Supplemental Digital Content is available in the text
Keywords: liver transplantation, multidrug-resistance, pediatric
Abstract
Bacterial infection has been identified as one of the most significant complications of liver transplantation (LT). Multidrug-resistant (MDR) gram-negative bacteria (GNB) infection remains problematic issue following LT in the adults. However, data in children are scarce. We aimed to examine the prevalence and associated factors of MDR-GNB infection among pediatric LT recipients.
We performed a single-center retrospectively study of 118 children who underwent LT between January 2010 and December 2018. Data on the prevalence, clinical characteristics, types, and sites of MDR-GNB infection within 3 months after LT as well as the treatment outcomes were collected. Multidrug resistance was defined as acquired non-susceptibility to at least 1 agent in 3 or more antibiotic classes.
In total, 64 (53.7%) patients developed 96 episodes of culture-proven bacterial infection with 93 GNB isolates. Moreover, there were 58 (62.4%) MDR-GNB isolates, with a predominance of Klebsiella pneumoniae (32.7%), Escherichia coli (31%), and Pseudomonas aeruginosa (10.3%). Interestingly, 10 (17.2%) isolates were determined to be carbapenem-resistant Enterobacteriaceae. The median time to MDR-GNB infection was 9 (interquartile range: 5–33) days. The most common type of infection was intra-abdominal infection (47.9%). In the multivariate analysis, the significant variables associated with post-LT MDR-GNB infection include exposure to third-generation cephalosporins (hazard ratio [HR]: 2.16, P = .023), operative time (hazard ratio [HR] 1.20, P = .009), and length of intensive care unit stay (HR 1.03, P = .049). With a focus on carbapenem-resistant Enterobacteriaceae infection, a pediatric end-stage liver disease score >21 was the only significant 6 variable in the multivariate analysis (HR 11.48, P = .024). The overall 3-month mortality rate was 6.8%.
This study has highlighted the high prevalence rate of MDR-GNB infection after pediatric LT. Therefore, caution on the emergence of MDR-GNB infection should be paid in at-risk children. Moreover, knowledge regarding the prevalence of MDR-GNB infection and resistant patterns is essential for guideline development to prevent and minimize the risk of MDR-GNB infection in this group of patients.
1. Introduction
Liver transplantation (LT) has become an effective treatment for pediatric patients with end-stage liver diseases. Despite advances in perioperative management, surgical techniques, postoperative medical care, infectious control, and immunosuppressive therapy, bacterial infections remain a significant cause of morbidity and mortality among LT recipients.[1] The incidence of bacterial infections is highest during the early postoperative period and is typically nosocomial origin. It has been reported that bacterial infections occur in more than 50% of adult LT recipients and mostly related to surgical procedures and postoperative complications.[1,2]
Multidrug-resistant organisms, particularly multidrug-resistant gram-negative bacilli (MDR-GNB), have emerged as predominant pathogens among LT recipients.[1,3] Infections caused by MDR-GNB are responsible for longer length of hospital stay, intensive care unit (ICU) admission, higher costs, increase in mortality and morbidity.[3,4] Liver transplant candidates are at risk for colonization and infection with MDR-GNB due to prolonged waiting times, which leads to repeated hospitalization and inevitable exposure to antibiotics. Previous studies among adult LT recipients have shown that risk factors for MDR-GNB infection included extended use of pre-transplant broad-spectrum antibiotics, prolonged pre-transplantation hospitalization, surgical complications, and the need for invasive devices.[1,5,6]
Incidence of bacterial infections has been reported to be 40% to 80% following pediatric LT.[7–11] Although risk factors for MDR-GNB infections have been well documented in adult LT recipients, data in the pediatric population are scarce. This retrospective study aimed to evaluate the incidence of early postoperative bacterial infections caused by MDR-GNB and to identify associated factors among pediatric LT recipients.
2. Patients and methods
We performed a retrospective study of all pediatric patients aged 1 month to 18 years who underwent LT at Faculty of Medicine Ramathibodi Hospital, a tertiary care university-based hospital in Bangkok, Thailand between 2010 and 2019. A total of 123 pediatric LT recipients were included in the study period. Patients’ information was reviewed from electronic medical records. The following information was recorded; age, gender, underlying diseases, pediatric end-stage liver disease (PELD) or model for end-stage liver disease (MELD) score, history of abdominal surgery, antibiotic exposure within 3 months before LT, on-going infection at the time of LT, history of infected biloma, operation time, cold ischemic time, operative complications, length of hospital stay, length of ICU stay, biliary complications, acute rejection, cytomegalovirus (CMV) and Epstein-Barr virus reactivation after LT, sites of infections, history of re-operation, and death. The incidence of bacterial infection or GNB infection were measured within 3 months after LT. The study was approved by the Institutional Review Board, Faculty of Medicine Ramathibodi Hospital, Bangkok, Thailand (COA. No. MURA2018/1044).
2.1. Immunosuppressive regimens
Postoperative immunosuppressive drugs comprised of a calcineurin inhibitor (tacrolimus), corticosteroid, and mycophenolate mofetil. If rejection occurred, the dosage of calcineurin inhibitor was increased, with or without administration of pulse methylprednisolone therapy.
2.2. Antimicrobial prophylaxis regimen
Intravenous ampicillin/sulbactam (200 mg/kg/d of ampicillin) and ceftriaxone (50 mg/kg/d) were used as standard preoperative antibiotic prophylaxis. Six patients received antibiotics other than routine preoperative antibiotics (piperacillin/tazobactam in 4 patients, ceftriaxone and metronidazole in 2 patients). Antibiotics were started at the beginning of surgery and continued for 7 days postoperation. Oral neomycin (10 mg/kg/dose) was given on the day before transplantation. For Pneumocystis jirovecii prophylaxis, trimethoprim-sulfamethoxazole was given orally 3 days/week for 6 months after surgery or until corticosteroids were discontinued. None of the patients received antifungal prophylaxis. For cytomegalovirus prevention strategies, pre-emptive therapy was used as the primary approach in all cases, including D+/R– patients. Weekly monitoring for plasma CMV viral load for 12 weeks after LT was implemented in all patients. During the study period, a viral load of more than 1000 copies/mL was used as a cut-off value for initiation of ganciclovir.
2.3. Microbiological monitoring
All patients were admitted in ICU following the transplant procedure. Surveillance bacterial cultures were taken from tracheal aspirate and rectal swab at the time of ICU admission. Abdominal drain fluid cultures, blood cultures, and urine cultures were performed twice a week during the first postoperative week. Afterwards, abdominal drain fluid cultures were performed once a week until all drains were removed. While patients were admitted, blood, urine, tracheal or bronchoalveolar lavage specimens were collected if clinically indicated.
2.4. Diagnosis of infection
Based on clinical manifestations and microbiological results, all infectious complications were categorized into one of the following; surgical site infections (including intra-abdominal and hepatobiliary tract), bloodstream (including catheter-related infections), pneumonia, and urinary tract infection (UTI). Intra-abdominal infection was defined as a positive culture from intra-abdominal fluid, peritoneal fluid, or bile. UTI was diagnosed by the presence of clinical signs and symptoms of UTI with a positive urine culture (≥104 cfu/mL or >105 cfu/mL if urine was collected by a catheter or mid-stream urine, respectively). Proven bacterial infections were defined as the presence of pathogens from a normally sterile sample. Isolation of organisms from non-sterile samples in the absence of clinical symptoms of infection was considered as colonization. Diagnosis of bacterial infections was defined and categorized according to the criteria of the Centers for Diseases Control and Prevention.[12,13]
Bacterial cultures were performed by standard microbiological procedures. Antimicrobial susceptibility was interpreted based on the Clinical and Laboratory Standards Institute guidelines. Drug resistance was defined based on the European Society of Clinical Microbiology and Infectious Diseases 2012.[14] MDR-GNB was defined as non-susceptible to at least 1 agent in 3 or more antimicrobial categories, XDR (extensively drug-resistant) was defined as non-susceptible to at least 1 agent in all but 2 or fewer antimicrobial categories. Carbapenem-resistant Enterobacteriaceae (CRE) was defined as strains of Enterobacteriaceae resistant to at least 1 carbapenem.
2.5. Statistical Analysis
STATA version 16 (StataCorp, College Station, TX) was used to perform all statistical analyses. Continuous variables were expressed as median and interquartile range (IQR) in non-normally distributed data. Comparison between groups was performed by Student test or Mann–Whitney U test. Categorical variables were expressed with frequencies and percentages, and comparison was performed by Pearson Chi-square or Fisher exact test. Kaplan–Meier survival analysis was used to identify the incidence of MDR infection after LT. Univariate and multivariate analyses were done with Cox proportional hazard modeling to identify associated factors for MDR-GNB infection and CRE-infection after LT. All the variables with a P-value < .05 in univariate analysis were used in multivariate analysis.
3. Results
3.1. Patients’ demographic and clinical characteristics
Over the study period, 123 pediatric patients underwent LT between January 2010 and December 2019. We excluded recipients who had incomplete data record (n = 4), and death during operation (n = 1). Thus, 118 patients were analyzed in the study. Fifty-two patients (44%) were male with a median age of 1.7 years (IQR 1.1_ 2.8 years). The majority of patients underwent living-donor LT (LDLT) (95.8%). The median PELD/MELD score before LT was 19 (IQR; 16–23). The most common underlying disease was biliary atresia (78.8%) with 62 patients (52.5%) having had Kasai procedure (hepatoportoenterostomy) before LT. Demographic and clinical characteristics of patients are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients (N = 118).
| Clinical characteristics | N (%) |
| Male sex | 52 (44) |
| Age (yr); median (IQR) | 1.7 (1.1–2.8) |
| Underlying diseases | |
| Biliary atresia | 93 (78.8) |
| Acute liver failure | 7 (5.9) |
| Allagile syndrome | 4 (3.4) |
| Other causes of cirrhosis | 14 (11.8) |
| PELD score; median (IQR) | 19 (16–23) |
| Previous abdominal surgery before LT | 62 (52.5) |
| Donor | |
| Living Donor | 113 (95.8) |
| Cadaveric Donor | 5 (4.2) |
| Antibiotic exposure within 3 mo before LT | 63 (53.4) |
| Operation time (h); median (IQR) | 11.1 (10.1–12.5) |
| Cold ischemic time (min); median (IQR) | 72 (52–92) |
| Intraoperative complications | |
| Massive bleeding∗ | 84 (84) |
| Bowel injury | 6 (6) |
| Adrenal gland injury | 3 (3) |
| Vascular injury | 5 (5) |
| Biliary injury | 2 (2) |
| Hospital stay; median (IQR) | 48 (34–65) |
| <1 mo | 20 |
| 1–3 mo | 80 |
| >3 mo | 18 |
| Length of ICU stay (d); median (IQR) | 8 (6–14) |
| Duration of endotracheal intubation (d); median (IQR) | 3 (2–7) |
| Duration of urinary catheter (d); median (IQR) | 5 (3–6) |
| Duration of central venous catheter (d); median (IQR) | 12 (8–17) |
| Duration of abdominal drain (d); median (IQR) | 27 (20–37) |
| Early postoperative complications | |
| Bile leak | 27 (33) |
| Hepatic artery stenosis/thrombosis | 18 (21.9) |
| Hepatic vein stenosis/thrombosis | 18 (21.9) |
| Portal vein stenosis/thrombosis | 19 (23.2) |
| Sites of infection | |
| Intra-abdominal infection | 46 (46.4) |
| Bloodstream infection | 23 (23.2) |
| Urinary tract infection | 11 (11.1) |
| Pneumonia | 16 (16.2) |
| CMV reactivation | 37 (31.4) |
| EBV reactivation | 5 (4.2) |
| Acute graft rejection | 21 (17.8) |
| Postoperative surgical intervention (ie, liver biopsy, abdominal drain insertion, PTBD insertion, dilate hepatic vein) | 35 (29.6) |
| Re-operation | 58 (49.1) |
| Death | 8 (6.7) |
3.2. Bacterial infections after LT
Among 118 LT recipients, 64 (53.7%) patients developed 96 episodes of a culture-proven bacterial infection within 3 months after LT. The median time to bacterial infections was 7 days after LT (IQR; 3–19). From Kaplan–Meier analysis, 50% of patients developed at least 1 episode of a bacterial infection within 47 days (Fig. 1). The most common site of infection was intra-abdomen (47.9%), followed by bloodstream infection (23.9%), pneumonia (16.7%), and urinary tract infection (11.5%). Thirty-five patients (54.7%) developed a single site infection, while the remaining experienced multiple sites of infection. Interestingly, of the 96 episodes, 93 GNB isolates were responsible for these infections. The most frequent GNB isolates were Escherichia coli, Klebsiella spp. and Pseudomonas aeruginosa (Supplement Table 1).
Figure 1.
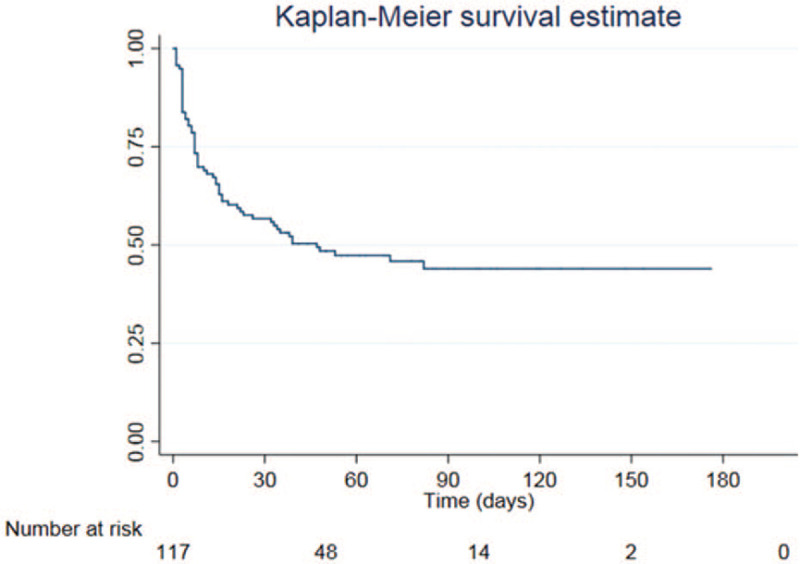
Kaplan–Meier curve of pediatric liver transplant recipients with bacterial infection.
3.3. Incidence of MDR-GNB infection after LT
Among 93 GNB isolates, a total of 58 MDR-GNB isolates (62.4%) were identified from 42 patients, with the predominance of Enterobacteriaceae (44 isolates, 75.9%). Thirteen patients had more than 1 episode of MDR-GNB infection (2 episodes in 10 patients and 3 episodes in 3 patients). The median time to the diagnosis of MDR-GNB infection following LT was 9 days (IQR; 5–33). From Kaplan–Meier analysis, the incidence rate of MDR-GNB infection was 6 per 1000 patient-days, and 25% of the patients developed at least 1 episode of MDR-GNB infection within 25 days postoperation (Fig. 2). The majority of MDR-GNB was extended-spectrum beta-lactamases-producing Enterobacteriaceae (34 isolates, 58.6%), followed by other MDR-GNB (14 isolates, 24.1%), and CRE (10 isolates, 17.2%). The details of MDR-GNB are shown in Table 2.
Figure 2.
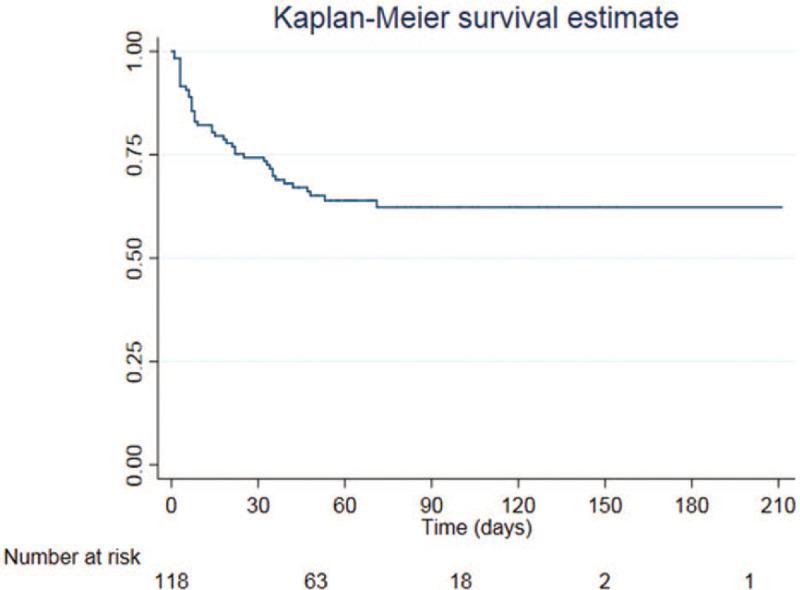
Kaplan–Meier curve of pediatric liver transplant recipients with multidrug-resistant gram-negative bacilli infection.
Table 2.
The details of multi-drug resistant gram-negative bacilli isolates from 42 patients.
| Species | Total number of isolates (N) |
| ESBL-producing organisms | |
| Escherichia coli | 16 |
| Klebsiella pneumoniae | 13 |
| Enterobacter cloacae | 3 |
| Klebsiella oxytoca | 1 |
| Enterobacter aerogenes | 1 |
| MDR-GNB | |
| Pseudomonas aeruginosa MDR | 6 |
| Acinetobacter baumannii MDR | 2 |
| Stenotrophomonas maltophilia MDR | 4 |
| Elizabethkingkia meningosepticum MDR | 2 |
| Carbapenem resistant Enterobacteriaceae (CRE) | |
| Escherichia coli | 2 |
| Klebsiella pneumoniae | 6 |
| Enterobacter cloacae | 2 |
3.4. Factors associated with MDR-GNB infection after LT
In univariate analysis, variables significantly associated with MDR-GNB infection were exposure to third-generation cephalosporins within 3 months before LT, operation time, length of ICU stay, re-operation, postoperative bile leakage, and CMV reactivation/infection. In multivariate analysis, exposure to third-generation cephalosporins within 3 months before LT (hazard ratio [HR] 2.20, confidence interval [CI] 95% 1.13–4.30, P = .021), operation time (HR 1.20 [CI 95% 1.05–1.38], P = .008), and length of ICU stay (HR 1.03 [CI 95% 1.00–1.06], P = .049), remained independent risk factors associated with MDR-GNB infection after LT (Table 3). Regarding infections caused by CRE, the only independent associated factor was PELD score of more than 21 (HR 11.48 [CI 95% 1.37–-96.18], P = .024) (Table 4).
Table 3.
Univariate and multivariate analyses of associated factors of multidrug-resistant gram-negative bacteria infection.
| Univariate analysis | Multivariate analysis | |||
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Sex, male | 1.16 (0.63–2.13) | .642 | ||
| Exposure to third-generation cephalosporins within 3 mo before LT | 2.40 (1.30–4.43) | .005∗ | 2.20 (1.13–4.30) | .021∗ |
| Operation time (h) | 1.22 (1.07–1.40) | .003∗ | 1.20 (1.05–1.38) | .008∗ |
| Cold ischemic time | 1.00 (0.99–1.00) | .888 | ||
| Length of ICU stay (d) | 1.04 (1.02–1.06) | <.001∗ | 1.03 (1.00–1.06) | .049∗ |
| Re-operation | 2.13 (1.14–3.98) | .017∗ | 1.27 (0.60–2.66) | .532 |
| Postoperative surgical intervention | 1.74 (0.94–3.23) | |||
| Bile leakage | 2.10 (1.11–4.01) | .023∗ | 1.20 (0.60–2.45) | .600 |
| CMV reactivation | 3.64 (1.60–8.32) | .002∗ | 0.98 (0.33–2.92) | .974 |
| EBV reactivation | 1.18 (0.29–4.90) | .816 | ||
| Acute graft rejection | 0.74 (0.31–1.75) | .491 | ||
Table 4.
Univariate and multivariate analyses of associated factors of carbapenem-resistant Enterobacteriaceae infection.
| Univariate | Multivariate | |||
| Factors | HR (95% CI) | P-value | HR (95% CI) | 0-value |
| PELD score ≥22 | 13.49 (1.62–112.08) | .016∗ | 11.48 (1.37–96.18) | .024∗ |
| Exposure to third-generation cephalosporins within 3 months before LT | 1.03 (1.00–1.06) | .045∗ | 1.02 (0.99–1.06) | .182 |
3.5. Impact of MDR-GNB infection on mortality
Eight patients died within the first 3 months after LT, representing the overall mortality rate of 6.8%. However, only 2 patients died due to uncontrolled intra-abdominal infection and sepsis. There was no significant difference in the 3-month mortality between recipients with a history of MDR-GNB infection and non-MDR- GNB infection (HR 3.09 [CI 95% 0.74–12.95], P = .122). In univariate analysis, the 3-month mortality rate was significantly higher in patients with a history of CRE infection. However, no significant difference was found in after adjustment with a length of ICU stay, graft rejection, and re-operation.
4. Discussion
Bacterial infections remain a significant complication following pediatric LT. Gram-negative bacilli, particularly MDR strains, have emerged as predominant organisms among adult LT recipients. In this study, we retrospectively reviewed 118 pediatric LT recipients and noted a high rate of MDR-GNB infection. The identified risk factors for MDR-GNB infection in this study were exposure to third-generation cephalosporins within 3 months before LT, operation time, and length of ICU stay. To the best of our knowledge, this is the first study to identify the incidence and associated factors of MDR-GNB infection among pediatric LT recipients.
The incidence of bacterial infections following pediatric LT has varied among transplant centers, ranging from 46% to 79%. [7–10,15] From Kaplan–Meier analysis, 54% of patients developed bacterial infections within 3 months after LT. Half of the patients in this study experienced at least 1 episode of a bacterial infection within 47 days, and 25% of the patients had an episode of MDR-GNB infection within 25 days postoperation. Although the results from this study were considerably concordant with other centers, it is worth mentioning that the duration of postoperative antibiotic prophylaxis in our center was longer, which could be 1 contributing factor for acquiring drug-resistant strains of bacterial infections.[7–11]
MDR-GNB have emerged as predominant pathogens and become a significant cause of morbidity and mortality following LT.[16] Studies on MDR-GNB infection in adult LT suggested that the prevalence of infection caused by MDR-GNB ranged from 42% to 63%.[16–19] In this study, we found that the rate of MDR-GNB infection was as high as in previous studies in adults (62.4%). This result supports the increase in the prevalence of MDR-GNB infections among LT recipients. Liver transplant recipients are usually more susceptible to infections caused by gram-negative bacteria since surgical procedures and complications can potentially cause spillage of bacteria into the peritoneum and hepatobiliary system.[20] Previous reports showed that common MDR-GNB following LT were Enterobacteriaceae, P aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. We found that Enterobacteriaceae were the predominant pathogens (75.9%, 44 in 58 isolates), similar to other studies.[9,21–23]
In multivariate analysis, independent risk factors associated with MDR-GNB infection were exposure to third-generation cephalosporins within 3 months before LT, operation time, and length of ICU stay. Children with end-stage liver diseases are likely to be hospitalized repeatedly and inevitably exposed to antibiotics. Exposure to broad-spectrum antibiotics has been recognized as a significant risk factor for MDR-GNB acquisition, particularly extended-spectrum beta-lactamases-producing organisms.[16,19,24] Our study found that recent exposure to third-generation cephalosporins was an independent predictor of MDR-GNB infection in LT recipients. Majority of these patients required antibiotic therapy before LT due to infected biloma, a common complication of BA patients, or spontaneous bacterial peritonitis as the consequence of their end-stage liver diseases. This finding suggests that antibiotic stewardship in patients with end-stage liver diseases is an important measure in order to prevent and reduce the incidence of MDR-GNB infection after LT.
Operative factors predispose to MDR-GNB infection among LT candidates in previous studies.[19,25] The length of operation reflects the technical complexity of the surgical procedures. It is well recognized that pediatric LT operation, particularly LDLT in small children, is a technically complicated than adult LT with whole LT. Previous abdominal surgery, Kasai operation, and abdominal infection cause the operation more difficult and taking longer operation time. Therefore, abdominal infection in pediatric LT recipients is often higher than adult LT.[26,27] Prolonged operation time was associated with an increase in the risk of postoperative complications, including surgical site infections.[25] In this study, we found that the length of operation appeared to increase the risk of post-transplant MDR-GNB infection.
ICU stay is generally associated with mechanical ventilation, vascular access, and retaining of catheters. Thus, critically ill patients are vulnerable to infections, particularly nosocomial infections, which are often caused by drug-resistant organisms. The previous study among LDLT recipients showed that longer ICU stay was an independent factor for postoperative bacterial infections.[23] Our study supported that patients with a longer length of ICU stay had a higher risk of infection caused by MDR-GNB.
Interestingly, the rate of CRE among MDR-GNB infection is considerably high in this study (17.2%). We found that a high PELD score at the time of transplantation was an independent factor associated with CRE infection following pediatric LT. This finding agreed with previous studies in adult recipients, which demonstrated that a high pre-LT MELD score was associated with post-LT CRE infection.[28,29] This finding might be explained by the severity of liver diseases, as measured by MELD or PELD score at the time of transplantation. A previous study in cirrhotic patients has shown that a higher MELD score was associated with longer hospitalization, hence increased probability of being colonized by these organisms.[30] Moreover, these patients were more vulnerable to infection and then more exposed to antibiotics. Prior antibiotic exposure has been recognized as a risk factor for CRE colonization and infection in adults and children.[31] As the result, the risk of CRE infection in patients with severely impaired hepatic function could be inevitably increased. Therefore, early LT in cirrhotic children before the patients became very sick would be another preventive measure for CRE infection. To the best of our knowledge, this is the first study to identify the incidence of CRE infection and its risk factors among pediatric LT recipients.
This study has certain limitations. First, the nature of retrospective, single-center study might limit the generalization of the results. Second, pre-LT colonization was not identified in all patients. Therefore, we were unable to access the association between pre-transplant colonization with MDR-GNB and post-LT MDR-GNB infection.
In conclusion, we highlighted the high rate of bacterial infections caused by MDR-GNB following pediatric LT. To the best of our knowledge, this is the first study to show the incidence and associated factors of MDR-GNB infection following pediatric LT. Pre-LT exposure to third-generation cephalosporins, prolonged operation time, and length of ICU stay was associated with increased risk of MDR-GNB infection. Additionally, high PELD score before LT is a significant risk factor for CRE infection. Our findings emphasized that antimicrobial stewardship program is crucial in order to prevent and reduce antimicrobial resistance. Besides, pre-LT screening for stool carriage of drug-resistant organisms and strict surveillance for the emergence of MDR-GNB infection in at-risk patients after LT should be implemented. Furthermore, the awareness of the prevalence and resistant pattern is essential for guideline development to minimize the risk of MDR-GNB infection in this group of patients.
Acknowledgment
The authors thank Pawin Numthavaj, MD, PhD, from Department of Clinical Epidemiology and Biostatistics for statistic consultation.
Author contributions
Conceptualization: Chanita Phichaphop, Sophida Boonsathorn, Nopporn Apiwattanakul.
Data curation: Chanita Phichaphop.
Investigation: Chanita Phichaphop.
Methodology: Chanita Phichaphop, Sophida Boonsathorn, Nopporn Apiwattanakul.
Resources: Suporn Treepongkaruna, Chollasak Thirapattaraphan.
Supervision: Sophida Boonsathorn, Nopporn Apiwattanakul, Chonnamet Techasaensiri, Chatmanee Lertudomphonwanit, Suporn Treepongkaruna.
Validation: Sophida Boonsathorn.
Visualization: Sophida Boonsathorn.
Writing – original draft: Chanita Phichaphop.
Writing – review & editing: Sophida Boonsathorn.
Supplementary Material
Footnotes
Abbreviations: CMV = cytomegalovirus, CRE = carbapenem-resistant Enterobacteriaceae, ICU = intensive care unit, IQR = interquartile range, LT = liver transplantation, MDR-GNB = multidrug-resistant gram-negative bacteria, MELD = model for end-stage liver disease, PELD = pediatric end-stage liver disease.
How to cite this article: Phichaphop C, Apiwattanakul N, Techasaensiri C, Lertudomphonwanit C, Treepongkaruna S, Thirapattaraphan C, Boonsathorn S. High prevalence of multidrug-resistant gram-negative bacterial infection following pediatric liver transplantation. Medicine. 2020;99:45(e23169).
Summary: Bacterial infections caused by gram-negative bacilli remain problematic issue after liver transplantation. To the best of the authors’ knowledge, this is the first study examining the incidence and associated factors of multidrug-resistant gram-negative bacterial infection among pediatric liver transplant recipients.
This work is supported by the Faculty of Medicine Ramathibodi Hospital (COA. No. MURA2018/1044).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
CMV = cytomegalovirus, EBV = Epstein Barr virus, ETT = endotracheal intubation, LT = liver transplantation, PELD = pediatric end-stage liver disease, PTBD = percutaneous trans-hepatic biliary drainage.
Massive bleeding is defined as rate of blood loss >150 mL/min or Blood loss >1.5 mL/kg/min in 20 min or replacement of 50% of total blood volume within 3 h.
ESBL = extended spectrum beta-lactamase, MDR-GNB = multidrug-resistant gram-negative bacilli.
CMV = cytomegalovirus, EBV = Epstein-Barr virus, HR = hazard ratio, ICU = intensive care unit, LT = liver transplantation.
Statistically significant by Cox proportional hazard modelling.
HR = hazard ratio, LT = liver transplantation, PELD = pediatric end-stage liver disease.
Statistically significant by Cox proportional hazard modelling.
References
- [1].Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol 2014;20:6211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fishman JA. Infection in organ transplantation. Am J Transplant 2017;17:856–79. [DOI] [PubMed] [Google Scholar]
- [3].Hand J, Patel G. Multidrug-resistant organisms in liver transplant: mitigating risk and managing infections. Liver Transpl 2016;22:1143–53. [DOI] [PubMed] [Google Scholar]
- [4].Carraro A, Montin U, Violi P, et al. Multidrug-resistant combined infections in a liver transplanted patient: case report. Exp Clin Transplant 2018;16:340–3. [DOI] [PubMed] [Google Scholar]
- [5].Freire MP, Villela Soares Oshiro IC, Bonazzi PR, et al. Surveillance culture for multidrug-resistant gram-negative bacteria: performance in liver transplant recipients. Am J Infect Control 2017;45:e40–4. [DOI] [PubMed] [Google Scholar]
- [6].Giannella M, Bartoletti M, Morelli MC, et al. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization. Am J Transplant 2015;15:1708–15. [DOI] [PubMed] [Google Scholar]
- [7].Dohna Schwake C, Guiddir T, Cuzon G, et al. Bacterial infections in children after liver transplantation: a single-center surveillance study of 345 consecutive transplantations. Transpl Infect Dis 2020;22:e13208. [DOI] [PubMed] [Google Scholar]
- [8].Pouladfar G, Jafarpour Z, Malek Hosseini SA, et al. Bacterial infections in pediatric patients during early post liver transplant period: a prospective study in Iran. Transpl Infect Dis 2019;21:e13001. [DOI] [PubMed] [Google Scholar]
- [9].Kim JE, Oh SH, Kim KM, et al. Infections after living donor liver transplantation in children. J Korean Med Sci 2010;25:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shoji K, Funaki T, Kasahara M, et al. Risk factors for bloodstream infection after living-donor liver transplantation in children. Pediatr Infect Dis J 2015;34:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bouchut JC, Stamm D, Boillot O, et al. Postoperative infectious complications in paediatric liver transplantation: a study of 48 transplants. Paediatr Anaesth 2001;11:93–8. [DOI] [PubMed] [Google Scholar]
- [12].Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- [13].Humar A, Michaels M. Monitoring AIWGoID. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 2006;6:262–74. [DOI] [PubMed] [Google Scholar]
- [14].Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- [15].Kukreti V, Daoud H, Bola SS, et al. Early critical care course in children after liver transplant. Crit Care Res Pract 2014;2014:725748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi SH, Kong HS, Xu J, et al. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis 2009;11:405–12. [DOI] [PubMed] [Google Scholar]
- [17].Qiao B, Wu J, Wan Q, et al. Factors influencing mortality in abdominal solid organ transplant recipients with multidrug-resistant gram-negative bacteremia. BMC Infect Dis 2017;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Men TY, Wang JN, Li H, et al. Prevalence of multidrug-resistant gram-negative bacilli producing extended-spectrum beta-lactamases (ESBLs) and ESBL genes in solid organ transplant recipients. Transpl Infect Dis 2013;15:14–21. [DOI] [PubMed] [Google Scholar]
- [19].Zhong L, Men TY, Li H, et al. Multidrug-resistant gram-negative bacterial infections after liver transplantation - spectrum and risk factors. J Infect 2012;64:299–310. [DOI] [PubMed] [Google Scholar]
- [20].Lim S, Kim EJ, Lee TB, et al. Predictors of postoperative infectious complications in liver transplant recipients: experience of 185 consecutive cases. Korean J Intern Med 2018;33:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abad CL, Lahr BD, Razonable RR. Epidemiology and risk factors for infection after living donor liver transplantation. Liver Transpl 2017;23:465–77. [DOI] [PubMed] [Google Scholar]
- [22].Kim YJ, Kim SI, Wie SH, et al. Infectious complications in living-donor liver transplant recipients: a 9-year single-center experience. Transpl Infect Dis 2008;10:316–24. [DOI] [PubMed] [Google Scholar]
- [23].Li C, Wen TF, Mi K, et al. Analysis of infections in the first 3-month after living donor liver transplantation. World J Gastroenterol 2012;18:1975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bert F, Larroque B, Dondero F, et al. Risk factors associated with preoperative fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in liver transplant recipients. Transpl Infect Dis 2014;16:84–9. [DOI] [PubMed] [Google Scholar]
- [25].Cheng H, Chen BP, Soleas IM, et al. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect (Larchmt) 2017;18:722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Keough WL, Michaels MG. Infectious complications in pediatric solid organ transplantation. Pediatr Clin North Am 2003;50:1451–69. [DOI] [PubMed] [Google Scholar]
- [27].Muiesan P, Vergani D, Mieli-Vergani G. Liver transplantation in children. J Hepatol 2007;46:340–8. [DOI] [PubMed] [Google Scholar]
- [28].Pereira MR, Scully BF, Pouch SM, et al. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl 2015;21:1511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Freire MP, Oshiro IC, Pierrotti LC, et al. Carbapenem-resistant enterobacteriaceae acquired before liver transplantation: impact on recipient outcomes. Transplantation 2017;101:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Martel-Laferriere V, Homberger C, Bichoupan K, et al. MELD score and antibiotics use are predictors of length of stay in patients hospitalized with hepatic encephalopathy. BMC Gastroenterol 2014;14:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chiotos K, Han JH, Tamma PD. Carbapenem-resistant Enterobacteriaceae infections in children. Curr Infect Dis Rep 2016;18:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


