Abstract
Background:
Functional dyspepsia (FD) is a common functional gastrointestinal disease. Acupuncture, including electroacupuncture (EA) is widely used as a complementary and alternative treatment for patients with FD. This study aimed to explore the effectiveness of EA for the treatment of FD.
Methods:
We searched Embase, PubMed, and the Cochrane Central Register of Controlled Trials (Cochrane Library) for randomized controlled trials of FD treated by EA from inception to February 3, 2020. Two reviewers will independently screen studies for data extraction and assess the quality and risk of bias. The Cochrane Collaboration's risk of bias tool, RevMan 5.3 software were used for meta-analysis. Data were pooled to calculate relative risk and 95% confidence intervals (CIs) of substantial improvement after treatment for dichotomous data and mean differences (SMDs) and 95% CIs for continuous data.
Results:
Seven randomized clinical trials included 853 patients. This meta-analysis investigated the effectiveness of EA alone in the treatment of FD relative to sham-EA or pharmacologic medication (PM). The results showed that EA could significantly improve clinical symptoms. Compared with sham-EA, EA was more effective in reducing symptom scores (SMD −3.44, 95% CI −4.21 to −2.67) and increasing normal slow waves of electrogastrogram (SMD 0.93, 95% CI −0.30 to1.55). When EA was combined with PM, there was no significant difference in reducing symptom scores (SMD −0.18, 95% CI −0.51 to 0.16), increasing the effective rate of clinical symptoms (risk ratio 1.04, 95% CI 0.96 to 1.13), enhancing the level of plasma motilin (SMD 0.93, 95% CI −0.30 to1.55), and reducing gastric half-emptying time (SMD 0.02, 95% CI −0.16 to 0.20). The results also showed that there were very few adverse events reported.
Conclusion:
This meta-analysis suggests that EA is better than the placebo (sham-EA) in treating FD, and the therapeutic effect of EA on FD is equivalent to that of PM on FD. Compared with PM, EA for FD is safer and has fewer adverse reactions. Despite limitations due to the quality and number of the included studies, EA might be used as an effective and safe treatment for FD.
Keywords: electroacupuncture, functional dyspepsia, meta-analysis, systematic review
1. Introduction
Functional dyspepsia (FD) is a common functional gastrointestinal disease. According to the Rome IV criteria, FD has 2 subgroups: postprandial distress syndrome (PDS) with postprandial fullness or early satiation, and epigastric pain syndrome (EPS) with epigastric pain or epigastric burning.[1] According to investigations, the global incidence of FD is between 11% and 29.2%.[2] Recent data showed that according to the Rome IV standard, the prevalence of FD in the adult population of the United States, Canada, and the United Kingdom is about 10%.[3] In addition to potentially seriously affecting the quality of life, FD also places a huge financial burden on the health-care system.[4] FD is a heterogeneous disease, and its pathophysiological mechanism is still unclear. Different symptoms may have different pathophysiological mechanisms. It is traditionally believed that FD is associated with abnormal brain and gut interactions and disorders of gastric physiological factors, such as gastric emptying disorders, impaired gastric fundus receptivity after the meal, high sensitivity to mechanical dilatation, high sensitivity of duodenum to gastric acid, fat and dilatation stimulation, gastroduodenal dyspnea, and autonomic nerve dysfunction.[5] At present, the main treatment for FD are antacids, prokinetics (mosapride/domperidone), antidepressants, anxiolytics, herbal preparations, and Helicobacter pylori eradication.[6] However, these treatments do not achieve satisfactory relief in many patients with FD. Therefore, exploring new treatment methods may help to effectively relieve the symptoms of the patients with FD and improve their quality of life, and reduce medical costs.
Electroacupuncture (EA) is an improvement on traditional acupuncture, which stimulates acupuncture points by electricity rather than manual manipulation, and it seems to have more consistent repeatable results in both clinical and research settings.[25,26] EA has been widely used in many gastrointestinal disorders including FD as an effective alternative therapy. The effectiveness of EA on FD has been investigated in several studies.[7,8] As far as we know, no systematic review or meta-analysis of trials of using EA alone for FD has been conducted to date. Accordingly, the purpose of this study was to conduct a meta-analysis of randomized clinical trials that test the effectiveness of EA on FD.
2. Materials and methods
All analysis results of this study were based on previously published literature and therefore did not require ethical approval or patient consent.
2.1. Literature research
We searched Embase, PubMed, and the Cochrane Central Register of Controlled Trials (Cochrane Library) for randomized controlled trials of FD treated by EA from inception to February 3, 2020. Search terms were “electro-acupuncture” and “functional dyspepsia.” Papers published in English or Chinese were evaluated. Only randomized controlled trials (RCTs) with full texts available were reviewed. The detailed search strategy was attached. The search strategy for Pubmed was as follows:
1# Search (Functional Dyspepsia [MeSH Terms]) OR Functional Dyspepsia [Title/Abstract]
2# Search (EA [MeSH Terms]) OR Electroacupuncture [Title/Abstract]
3# Search (((Functional Dyspepsia [MeSH Terms]) OR Functional Dyspepsia [Title/Abstract])) AND ((Electroacupuncture [MeSH Terms]) OR Electroacupuncture [Title/Abstract]).
2.2. Inclusion criteria
Studies meeting the following criteria were considered to be eligible for inclusion:
-
(1)
inclusion only of patients diagnosed with FD;
-
(2)
the type of publication must be an RCT;
-
(3)
using EA alone to intervene;
-
(4)
comparison of EA with pharmacologic medication (PM) or sham-EA;
-
(5)
complete experimental and control data.
Any disagreement during the course will be resolved by discussion between the 2 reviewers (Mengxue Luo and Yuyan Pan). If a consensus cannot be achieved, an independent reviewer (Tao Zhang) will be consulted. Details of the selection procedure for studies are shown in a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart.
2.3. Exclusion criteria
The excluded studies with the following reasons:
-
(1)
the studies did not meet the above criteria;
-
(2)
the treatment group combined with other therapies;
-
(3)
the studies were conducted in the form of letters, abstracts, reviews, meta-analysis, animal experiments or comments;
-
(4)
the studies could not extract relevant data;
-
(5)
duplicate articles.
2.4. Data extraction
The internal consistency of all data was checked, and disagreements were resolved by discussion between reviewers. From the final selection of papers, general study information on first author's name, year of publication, study location, the number of patients, criteria for diagnosis, experimental and control intervention, intervention time, adverse events and results relating to outcome measures will be extracted.
2.5. Quality assessment
Two investigators (Qian Liu and Yang Yang) independently extracted data and assessed the risk of bias using the Cochrane Collaboration's risk of bias tool,[9] which consists of the following 7 domains that may bring the potential risks of overestimating or underestimating an intervention effect: sequence generation, blinding of participants, blinding of outcome assessors, allocation concealment, incomplete outcome data, selective outcome reporting, and other sources of bias. The assessment will rank the risk levels according to the categories of low risk, high risk, and unclear risk.
2.6. Outcome measures
Symptom score was the main outcome measure, and the secondary outcome measures were effective rate, electrogastrogram (EGG), gastric empty, and motilin level.
2.7. Data synthesis and statistical analysis
RevMan 5.3 (Cochrane, London) was used to analyze the data. Dichotomous data were expressed as relative risk (RR) and continuous variables as standardized mean difference (SMD) with 95% confidence interval (CI). Both the x2 test and I2 statistics were used for the assessment of heterogeneity.[10] A fixed-effects model was used if there was no obvious heterogeneity (I2 < 50% or P > .1) and a random-effects model was used if significant heterogeneity existed (50% < I2 < 80% or P < .05). A descriptive analysis was implemented if the heterogeneity was substantial (I2 > 80% or P < .01).[11] When the necessary data is available, a subgroup analysis of different treatment methods were performed. Analysis of funnel plot symmetry was used to identify the existence of publication bias.
3. Results
3.1. Literature search and study sample characteristics
A total of 84 citations were identified during the initial search and 7 articles were finally selected (Fig. 1). One[12] of the articles included a chronic clinical trial (CCT) and an acute clinical trial (ACT). The 7 articles[12–18] included 8 RCT with a total of 853 patients (426 and 427 in the treatment and control groups, respectively). 5 articles[12–16] were published in English and 2 articles[17,18] were published in Chinese. All of the trials conducted in China. In all the trials, the intervention was EA alone in the experimental group and Sham-EA or PM in the control group. Detailed information is provided in Table 1 (see online supplementary material).
Figure 1.

Included eligibility screening identification. Flow diagram of the study selection process. Identification of 7 eligible randomized, placebo-controlled, clinical trials.
Table 1.
Characteristics of the included studies.
| Diagnostic | Treatment | Interventions | Outcome | ||||
| Authors | County | patients (T/C) | criteria | Duration | T | C | measurements |
| Zheng et al (2018)[13] | China | 100/100 | Rome III | 4 wk | EA | Sham-EA | 1. Effective rate |
| 2. LDQ scores | |||||||
| 3. NDI | |||||||
| Qiang et al (2018)[14] | China | 32/32 | Rome III | 30 d | EA | Mosapride | 1. Effective rate |
| 2. LDQ scores | |||||||
| 3. FDDQL scores | |||||||
| 4. Ghrelin, CGRP and GLP-1 level | |||||||
| Zhang et al (2015)[15] | China | 159/160 | Rome III | 4 wk | EA | Mosapride | 1. Effective rate |
| 2. Symptom scores | |||||||
| 3. SF-36 scores | |||||||
| 4. Plasma motilin level | |||||||
| 5. EGG | |||||||
| 6. Gastric empty | |||||||
| Xu et al (2015)[16] | China | 8/8 | Rome III | 30 min | EA | Sham-EA | 1. EGG |
| 2. HRV | |||||||
| 3. Symptom scores | |||||||
| Guo et al (2011)[17] | China | 80/80 | Rome III | 6 wk | EA | Mosapride, | 1. Symptom scores Omeprazole, |
| 2. Effective rate Amitriptyline | |||||||
| 3. NDSI scores | |||||||
| 4. SF-36 scores | |||||||
| 5. EGG | |||||||
| 6. Plasma motilin | |||||||
| 7. Gastric empty | |||||||
| Peng et al (2008)[18] | China | 20/20 | Rome III | 2 wk | EA | Domperidone | 1. Symptom scores |
| 2. SAS | |||||||
| 3. SDS | |||||||
| 4. HRV | |||||||
| 5. EGG | |||||||
| 6. Neuropeptide level | |||||||
| Liu et al(ACT) (2008)[12] | China | 27/27 | Rome II | 30 min | EA | Sham-EA | 1. HRV |
| 2. EGG | |||||||
| Liu et al(CCT) (2008)[12] | China | 27/27 | Rome II | 2 wk | EA | Sham-EA | 1. Symptom scores |
| 2. HRV | |||||||
| 3. EGG | |||||||
| 4. Neuropeptide level | |||||||
| 5. Plasma motilin level | |||||||
3.2. Risk of bias
All articles included in the analysis were designed as randomized clinical trial studies. Five studies[12–15,17] used random number tables or lists, while the others did not detail the specific methods of randomization. An attempt to contact the authors to clarify the method did not generate any responses. There was 3 sham intervention (sham-EA)[12,13,16] in the included studies and participant blinding was deemed to be applicable. Three trials[13–15] reported dropout rates of patients. All trials reported all outcome measurements mentioned in the methods and were therefore deemed to be at low risk of attrition bias. For other sources of bias, all studies were rated as an unclear risk because of the lack of registration information. (Fig. 2)
Figure 2.

Risk of bias graph.
3.3. Meta-analysis results
3.3.1. Symptom score
3.3.1.1. EA vs. Sham-EA
Two trials[12,16] reported the symptom scores. A total of 70 patients (35 and 35 in the EA and the Sham-EA groups, respectively) were included in the analysis. When EA was compared with sham-EA, there was a significant improvement in symptom scores (SMD −3.44, 95% CI−4.21 to −2.67; P = .30) with no significant heterogeneity (I2 = 0%, P = .43). (Fig. 3A)
Figure 3.

A, Forest plots of symptom score in the EA vs Sham-EA. B, Forest plots of symptom score in the EA vs. PM (subgroup analysis).
3.3.1.2. EA vs. PM
Three trials[15,17,18] reported the symptom scores. A total of 519 patients (259 and 260 in the EA and PM, respectively) were included in the analysis. The pooled results showed that EA was equivalent to PM in reducing symptom scores of FD (SMD −0.18, 95% CI −0.51 to 0.16; P = .30) with high heterogeneity (I2 = 63%, P = .07). (Fig. 3B) Sensitivity analysis and subgroup analysis were performed to identify the source of heterogeneity.
3.3.2. Sensitivity analysis
The included studies will be excluded 1 by 1 for sensitivity analysis. The heterogeneity of meta-analysis result was decreased when 2008 Peng[18] was excluded (SMD −0.03, 95% CI −0.21 to 0.15; I2 = 0%, P = .97), suggesting that the meta-analysis results were not robust and 2008 Peng[18] was 1 of the sources of heterogeneity (Table 2).
Table 2.
Sensitivity analysis of symptom scores.
3.3.3. Subgroup analysis
Figure 3B shows the results of the meta-analyses of trials comparing EA alone versus prokinetic agents alone (mosapride, domperidone)[15,18] and drug combination (mosapride, omeprazole, amitriptyline) respectively.[17] The pooled results showed that EA was equivalent to prokinetic agents in reducing symptom scores of FD (SMD −0.37, 95% CI −1.15 to 0.40) with high heterogeneity (I2 = 81%, P = .02).
3.3.4. Dominant frequency of EGG
Three trials[12,16] reported the dominant frequency of EGG. A total of 124 patients (62 and 62 in the EA and the Sham-EA groups, respectively) were included in the analysis. The pooled results showed EA was equivalent to sham-EA in improving dominant frequency of EGG (SMD −0.69, 95% CI −3.02 to 4.40; P = .71) with high heterogeneity (I2 = 97%, P < .00001). (Fig. 4) Sensitivity analysis was conducted by eliminating studies 1 by 1, and no significant changes were observed after combining the results, indicating that the results of the study were relatively stable. (Fig. 4).
Figure 4.

Forest plot of the dominant frequency of EGG (EA vs Sham-EA).
3.3.5. Percentage of normal slow waves of EGG
Three trials[12,16] reported the normal slow waves of EGG. A total of 124 patients (62 and 62 in the EA and the Sham-EA groups, respectively) were included in the analysis. The pooled results showed EA could increase normal slow waves of EGG compared to the sham-EA (SMD 0.93, 95% CI −0.30 to1.55; P = .004) with high heterogeneity (I2 = 57%, P = .10). (Fig. 5)
Figure 5.

Forests plot of percentage of normal slow waves of EGG (EA vs Sham-EA).
3.3.6. Effective rate
Three trials[14,15,17] reported the normal slow waves of EGG. A total of 543 patients (271 and 272 in the EA and PM groups, respectively) were included in the analysis. The pooled results showed EA was equivalent to PM in improving effective rate of clinical symptoms (RR 1.04, 95% CI 0.96 to 1.13; P = .35) with low heterogeneity (I2 = 45%, P = .16). (Fig. 6)
Figure 6.
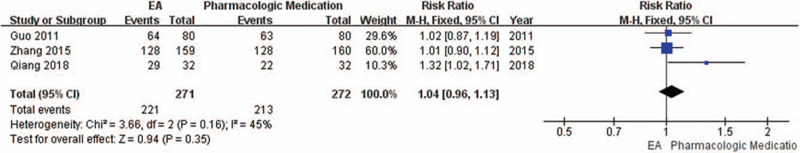
Forest plot of effective rate (EA vs PM).
3.3.7. Plasma motilin level
Three trials[15,17,18] reported the plasma motilin. A total of 519 patients (259 and 260 in the EA and PM groups, respectively) were included in the analysis. The pooled results showed EA was equivalent to PM in improving level of plasma motilin (SMD 0.93, 95% CI −0.30 to1.55; p = 0.50) with no significant heterogeneity (I2 = 0%, P = .61). (Fig. 7)
Figure 7.

Forest plot of plasma motilin level (EA vs PM).
3.3.8. Gastric half-emptying time
Two trials[15,17] reported the gastric half-emptying time. A total of 479 patients (239 and 240 in the EA and PM groups, respectively) were included in the analysis. The pooled results showed EA was equivalent to PM in reducing gastric half-emptying time (SMD 0.02, 95% CI −0.16 to 0.20; P = .84) with no significant heterogeneity (I2 = 0%, P = .93). (Fig. 8)
Figure 8.

Forest plot of gastric half-emptying time (EA vs PM).
3.3.9. Adverse events
Adverse events were reported in 3 of the studies. Zhang et al[13] reported 5 events, including subcutaneous hemorrhage (3 events), faint during acupuncture needling (1 event), and marasmus during acupuncture (1 event). The rate of adverse events was not significantly different between the EA and the sham-EA group. Zhang et al[15] reported mild diarrhea in 2 patients receiving PM during the initial stages of treatment, which spontaneously resolved within 5 days. Guo et al[17] reported zero adverse events in their trial. By contrast, other studies[12,14,16,18] did not report adverse events.
3.3.10. Publication bias
As shown in Figure 9, our funnel plot analysis of symptom scores[12,15–18] was asymmetrical, which suggested that there may be some publication bias among the included studies.
Figure 9.

Funnel plot.
4. Discussion
4.1. Main findings
This meta-analysis investigated the effectiveness of EA (used alone) in the treatment of FD relative to sham-EA or PM. The results showed that EA could significantly improve clinical symptoms. When EA was compared with sham-EA, there was a significant increase in symptom scores improvement (SMD −3.44, 95% CI −4.21 to −2.67; P = .30) and normal slow waves of EGG (SMD 0.93, 95% CI −0.30 to1.55; P = .004). When EA was compared with PM, there was no significant difference in reducing symptom scores (SMD −0.18, 95% CI −0.51 to 0.16; P = .30), increasing effective rate of clinical symptoms (RR 1.04, 95% CI 0.96 to 1.13; P = 0.35) enhancing level of plasma motilin (SMD 0.93, 95% CI −0.30 to1.55; P = .50) and reducing gastric half-emptying time (SMD 0.02, 95% CI −0.16 to 0.20; P = .84). Furthermore, results also suggest that there were minimal reported adverse events.
4.2. Interpretation
Functional dyspepsia is 1 of the most common functional gastrointestinal diseases, which includes 3 subtypes that may have different pathophysiology and etiology: PDS, EPS, and a subtype with overlapping PDS and EPS characteristics. Symptoms of functional dyspepsia may be caused by dyspepsia, gastric paresthesia, or inflammation of the stomach and duodenum. Besides, comorbidity, psychopathology, and personality traits may play a role. Among them, decreased gastric motility (delayed gastric emptying) and disorders of gut-brain interaction are important physiological characteristics.[19] Due to the heterogeneity of FD, drugs such as eradication of helicobacter pylori, inhibition of gastric acid, improvement of gastrointestinal peristalsis, and anti-depressants have limited effects, and long-term use of these drugs may also lead to adverse reactions.[20] According to the body surface gastric electrical signals, the EGG, which can show the electrical activity of the gastric smooth muscle, indirectly reflects the ionization activity and the relaxation and contraction situation of the gastric wall muscle, and objectively reflects the gastrointestinal dynamic status.[21] In the study published in the current issue, Zheng et al found that 16-week EA treatment was more effective than 16-week sham EA treatment: a higher percentage of patients had no symptoms of dyspepsia at all or improved symptoms of dyspepsia.[13] Previously, many studies have explored the influence of EA on FD. A recent meta-analysis of 20 studies and 1,423 patients suggests that the role of EA in the treatment of FD is positive compared to the sham treatment.[22] At the same time, transcutaneous electroacupuncture can improve gastric pacing activity, increase neuropeptide Y and motilin gastrointestinal hormones, enhance vagus nerve activity.[12] In a similar clinical study, transcutaneous electroacupuncture was found to significantly improve FD symptoms and quality of life as well as gastric regulation and gastric emptying.[23] In FD patients with gastroparesis, EA at ST36 and PC6 can accelerate gastric solid emptying by scintigraphy.[27] Zhou J et al found that auricular EA can improve the gastric sensitivity of FD rats by improving the balance of sympathetic vagus nerve.[24] Various clinical studies have reported that the improvement effect of EA on gastric dysrhythmia is consistent and repeatable, suggesting that EA has a strong effect on the treatment of gastric slow-wave dysrhythmia.[28–30]
At present, PMs mainly play a role in relieving the symptoms of FD, commonly including prokinetic drugs, acid suppressants, and psychotropic drugs, such as mosapride, omeprazole, and amitriptyline. There is a common problem existed in these drugs, they tend to have a single effect and are not safe for long-term use. Through analysis of the included literature, it was found that EA can better relieve FD-related symptoms compared with PM. The main points that may be selected are Zusanli (ST 36), Zhongwan (CV 12), Neiguan (PC 6), Taichong (LR 3), and Gongsun (SP 4). The treatment plan may be a stimulation frequency of 2 Hz/100 Hz, 2 times a day, each lasting 30 minutes, continuous treatment for 4 weeks.[13,14] However, there is greater heterogeneity, which is due to the insufficient literature and sample size included, which requires more high-quality clinical studies to verify.
4.3. Strengths and limitations
There are several strengths to our study. To the best of our knowledge, this is the first published systematic review and meta-analysis to investigate the effectiveness and safety of EA alone in the treatment of FD. Several outcome measures were used to comprehensively evaluate effectiveness and safety. However, there are also limitations to this meta-analysis.
-
(1)
The trials included in the analysis were limited and the sample sizes were relatively small.
-
(2)
All of the trials were carried out in China and 2 studies were published in Chinese. Accordingly, there is a high risk of publication bias (as suggested by our funnel plot analysis).
-
(3)
the effect values of HRV, NDI, and other outcomes cannot be combined due to different control groups and assessment criteria.
-
(4)
But not least, the methodological quality of some included studies was poor.
4.4. Implications for further research
Based on this meta-analysis, several issues need to be addressed to improve the methodological quality of future clinical studies.
-
(1)
The randomization procedure, allocation concealment and blinding methods should be explicitly described and fully reported.
-
(2)
Withdrawal/dropout and adverse events during the study should be clearly reported.
-
(3)
Diagnostic and evaluation criteria should be unified and standardized.
-
(4)
All clinical trials should be prospectively registered and a link to the protocol should be provided in the published article.
5. Conclusions
This meta-analysis suggests that EA is better than the placebo (sham-EA) in treating FD, which is equivalent to PM. And it has higher safety and fewer adverse reactions. Due to the generally small sample size and poor methodological quality of the included studies, it is impossible to draw a definitive conclusion and hence our findings should be interpreted with caution. Therefore, a large number of RCTs with high quality, large samples, multi-centers, and relatively uniform evaluation criteria are still needed to further verify the results and provide strong evidence for the superiority of EA in the treatment of FD.
Author contributions
Conceptualization: Jiande JD Chen, Peijing Rong, Wei Wei.
Data curation: Xiaolan Su, Yang Yang.
Formal analysis: Wenchao Ni, Sijing Du.
Investigation: Tao Zhang, Qian Liu.
Methodology: Yu Guo, Song Guo.
Resources: Mengxue Luo, Yuyan Pan.
Software: Song Guo, Baoqi Wu.
Writing – original draft: Xinyong Mao, Song Guo.
Writing – review & editing: Xinyong Mao, Song Guo.
Footnotes
Abbreviations: CI = confidence interval, EA = electroacupuncture, EGG = electrogastrogram, EPS = epigastric pain syndrome, FD = functional dyspepsia, PDS = postprandial distress syndrome, PM = pharmacologic medication, RCTs = randomized controlled trials, RR = risk ratio, SMD = standardized mean difference.
How to cite this article: Mao X, Guo S, Ni W, Zhang T, Liu Q, Du S, Luo M, Pan Y, Wu B, Su X, Yang Y, Guo Y, Chen JJ, Rong P, Wei W. Electroacupuncture for the treatment of functional dyspepsia: A systematic review and meta-analysis. Medicine. 2020;99:45(e23014).
XM, SG, and WN contributed equally to this work and should be considered co-first authors.
This research was supported by the National Natural Science Foundation of China (Grant nos. 81820108033).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
C = control group, CGRP = calcitonin gene-related peptide, EA = electroacupuncture, EGG = electrogastrogram, FDDQL = functional digestive disorder quality of life, HRV = heart rate variability, LDQ = Leeds Dyspepsia Questionnaire, NDI = Nepean dyspepsia index, SAS = Self-rating Anxiety Scale, SDS = Selfrating Depression Scale, SF-36 = 36-item Short Form Health Survey, T = trial group.
SMD = standardized mean difference.
References
- [1].Vincenzo S, Francis KL, Chan, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380–92. [DOI] [PubMed] [Google Scholar]
- [2].Mahadeva S, Goh KL. Epidemiology of functional dyspepsia:a global perspective. World J Gastroenterol 2006;12:2661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aziz I, Palsson OS, Törnblom H, et al. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study. Lancet Gastroenterol Hepatol 2018;3:252–62. [DOI] [PubMed] [Google Scholar]
- [4].Brook RA, Kleinman NL, Choung RS, et al. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol 2010;8:498–503. [DOI] [PubMed] [Google Scholar]
- [5].Carbone F, Tank J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig Dis Sci 2014;32:222–9. [DOI] [PubMed] [Google Scholar]
- [6].Imke Masuy, Lukas Van Oudenhove, Jan Tack. Review article: treatment options for functional dyspepsia. Aliment Pharmacol Ther 2019;49:1134–72. [DOI] [PubMed] [Google Scholar]
- [7].Zeng F, Lan L, Tang Y, et al. Cerebral responses to puncturing at different acupoints for treating meal-related functional dyspepsia. Neurogastroenterol Motil 2015;27:559–68. [DOI] [PubMed] [Google Scholar]
- [8].Robin ST Ho, Charlene HL Wong, Vincent CH Chung. Medical synopsis: can acupuncture be an alternative treatment option for patients with refractory functional dyspepsia? Advances in Intergrative Medicine 2015;2:143–5. [Google Scholar]
- [9].Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration 2011; Online document Available at: http://handbook.cochrane.org/. [Google Scholar]
- [10].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [12].Liu S, Peng S, Hou X, et al. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol Motil 2008;20:1204–11. [DOI] [PubMed] [Google Scholar]
- [13].Zheng H, Xu J, Sun X, et al. Electroacupuncture for patients with refractory functional dyspepsia: a randomized controlled trial. Neurogastroenterol Motil 2018;30:e13316. [DOI] [PubMed] [Google Scholar]
- [14].Liming Qiang, Yuan JIANG. Electroacupuncture for functional dyspepsia and the influence on serum Ghrelin, CGRP and GLP-1 levels. World Journal of Acupuncture - Moxibustion 2018;28:86–90. [Google Scholar]
- [15].Zhang CX, Guo LK. Dalitong granule combined with electroacupuncture in the treatment of functional dyspepsia: a randomized controlled trial. Chin J Integr Med 2015;21:743–50. [DOI] [PubMed] [Google Scholar]
- [16].Xu F, Tan Y, Huang Z, et al. Ameliorating effect of transcutaneous electroacupuncture on impaired gastric accommodation in patients with postprandial distress syndrome-predominant functional dyspepsia: a Pilot Study. Evid Based Complement Alternat Med 2015;2015:168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li-ke GUO, Chao-xian ZHANG, G.U.O Xiao-feng. Long-term efficacy and safety research on functional dyspepsia treated with electroacpuncture and Zhizhu Kuangzhong capsule. Chinese Acupuncture & Moxibustion 2011;12:1071–7. [PubMed] [Google Scholar]
- [18].Sui-Feng Peng, Jia-Yao Yang, Zhao-Hong Shi. Electroacupuncture improves gastric motility, autonomic nerve activity and psychological state in patients with functional dyspepsia. World Chinese Journal of Digestology 2008;16:4105–9. [Google Scholar]
- [19].Paul Enck, Fernando Azpiroz, Guy Boeckxstaens, et al. Functional dyspepsia. Nat Rev Dis Primers 2017;3:17081. [DOI] [PubMed] [Google Scholar]
- [20].Moayyedi PM, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol 2017;112:988–1013. [DOI] [PubMed] [Google Scholar]
- [21].Agrusa AS, Gharibans AA, Allegra A, et al. A deep convolutional neural network approach to classify normal and abnormal gastric slow wave initiation from the high resolution electrogastrogram. IEEE Trans Biomed Eng 2019;12.doi: 10.1109/TBME. [DOI] [PubMed] [Google Scholar]
- [22].Kim KN, Chung SY, Cho SH. Efficacy of acupuncture treatment for functional dyspepsia: a systematic review and meta-analysis. Complement Ther Med 2015;23:759–66. [DOI] [PubMed] [Google Scholar]
- [23].Ji T, Li X, Lin L, et al. An alternative to current therapies of functional dyspepsia: self-administrated transcutaneous electroacupuncture improves dyspeptic symptoms. Evid Based Complement Alternat Med 2014;2014:832523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou J, Li S, Wang Y, et al. Effects and mechanisms of auricular electroacupuncture on gastric hypersensitivity in a rodent model of functional dyspepsia. PLoS One 2017;12:e0174568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li Y, Tougas G, Chiverton SG, et al. The effect of acupuncture on gastrointestinal function and disorders. Am J Gastroenterol 1992;87:1372–81. [PubMed] [Google Scholar]
- [26].Lux G, Hagel J, Backer P, et al. Acupuncture inhibits vagal gastric acid secretion stimulated by sham feeding in healthy subjects. Gut 1994;35:1026–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu S, Hou X, Zha H, et al. Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia. Dig Dis Sci 2006;51:2154–9. [DOI] [PubMed] [Google Scholar]
- [28].Chang CS, Chou JW, Ko CW, et al. Cutaneous electrical stimulation of acupuncture points may enhance gastric myoelectrical regularity. Digestion 2002;66:106–11. [DOI] [PubMed] [Google Scholar]
- [29].Chou JW, Chang YH, Chang CS, et al. The effect of different frequency electrical acustimulation on gastric myoelectrical activity in healthy subjects. Hepatogastroenterology 2003;50:582–6. [PubMed] [Google Scholar]
- [30].Shiotani A, Tatewaki M, Hoshino E, et al. Effects of electroacupuncture on gastric myoelectrical activity in healthy humans. Neurogastroenterol Motil 2004;16:293–8. [DOI] [PubMed] [Google Scholar]


