 95% confidence interval |
| High nutrition level∗ | −0.800 | 0.240 | 11.166 | .001 | 0.449 | 0.281∼0.718 |
Table 5.
Multiple logistic regression analysis of related factors of post-operative weariness.
| Variables | β | SD (β) | WALD | P value | Odds Ratio | 95% confidence interval |
| Duration of procedure∗ | 0.010 | 0.004 | 7.475 | .006 | 1.011 | 1.003∼1.018 |
| Sum of treated teeth | 0.052 | 0.035 | 2.214 | .137 | 1.053 | 0.984∼1.128 |
| Pulpectomy or root canal therapy-primary teeth | 0.030 | 0.042 | 0.506 | .477 | 1.030 | 0.949∼1.118 |
| Stainless steel crown—primary molars | 0.132 | 0.088 | 2.243 | .134 | 1.142 | 0.960∼1.358 |
| Dose of propofol (mg/kg) | −0.162 | 0.131 | 1.535 | .215 | 0.850 | 0.658∼1.099 |
4. Discussion
Although cooperation in pediatric dental clinics can be achieved in most young patients through non-pharmacological dental behavior management techniques and sedation, there are limitations in applying these to all children. The primary reasons for healthy children to undergo DGA were S-ECC and dental anxiety or phobia. General anesthesia could be the best option to enable quality medical care by reducing the number of hospital visits and providing psychological security to patients, guardians, and dentists, especially for children with disabilities. All the children enrolled in this study reported at least one postoperative complication. However, severe complications were not reported.
4.1. Postoperative pain
Postoperative pain was the most prevalent complaint, with a prevalence of 62.70% in the present study. Not all the patients complaining of pain had severe pain, and 36.7% of children exhibited FPS-R scores >4. Earlier studies found that the incidence of postoperative pain ranged from 36% to 95%.[4–6,11–14] Significant differences between studies might be attributed to differences in enrolled subjects, dental procedures, use of local anesthesia, or pain assessment tools. Pain is a subjective phenomenon that varies from person to person. The gold standard to assess pain is self-report of pain. Considering the limited ability of young patients to express themselves, with a mean age of 4.38 ± 0.77 years in the present study, we employed FPS-R, validated for postoperative pain assessment in 4 to 10-year-old children.[10] Different results might have been obtained if children under six years of age had been selected. However, a study of this nature would probably require a different set of outcome variables that do not rely as heavily on individual self-reports. This could be the subject of further investigations. Atan et al reported an incidence of 95% of their patients (2–10 years old) using Faces, Legs, Activity, Cry, and Consolability scale (FLACC scale).[5] Nevertheless, the FLACC scale has been validated to assess postoperative pain in children between the ages of two months and seven years.[10]
The results of this study showed that the duration of the procedure was significantly related to postoperative pain. Long duration means rendering complex treatments, such as pulp therapy or extractions. We speculate that the longer the duration is, the more difficult the procedure is. The accumulation of discomfort causes the child to feel pain. It is suggested that it is necessary to achieve a detailed preoperative evaluation, careful consideration, and close cooperation among the anesthetists, dentists, and nurses to minimize the length of surgery and reduce the incidence and severity of postoperative pain. The male gender was a protective factor for postoperative pain in univariate analysis. However, there was no statistical difference in multiple logistic regression analysis in this study. It is speculated that postoperative pain is affected by social, psychological, and other factors in children.[15] Ersin et al reported that pain was significantly more common in the group in which sevoflurane was used as an anesthetic agent than the halothane group.[13] In this study, sevoflurane was used for the induction and maintenance of anesthesia, and analgesics were not given before surgery for prophylaxis, which might be responsible for the relatively high incidence of postoperative pain reported. However, the multivariate analysis results did not show the effect of anesthetic procedures on postoperative pain, even when the children underwent standardized general and local anesthesia procedures.
4.2. Weariness and drowsiness
Overall, 47.57% and 36.49% of the patients felt weary and drowsy, respectively, close to the incidence reported by Needleman[11] (43%) but lower than that by Atan[5] and Faisi[16] (84% and 71%, respectively). It showed that the procedure duration was attributed to postoperative drowsiness in the present study. Needleman et al[11] reported that an increase in the procedure's duration is related to weariness. Atan et al[5] showed that weariness was related to the anesthetic time. For every 10-minute increase in anesthetic time, the patient had 15% increased odds of feeling weary. In addition, females and those undergoing local anesthesia were more likely to feel weary. The incidence of weariness reported by Enever[17] was only 13%, which might be attributed to the age of enrolled children (3–17 years old, 10.7 years old on average).
4.3. Fever
Fever (27.03%) was not included in the top three most common postoperative complications in the present study. However, it is the hot spot worrying the parents. The incidence of fever after DGA ranged from 1% to 50% in previous studies.[11,16,18–22] However, it ranged from 19.2% to 71% after head and neck and other maxillofacial operations in children.[23–25] Some factors can cause postoperative fever in children, including bacteremia, tissue trauma, dehydration, pulmonary atelectasis, drugs such as atropine, and environmental factors like room temperature and the draping of the patient during surgery. Multivariate analysis showed that low nutritional levels were a risk factor for postoperative fever. It is speculated that children with low nutritional levels appear hypoimmune and are more likely to develop postoperative fever due to bacteremia, soft and hard tissue damage, and dehydration. In the present study, most fever cases developed within 24 hours postoperatively, significantly decreasing between 24 and 72 hours postoperatively. None of the children developed a severe infection, which could be explained by the longer duration of children's preoperative fasting, regardless of their age and inability to eat postoperatively. Those 2 reasons might lead to children's postoperative dehydration and fever; even the preoperative fasting and water-deprivation time were expected for 6 hours in this study.
Clinical trials have already confirmed a strong correlation between dehydration and fever after general anesthesia.[26] Liang et al[23] reported that the method of anesthesia, duration of surgery, and duration of anesthesia were risk factors for postoperative fever in children following cleft lip and palate repair surgery. However, considering the differences in the surgical site, treatment method, and children's age, further studies are necessary to assess whether these factors affect fever after DGA in children with S-ECC. By considering the nutritional level and duration of anesthesia, the incidence of postoperative fever can be estimated. Parents were advised to improve the monitoring of children's body temperature after DGA and respond actively.
4.4. Other postoperative complications
Other discomforts also exhibited considerable incidence in this study; however, there was no significant difference in the factors according to the multiple logistic regression analysis.
Agitation is a common complication of general anesthesia.[27] The incidence of agitation after DGA was from 26% to 76%,[11,13] with 45.14% in the present study. As part of our DGA protocol, anesthesia was induced with sevoflurane in the patients, which we inferred to lead to agitation. A meta-analysis by Costi et al,[28] including 14045 children from 158 studies, showed that children in which sevoflurane was used as an inhalation anesthetic during general anesthesia were more likely to exhibit postoperative irritability compared to the halothane group. Studies by Xu et al[27] showed that children's low adaptability to environmental changes, hunger, and thirst caused by long-term fasting and drinking, and parent-child separation might all result in irritability after surgery. The young children aged 4.38 ± 0.77 years in this study might have had less tolerance for fasting and parent-child separation.
Overall, 42.43% of the enrolled children complained about masticatory problems in the first 3 days after DGA, which was lower than 53.8% and 86% reported by Cantekin et al[20] and Farsi et al,[16] respectively. However, the parents reported that nearly all the masticatory problem disappeared in about one week. The average number of stainless steel crowns (SSC) for each child was about 7.2 in this study. Almost all the primary molars of S-ECC children were restored with SSC under DGA, conforming to the Guidelines of AAPD.[9] A complete dental occlusal relationship was reconstructed, which requires children to adjust and adapt during mastication gradually. Studies have shown that it would take an average of 7 to 14 days to accommodate the new occlusal relationship, with no temporomandibular joint disorders.[29] A fixed or semi-fixed space maintainer was also applied immediately after extraction in most children (31.98%) with primary molars being extracted during the operation, increasing the feeding difficulty. Based on the number of repaired SSC and the difference in individual sensitivity after surgery, the parents were advised to gradually change children's diet from soft to hard and encourage them to chew.
Surprisingly, although 30.27% of the children had postoperative oral bleeding on the first day after the procedure, there was no relationship between postoperative pain and dental bleeding and the number of extracted teeth, which is different from previous studies[30] and might be attributed to the vasoconstrictor in the local anesthesia used before extraction and the surgical filling of the alveolar socket after extraction.
Approximately one-third of the patients (27.57%) reported coughing, higher than the 12% incidence reported by Farsi;[16] 7.9% complained of a sore throat, lower than the rate in earlier studies (27%–34%),[11,16,20] with7.84% experiencing epistaxis, which is lower than the 12.8% rate reported by Cantekin.[20] Nasotracheal intubation would inevitably stimulate and even hurt the respiratory tract's mucosa, leading to a sore throat, coughing, and epistaxis. Fortunately, all the children were successfully intubated under one-time photopic vision; therefore, the mucosa's irritation was transient, and the symptoms were mild and resolved easily, consistent with numerous studies carried out under non-dental general anesthesia.[31,32]
A small number of our patients reported nausea and/or vomiting (9.46% and 7.57, respectively); the incidence of these conditions was 6% to 26% in previous studies.[11,17,21] A meta-analysis by Xu et al showed that a shortened fasting time reduced postoperative nausea and vomiting after laparoscopic cholecystectomy under general anesthesia.[33] It is speculated that children's gastrointestinal tracts might be in a state of stress due to long fasting time, which was at least 6 hours in our study. Atan et al[5] showed that for every 10-minute increase in anesthetic time, patients aged 6 to 16 years exhibited a 15% increase in the odds of feeling sleepy and a 19% increase in the odds of feeling nauseous following DGA. Opioid analgesics adopted in general anesthesia could increase the incidence of postoperative nausea or vomiting.[34] We employed sufentanil in this study, which might be responsible for postoperative nausea or vomiting.
5. Conclusion
The anesthetic procedures did not affect postoperative morbidity significantly as the anesthetist executed a standardized protocol in this study. The most prevalent complication after the DGA in children is postoperative pain. It has the potential to decrease postoperative complications by merely reducing the operation duration. Children with low nutritional levels should be closely monitored, and their postoperative body temperature should be controlled. However, further research is necessary to precisely evaluate factors influencing postoperative complications, in order to eliminate children's discomfort, reduce parents’ anxiety, and provide instructions for the postoperative management of children's oral and general health.
Author contributions
Conceptualization: Jing Zou.
Data curation: Qiong Zhang.
Formal analysis: Qiong Zhang.
Investigation: Qiong Zhang, Xiaoyu Deng.
Supervision: Jing Zou.
Validation: Yan Wang.
Visualization: Qiong Zhang, Xiaoyu Deng, Yan Wang.
Writing – original draft: Qiong Zhang.
Writing – review & editing: Ruijie Huang, Ran Yang, Jing Zou.
Footnotes
Abbreviations: AAPD = American Academy of Pediatric Dentistry, DGA = dental general anesthesia, ECC = early childhood caries, FLACC scale = faces, legs, activity, cry and consolability scale, FPS-R = face pain scale-revised, S-ECC = severe early childhood caries, SSC = stainless steel crown.
How to cite this article: Zhang Q, Deng X, Wang Y, Huang R, Yang R, Zou J. Postoperative complications in Chinese children following dental general anesthesia: a cross-sectional study. Medicine. 2020;99:45(e23065).
This research was supported by the Applied Basic Research Project of Science and Technology Department of Sichuan Province (2020YJ0296), and the Innovation and Collaborative Project of Science and Technology Department of Sichuan Province (2019YFH0025).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
P < .1.
SD = standard deviation.
P < .05.
SD = standard deviation.
P < .05.
SD = standard deviation.
P < .05.
References
- [1].Wang X. The fourth national oral health epidemiological survey report. 2018;Beijing: People's Medical Publishing House, 13-14. [Google Scholar]
- [2].Pham L, Tanbonliong T, Dizon MB, et al. Trends in general anesthesia utilization by board-certified pediatric dentists. Pediatr Dent 2018;40:124–30. [PubMed] [Google Scholar]
- [3].Tanner ACR, Kressirer CA, Rothmiller S, et al. The caries microbiome: implications for reversing dysbiosis. Adv Dent Res 2018;29:78–85. [DOI] [PubMed] [Google Scholar]
- [4].Holt RD, Chidiac RH, Rule DC. Dental treatment for children under general anaesthesia in day care facilities at a London dental hospital. Br Dent J 1991;170:262–6. [DOI] [PubMed] [Google Scholar]
- [5].Atan S, Ashley P, Gilthorpe MS, et al. Morbidity following dental treatment of children under intubation general anaesthesia in a day-stay unit. Int J Paediatr Dent 2004;14:9–16. [DOI] [PubMed] [Google Scholar]
- [6].Hu YH, Tsai A, Ou-Yang LW, et al. Postoperative dental morbidity in children following dental treatment under general anesthesia. BMC Oral Health 2018;18:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Almaz ME, Oba AA, Sonmez IS. Postoperative morbidity in pediatric patients following dental treatment under general anesthesia. Eur Oral Res 2019;53:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Waggoner W F, Nelson T. Restorative Dentistry for the Primary Dentition. Pediatric Dentistry: infancy through adolescence. 6th Edition. Philadelphia, PA. 2019; Elsevier, 304–328. [Google Scholar]
- [9].Dentistry AA. Pediatric Restorative Dentistry. Pediatr Dent 2017;39:312–24. [PubMed] [Google Scholar]
- [10].Beltramini A, Milojevic K, Pateron D. Pain assessment in newborns, infants, and Children. Pediatr Ann 2017;46:387–95. [DOI] [PubMed] [Google Scholar]
- [11].Needleman HL, Harpavat S, Wu S, et al. Postoperative pain and other sequelae of dental rehabilitations performed on children under general anesthesia. Pediatr Dent 2008;30:111–21. [PubMed] [Google Scholar]
- [12].Fung DE, Cooper DJ, Barnard KM, et al. Pain reported by children after dental extractions under general anaesthesia: a pilot study. Int J Paediatr Dent 1993;3:23–8. [DOI] [PubMed] [Google Scholar]
- [13].Ersin NK, Önçağ Ö, Cogulu D, et al. Postoperative morbidities following dental care under day-stay general anesthesia in intellectually disabled children. J Oral Maxillofac Surg 2005;63:1731–6. [DOI] [PubMed] [Google Scholar]
- [14].Hosey MT, Macpherson LM, Adair P, et al. Dental anxiety, distress at induction and postoperative morbidity in children undergoing tooth extraction using general anaesthesia. Br Dent J 2006;200:39–43. [DOI] [PubMed] [Google Scholar]
- [15].Sorge RE, Totsch SK. Sex differences in pain. J Neurosci Res 2017;95:1271–81. [DOI] [PubMed] [Google Scholar]
- [16].Farsi N, Ba’Akdah R, Boker A, et al. Postoperative complications of pediatric dental general anesthesia procedure provided in Jeddah hospitals, Saudi Arabia. Bmc Oral Health 2009;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Enever GR, Nunn JH, Sheehan JK. A comparison of post-operative morbidity following outpatient dental care under general anaesthesia in paediatric patients with and without disabilities. Int J Paediatr Dent 2000;10:120–5. [DOI] [PubMed] [Google Scholar]
- [18].Holan G, Kadari A, Engelhard D, et al. Temperature elevation in children following dental treatment under general anesthesia with or without prophylactic antibiotics. Pediatr Dent 1993;15:99–103. [PubMed] [Google Scholar]
- [19].Morrow JW, Seale NS, Berry CW, et al. Incidence of temperature elevations after a full mouth dental rehabilitation under general anesthesia. ASDC J Dent Child 1986;53:420–4. [PubMed] [Google Scholar]
- [20].Cantekin K, Yildirim MD, Delikan E, et al. Postoperative discomfort of dental rehabilitation under general anesthesia. Pak J Med Sci 2014;30:784–8. [PMC free article] [PubMed] [Google Scholar]
- [21].Vinckier F, Gizani S, Declerck D. Comprehensive dental care for children with rampant caries under general anaesthesia. Int J Paediatr Dent 2001;11:25–32. [DOI] [PubMed] [Google Scholar]
- [22].Beskow A, Westrin P. Sevoflurane causes more postoperative agitation in children than does halothane. Acta Anaesthesiol Scand 1999;43:536–41. [DOI] [PubMed] [Google Scholar]
- [23].Liang HH, Zhang MX, Wen YM, et al. The incidence of and risk factors for postoperative fever after cleft repair surgery in children. J Pediatr Nurs 2019;45:e89–94. [DOI] [PubMed] [Google Scholar]
- [24].Al Sebeih K, Hussain J, Albatineh AN. Postoperative complications following tonsil and adenoid removal in Kuwaiti children: a retrospective study. Ann Med Surg (Lond) 2018;35:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].El-Saied S, Joshua BZ, Abu Tailakh M, et al. Early postoperative fever in paediatric patients undergoing cochlear implant surgery. Clin Otolaryngol 2018;43:385–8. [DOI] [PubMed] [Google Scholar]
- [26].Jui-Lin F, Cotter JD, Lucas RAI, et al. Human cardiorespiratory and cerebrovascular function during severe passive hyperthermia: effects of mild hypohydration. J Appl Physiol 2008;105:433–45. [DOI] [PubMed] [Google Scholar]
- [27].Xu H, Mei XP, Xu LX. Cause analysis, prevention, and treatment of postoperative restlessness after general anesthesia in children with cleft palate. J Dent Anesth Pain Med V 17 2017;13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Costi D, Cyna AM, Ahmed S, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev 2014;Cd007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Innes NPT, Evans DJP, Bonifacio CC, et al. The Hall Technique 10 years on: Questions and answers. Brit Dent J 2017;222:478–83. [DOI] [PubMed] [Google Scholar]
- [30].Coulthard P, Rolfe S, Mackie I, et al. Intraoperative local anaesthesia for paediatric postoperative oral surgery pain–a randomized controlled trial. Int J Oral Maxil Surg 2006;35:1114–9. [DOI] [PubMed] [Google Scholar]
- [31].El-Boghdadly K, Bailey CR, Wiles MD. Postoperative sore throat: a systematic review. Anaesthesia 2016;71:706–17. [DOI] [PubMed] [Google Scholar]
- [32].Chang JE, Kim H, Han SH, et al. Effect of endotracheal tube cuff shape on postoperative sore throat after endotracheal intubation. Anesth Analg 2017;125:1240–5. [DOI] [PubMed] [Google Scholar]
- [33].Xu D, Zhu X, Xu Y, et al. Shortened preoperative fasting for prevention of complications associated with laparoscopic cholecystectomy: a meta-analysis. J Int Med Res 2017;45:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Boer HDD, Detriche O, Forget P. Opioid-related side effects: Postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol 2017;31:499–504. [DOI] [PubMed] [Google Scholar]


