Summary:
Gynecomastia is a graded condition characterized by enlargement of the male breast that affects a significant proportion of the male population. A plethora of varying surgical approaches currently exists in the literature; thus this comprehensive review sought to analyze surgical practice patterns and trends as they pertain to gynecomastia grade and severity. The current literature was queried utilizing the PubMed and MEDLINE databases—based on predefined parameters and individual review, 17 studies were ultimately included. Key data points included gynecomastia grade, surgical intervention, rate of complication, including hematoma, seroma, infection, and necrosis, and drain use. Two-sample t test was utilized for further analysis. A total of 1112 patients underwent surgical treatment for gynecomastia. Skin-sparing mastectomy with or without liposuction was the most frequently used procedure followed by mastectomy with skin reduction. Major complication rates ranged from 0% to 33%, with hematoma formation being most common (5.8%) followed seroma (2.4%). There was a higher rate of hematoma/seroma formation among authors who routinely utilized drain placement (9.78% versus 8.36%; P = 0.0051); however, this is likely attributable to the large discrepancy in percentage of grade III patients found in each group (50.23% versus 4.36%; P = 0.0000). As a wide variety of surgical techniques exist for the treatment of gynecomastia, an individualized approach based upon gynecomastia grade and patient preference may assist the surgeon in providing optimal outcomes. This senior author’s preferred method for treatment of gynecomastia is illustrated in the included algorithm.
INTRODUCTION
Gynecomastia is a condition characterized by enlargement of the male breast secondary to a proliferation of ductal, stromal, and/or fatty tissue.1 This condition is well known to the plastic surgeon as it has been reported to affect 32%–65% of all men; a significant portion of these men desire surgical correction.1–5 Definitive surgical intervention has been shown to provide benefits—most pronounced in the adolescent population—such as improved physical and psychosocial functioning.6 Overall patient satisfaction following surgical correction has been reported as high as 84.5%–100%.7–9
Three age distributions have been identified regarding development of gynecomastia, all of which correspond to times of physiologic hormonal change in men.7,10–12 The first peak is found in the neonatal period, when an estimated 60%–90% of men develop transient palpable breast tissue secondary to trans-placental passage of estrogen. Neonatal gynecomastia usually regresses by the age of 1 year.11 The next age distribution is found during puberty (ages 10–17 years) when palpable breast tissue has been reported to occur in as high as 69% of individuals.11 The final peak is found in older men (between 50 and 80 years), with the most frequent causes in this age group being hypogonadism and pharmaceutical drug use.13
The most common etiology of gynecomastia is idiopathic; however, other causative factors include drug use (spironolactone, ketoconazole, calcium channel blockers, and/or marijuana) or pathologic etiologies such as cirrhosis, testicular or adrenal neoplasms, and hypogonadism.1,4 To properly manage the male patient suffering from gynecomastia, a detailed work-up to determine the specific etiology must be completed that is tailored to the patient’s age and presenting symptoms—this often requires a multidisciplinary approach. Once the underlying cause has been identified and addressed, surgical correction may still be required for symptomatic relief or improvement in psychosocial functioning.6
Initial evaluation by the plastic surgeon should consist of a thorough history and physical, which includes duration of symptoms, familial history of male and female breast cancer, presence of nipple discharge, laterality of disease, evaluation for hepatomegaly, and testicular exam.14 Imaging may play a role in the complete evaluation for patients in which malignancy cannot be excluded. Fentiman14 proposed an algorithm to guide the clinician regarding the need for imaging in this patient population. Clinical examination findings with a high probability of benign disease may be initially evaluated by breast ultrasound alone. In contrast, clinical examination findings concerning for malignancy should be further evaluated with ultrasound in conjunction with core needle biopsy. Patients with core needle biopsy showing malignancy should be further evaluated with bilateral mammography.14
Several classification systems have been described in the literature that characterize the severity of male breast hypertrophy. Of these, the 2 most often cited are those described by Simon et al15 and Rohrich et al.16 The Simon classification system was described in 1973 and focused on a qualitative assessment of skin redundancy and breast volume15 (Table 1). Rohrich et al16 proposed a new classification system in 2003, which focused on estimates of total mass requiring excision—these categories were then further divided based upon tissue-type predominance.
Table 1.
Simon Classification of Gynecomastia
| Grade | Description |
|---|---|
| I | Small enlargement, no excess skin |
| IIa | Moderate enlargement, no excess skin |
| IIb | Moderate enlargement, excess skin present |
| III | Marked enlargement with excess skin present |
Multiple surgical approaches have been described for the treatment of gynecomastia and may be categorized into the following groups: (1) minimally invasive techniques, including liposuction, vacuum-assisted mastectomy (VAM), and endoscopic mastectomy (ESCM); (2) skin-sparing mastectomy (SSPM), utilizing a single small incision without resection of skin; (3) mastectomy with skin resection (MSR); (4) breast amputation/simple mastectomy with free nipple graft; and (5) any combination of previously described treatment. The purpose of this review is to provide a comprehensive review of the current literature regarding various surgical approaches in the management of gynecomastia based on severity, as defined by Simon grade.
METHODS
A comprehensive review of the current literature was executed via use of the MEDLINE/PubMed database accessed through the Loma Linda University Health network utilizing a Reviews and Meta-analyses (PRISMA)-guided approach. Key search terms entered into the database included surgical, management, and gynecomastia, which yielded a total of 219 articles. Of the 219 available articles, only those published between the years 2000 and 2020 and available in the English language were further reviewed, resulting in 152 possible articles for inclusion.
These 152 publications were then individually reviewed, and studies reporting greater than 5 subjects and those that provided specific recommendations regarding surgical management of gynecomastia stratified by the Simon grade were considered. For the purpose of this review, the Simon classification system was chosen as our preferred system over the Rohrich system to allow for a maximum inclusion of published studies, as more authors provided demographic data based on the Simon grade. The decision to exclude studies using only the Rohrich classification system was made in an effort to maintain consistency when drawing conclusions based on data stratified by grade. Based on the above criteria, a total of 9 studies were initially included in this review. After additional review of the MEDLINE/PubMed database was conducted to include any relevant studies missed by the specific search criteria, 8 more studies were identified. These 8 studies were identified based on search terms including treatment, male breast, and surgical management of gynecomastia. A total of 17 studies were ultimately included in this review. Key data points included patient grade of gynecomastia, type of surgical intervention (including location of incision), rate of complication [hematoma, seroma, infection, necrosis, revision rate, and nipple–areolar complex (NAC) hypoesthesia], and drain use. Two-sample t test was used to compare the average rates of hematoma/seroma formation and average percentage of patients with grade III gynecomastia with regard to routine surgical drain use.
RESULTS
A total of 1112 patients received surgical treatment for various grades of gynecomastia. An estimated 334 patients were categorized as Simon grade I (20 of which were listed as Simon grade I/II), 581 patients were categorized as Simon grade II, including IIa and IIb (26 of which were listed as Simon grade II/III and 20 of which were listed as Simon grade I/II), and 224 patients were categorized as Simon grade III (26 of which were listed as Simon grade II/III) (Table 2). The majority of authors specifically listed standard use of drain placement in all patients regardless of surgical technique, with the exception of Wyrick et al (who reported drain usage based on surgeon preference), Li et al (who did not utilize drain placement in patients with grade I gynecomastia who received SSPM ± liposuction), and Shirol, Khalil et al, Coskun et al, Sim et al, and Akhtar et al (who did not routinely utilize drain placement in any patients).8,9,17,20,23,24,26–31
Table 2.
Surgical Technique by Gynecomastia Grade
| Author | Year | Total Patients | Patients by Grade | Proposed Treatment | Incision |
|---|---|---|---|---|---|
| Coskun et al17 | 2001 | 32 | 12-I | SSPM | IA |
| 20-II | SSPM | IA versus extended IA | |||
| Wiesman et al18 | 2004 | 174 | 65-I | SSPM, SSPM + lipo, lipo only | IA |
| 74-II | SSPM, SSPM + lipo, lipo only, MSR | IA for SSPM, IV-T versus lateral wedge for MSR | |||
| 35-III | SSPM, SSPM + lipo, lipo only, MSR, MSR + lipo | IA for SSPM, IV-T versus lateral wedge for MSR | |||
| Handschin et al19 | 2007 | 100 | 3-I | SSPM | IA |
| 42-IIa | SSPM, SSPM + lipo, lipo only | IA | |||
| 31-IIb | SSPM, SSPM + lipo, MSR, lipo only | IA, CC versus IV-T | |||
| 24-III | SSPM + lipo, MSR, lipo only | IA, CC versus IV-T | |||
| Tashkandi et al20 | 2004 | 24 | 24-III | MSR-central subdermal plexus pedicle* | CC |
| Fan et al21 | 2009 | 65 | 16-IIB | ESCM | Axilla (2 cm), MA (5–10 mm), inferolateral (5–10 mm) |
| 49-III | ESCM | Axilla (2 cm), MA (5–10 mm), inferolateral (5–10 mm) | |||
| Murali et al22 | 2011 | 20 | 20-I/II | Lipo only, SSPM + lipo | IA |
| Li et al23 | 2012 | 41 | 7-I | SSPM ± lipo | IA 2 cm |
| 15-IIa | Lipo + SSPM | IA 2 cm | |||
| 14-IIb | Lipo + SSPM | IA 2 cm | |||
| 5-III | Lipo + SSPM vs. MSR + lipo + nipple repositioning | IA 2 cm versus CC | |||
| Kasielska and Antoszewski24 | 2013 | 113 | 50-I | SSPM | IA |
| 33-IIa | SSPM | IA | |||
| 23-IIb | SSPM | IA | |||
| 7-III | MSR versus breast amputation, FNG | IV-T versus CC + IMF | |||
| Sarkar et al25 | 2014 | 12 | 12-IIb/III | MSR + lipo | CC |
| Shirol9 | 2016 | 20 | 8-IIa | Lipo + SSPM (Orange peel pull through) | IA 6–8 mm |
| 10-IIb | Lipo + SSPM (Orange peel pull through) | IA 6–8 mm | |||
| 2-III | Lipo + SSPM (Orange peel pull through) | IA 6–8 mm | |||
| Khalil et al26 | 2017 | 52 | 10-I | Lipo + SSPM (Direct pull through) | ILQ 8–10 mm |
| 25-IIa | Lipo + SSPM (Direct pull through) | ILQ 8–10 mm | |||
| 17-IIb | Lipo + SSPM (Direct pull through) | ILQ 8–10 mm | |||
| Thiénot et al27 | 2017 | 9 | 9-III | MSR-inferolateral subdermal plexus pedicle | CC and IMF |
| Wyrick et al28 | 2018 | 52 | 38-I | MSR-central subdermal plexus pedicle versus SSPM | CC versus IA 1/3 NAC circumference |
| 14-II/III | MSR-central subdermal plexus pedicle versus SSPM | CC versus IA 1/3 NAC circumference | |||
| Akhtar et al29 | 2019 | 60 | 26-IIA | SSPM + lipo versus VAM + lipo | IA versus 3 mm lateral IMF |
| 34-IIB | SSPM + lipo versus VAM + lipo | IA versus 3 mm lateral IMF | |||
| Varlet et al30 | 2019 | 12 | 8-IIb | ESCM | MA trocar site 10 mm |
| 4-III | ESCM | MA trocar site 10 mm | |||
| Yao et al8 | 2019 | 22 | 3-I | VAM | ILQ 3 mm |
| 19-IIa | VAM | ILQ 3 mm | |||
| 8-IIb | VAM | ILQ 3 mm | |||
| 3-III | VAM | ILQ 3 mm | |||
| Sim et al31 | 2020 | 304 | 126-I | MELT, lipo only, SSPM, SSPM + lipo | IA |
| 112-II | MELT, lipo only, SSPM, SSPM + lipo | IA | |||
| 36-III | MELT, lipo only, SSPM, SSPM + lipo | IA |
FNG, free nipple graft; IA, infra-areolar; IV-T, inverted-T; Lipo, liposuction; LQ, inferolateral quadrant; MA, mid-axillary.
Across all grades, SSPM alone or in combination with liposuction was the most commonly reported technique, described by 10 of the 17 articles included in this study.9,17–19,22–24,26,29,31 The next most common surgical procedure was MSR, which was performed by 8 of the authors.18–20,23–25,27,28 MSR was most frequently reported to be used in patients with Simon grades II–III gynecomastia, with the exception of Wyrick et al28 performing this technique in some grade I patients. In contrast, only 4 authors reported the use of liposuction alone.18,19,22,31 Not all authors reported specific surgical technique utilized in each patient stratified by Simon grade of gynecomastia; however, of those that did, percentage of techniques employed by grade may be seen in Table 3.
Table 3.
Percentage of Surgical Treatment by Simon Grade
| Grade | Total Patients | Surgical Technique | Percentage |
|---|---|---|---|
| I | 158 | ||
| 17 | Lipo only | 10.8 | |
| 3 | VAM | 1.9 | |
| 55 | MELT | 35.0 | |
| 49 | SSPM | 31.0 | |
| 34 | SSPM + lipo | 21.5 | |
| II | 277 | ||
| 18 | Lipo only | 6.5 | |
| 27 | VAM | 9.7 | |
| 72 | MELT | 26.0 | |
| 24 | ESCM | 8.7 | |
| 26 | SSPM | 9.4 | |
| 105 | SSPM + lipo | 46.3 | |
| 5 | MSR + lipo | 1.8 | |
| III | 139 | ||
| 11 | Lipo only | 7.9 | |
| 3 | VAM | 2.2 | |
| 18 | MELT | 12.9 | |
| 53 | ESCM | 38.1 | |
| 1 | SSPM | 0.7 | |
| 8 | SSPM + lipo | 5.8 | |
| 33 | MSR | 23.7 | |
| 12 | MSR + lipo | 8.6 |
Lipo, liposuction.
Major complication rates (including seroma, hematoma, NAC necrosis, and dehiscence/infection) were cited as 0%–33% in these studies. Transient NAC hypoesthesia was not considered a major complication for the purpose of this study; however, of those articles that reported NAC hypoesthesia occurrence (6), the rate ranged from 3% to 19.2%.8,17,19,23,24,26 Revision rate was specifically reported by 8 authors and ranged from 0% to 14.1%.8,9,19,20,23,26,29,31 Hematoma was the most often cited major complication occurring in 5.8% of cases, which is consistent with what has been previously stated in the literature (4.6%).10,11 Seroma formation was the next most common complication occurred in 2.4% of cases (Table 4).10,11 A further analysis was conducted to evaluate the average rate of hematoma/seroma formation with regard to surgical drain use. The average rate of hematoma/seroma formation reported by authors who routinely utilize surgical drains was 9.78% versus 8.36% in those who do not (P = 0.0051). However, there was a disproportionate percentage of patients with grade III gynecomastia reported in the group of authors who routinely use surgical drains when compared with those who do not (50.23% versus 4.36%; P = 0.0000), which likely attributed to this discrepancy. There were not enough data to stratify rate of complication by grade between these 2 groups.
Table 4.
Complications Data
| Author | Year | Patients | Drains | Complication Rate | Hematoma | Seroma | NAC Necrosis/Epidermolysis | Dehiscence/Infection | Revision Rate | Transient Hypoesthesia |
|---|---|---|---|---|---|---|---|---|---|---|
| Coskun et al17 | 2001 | 32 | N | 33% (8/32) | * | * | 1 | NR | NR | 1 (3%) |
| Wiesman et al18 | 2004 | 174 | NR | 28.7% (50/174) | * | * | 0 | 2 | NR | NR |
| Handschin et al19 | 2007 | 100 | Y | 25% (25/100) | 9 | 8 | 7 | 1 | 7 (7%) | 6 (6%) |
| Tashkandi et al20 | 2004 | 24 | Y | 0% (0/24) | 0 | 0 | 0 | 0 | 0 (0%) | NR |
| Fan et al21 | 2009 | 65 | Y | 4.6% (3/65) | 0 | 1 | 2 | 0 | NR | NR |
| Murali et al22 | 2011 | 20 | Y | 10% (2/20) | 1 | 1 | NR | NR | NR | NR |
| Li et al23 | 2012 | 41 | Y | 12.1% (5/41) | 4 | 1 | NR | NR | 2 (4.87%) | 4 (9.75%) |
| Kasielska and Antoszewski24 | 2013 | 113 | Y | 12.4% (14/113) | 8 | 4 | 1 | 1 | NR | 11 (9.7%) |
| Sarkar et al25 | 2014 | 12 | Y | 25% (3/12) | 1 | 2 | NR | NR | NR | NR |
| Shirol9 | 2016 | 20 | N | 5% (1/20) | 1 | NR | NR | NR | 1 (5%) | NR |
| Khalil et al26 | 2017 | 52 | N | 0% (0/52) | 0 | 0 | 0 | 0 | 1 (1.9%) | 10 (19.2%) |
| Thiénot et al27 | 2017 | 9 | Y | 22.2% (2/9) | 1 | 0 | 0 | 1 | NR | NR |
| Wyrick et al28 | 2018 | 52 | Y/N | 11.5% (6/52) | 2 | 4 | 0 | 0 | NR | NR |
| Akhtar et al29 | 2019 | 60 | N | 8.3% (5/60) | 5 | 0 | 0 | NR | 2 (3.3%) | NR |
| Varlet et al30 | 2019 | 12 | Y | 8.3% (1/12) | 0 | 1 | 0 | 0 | NR | NR |
| Yao et al8 | 2019 | 22 | Y | 4.5% (1/22) | 1 | 0 | 0 | 0 | 0 (0%) | 1 (4.5%) |
| Sim et al31 | 2020 | 304 | N | 6.6% (20/304) | 20 | 0 | NR | NR | 43 (14.1%) | NR |
| Total | 1112 | 13.1% (146/1112) | 5.8% (53/906) | 2.4% (22/906) |
As hematoma and seroma rates were reported together, these values did not contribute to the calculated overall seroma and hematoma complication rates.
NR, not recorded.
Most authors comment on the importance of leaving a retro-areolar disc of tissue to prevent indentation or necrosis of the NAC; however, only a few comment on specific thickness. Of those who did, thickness ranged from 2 to 10 mm.8,9,28,30
DISCUSSION
Gynecomastia is the most common benign condition of the male breast, affecting 32%–65% of all men.1–5 This condition may be physically and psychologically detrimental to the patient, thus surgical correction is warranted in many circumstances. As several grading classification systems and surgical approaches exist in the literature, the plastic surgeon is tasked with determining the most appropriate surgical approach for each individual patient. This review sought to build a comprehensive collection of various surgical approaches according to severity as defined by Simon’s grade.
Methods listed for surgical treatment of grade I gynecomastia vary and include SSPM only, SSPM in combination with liposuction, VAM, or microdebrider excision and liposuction technique (MELT). Most authors agree that skin resection is not indicated for patients with grade I gynecomastia. The exception to this is detailed in the study published by Wyrick et al,28 who used MSR using a concentric circumareolar (CC) incision in 6 patients with grade I gynecomastia. While the author agreed MSR is not usually indicated in patients with grade I gynecomastia, Wyrick et al28 advocated for use of this technique when reduction of NAC size is desired.
SSPM was described using various techniques, including a traditional, larger, 2-cm infra-areolar incision for direct excision, or a small 6–8 mm infra-areolar or inferolateral quadrant incision for “pull through” or “orange peel” techniques for direct excision.9,26 Both techniques include grasping of the breast tissue through a small incision with removal of the breast tissue from surrounding structures under direct visualization. VAM for treatment of gynecomastia used a 3-mm incision at the intersection between the mid-axillary and trans-areolar lines.8 A dilute epinephrine solution was infiltrated into the tissue via this incision followed by insertion of a vacuum-assisted rotation needle into the posterior space between the superficial fascia of the pectoralis major muscle and the breast tissue. This technique has been primarily used for breast mass biopsy but has been adopted by Yao et al8 for the treatment of gynecomastia across all Simon grades. Similarly to VAM, MELT, as described by Sim et al,31 uses a microdebrider for removal of breast tissue; however, their preferred location for incision lies within the NAC due to the tendency for hypertrophic scarring in their specific patient population.
A large variety of surgical approaches was also noted for treatment of grade II gynecomasta, including SSPM, SSPM + liposuction, VAM, MELT, ESCM, and MSR. Varlet et al30 and Fan et al21 used ESCM for treatment of patients with grade IIb and III gynecomastia via an incision made at the intersection of the mid-axillary and trans-areolar lines. A trocar was then inserted with insufflation of CO2 at 8 mm Hg of pressure. Dissection and removal of the breast tissue was conducted with an ultrasonic scalpel or tissue clipper.21,30 Although the techniques described by Varlet et al30 and Fan et al31 do not address the excess skin characteristic of grade IIb gynecomastia, this may be a viable alternative for patients who scar poorly or prefer skin redundancy over the presence of visible scars.
MSR for grade II gynecomastia was achieved through the use of CC incisions (dubbed the “double donut technique” by Wyrick et al).28 The intervening skin between the CC incisions was de-epithelialized, leaving the dermis intact to protect the vascular supply to the NAC via the subdermal plexus.28 A transdermal incision was then made along the inferior portion of the de-epithelialized skin to gain access to the breast tissue for direct excision. The procedure was then concluded with approximation of the NAC to the surrounding epithelialized skin. This technique is a viable option for patients in need of excess skin resection or reduction of the NAC size who are willing to accept the presence of a periareolar scar.
An even wider variety of surgical techniques exists for grade III gynecomastia, including MSR with central subdermal plexus pedicle (as previously described), MSR with postero-inferior subdermal plexus pedicle, liposuction + SSPM, SSPM alone, breast amputation through inframammary fold (IMF) approach with free nipple graft, ESCM, and VAM. Several of these surgical approaches have been previously detailed in this report for treatment of grades I and II gynecomastia.
Thiénot et al27 described a technique new to this category yet similar to the Wise pattern approach that utilized MSR through an IMF approach with a de-epithelialized postero-inferior pedicle at least 6 cm in width. New nipple position was positioned generally at the junction of the upper and middle third of the arm or along the breast meridian 15–17 cm from the clavicle.27 Liposuction of the breast tissue was performed in all areas except the location of the pedicle, which was then de-epithelialized from the areola to the IMF. Breast tissue was then resected, creating a de-epithelialized areolar flap and a superior thoracic flap. The superior thoracic flap was then pulled over the areolar flap, secured to the IMF, and the skin overlying the newly positioned NAC was incised.27 This technique allows for significant skin resection with preservation of NAC neurovascular function. The author notes that a disadvantage of this technique is the remaining thickness of the gland due to the presence of the pedicle, which may prolapse with time.
No definitive, universally accepted algorithm exists regarding the ideal surgical approach for treatment of gynecomastia based on severity. Each patient must be considered individually, and the treatment decided upon should be tailored as such. Given the wide variety of acceptable surgical techniques available regardless of patient severity, several factors should be considered. These include patient concern for scarring/tendency for poor scarring, patient comfort with the possibility of revision, patient comfort with the presence of skin redundancy, patient refusal to accept insensate NAC, and other specific circumstances such as the presence of tuberous breast deformity, size of the NAC, and large nipple-to-IMF distances, as seen in massive weight loss patients.
Based on the above referenced literature and personal experience, this senior author provides the following surgical algorithm based on the Simon grading system to assist the plastic surgeon in determining the most appropriate surgical approach for each individual patient (Fig. 1). The first step in determination of appropriate surgical treatment should be characterization of the patient’s gynecomastia based on Simon grade. For patients with grade I gynecomastia, patient’s previous scars should be examined. If the patient is known to form hypertrophic scars or keloids, excision of glandular tissue through SSPM should be avoided as an unsightly periareolar scar may cause distress to the patient. In this case, liposuction alone should be chosen as the modality treatment of choice. Conversely, if that patient does not scar poorly, liposuction in addition to SSPM may be chosen. For patients with grade II gynecomastia, the question regarding the presence of excess skin should be considered. If that patient does not have excess skin but does have an enlarged NAC, MSR ± liposuction may be utilized. If the patient does not have excess skin and NAC is not enlarged, we advocate for the use of SSPM + liposuction. If significant excess skin exists regardless of NAC size, we advocate for the use of MSR. Finally, for patients with grade III gynecomastia, the primary question we consider pertains to the nipple-to-IMF distance. If that distance is >10 cm, we advocate for the use of simple mastectomy via the IMF approach with free nipple grafting, as the amount of resection necessary to achieve an acceptable contour would likely devascularize the NAC. If the nipple-to-IMF distance is <10 cm, we advocate for the use of MSR ± liposuction.
Fig. 1.
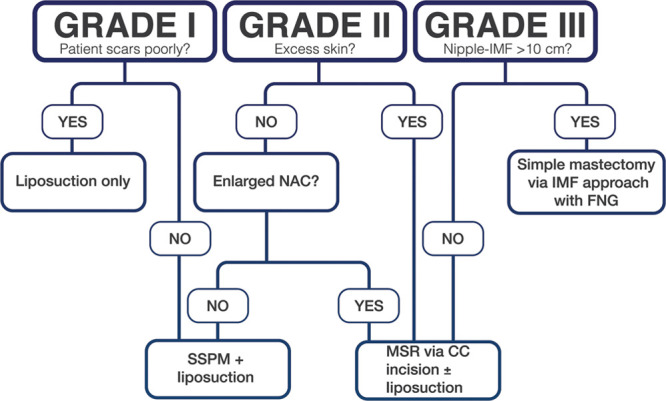
–The proposed algorithm for surgical management of gynecomastia based on the Simon grade.
Footnotes
Published online 29 October 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Brown RH, Chang DK, Siy R, et al. Trends in the surgical correction of gynecomastia. Semin Plast Surg. 2015;29:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagerlund A, Lewin R, Rufolo G, et al. Gynecomastia: a systematic review. J Plast Surg Hand Surg. 2015;49:311–318. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Byun IH, Lee WJ, et al. Surgical management of gynecomastia: subcutaneous mastectomy and liposuction. Aesthetic Plast Surg. 2016;40:877–884. [DOI] [PubMed] [Google Scholar]
- 4.Waltho D, Hatchell A, Thoma A. Gynecomastia classification for surgical management: a systematic review and novel classification system. Plast Reconstr Surg. 2017;139:638e–648e. [DOI] [PubMed] [Google Scholar]
- 5.Innocenti A, Melita D, Mori F, et al. Management of gynecomastia in patients with different body types: considerations on 312 consecutive treated cases. Ann Plast Surg. 2017;78:492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuzzi LC, Firriolo JM, Pike CM, et al. The effect of surgical treatment for gynecomastia on quality of life in adolescents. J Adolesc Health. 2018;63:759–765. [DOI] [PubMed] [Google Scholar]
- 7.Fricke A, Lehner GM, Stark GB, et al. Long-term follow-up of recurrence and patient satisfaction after surgical treatment of gynecomastia. Aesthetic Plast Surg. 2017;41:491–498. [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Yang Y, Liu J, et al. Vacuum-assisted minimally invasive surgery—an innovative method for the operative treatment of gynecomastia. Surgery. 2019;166:934–939. [DOI] [PubMed] [Google Scholar]
- 9.Shirol SS. Orange peel excision of gland: a novel surgical technique for treatment of gynecomastia. Ann Plast Surg. 2016;77:615–619. [DOI] [PubMed] [Google Scholar]
- 10.Lapid O, Jolink F. Surgical management of gynecomastia: 20 years’ experience. Scand J Surg. 2014;103:41–45. [DOI] [PubMed] [Google Scholar]
- 11.Lemaine V, Cayci C, Simmons PS, et al. Gynecomastia in adolescent males. Semin Plast Surg. 2013;27:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorvetzian J, Funderburk C, Copeland-Halperin LR, et al. Correction of the tuberous breast deformity in a prepubescent male patient: a surgical approach to an unusual problem. JPRAS Open. 2019;19:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costanzo PR, Pacenza NA, Aszpis SM, et al. Clinical and etiological aspects of gynecomastia in adult males: a multicenter study. Biomed Res Int. 2018;2018:8364824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fentiman IS. Managing male mammary maladies. Eur J Breast Health. 2018;14:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon BE, Hoffman S, Kahn S. Classification and surgical correction of gynecomastia. Plast Reconstr Surg. 1973;51:48–52. [DOI] [PubMed] [Google Scholar]
- 16.Rohrich RJ, Ha RY, Kenkel JM, et al. Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction. Plast Reconstr Surg. 2003;111:909–923; discussion 924–925. [DOI] [PubMed] [Google Scholar]
- 17.Coskun A, Duzgun SA, Bozer M, et al. Modified technique for correction of gynaecomastia. Eur J Surg. 2001;167:822–824. [DOI] [PubMed] [Google Scholar]
- 18.Wiesman IM, Lehman JA, Jr, Parker MG, et al. Gynecomastia: an outcome analysis. Ann Plast Surg. 2004;53:97–101. [DOI] [PubMed] [Google Scholar]
- 19.Handschin AE, Bietry D, Hüsler R, et al. Surgical management of gynecomastia—a 10-year analysis. World J Surg. 2008;32:38–44. [DOI] [PubMed] [Google Scholar]
- 20.Tashkandi M, Al-Qattan MM, Hassanain JM, et al. The surgical management of high-grade gynecomastia. Ann Plast Surg. 2004;53:17–20; discussion 21. [DOI] [PubMed] [Google Scholar]
- 21.Fan L, Yang X, Zhang Y, et al. Endoscopic subcutaneous mastectomy for the treatment of gynecomastia: a report of 65 cases. Surg Laparosc Endosc Percutan Tech. 2009;19:e85–e90. [DOI] [PubMed] [Google Scholar]
- 22.Murali B, Vijayaraghavan S, Kishore P, et al. Cross-chest liposuction in gynaecomastia. Indian J Plast Surg. 2011;44:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CC, Fu JP, Chang SC, et al. Surgical treatment of gynecomastia: complications and outcomes. Ann Plast Surg. 2012;69:510–515. [DOI] [PubMed] [Google Scholar]
- 24.Kasielska A, Antoszewski B. Surgical management of gynecomastia: an outcome analysis. Ann Plast Surg. 2013;71:471–475. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar A, Bain J, Bhattacharya D, et al. Role of combined circumareolar skin excision and liposuction in management of high grade gynaecomastia. J Cutan Aesthet Surg. 2014;7:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil AA, Ibrahim A, Afifi AM. No-drain single incision liposuction pull-through technique for gynecomastia. Aesthetic Plast Surg. 2017;41:298–303. [DOI] [PubMed] [Google Scholar]
- 27.Thiénot S, Bertheuil N, Carloni R, et al. Postero-inferior pedicle surgical technique for the treatment of grade III gynecomastia. Aesthetic Plast Surg. 2017;41:531–541. [DOI] [PubMed] [Google Scholar]
- 28.Wyrick DL, Roberts M, Young ZT, et al. Changing practices: the addition of a novel surgical approach to gynecomastia. Am J Surg. 2018;216:547–550. [DOI] [PubMed] [Google Scholar]
- 29.Akhtar A, Eitezaz F, Rashid M, et al. Liposuction in gynecomastia: an assessment of the suction-assisted arthroscopic shaver versus open disc excision techniques. Cureus. 2019;11:e5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varlet F, Raia-Barjat T, Bustangi N, et al. Treatment of gynecomastia by endoscopic subcutaneous mastectomy in adolescents. J Laparoendosc Adv Surg Tech A. 2019;29:1073–1076. [DOI] [PubMed] [Google Scholar]
- 31.Sim N, Tan G, Tan BK, et al. Review of the microdebrider excision and liposuction technique (MELT) for the treatment of gynecomastia. J Plast Reconstr Aesthet Surg. 2020;73:303–312. [DOI] [PubMed] [Google Scholar]


