Abstract
Type A acute aortic dissection (TAAAD) carries a high mortality rate in the absence of surgical treatment. This study sought to determine whether combining the assessment of clinical and computed tomography (CT) findings can be used to predict the long-term all-cause mortality rate of patients with TAAAD.
Eighty-five consecutive patients with TAAAD who had undergone CT imaging and surgery were retrospectively reviewed. For the clinical and CT findings, univariate testing followed by multivariate logistic regression analysis was conducted to identify independent predictors of death. Then, the area under the receiver operating characteristic curve of the combined prediction model was calculated.
The long-term mortality rate was 34.1% in our cohort (a median follow-up period of 60 months). Multivariate logistic regression analysis identified the following presenting variables as predictors of death: male sex (odds ratio [OR]: 6.67; 95% confidence interval [CI]: 1.67–25.0; P = .007), kidney malperfusion (OR: 2.18; 95% CI: 1.16–4.1; P = .02), and descending aorta size (OR: 1.12; 95% CI: 1.00–1.25; P = .05). Receiver operating characteristic curve analysis revealed an area under the receiver operating characteristic curve of 0.84 when using the combined model for prediction of long-term all-cause mortality (P ≤ .01).
The combined assessment of clinical and CT findings can reasonably predict the long-term prognosis of TAAAD with surgery.
Keywords: computed tomography, long-term prognosis, type A acute aortic dissection
1. Introduction
Although type A acute aortic dissection (TAAAD) patients have shown improved mortality outcomes over the past 2 decades, TAAAD still carries a high mortality rate in the absence of surgical treatment.[1–3] Reportedly, the in-hospital mortality rate of patients presenting with type A is 22% and the surgical mortality rate is 18%.[1]
Computed tomography (CT) has been extensively used for diagnosis in patients with acute aortic dissection.[1] TAAAD is defined as involving the ascending aorta irrespective of the site of origin and requires surgery for resolution. Approximately one-third of TAAAD patients develop preoperative malperfusion syndrome.[4,5] Malperfusion syndromes may be diagnosed when patients with TAAAD display symptoms and signs attributable to a disturbance in the blood flow to defined end-organ systems. CT imaging has been also used for diagnosis in TAAAD patients with malperfusion syndromes. Malperfusion is frequently associated with short-term death in patients undergoing surgery for TAAAD.[6] Moreover, several prognostic factors for TAAAD patients with surgery have been reported[6–9] but, to our knowledge, there have been no reports made of the influence of composite parameters combining CT findings and clinical data on the long-term prognosis after surgery.
The purpose of this study was therefore to determine whether a comprehensive assessment of CT findings and clinical data could be used to predict long-term all-cause mortality among patients with TAAAD.
2. Materials and methods
2.1. Study population
One hundred one consecutive patients diagnosed with TAAAD using CT were retrospectively reviewed between January 2009 and December 2013 (Fig. 1). We excluded 16 patients with TAAAD who were treated medically for a variety of reasons, such as advanced age (age 90 and older) (n = 8), severe comorbid illness (n = 6), or refusal to undergo any surgical intervention (n = 2). Of the remaining 85 patients, 57 (67.1%) underwent hemiarch replacement, 20 (23.5%) underwent total arch replacement, 3 (3.5%) underwent hemiarch replacement combined with aortic root replacement, 3 (3.5%) underwent hemiarch replacement combined with aortic valve replacement, 1 (1.2%) underwent aortic root replacement combined with coronary artery bypass grafting, and 1 (1.2%) underwent aortic root replacement. All 85 TAAAD patients were included in this study following surgery.
Figure 1.
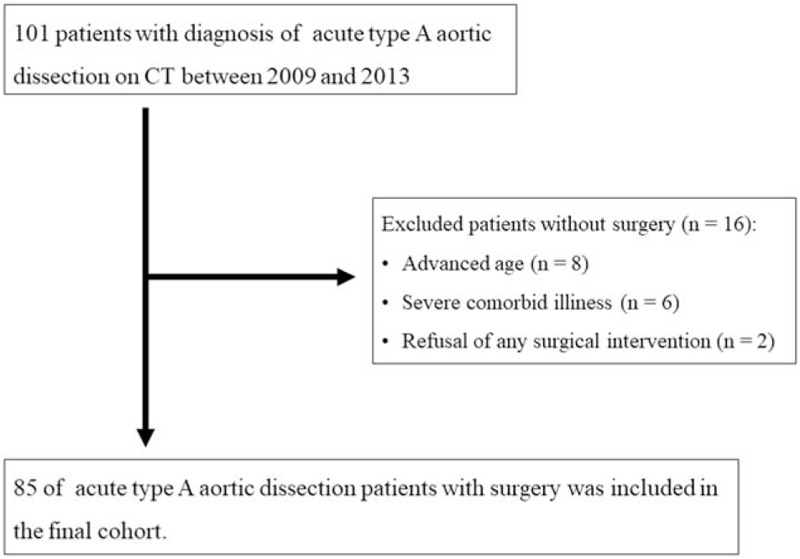
Flowchart of the study population enrollment process.
For our study, acute aortic dissection was defined as a dissection operated on no later than 14 days after symptom onset. Our institutional review committee approved this retrospective study and the need for informed consent was waived.
2.2. CT imaging
In all 85 patients, CT studies were performed using a multi-detector CT system (Aquilion 64; Canon Medical Systems, Otawara, Japan) within 24 hours of hospital arrival. CT studies were performed involving both nonenhanced and contrast-enhanced CT in all patients. Contrast-enhanced CT was conducted using a bolus intravenous injection of 60 to 90 mL of nonionic iodinated contrast material using a power injector, and CT data acquisition began at 20 to 30 seconds and 70 to 90 seconds (2 phases) after the start of contrast material injection. The image reconstruction section thickness and the section interval were 5.0 and 5.0 mm for routine axial image reconstruction, and 1.0 and 1.0 mm for multiplanar reformations and 3D image reconstruction, respectively
2.3. Data analysis
Data were collected by using a standardized data form with 17 clinical and CT variables, including patient demographics, history, clinical presentation, physical findings, and CT findings (Table 1). All CT findings were examined by 2 investigator (a board-certified radiologists with 10 and 14 years of experience with cardiovascular CT) who were blinded to all other clinical data. Based on a previous study, the segmental extent of aortic involvement from the proximal to distal boundary was assessed (ie, ascending, ascending to arch, ascending to descending, ascending to abdomen or ascending to iliac).[10] Referring to previous research,[7,11] severe malperfusion was determined only when signs were observed (ie, true lumen collapse > 70% or decreased contrast-enhancement of organs) (Fig. 2A). Meanwhile, mild malperfusion was defined as true lumen collapse ≤70% or the non-decreased contrast-enhancement of organs. Evaluation parameters included the superior mesenteric artery and bilateral renal arteries. The largest short-axial diameter of the outer contour of the affected segment of the aorta was measured (Fig. 2B). In cases of interobserver disagreement, final decisions were reached with consensus. Visible pericardial effusion with a CT number of 50 Hounsfield Unit or more was defined as bloody pericardial effusion.
Table 1.
Patient characteristics in the total study population.
| Variable | Overall (n = 85) | Survived (n = 56) | Died (n = 29) | P-value |
| Age | 69.7 ± 12.7 | 67.2 ± 10.9 | 74.3 ± 14.6 | .02 |
| Male sex | 37 (43.5) | 19 (33.9) | 18 (62.1) | .02 |
| Prior thoracic surgery | 7 (8.1) | 2 (3.6) | 5 (17.2) | .03 |
| Dyspnea | 15 (17.4) | 7 (12.5) | 8 (27.6) | .08 |
| Coma | 11 (12.9) | 7 (12.5) | 4 (13.8) | .87 |
| PLT | 15.6 ± 4.3 | 16.6 ± 4.4 | 13.9 ± 3.8 | <.01 |
| eGFR | 58.3 ± 19.1 | 62.3 ± 19.3 | 51.4 ± 16.5 | <.01 |
| LDH | 287 ± 105 | 271 ± 74 | 308 ± 145 | .12 |
| Cardiac tamponade | 44 (51.8) | 21 (37.5) | 13 (44.8) | .51 |
| Mediastinal hematoma | 19 (22.0) | 9 (16.1) | 10 (34.5) | .05 |
| Ascending aorta size (mm) | 50.0 ± 5.1 | 49.4 ± 4.2 | 51.1 ± 6.4 | .22 |
| Descending aorta size (mm) | 34.3 ± 4.8 | 33.4 ± 4.0 | 36.3 ± 5.8 | .03 |
| DeBakey type II | 8 (9.4) | 3 (5.4) | 5 (19.2) | .08 |
| Distal extent of dissection | .27 | |||
| Ascending | 8 (9.4) | 3 (5.4) | 5 (19.2) | |
| Ascending to arch | 18 (21.2) | 11 (19.6) | 7 (26.9) | |
| Ascending to descending | 9 (10.6) | 7 (12.5) | 2 (7.7) | |
| Ascending to abdomen | 15 (17.6) | 11 (19.6) | 4 (15.4) | |
| Ascending to iliac | 35 (41.2) | 24 (42.9) | 11 (42.3) | |
| False lumen thrombosis | .94 | |||
| Complete thrombosis | 25 (29.4) | 18 (32.1) | 7 (24.1) | |
| Partial thrombosis | 37 (43.5) | 21 (37.5) | 16 (55.2) | |
| Patent | 23 (27.1) | 17 (30.4) | 6 (20.7) | |
| Kidney malperfusion | .51 | |||
| Mild | 21 (24.7) | 17 (30.4) | 4 (15.4) | |
| Severe | 10 (11.8) | 3 (5.4) | 7 (26.9) | |
| Intestinal malperfusion | .78 | |||
| Mild | 8 (9.3) | 6 (10.7) | 2 (6.9) | |
| Severe | 2 (2.3) | 0 | 2 (6.9) |
Figure 2.
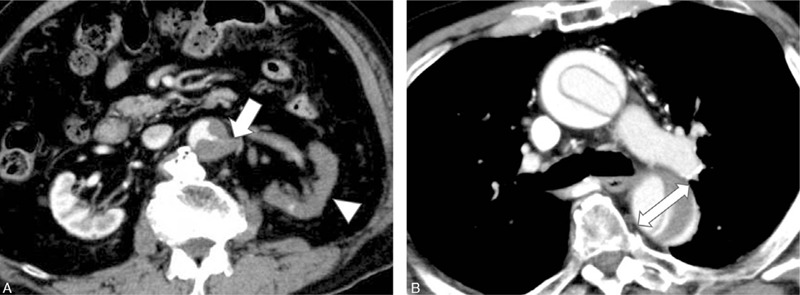
CT images show severe kidney malperfusion (A) and the descending aorta size (B) of an 88-yr-old male with TAAAD. (A) Left kidney artery occlusion (arrow) and decreased contrast-enhancement of the left kidney (arrowhead) can be seen. (B) The largest short-axial diameter (double arrow) of the descending aorta was dilated to 41.5 mm at the time of measurement. The patient received dialysis after hemiarch replacement and died 13 mo later. CT = confidence interval.
2.4. Statistical analysis
Statistical analysis was performed using the JMP software version 9.0.2 (SAS Institute, Cary, NC). Continuous variables are expressed as means ± standard deviations, while categorical variables are presented as frequencies with corresponding percentages. Differences between groups were tested by means of the Chi-squared test, Student t test, or Mann–Whitney U test where appropriate. To identify predictors of risk factors for mortality, univariate and multivariate logistic regression analyses were performed. Variables with a significance level of P ≤ .20 in the univariate analyses were then entered into a multivariate analysis via binary logistic regression and were considered independently significant when P ≤ .05. Results are displayed as odds ratio (ORs) with 95% confidence interval (CIs) and P-values. According to the multivariate model, we developed a combined logistic regression model including all variables associated with significant P-values in the previous models, then calculated the area under the receiver operating characteristic curve (AUC) of the combined prediction model. Kaplan–Meier curves plotted for cumulative all-cause mortality of patients (time between CT and death) were analyzed using log-rank analysis with right censoring.
3. Results
3.1. Overall characteristics of patients
The patients’ characteristics are summarized in Table 1. Among the 85 patients with TAAAD (48 women and 37 men), the mean age was 69.7 ± 12.7 years. During a median follow-up period of 60 months, we observed a total of 29 deaths after surgery. The cause of death was not specified in 21 of these individuals, followed by aortic rupture (n = 2), cerebrovascular disease (n = 2), mediastinitis (n = 2), visceral ischemia (n = 1), and kidney failure (n = 1) in order of most to least frequently reported. Age and the prevalence of male sex were significantly higher among the patients who died. The deceased patients also tended to have characteristics such as prior thoracic surgery, mediastinal hematoma, descending aorta size, and lower platelet count and estimated glomerular filtration rate relative to the ones surviving postsurgery.
3.2. Predictors of overall death
Table 2 summarizes the results of the univariate analyses to identify predictors of overall death. Of 17 variables, 13 were considered to be significant predictors (P ≤ .20) for long-term prognosis during univariate analysis, including age, male sex, prior thoracic surgery, dyspnea, platelet count, lactate dehydrogenase, mediastinal hematoma, ascending aorta size, descending aorta size, DeBakey type II, distal extent of dissection, and kidney malperfusion. A multivariate analysis involving these 13 factors was conducted and identified the following as predictors of death: male sex (OR: 6.67; 95% CI: 1.67 –25.0; P = .007), kidney malperfusion (OR: 2.18; 95% CI: 1.16–4.1; P = .02), and descending aorta size (OR: 1.12; 95% CI: 1.00–1.25; P = .05) (Table 3). Patients categorized by sex, kidney malperfusion, descending aorta size (37.7 mm) differed significantly in survival probability (log rank P = <.01) (Fig. 3).
Table 2.
Univariate analysis for predictors of mortality.
| Variable | OR (95% CI) | P-value |
| Age | 1.05 (1.01–1.10) | .02 |
| Male sex | 3.23 (1.25–26.9) | .01 |
| Prior thoracic surgery | 5.63 (1.02–31.1) | .05 |
| Dyspnea | 2.67 (0.86–8.3) | .09 |
| Coma | 1.12 (0.30–4.19) | .87 |
| PLT | 0.85 (0.74–0.96) | .01 |
| eGFR | 0.96 (0.94–0.99) | .01 |
| LDH | 1.00 (1.00–1.01) | .08 |
| Bloody pericardial effusion | 1.35 (0.55–3.36) | .51 |
| Mediastinal hematoma | 2.75 (0.97–7.83) | .06 |
| Ascending aorta size | 1.07 (0.97–1.17) | .17 |
| Descending aorta size | 1.12 (1.01–1.24) | .02 |
| DeBakey type II | 3.68 (0.81–16.7) | .09 |
| Distal extent of dissection | 0.81 (0.59–1.1) | .18 |
| False lumen thrombosis | 0.97 (0.53–1.76) | .92 |
| Kidney malperfusion | 1.49 (0.95–2.35) | .08 |
| Intestinal malperfusion | 1.75 (0.61–5.01) | .30 |
Table 3.
Multivariate analysis for predictors of mortality.
| Variable | OR (95% CI) | P-value |
| Age | 1.05 (1.00–1.12) | .07 |
| Male sex | 6.67 (1.67–25.0) | <.01 |
| Prior thoracic surgery | 1.98 (0.23–17.1) | .54 |
| Dyspnea | 2.84 (0.72–11.3) | .14 |
| PLT | 0.88 (0.76–1.02) | .08 |
| eGFR | 0.99 (0.95–1.03) | .55 |
| LDH | 1.00 (1.00–1.01) | .12 |
| Mediastinal hematoma | 1.65 (0.34–7.86) | .53 |
| Ascending aorta size | 1.03 (0.93–1.14) | .58 |
| Descending aorta size | 1.12 (1.00–1.25) | .05 |
| DeBakey type II | 1.45 (0.18–11.5) | .72 |
| Distal extent of dissection | 0.66 (0.39–1.13) | .13 |
| Kidney malperfusion | 2.18 (1.16–4.10) | .02 |
Figure 3.
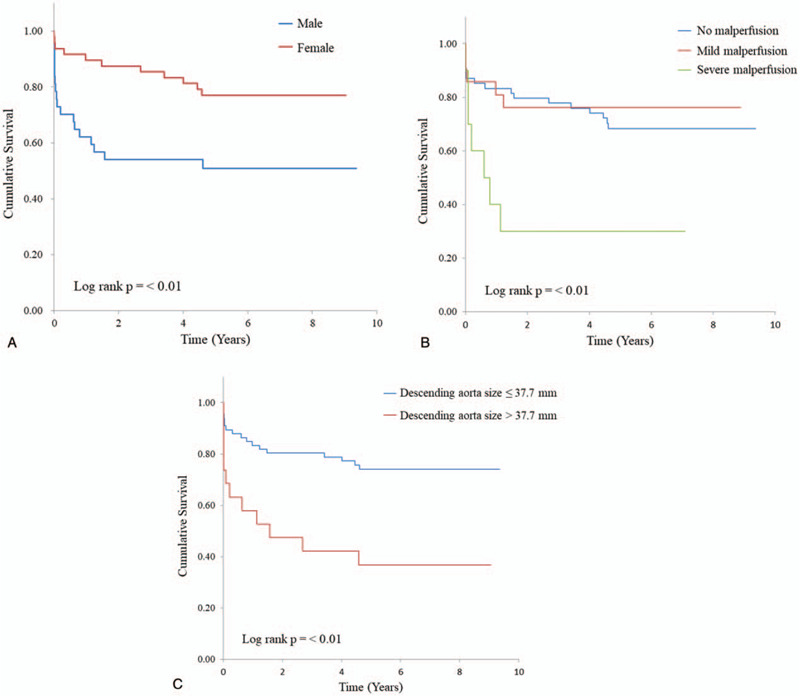
Kaplan–Meier survival curves for sex, kidney malperfusion, and descending aorta size. Patients categorized by sex (A), kidney malperfusion (B), descending aorta size (37.7 mm) (C) differed significantly in survival probability (log rank P = <.01).
3.3. Prediction model for overall death
A multivariate logistic regression model using the significant predictors yielded an AUC of 0.84 for the prediction of long-term all-cause mortality (P < .01) (Fig. 4).
Figure 4.
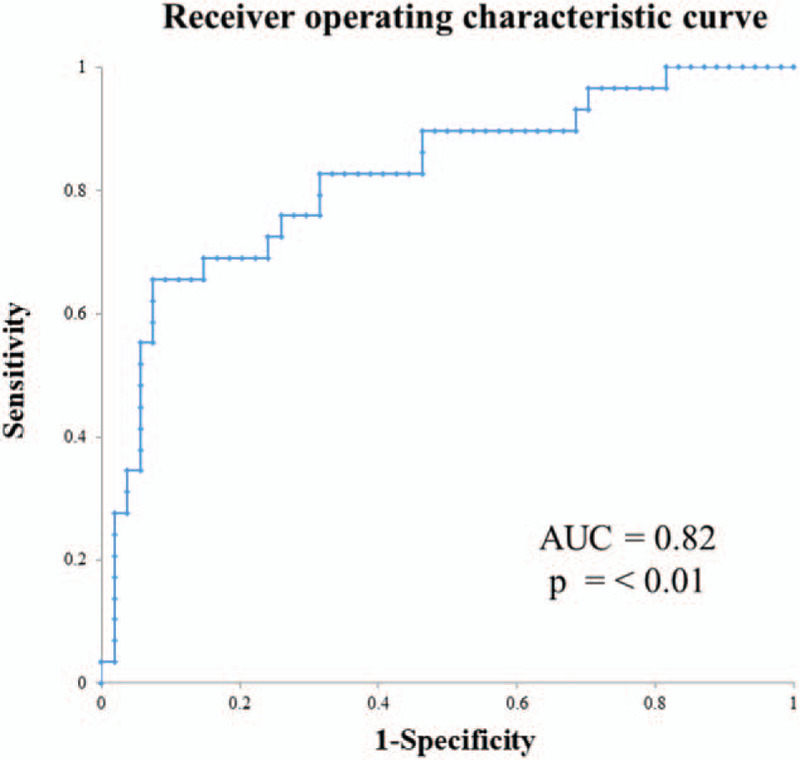
Receiver operating characteristic curve for the prediction of long-term death in patients with TAAAD. The combined model yielded an AUC of 0.82 (P ≤ .01). AUC = area under the receiver operating characteristic curve, TAAAD = type A acute aortic dissection.
4. Discussion
In the present cohort study, roughly 34.1% of patients with TAAAD who underwent surgery died during a median follow-up period of 60 months. Male sex, kidney malperfusion, and descending aorta size were associated with long-term all-cause mortality. The predictive ability of the combined model of clinical and CT findings was relatively high, with an AUC of 0.84. Our results suggest that, in real-world clinical practice, it is important to evaluate both clinical and CT findings comprehensively rather than individually. Improving the accuracy of long-term prognosis prediction using this comprehensive approach could support more effective clinical decision-making for patient management.
Previous studies have reported higher age, prior thoracic surgery, and false lumen thrombosis to be predictors of long-term death in patients with TAAAD.[8,12,13] Other previous research suggested that higher age, mesenteric ischemia, cardiac tamponade, coma, and kidney failure may be predictors of in-hospital death in this population.[14–16] Of note, the variables predicting death in these prior reports were not associated with long-term mortality in the present study. Possible causes for this discrepancy include variations in the patient population and surgical indications.
Our study suggested that male sex is an important predictor of long-term death in patients with TAAAD. Although female sex is listed as one of the risk factors for adverse outcomes of cardiac surgery such as coronary artery bypass grafting in the risk models of both the Society of Thoracic Surgeons and the European System for Cardiac Operative Risk Evaluation,[17,18] there have been few reports published on sex differences in patients undergoing surgical repair of TAAAD. A previous study postulated that there are no differences in both short- and long-term outcomes between male and female patients undergoing surgery for TAAAD.[19] Thus, 1 reason for the difference in our results may be a disparity in patient age.
Kidney malperfusion independently predicted an increased risk of long-term death after surgery in our study. In contrast, a previous study reported that the risk for mortality was not increased in asymptomatic patients with kidney malperfusion. However, in this prior research, kidney malperfusion was defined by an elevated serum creatinine level,[7] while our definition of kidney malperfusion was based on contrast-enhanced CT findings, which may constitute an improved criterion.
It was also reported that the widest diameter of the ascending aorta did not differ between those patients who survived through the follow-up period and those who died in a previous study.[12] In our study, the ascending aorta size was not associated with long-term death. On the other hand, the descending aorta size was associated with worse outcomes. Perhaps a reason why ascending aorta size is not associated with long-term mortality is because that segment is replaced at surgery. Previous studies have reported the descending aorta size to be an important predictor of death in patients with acute type B dissection.[20] Thus, descending aorta size may independently predict a greater risk of long-term death in patients with TAAAD.
In our study population, only a few cases had previous CT before onset of TAAAD. As far as their previous CT was checked, no special features other than arteriosclerosis were observed. Even so, if CT findings before onset of TAAAD can predict the future onset and the long-term prognosis, it will be of great benefit to the patients. Further verification is needed in this regard.
Importantly, our study had some limitations that must be kept in mind. First, it was a single-center retrospective study and the study population was relatively small. Our results should be validated in large-scale clinical studies. Second, we did not evaluate long-term mortality by surgical methods due to an insufficient number of cases with which to attempt to do so. Lastly, since an electrocardiogram synchronization technique was not applied during CT, coronary artery and entry tears were not evaluated.
In conclusion, the combined assessment of clinical and CT findings may predict the long-term prognosis of patients TAAAD following surgery. Male sex, kidney malperfusion, and descending aorta size were associated with increased long-term mortality in this cohort.
Author contributions
Conceptualization: Seitaro Oda.
Data curation: Kenichiro Hirata.
Formal analysis: Kenichiro Hirata, Seitaro Oda.
Investigation: Seitaro Oda, Takeshi Sugahara.
Methodology: Kenichiro Hirata, Seitaro Oda.
Supervision: Ryusuke Suzuki.
Writing – original draft: Kenichiro Hirata.
Writing – review & editing: Seitaro Oda, Takeshi Sugahara.
Footnotes
Abbreviations: AUC = area under the receiver operating characteristic curve, CI = confidence interval, CT = computed tomography, OR = odds ratio, TAAAD = type A acute aortic dissection.
How to cite this article: Hirata K, Oda S, Suzuki R, Sugahara T. Long-term prognostic value of the combined assessment of clinical and computed tomography findings in type: an acute aortic dissection. Medicine. 2020;99:45(e23008).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data are shown as n (%) or mean ± standard deviation.
eGFR = estimated glomerular filtration rate, LDH = lactate dehydrogenase, PLT = platelet.
CI = confidence interval, eGFR = estimated glomerular filtration rate, LDH = lactate dehydrogenase, OR = odds ratio, PLT = platelet.
CI = confidence interval, eGFR = estimated glomerular filtration rate, LDH = lactate dehydrogenase, OR = odds ratio, PLT = platelet.
References
- [1].Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol 2015;66:350–8. [DOI] [PubMed] [Google Scholar]
- [2].Landenhed M, Engstrom G, Gottsater A, et al. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J Am Heart Assoc 2015;4:e001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Trimarchi S, Eagle KA, Nienaber CA, et al. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg 2010;140:784–9. [DOI] [PubMed] [Google Scholar]
- [4].Girdauskas E, Kuntze T, Borger MA, et al. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg 2009;138:1363–9. [DOI] [PubMed] [Google Scholar]
- [5].Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol 2011;58:2455–74. [DOI] [PubMed] [Google Scholar]
- [6].Czerny M, Schoenhoff F, Etz C, et al. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cardiol 2015;65:2628–35. [DOI] [PubMed] [Google Scholar]
- [7].Cho YH, Sung K, Kim WS, et al. Malperfusion syndrome without organ failure is not a risk factor for surgical procedures for type A aortic dissection. Ann Thorac Surg 2014;98:59–64. [DOI] [PubMed] [Google Scholar]
- [8].Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244–50. [DOI] [PubMed] [Google Scholar]
- [9].Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005;129:112–22. [DOI] [PubMed] [Google Scholar]
- [10].Dake MD, Thompson M, van Sambeek M, et al. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg 2013;46:175–90. [DOI] [PubMed] [Google Scholar]
- [11].Sueyoshi E, Sakamoto I, Hayashi K, et al. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation 2004;110: 11 Suppl 1: I256–61. [DOI] [PubMed] [Google Scholar]
- [12].Bajona P, Quintana E, Schaff HV, et al. Aortic arch surgery after previous type A dissection repair: results up to 5 years. Interact Cardiovasc Thorac Surg 2015;21:81–5. [DOI] [PubMed] [Google Scholar]
- [13].Tsai TT, Evangelista A, Nienaber CA, et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114: 1 Suppl: I350–6. [DOI] [PubMed] [Google Scholar]
- [14].Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002;105:200–6. [DOI] [PubMed] [Google Scholar]
- [15].Berretta P, Patel HJ, Gleason TG, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016;5:346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bossone E, Gorla R, LaBounty TM, et al. Presenting systolic blood pressure and outcomes in patients with acute aortic dissection. J Am Coll Cardiol 2018;71:1432–40. [DOI] [PubMed] [Google Scholar]
- [17].Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–44. [DOI] [PubMed] [Google Scholar]
- [18].Shroyer AL, Coombs LP, Peterson ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg 2003;75:1856–64. [DOI] [PubMed] [Google Scholar]
- [19].Fukui T, Tabata M, Morita S, et al. Gender differences in patients undergoing surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2015;150:581–7.e1. [DOI] [PubMed] [Google Scholar]
- [20].Tolenaar JL, Froehlich W, Jonker FH, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation 2014;130: 11 Suppl 1: S45–50. [DOI] [PubMed] [Google Scholar]


