Supplemental Digital Content is available in the text
Keywords: breast cancer, meta-analysis, sunscreen, ultraviolet radiation
Abstract
The relationship between solar ultraviolet radiation and the risk of breast cancer is conflicting. The purpose of our study was to quantitatively assess the relationship between solar ultraviolet radiation and breast cancer risk and to analyze related factors such as age and sunscreen use.
Articles indexed in PubMed and Embase and published between January 2005 and March 2020 were searched for relevant keywords. The relative risk was calculated using random-effect or fixed-effect models in the meta-analysis and dose-response meta-analysis, which were conducted according to the Meta-Analyses of Observational Studies in Epidemiology reporting guidelines. Sensitivity analyses for heterogeneity and publication bias were evaluated.
Six studies were eligible for inclusion in the meta-analysis, and three of these were included in the dose-response analysis. We found a correlation between exposure to solar ultraviolet radiation and breast cancer risk (relative risk: 0.70, 95% confidence interval: 0.65, 0.75). We also found a linear dose-response relationship between the exposure and breast cancer risk (relative risk: 0.86, 95% confidence interval: 0.81, 0.91) in women over 40. Not tanning and covering the limbs were associated with breast cancer risk, but sunscreen use was not.
Exposure to solar ultraviolet radiation is negatively correlated with breast cancer risk, and the association is linear in women over 40. This is the first dose-response meta-analysis on the topic, and the influence of factors such as estrogen receptor status, occupational exposure, and ethnicity requires in-depth study.
1. Introduction
Breast cancer is one of the most common malignancies in women, accounting for approximately 30% of tumors in women in the United States in 2019.[1] Studies have shown that age, family history, age at first menstruation and at menopause, use of progesterone and related products, gene mutations, smoking, and other lifestyle factors affect the risk of developing breast cancer.[2]
Exposure to ultraviolet radiation is associated with the development of a number of diseases, such as actinic keratosis and skin cancer.[3,4] 50% to 70% of squamous cell carcinomas and 50% to 90% of basal cell carcinomas in Caucasians are associated with ultraviolet radiation,[5] but certain wavelengths of sunlight (290–310 nm) may reduce the risk of breast cancer and some other tumors.[6] In a cohort study on the relationship between solar ultraviolet radiation and breast cancer, exposure was not associated with the overall risk of invasive breast cancer or with estrogen receptor status.[7] Other ecological and cohort studies have suggested that exposure to sunlight may actually reduce the risk of breast cancer.[8–10]
A previous meta-analysis showed that exposure to ultraviolet radiation may reduce the risk of breast cancer[11] but did not assess the dose-response relationship and did not explicitly describe the relationship between sunscreen use, limb coverage, and breast cancer risk. In addition, the role of age in modifying such as association is unknown. Therefore, we undertook to quantitatively assess the association between exposure to solar ultraviolet radiation and breast cancer risk.
The dose-response meta-analysis offers unique advantages over the traditional meta-analysis, as it can assess a relationship quantitatively and graphically. The purpose of this study was to quantitatively analyze the relationship between exposure to solar ultraviolet radiation and breast cancer risk. We extracted data from case-control studies, evaluated the quality of the studies, and conducted a dose-response meta-analysis stratified by age. Next, we analyzed risk factors for breast cancer that are related to the exposure, such as sunscreen use and limb coverage. Finally, we investigated whether estrogen receptor or progesterone receptor status affected the relationship.
2. Methods
2.1. Search strategy
We searched the PubMed and Embase databases using the following keywords in the search bar: “sunlight,” “ultraviolet,” “breast cancer,” “sunscreen,” “sun protection,” and “risk.” We limited the publication dates to January 2005 to March 2020. We also collected articles from reference lists, observing the following criteria: the study is a case-control study; the article must include the term “breast cancer”; exposure factors must include ultraviolet light; the article must specify the relative risk, advantage ratio, or risk ratio adjusted for confounders, and the corresponding confidence interval (CI) can be obtained or calculated from the article. We conducted the meta-analysis following the Meta-Analyses of Observational Studies in Epidemiology reporting guidelines.
2.2. Data extraction
We obtained the following information from the articles included: first author's last name, year of publication, geographical location, sample size, how ultraviolet exposure was measured, and confounding factors assessed in each study. The CIs and effect quantities (relative risk and odds ratio) under various exposure categories were extracted. An approximate relative risk value can also be obtained from the odds ratio in case-control studies.[12] If a study reported multiple exposure periods (ages), the information was recorded for each exposure period. All information was stored in a structured table.
2.3. Statistical analysis
In the meta-analysis, we set the minimum exposure interval from the articles as a reference, and compared the maximum exposure intervals to assess the effect on breast cancer risk. Other factors, such as sunscreen use and limb coverage, were also set as references and used for comparisons. The heterogeneity of effects between studies was evaluated by the I2 statistic, and I2 < 50 or a Q test P-value < .1 indicated significant heterogeneity.[13] We used both random-effect and fixed-effect models. A sensitivity analysis was conducted by excluding 1 study at a time (“leave one out” approach) to assess whether the effect values changed significantly. Funnel plots, Begg test, and Egger test were used to assess publication bias.
We performed a dose-response analysis of the relationship between the exposure dose of solar ultraviolet radiation and breast cancer, took the midpoint of the upper and lower limits of the exposure dose for each interval as the average exposure dose, and assumed that the threshold value of the open interval would have the same range as the interval of the nearest category. The general least-squares method proposed by Greenland and Longnecker was used to perform the dose-response analysis.[14,15] We investigated the association between the exposure dose and breast cancer risk using a restricted cubic spline model with 4 fixed percentiles (5%, 35%, 65%, and 95%) of exposure distribution.[16] The between-study variance of the linear dose response and the between-study covariance matrices of the non-linear dose response were evaluated by the Greenland and Longnecker method. The nonlinear P-value was calculated after checking that the coefficient of the second spline was zero.[17] All analyses were performed in Stata 14.0 (StataCorp, College Station, TX), and the “glst” and “xblc” packages were used for dose-response data analysis and graphing.
2.4. Assessment of study quality
We used the Newcastle–Ottawa Scale (NOS) for observational studies to assess the quality of the studies and the risk of bias (Supplemental files “NOS questions” and “NOS scoring, ”). NOS evaluates, study quality and the risk of bias from the selection of cases and controls, the comparability of cases and controls, and the ascertainment of exposure and non-response rates.[18] We modified the scoring criteria to account for adjustments for age and other confounders. Studies that adjusted for age and 5 or more confounders received 2 stars, studies that only adjusted for age or had ambiguous confounders received 1 star, and studies that did not adjust for confounders received no stars. Standards in selection and ascertainment earned the studies more stars. We used this format because a simple quality score based on the level of evidence does not provide sufficient stratification. The maximum NOS score is 9 stars. Studies with 5 or more stars were considered to be of high quality.[19]
3. Results
3.1. Literature search and study selection
We searched PubMed and Embase using the designated keywords and obtained articles from references. A total of 594 records were retrieved, 144 duplicate articles were excluded, and 436 articles were excluded after review of titles or abstracts (Fig. 1). The full texts of 14 articles were read and 8 records were excluded because they were not case-control studies. Six articles were included in the analysis (Table 1).[20–25]
Figure 1.
Flow chart of study selection.
Table 1.
Characteristics of eligible studies investigating the association between exposure to solar ultraviolet radiation and breast cancer risk.
| Article | Country | Sample size (cases) | Exposure | Adjustments | Study quality (star rating) |
| John 2007[20] | USA | 4183 (2054) | Self-reported lifetime outdoor activity | Age, race/ethnicity, education, family history of breast cancer, personal history of benign breast disease, age at menarche, number of full-term pregnancies, breastfeeding, body mass index, height, physical activity, and alcohol consumption | ∗∗∗∗∗∗∗∗ |
| Knight 2007[21] | Canada | 2107 (972) | Time spent outdoors | Reference age, ethnicity, family history in first-degree relatives, ever breast-fed, education, age menarche, and age at first birth | ∗∗∗∗∗∗∗∗ |
| Blackmore 2008[22] | Canada | 1894 (759) | Outdoor activity | Age, ethnicity, family history, ever breastfed, education, age menarche, age at first birth | ∗∗∗∗∗∗∗∗ |
| Aderson 2011[23] | Canada | 6521 (3101) | Time spent in the sun | Age, marital status, education, ethnicity,body mass index,smoking status and packyears, breastfeeding, lactation, age at menarche, Oral Contraceptive use, Oral Contraceptive duration,parity, age at first live birth, age at last menstruation, duration of Hormone Replacement Therapy use, history of benign breast disease, family history of breast cancer,screening mammogram,alcohol intake, fat intake,calorie intake, physical activity, phytoestrogen intake, vitamin D and calcium intake | ∗∗∗∗∗∗∗∗ |
| Bidgoli 2014[24] | Iran | 176 (60) | Daily sunlight exposure | Not specified | ∗∗∗∗∗∗∗ |
| Qin 2019[25] | USA | 1402 (1015) | Time spent outdoors | Age, education, age at menarche, menopausal status, age at first birth, ever breastfeeding, first-degree family history of breast cancer, history of benign breast disease, body mass index, vigorous physical activity, total energy intake, and total vitamin D intake. | ∗∗∗∗∗∗∗∗ |
3.2. Summary relative risk for the highest versus lowest category of exposure to solar ultraviolet radiation
Three of the articles[21–23] contained 16 sub-studies; we integrated the sub-studies as separate research results (Fig. 2). The overall relative risk of breast cancer in the highest and the lowest exposure dose categories was 0.70 (95% CI: 0.65, 0.75), and no significant heterogeneity was observed (I2 = 0%, P = .479).
Figure 2.
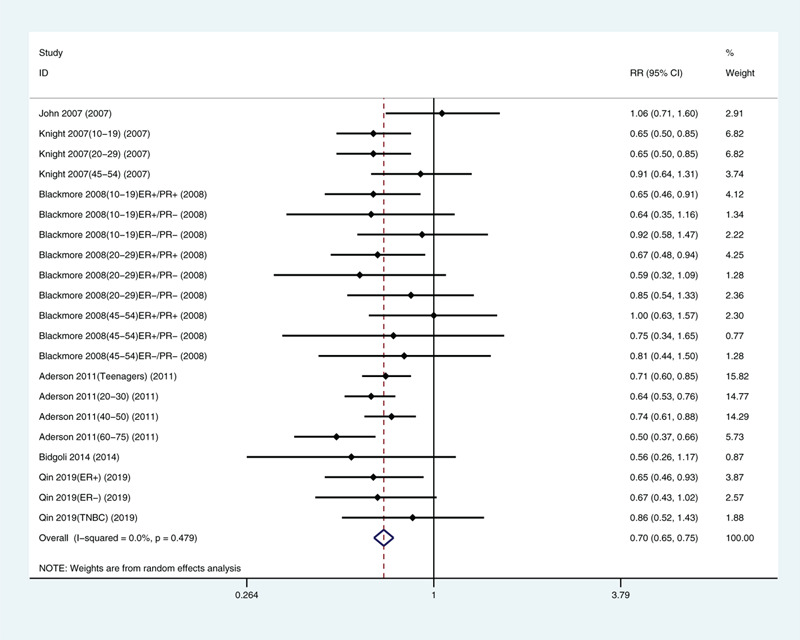
Multivariate-adjusted risk of breast cancer for the highest versus lowest categories of exposure to solar ultraviolet radiation.[20–25] CI = confidence interval, TNBC = triple-negative breast cancer.
3.3. Analysis of other factors
Because we included only a few articles, we only analyzed limb coverage, tanning, sunscreen use, and traveling to sunny areas in winter. We found correlations with limb coverage (relative risk: 1.32, 95% CI: 1.16, 1.50; Fig. 3) and not tanning (relative risk: 1.25, 95% CI: 1.09, 1.42; Fig. 4). The data did not support a correlation between sunscreen use (relative risk: 0.95, 95% CI: 0.88, 1.04; Fig. 5) or winter travel to sunny areas (relative risk: 0.98, 95% CI: 0.91, 1.06; Fig. 6).
Figure 3.
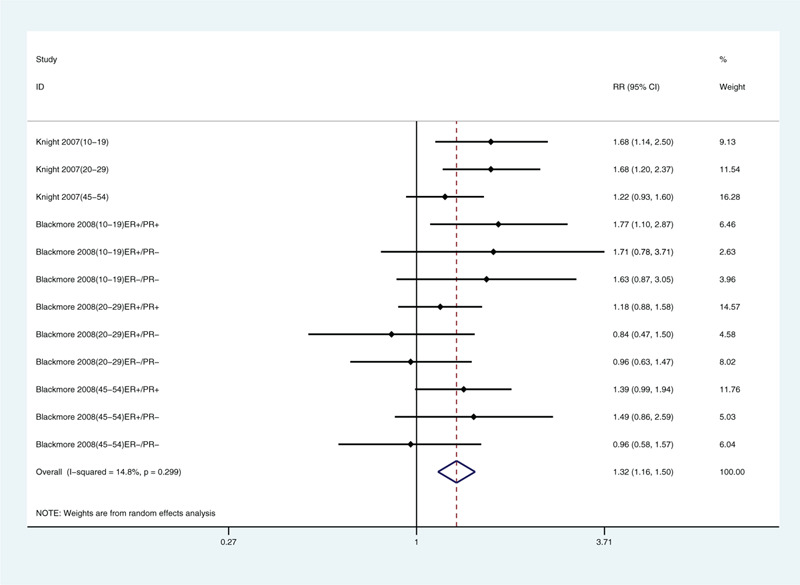
Multivariate-adjusted association of limb coverage and risk of breast cancer.[21,22] CI = confidence interval, RR = relative risk.
Figure 4.
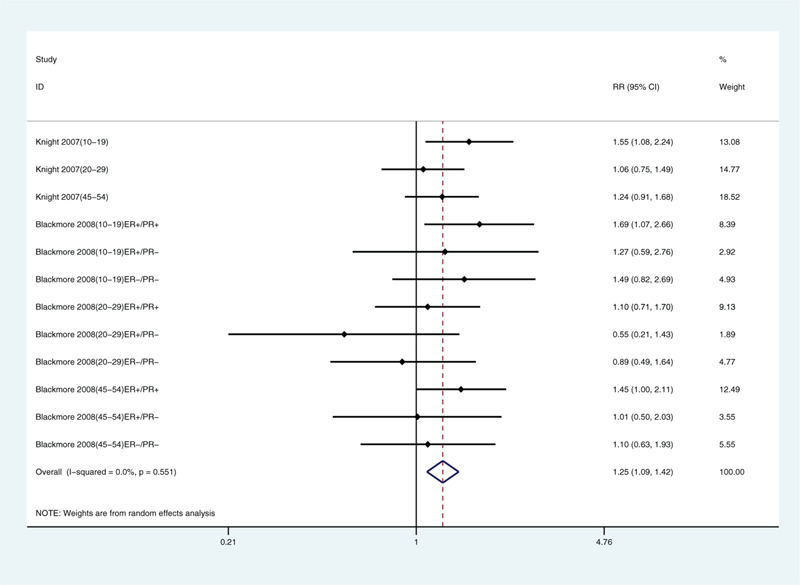
Multivariate-adjusted association of not tanning and risk of breast cancer.[21,22] CI = confidence interval, RR = relative risk.
Figure 5.
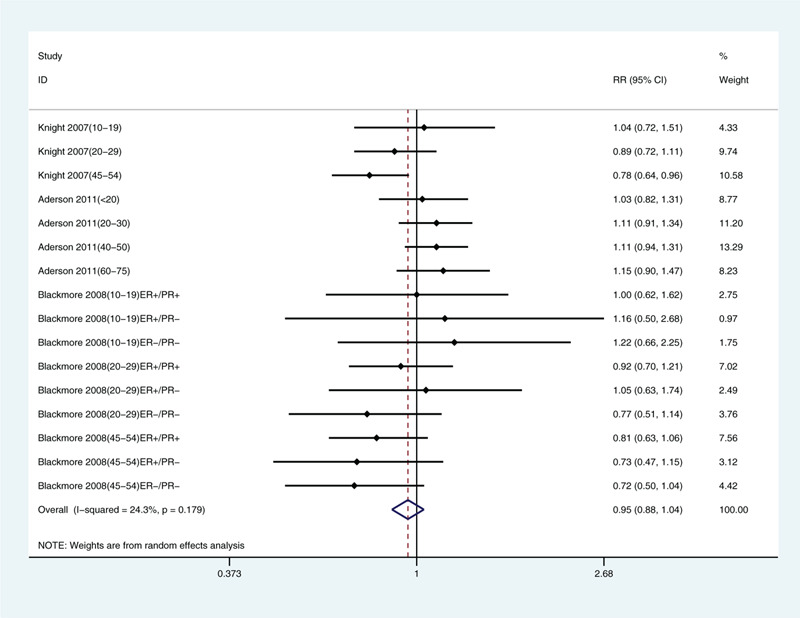
Multivariate-adjusted association of sunscreen use and risk of breast cancer.[21–23] CI = confidence interval, RR = relative risk.
Figure 6.
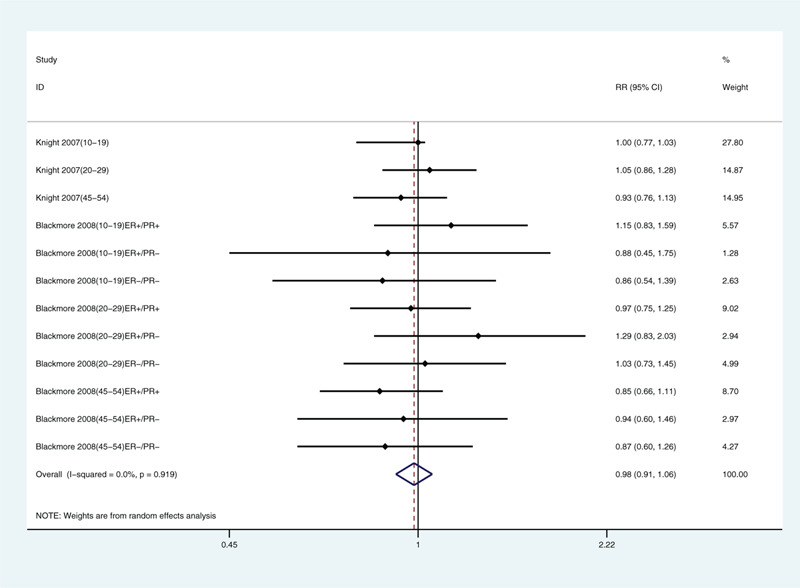
Multivariate-adjusted association of traveling to sunny areas in winter and risk of breast cancer.[21,22] CI = confidence interval, RR = relative risk.
We also analyzed breast cancer incidence by type[22,25] and found that sunlight exposure was negatively correlated with estrogen receptor-positive and progesterone receptor-positive breast cancer (relative risk: 0.72, 95% CI: 0.57, 0.92) and with estrogen receptor-positive and progesterone receptor-negative breast cancer (relative risk: 0.64, 95% CI: 0.44, 0.94; Fig. 7). No correlation was found for estrogen receptor-negative and progesterone receptor-negative breast cancer (relative risk: 0.87, 95% CI: 0.67, 1.11).
Figure 7.
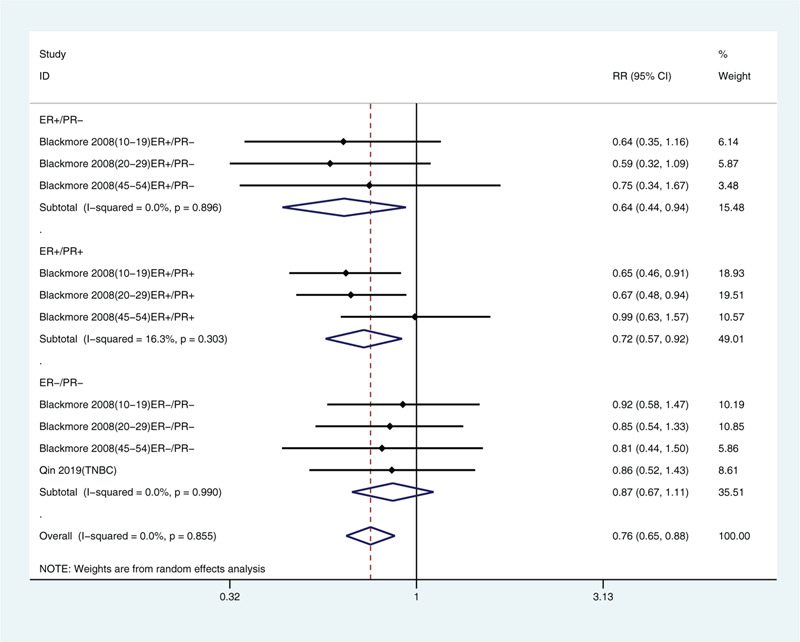
Multivariate-adjusted risk of breast cancer for the highest vs. lowest categories of exposure to solar ultraviolet radiation stratified by estrogen receptor (ER) and progesterone receptor (PR) status.[22,25] CI = confidence interval, RR = relative risk, TNBC = triple-negative breast cancer.
3.4. Dose-response analysis
We conducted a dose-response analysis of 3 case-control studies that included 16 sub-studies[21–23] with a total of 4832 breast cancer patients and stratified the findings by age. A non-linear relationship was observed between the exposure dose and the incidence of breast cancer (non-linear P = .02). In women aged 20 to 30 years, breast cancer risk declined as the radiation dose increased (relative risk: 0.92, 95% CI: 0.90, 0.95; Figs. 8 and 9). The articles included in the study did not provide data for women aged 31–40 years. An insignificant linear relationship was seen (non-linear P = .07; relative risk: 0.89, 95% CI: 0.85, 0.93; Figs. 10 and 11), and in women over 40, the exposure dose and breast cancer risk had a linear dose-response relationship (nonlinear P = .46, relative risk: 0.86, 95% CI: 0.81, 0.91; Figs. 12 and 13). For each increase of 1000 mW/m2 hours in the dose of sunlight, breast cancer risk was reduced by 10%.
Figure 8.
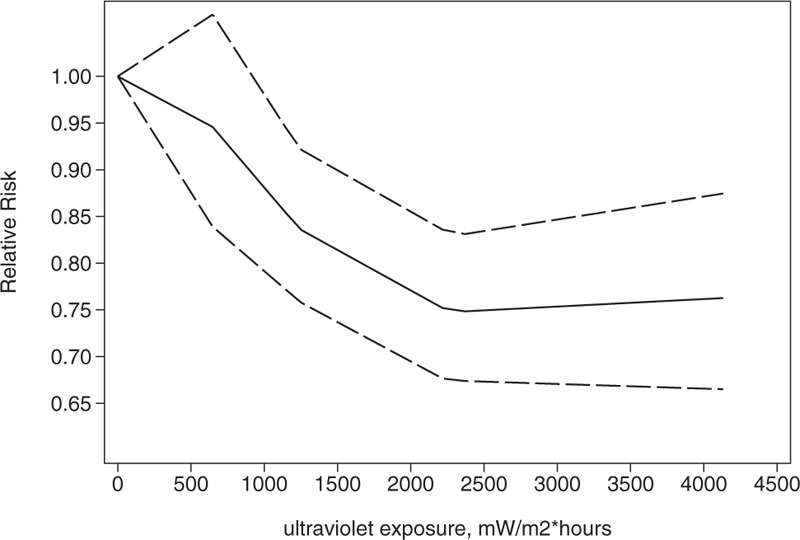
Association between exposure to solar ultraviolet radiation and risk of breast cancer among women aged 10 to 19 yr in a nonlinear model.[21–23]
Figure 9.
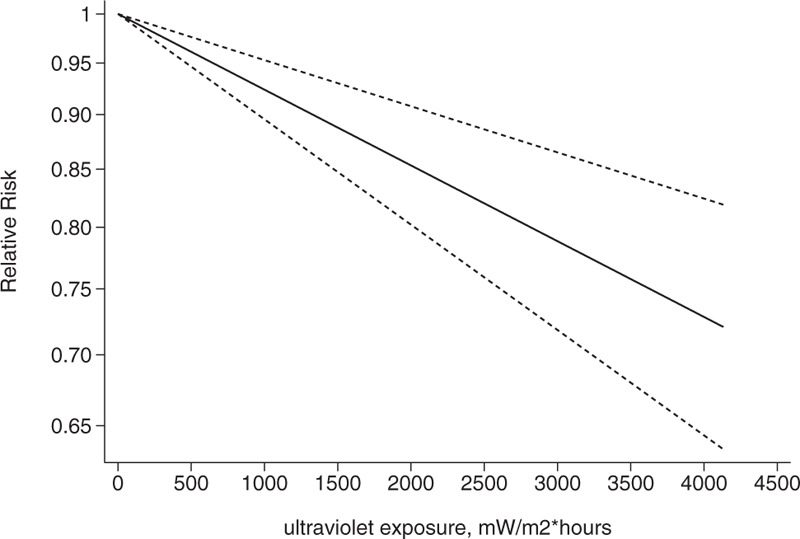
Association between exposure to solar ultraviolet radiation and risk of breast cancer among women aged 10 to 19 yr in a linear model.[21–23]
Figure 10.
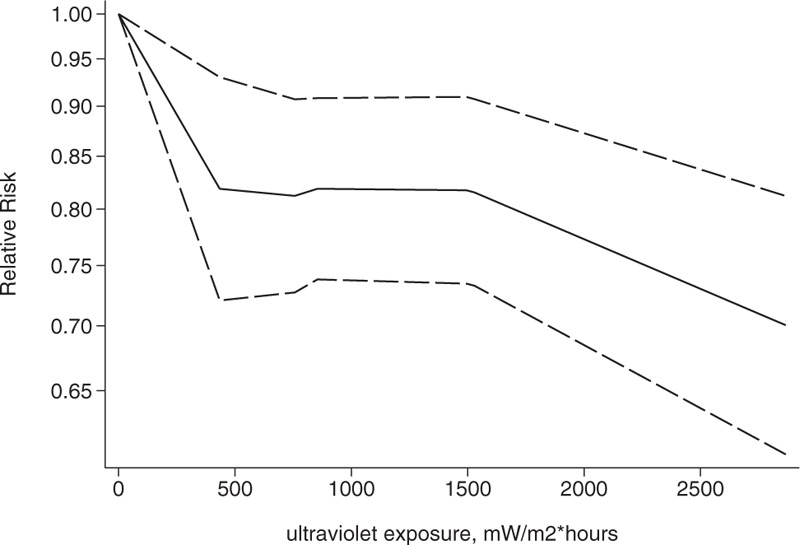
Association between exposure to solar ultraviolet radiation and risk of breast cancer among women aged 20 to 30 yr in a nonlinear model.[21–23]
Figure 11.
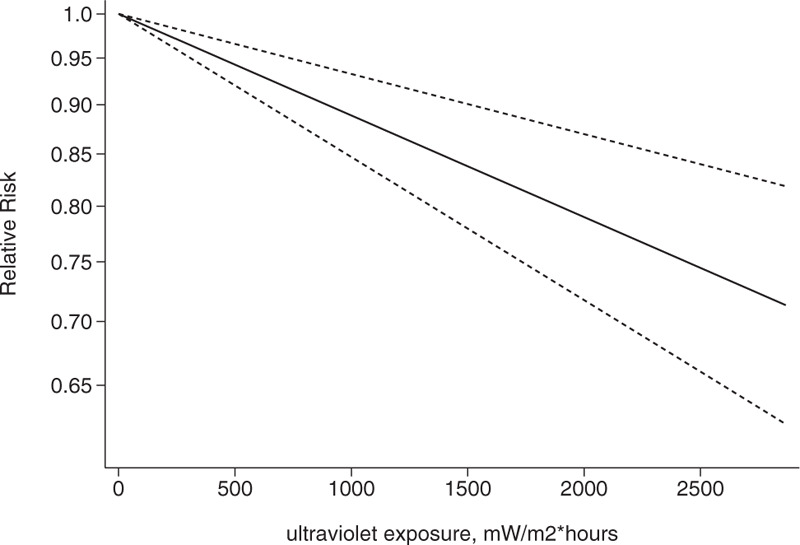
Association between exposure to solar ultraviolet radiation and risk of breast cancer among women aged 20 to 30 yr in a linear model.[21–23]
Figure 12.
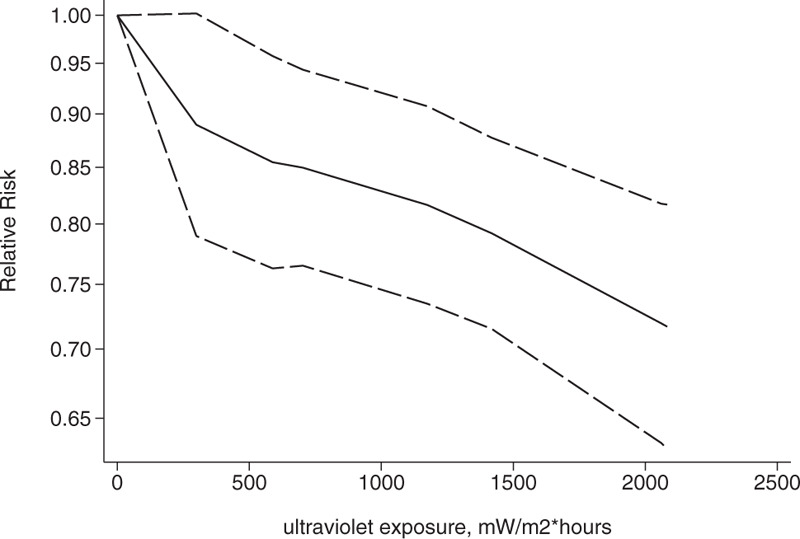
Association between exposure to solar ultraviolet radiation and risk of breast cancer among women aged over 40 in a nonlinear model.[21–23]
Figure 13.
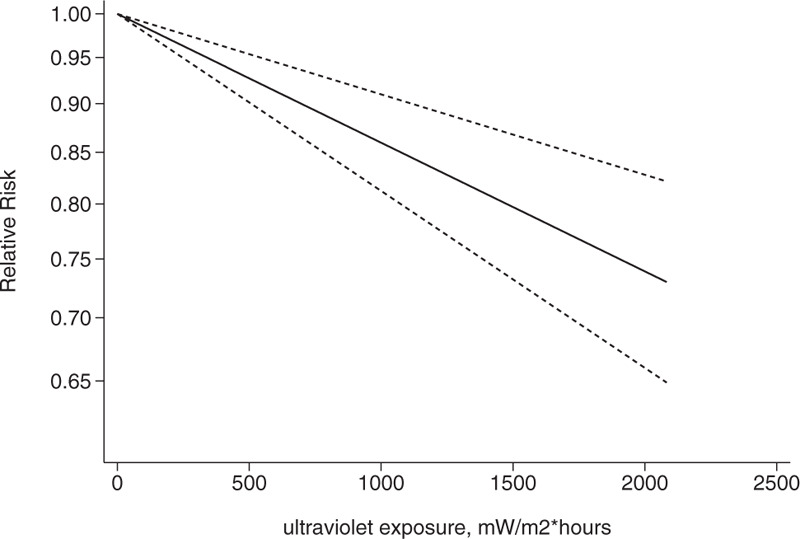
Association between exposure to solar ultraviolet radiation and risk of breast cancer among women aged over 40 in a linear model.[21–23]
3.5. Evaluation of study quality and risk of bias
All articles scored more than 5 stars. Five articles scored 8 stars[20–23,25] and 1 article scored 7 stars,[24] indicating no significant risk of bias (Table 1). A sensitivity analysis (Fig. 14) showed that no study had a significant impact on the relationship between exposure to solar ultraviolet radiation and breast cancer risk, and no substantial publication bias was found (Fig. 15; Egger test P = .22; Begg test P = .25).
Figure 14.
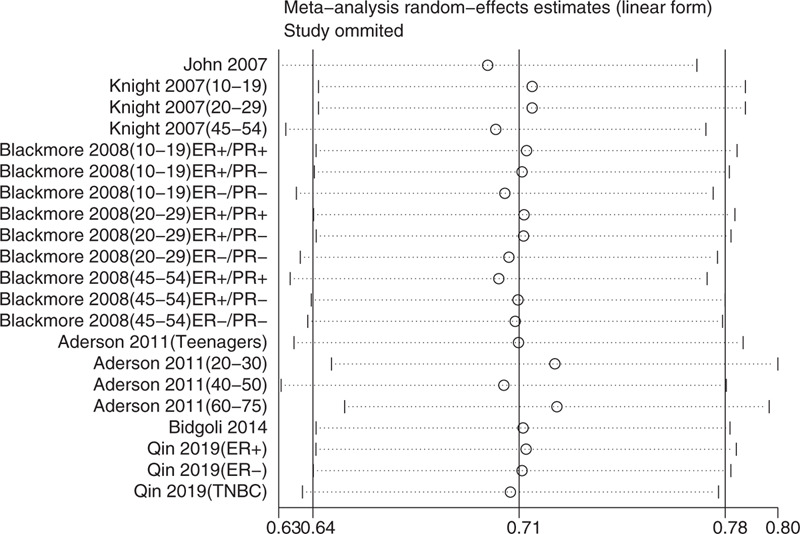
Sensitivity analysis (random-effects model).
Figure 15.
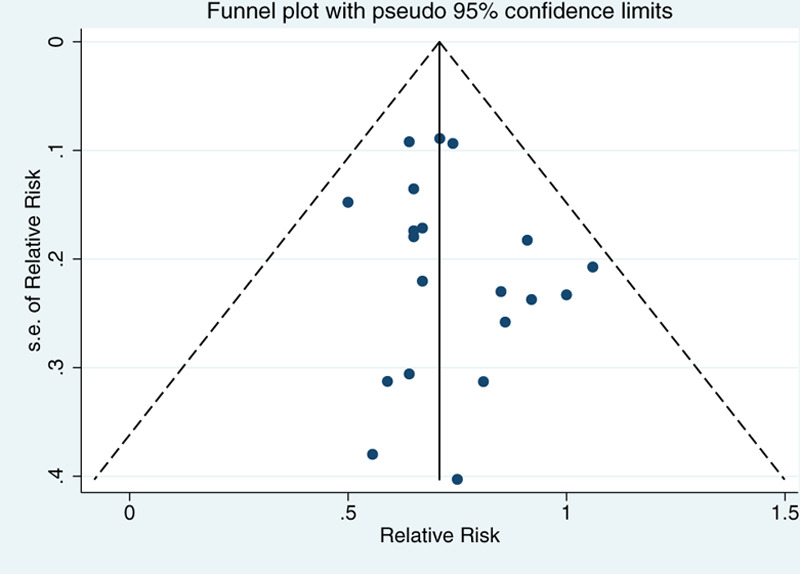
Funnel plot with pseudo-95% confidence limits.
3.6. Ethical approval
We did not collect original epidemiological or clinical data in this study. This study analyzed anonymized published data from other studies.
4. Discussion
We conducted a meta-analysis to study the relationship between exposure to solar ultraviolet radiation and breast cancer risk, and found that overall, exposure was negatively correlated with breast cancer risk, consistent with the conclusion drawn by the previous meta-analysis.[11] When stratifying by age, we found a negative and linear dose-response relationship in women over 40, contrasting with the conclusions of previous meta-analyses. We also analyzed other factors related to exposure to sunlight, and found no significant correlation between sunscreen use and breast cancer risk. Not tanning and limb coverage were positively correlated with breast cancer risk.
Vitamin D has been shown to be protective against liver cancer.[26] Vitamin D inhibits the release of tumor necrosis factor α induced by pancreatic ductal adenocarcinoma and reactivates the nuclear factor kappa B signaling pathway for an anti-tumor effect.[27] In the present assessment, we found an overall negative association between exposure to solar ultraviolet radiation and breast cancer risk, perhaps because sunlight (at 280–315 nm) catalyzes the synthesis of vitamin D,[28] which can reduce breast cancer risk.[23] Vitamin D can inhibit the proliferation of breast cancer cells by inducing apoptosis and down-regulating the expression of kv10.1 channels.[29–31] Vitamin D has been shown to inhibit the transcription and expression of KCa1.1 channels in mda-mb-453 breast cancer cells, inhibiting their proliferation.[32] Ultraviolet radiation can increase the content of 25-hydroxy vitamin D in the lobular epithelium of the terminal ducts of the breast, reducing cancer risk.[33] In addition, vitamin D can enhance the effect of certain anti-cancer drugs. For example, astemizole and vitamin D can synergize to decrease the expression of ki-67 (a cell proliferation marker) and kv10.1, inhibiting the proliferation of human breast cancer cells.[34] The combination of paclitaxel and vitamin D can enhance the efficacy of taxanes and reduce the incidence of paclitaxel-induced peripheral neuropathy.[35,36]
We found an association between not tanning and breast cancer risk, suggesting a connection to melanin formation, perhaps via melanin's scavenging of free radicals.[37] Less skin pigmentation has been correlated with higher levels of estrogen, and estrogen stimulates the division of breast epithelial cells, increasing breast cancer risk.[38]
In our meta-analysis, we also found a correlation between limb coverage and breast cancer risk, perhaps via reduction of the area exposed to sunlight, thereby inhibiting the synthesis of vitamin D and melanin.[38,39]
As for the relationship between exposure and breast cancer subtypes, we found that exposure was negatively correlated with estrogen receptor-positive breast cancer, which was consistent with the conclusion of the previous meta-analysis.[11] However, because there are few related studies and the findings may be modified by other factors, such as age and menstruation status, the underlying reason is unclear.
Obesity and ethnicity can also affect breast cancer risk. Obesity may affect the levels of circulating free estradiol, contributing to the development of breast cancer.[40] Estrogen has been linked to DNA damage and angiogenesis, promoting tumorigenesis and tumor metastasis.[41,42] Elevated levels of reactive oxygen species in obese individuals have been associated with DNA damage and the incidence of breast cancer.[41,43] Genetic predisposition to cancer may also be involved, and ethnic differences in the levels of secreted protein acidic and rich in cysteine[44,45] may decrease survival time in breast cancer patients and increase the recurrence rate.[46] The impact of other gene expression differences on breast cancer risk may perhaps be elucidated using comparative data from tools such as next-generation sequencing.[47] Lifestyle factors such as diet, chronic diseases, and smoking may also affect the development of breast cancer.[41,48] Occupational exposures, such as to diesel exhaust,[49] may increase the risk of breast cancer.
We performed—to our knowledge, for the first time—a meta-analysis of case-control studies and a quantitative dose-response analysis of the relationship between exposure to ultraviolet radiation and breast cancer incidence and stratified the results by age. Our findings can provide guidance for clinicians on advising sun protection strategies.
Several limitations should be noted. Although no overt heterogeneity was found, the number of case-control studies included was small and the study populations were mainly North American, limiting the generalizability of the findings. Evidence is also lacking on the relationship between the exposure and breast cancer subtypes. In addition, there was no information on exposure and breast cancer risk in women aged 31 to 40 years. Moreover, none of the studies accounted for occupational exposures or ethnic factors, potentially biasing their results.
5. Conclusion
Our meta-analysis found that exposure to solar ultraviolet radiation reduces the risk of breast cancer, especially in women over 40. No significant association was found for sunscreen use. This is the first quantitative dose-response meta-analysis on this topic, and the findings may be useful for dermatologists and oncologists. Estrogen receptor status, tanning, and limb coverage may affect the risk of developing breast cancer.
Acknowledgment
The authors thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
Data curation: Yilun Li, Li Ma.
Investigation: Li Ma.
Methodology: Yilun Li, Li Ma.
Software: Yilun Li, Li Ma.
Writing – original draft: Yilun Li.
Writing – review & editing: Yilun Li, Li Ma.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, NOS = Newcastle–Ottawa scale.
How to cite this article: Li Y, Ma L. Exposure to solar ultraviolet radiation and breast cancer risk: a dose-response meta-analysis. Medicine. 2020;99:45(e23105).
This work was supported by the Clinical Medical Talent Support Program of the Hebei Provincial Department of Finance [201746].
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Sun YS, Zhao Z, Yang ZN, et al. Risk factors and preventions of breast cancer. Int J Biol Sci 2017;13:1387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].John SM, Trakatelli M, Gehring R, et al. CONSENSUS REPORT: recognizing non-melanoma skin cancer, including actinic keratosis, as an occupational disease - a call to Action. J Eur Acad Dermatol Venereol 2016;30: Suppl 3: 38–45. [DOI] [PubMed] [Google Scholar]
- [4].Vimercati L, De Maria L, Caputi A, et al. Non-melanoma skin cancer in outdoor workers: a study on actinic keratosis in Italian navy personnel. Int J Environ Res Public Health 2020;17:2321.doi:10.3390/ijerph17072321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Larese Filon F, Buric M, Fluehler C. UV exposure, preventive habits, risk perception, and occupation in NMSC patients: A case-control study in Trieste (NE Italy). Photodermatol Photoimmunol Photomed 2019;35:24–30. [DOI] [PubMed] [Google Scholar]
- [6].van der Rhee H, Coebergh JW, de Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer 2013;49:1422–36. [DOI] [PubMed] [Google Scholar]
- [7].VoPham T, Bertrand KA, DuPre NC, et al. Ultraviolet radiation exposure and breast cancer risk in the Nurses’ Health Study II. Environ Epidemiol 2019;3: doi:10.1097/ee9.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Engel LS, Satagopan J, Sima CS, et al. Sun exposure, vitamin D receptor genetic variants, and risk of breast cancer in the Agricultural Health Study. Environ Health Perspect 2014;122:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grant WB. Role of solar UVB irradiance and smoking in cancer as inferred from cancer incidence rates by occupation in Nordic countries. Dermato-endocrinology 2012;4:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grant WB. A multicountry ecological study of cancer incidence rates in 2008 with respect to various risk-modifying factors. Nutrients 2013;6:163–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hiller TWR, O'Sullivan DE, Brenner DR, et al. Solar ultraviolet radiation and breast cancer risk: a systematic review and meta-analysis. Environ Health Perspect 2020;128:16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li XJ, Ren ZJ, Qin JW, et al. Coffee consumption and risk of breast cancer: an up-to-date meta-analysis. PloS One 2013;8:e52681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- [15].Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40. [Google Scholar]
- [16].Ying H, Jianping C, Jianqing Y, et al. Cognitive variations among vascular dementia subtypes caused by small-, large-, or mixed-vessel disease. Arch Med Sci 2016;12:747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang W, Wu Y, Jiang X. Coffee and caffeine intake and breast cancer risk: an updated dose-response meta-analysis of 37 published studies. Gynecol Oncol 2013;129:620–9. [DOI] [PubMed] [Google Scholar]
- [18].Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet (London, England) 2001;358:870–5. [DOI] [PubMed] [Google Scholar]
- [19].Zhao R, Gao Q, Wang S, et al. The effect of maternal seafood consumption on perinatal outcomes: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr 2020;1–4. doi:10.1080/10408398.2020.1802573. [DOI] [PubMed] [Google Scholar]
- [20].John EM, Schwartz GG, Koo J, et al. Sun exposure, vitamin D receptor gene polymorphisms, and breast cancer risk in a multiethnic population. Am J Epidemiol 2007;166:1409–19. [DOI] [PubMed] [Google Scholar]
- [21].Knight JA, Lesosky M, Barnett H, et al. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2007;16:422–9. [DOI] [PubMed] [Google Scholar]
- [22].Blackmore KM, Lesosky M, Barnett H, et al. Vitamin D from dietary intake and sunlight exposure and the risk of hormone-receptor-defined breast cancer. Am J Epidemiol 2008;168:915–24. [DOI] [PubMed] [Google Scholar]
- [23].Anderson LN, Cotterchio M, Kirsh VA, et al. Ultraviolet sunlight exposure during adolescence and adulthood and breast cancer risk: a population-based case-control study among Ontario women. Am J Epidemiol 2011;174:293–304. [DOI] [PubMed] [Google Scholar]
- [24].Bidgoli SA, Azarshab H. Role of vitamin D deficiency and lack of sun exposure in the incidence of premenopausal breast cancer: a case control study in Sabzevar, Iran. Asian Pac J Cancer Prev 2014;15:3391–6. [DOI] [PubMed] [Google Scholar]
- [25].Qin B, Xu B, Ji N, et al. Intake of vitamin D and calcium, sun exposure, and risk of breast cancer subtypes among black women. Am J Clin Nutr 2020;111:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang Y, Jiang X, Li X, et al. Serum vitamin D levels and risk of liver cancer: a systematic review and dose-response meta-analysis of cohort studies. Nutrition and Cancer 2020;1–9. doi:10.1080/01635581.2020.1797127. [DOI] [PubMed] [Google Scholar]
- [27].Moz S, Contran N, Facco M, et al. Vitamin D prevents pancreatic cancer-induced apoptosis signaling of inflammatory cells. Biomolecules 2020;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- [29].Fleet JC, DeSmet M, Johnson R, et al. Vitamin D and cancer: a review of molecular mechanisms. Biochem J 2012;441:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shao T, Klein P, Grossbard ML. Vitamin D and breast cancer. Oncologist 2012;17:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Restrepo-Angulo I, Banuelos C, Camacho J. Ion channel regulation by sex steroid hormones and vitamin d in cancer: a potential opportunity for cancer diagnosis and therapy. Front Pharmacol 2020;11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khatun A, Fujimoto M, Kito H, et al. Down-regulation of Ca(2+)-activated K(+) channel KCa1.1 in human breast cancer MDA-MB-453 cells treated with vitamin D receptor agonists. Int J Mol Sci 2016;17:2083.doi:10.3390/ijms17122083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol 2007;103:708–11. [DOI] [PubMed] [Google Scholar]
- [34].Garcia-Quiroz J, Garcia-Becerra R, Barrera D, et al. Astemizole synergizes calcitriol antiproliferative activity by inhibiting CYP24A1 and upregulating VDR: a novel approach for breast cancer therapy. PloS One 2012;7:e45063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hershberger PA, Yu WD, Modzelewski RA, et al. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res 2001;7:1043–51. [PubMed] [Google Scholar]
- [36].Jennaro TS, Fang F, Kidwell KM, et al. Vitamin D deficiency increases severity of paclitaxel-induced peripheral neuropathy. Breast Cancer Res Treat 2020;180:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].LaBerge GS, Duvall E, Grasmick Z, et al. Recent advances in studies of skin color and skin cancer. Yale J Biol Med 2020;93:69–80. [PMC free article] [PubMed] [Google Scholar]
- [38].Manning JT, Caswell N. Constitutive” skin pigmentation: a marker of breast cancer risk? Medical Hypotheses 2004;63:787–9. [DOI] [PubMed] [Google Scholar]
- [39].Engelsen O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010;2:482–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dashti SG, Simpson JA, Karahalios A, et al. Adiposity and estrogen receptor-positive, postmenopausal breast cancer risk: quantification of the mediating effects of fasting insulin and free estradiol. Int J Cancer 2020;146:1541–52. [DOI] [PubMed] [Google Scholar]
- [41].Atoum MF, Alzoughool F, Al-Hourani H. Linkage between obesity leptin and breast cancer. Breast Cancer 2020;14:1178223419898458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zare H. Effects of Salvia Officinalis extract on the breast cancer cell line. SciMedicine J 2019;1:25–9. [Google Scholar]
- [43].Epingeac ME, Gaman MA, Diaconu CC, et al. The evaluation of oxidative stress levels in obesity. Rev Chim 2019;70:2241–4. [Google Scholar]
- [44].Gad MM, Găman MA, Saad AM, et al. Temporal trends of incidence and mortality in Asian-Americans with pancreatic adenocarcinoma: an epidemiological study. Ann Gastroenterol 2020;33:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gundewar C, Sasor A, Hilmersson KS, et al. The role of SPARC expression in pancreatic cancer progression and patient survival. Scand J Gastroenterol 2015;50:1170–4. [DOI] [PubMed] [Google Scholar]
- [46].Zhu A, Yuan P, Du F, et al. SPARC overexpression in primary tumors correlates with disease recurrence and overall survival in patients with triple negative breast cancer. Oncotarget 2016;7:76628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kosvyra A, Maramis C, Chouvarda I. Developing an integrated genomic profile for cancer patients with the use of NGS data. Emerg Sci J 2019;3:157–67. [Google Scholar]
- [48].Gordon-Dseagu VL, Devesa SS, Goggins M, et al. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol 2018;47:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Videnros C, Selander J, Wiebert P, et al. Postmenopausal breast cancer and occupational exposure to chemicals. Scand J Work Environ Health 2019;45:642–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


