Introduction:
Migraine headache (MH) is one of the most common diseases worldwide and pharmaceutical treatment is considered the gold standard. Nevertheless, one-third of patients suffering from migraine headaches are unresponsive to medical management and meet the criteria for “refractory migraines” classification. Surgical treatment of MH might represent a supplementary alternative for this category of patients when pharmaceutical treatment does not allow for satisfactory results. The goal of this article is to provide a comprehensive review of the literature regarding surgical treatment for site I migraine management.
Methods:
A literature search using PubMed, Medline, Cochrane and Google Scholar database according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines was conducted using the following MeSH terms: “frontal neuralgia,” “frontal trigger site treatment,” “frontal migraine surgery” and “frontal headache surgery” (period: 2000 -2020; last search on 12 March 2020).
Results:
Eighteen studies published between 2000 and 2019, with a total of 628 patients, were considered eligible. Between 68% and 93% of patients obtained satisfactory postoperative results. Complete migraine elimination rate ranged from 28.3% to 59%, and significant improvement (>50% reduction) rates varied from 26.5% to 60%.
Conclusions:
Our systematic review of the literature suggests that frontal trigger site nerve decompression could possibly be an effective strategy to treat migraine refractory patients, providing significant improvement of symptoms in a considerable percentage of patients.
INTRODUCTION
Migraine headache (MH) is one of the most common diseases worldwide, reportedly afflicting more than 11% of the adult population, approximately 35 million Americans in the United States.1 MH affects working-aged female patients (25- to 50-year-olds), resulting more commonly in an annual economic loss of approximately $14 billion in the United States.2,3 Pharmaceutical and behavioral treatment is considered the gold standard. Despite this, between 5% and one-third of MH patients meet criteria for “refractory migraines” demonstrating unresponsiveness to medical management or intolerance to pharmacological side effects.4–6 Surgical treatment of MH might represent a supplementary alternative for this category of patients when pharmaceutical treatment does not allow for satisfactory results. Surgical strategy is based on the decompression of peripheral sensory nerve branches considered to be migraine trigger points. Since 2000,7 the efficacy and cost-effective modality of surgical deactivation has been confirmed in over 40 scientific studies published by various centers.8–19 The migraine trigger points correspond to branches of the trigeminal and the greater occipital nerves in 3 different craniofacial regions (frontal, temporal, and occipital) corresponding to 6 different sites (Table 1).
Table 1.
Six Different Migraine Trigger Sites Corresponding to Branches of the Trigeminal and the Greater Occipital Nerves in 3 Different Craniofacial Regions
| Trigger Site | Trigger Site | Corresponding Nerve |
|---|---|---|
| Site I | Frontal | Supratrochlear and supraorbital nerves |
| Site II | Temporal | Zygomatico-temporal branch of the trigeminal nerve |
| Site III | Septo-nasal | – |
| Site IV | Occipital | Great occipital nerve |
| Site V | Auricolo-temporalis | Auriculo-temporal nerve |
| Site VI | Lesser occipital | Lesser occipital nerve |
Trigger site I migraine is most common20 and originates from the irritation of the supraorbital (SON) and supratrochlear nerves (STN), as well as the terminal branches of the frontal nerve. Different anatomical studies have been conducted to better understand supraorbital and supratrochlear nerve anatomy and to identify their possible irritation points.21–24 The supratrochlear nerve exits the orbit medially, runs along its medial roof, and penetrates into the corrugator at about 1.8 cm from the midline, exiting the muscle approximately 2 cm from the midline.24,25 In most cases, the nerve splits into 2 branches in the retro-orbicularis oculi fat pad before penetrating the muscle.25 The supraorbital nerve exits the orbit via a supraorbital notch or via a foramen, splitting in a superficial and in a deep branch.21–23 Four different patterns of branching were identified based on their interaction with the corrugator muscle.24 However, different studies show that the mean distance of supraorbital nerve entrance into the brow and the midline is about 2.7 cm.21,22,25 The irritation mechanism depends on the compression of the nerve structures by either their arteries, the glabellar muscles group (procerus, depressor, and corrugator supercilii), the supraorbital foramen, or by a fascial band present at the supraorbital notch.
Clinically, patients affected by trigger site I MH usually report pain starting above the eyebrows and show deep frown lines and corrugator muscles hypertrophy or eyelid ptosis.26 Often, clinical history and clinical examination (tenderness of the trigger point at manual compression) are sufficient to clearly identify the MH trigger site.19 Complementary signs can be an audible vessel signal using a handheld Doppler on the trigger point, botulinum toxin-A injection27 (useful only in case of “non-vascular” etiology), or local anesthetic injection if the patient examination is contextual to a pain episode.26
Currently, frontal trigger site deactivation is performed through a transpalpebral or an endoscopic approach, under local anesthesia, sedo-analgesia, or general anesthesia, including different surgical procedures. The goal of this article is to provide a comprehensive review of the literature about surgical treatment for site I migraine management.
METHODS
A literature search using PubMed, Medline, Cochrane, and Google Scholar database according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines was conducted to perform a review of the different surgical techniques and to evaluate the outcomes of surgical deactivation of frontal trigger site migraines. The following MeSH terms were used: “frontal neuralgia,” “frontal trigger site treatment,” “frontal migraine surgery,” and “frontal headache surgery” (period: 2000–2020; last search conducted on March 12, 2020). Two independent reviewers performed two-stage screening and data extraction. Abstracts were screened to identify eligible papers. Reference lists of relevant articles were searched for additional studies. The search strategy is shown in the form of a flow chart (Fig. 1).
Fig. 1.
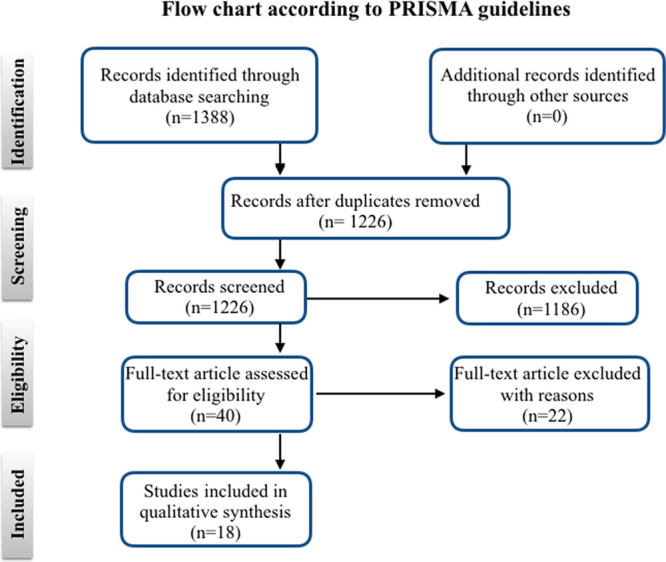
PRISMA guidelines.
Inclusion and Exclusion Criteria
Studies were selected based on the following inclusion criteria: (i) studies selectively investigating surgical treatment of frontal headache; (ii) studies that include more than 10 patients; (iii) full text available in English.
Studies were excluded due to any one of the following criteria: (i) review articles; (ii) case report; (iii) articles reporting only radiologic anatomic data; (iv) studies that included fewer than 10 patients; (v) studies investigating simultaneous decompression of multiple sites surgical site I decompression (vi) contextual to other sites decompression; (vii) non-referenced articles; and (viii) expert opinion (Level V).
Data Collection
Extracted data included: number of patients, sex, mean age, surgical strategy (incision type, myotomy versus muscle, foraminotomy, fasciotomy, and vessel obliteration), mean follow-up time, method of outcome measurements used, outcomes after surgical treatment (including resolution of migraine headache and postoperative complication).
Statistical Analysis
Statistical analyses were performed using SPSS statistical software (version 24.0; IBM Corporation, Somers, N.Y.).
RESULTS
After duplicate exclusion, 1266 articles were identified. Two different reviewers analyzed all the records by titles and abstracts. Forty full-text articles were examined for eligibility. Eighteen studies published between 2000 and 2019 were considered eligible and included in this systematic review based on appropriateness, relevance, and actuality7, 28–44 (Fig. 1).
From the 16 selected studies, 6 were retrospective studies,7,32–34,37,39 11 were prospective studies,28–31,35,36,38,40–42 of which 1 was a blinded randomized cohort study,41 1 was a double-blind, sham surgery, controlled clinical trial,30 and 1 was a cross sectional study.40 A total of 628 patients were included in the review, and the sample size of each study ranged from 10 to 132 patients. Ten of 16 studies reported patient gender showing female prevalence ranged from 68.14% to 100%. Twelve of 16 studies reported the age of patients (as mean or as range). Moreover, two additional studies investigating supraorbital region anatomy were included43,44: one study43 described the supraorbital anatomy in 30 cadavers and the other44 reported the intraoperative findings in 61 patients who had undergone multiple site deactivation surgery.
Concerning surgical techniques, 2 different approaches were mentioned. Seven studies reported a transpalpebral approach28–30,39,40,43,44: 6 studies reported an endoscopic approach31,35–38,42 and 5 studies reported both.27,31–34,41 Studies further mentioned that among endoscopic techniques from 1 single incision (1.5 cm) to 5 incisions, all were positioned behind the hairline. Moreover, the tip to place three surgical sutures bilaterally in the superciliary region to lift the frontal skin allowing for better visualization of the SON, STN, and the surrounding muscles36–38 was given. Among transpalpebral approaches, 5 studies referenced the transposition of fat from the medial compartment of the upper eyelid to fill any defect left by the excised muscles.31–34,40 One paper34 consisted of a comparative study between the two approaches and reported significantly higher success and elimination rates in the endoscopic decompression group than in the transpalpebral decompression group (89% versus 79%, P < 0.05 and 67% versus 52%, P < 0.03, respectively). In any case, the surgical approach corrugator and depressor supercilii resections or myotomies and careful preservation of SON and STN were described in all the procedures. Procerus muscle weakening was reported in 10 articles.29,30,32–38,40 Three articles expressly mentioned vessels coagulation or arterectomy.28,34,41 Three articles32,34,41 expressly mentioned foraminotomy using a percutaneous 2 mm osteotome to perform a supraorbital foramen release,32 and 2 articles expressly mentioned fasciotomy.34,41
The anatomical cadaveric study included in the review43 reported the presence of a supraorbital foramen or a supraorbital notch in 26.6% and 83.3% of the sample, respectively. Moreover, this study43 documented the existence of a fascial band encasing the supraorbital neurovascular bundle in 86% of the supraorbital region that contained a notch. A recent study44 describing the intraoperative anatomy of the supraorbital region on 118 sites reported a supraorbital nerve foramen and a supraorbital nerve notch prevalence pair to 41% and 49%, respectively. In addition, SON or STN compression appeared macroscopically evident in 95% of cases. Another interesting finding was the presence of nerve edema, nerve flattening, or nerve discoloration in 74% of patients.
Table 2 shows the study characteristics and data collection regarding surgical strategies (surgical approach, incision type, glabellar muscle resection, formaninotomy, fasciotomy, or arterectomy).
Table 2.
Study Characteristics and Data Collection Regarding Surgical Strategies
| Study | Type | Sample (patients) | Surgical Incision | Surgical Strategy |
|---|---|---|---|---|
| Guyuron et al7 | Retrospective analysis | 39 | TP or E or open | Resection of the corrugator and depressor muscles |
| Dirnberger and Becker28 | Prospective | 60 Female: 78.3% |
TP | Resection of the corrugator and depressor muscles, vessels coagulation by bipolar diathermy |
| Bearden and Anderson29 | Prospective | 12 | TP (combined with blepharoplasty) |
Resection of the corrugator and depressor muscles, procerus muscle weakening |
| Guyuron et al30 | Double-blind, sham surgery, controlled clinical trial | 29 Actual surgery: 19 Sham surgery: 10 |
TP | Resection of corrugator, depressor and procerus muscle + fat from medial compartment of the upper eyelid to fill any defect left by the excised muscles |
| de Ru et al31 | Prospective | 10 Mean age: 30.7 y Female: 100% |
E 3 small incisions above the hairline |
Cleavage of the corrugator muscle |
| Chepla et al32 | Retrospective analysis | 86 Mean age: 42.5 y versus 46.4 y Female: 97.67% |
TP or E | Group 1: glabellar myectomy Group 2: glabellar myectomy + supraorbital foraminotomy |
| Lee et al33 | Retrospective analysis | 132 Mean age: 44.6 y versus 44.7 y |
TP or E | Resection of corrugator, depressor, and lateral portion of the procerus + fat from medial compartment of the upper eyelid to fill any defect left by the excised muscles |
| Liu et al34 | Retrospective analysis | 35 Mean age: 45.3 y versus 44.7 y Female: 89.3% |
TP or E | – Resection of corrugator, depressor and lateral portion of the procerus + fat from medial compartment of the upper eyelid to fill any defect left by the excised muscles – Removal of the vessels accompanying the nerves – Foraminotomy when a foramen was present – Release of the fibrous bands across the supraorbital notch when a notch was present |
| Caruana et al35 | Prospective | 54 Age range: 18–75 y |
E | Frontal bilateral selective myotomy procedure of procerus, depressor, and corrugator muscles |
| Caruana et al36 | Prospective | 16 Age range: 27–72 y Female: 80% |
E 2 incisions (1.5 cm) above the hairline, positioned 4 cm from the midline Placement of 3 surgical sutures in the superciliary region to lift the frontal skin |
Resection of corrugator, depressor, and procerus muscles |
| Polotto et al37 | Retrospective analysis | 43 Age range: 18–72 y Female: 88.3% |
E One midline scalp incision (length: 1.5 cm) |
Selective myotomies of corrugator, depressor, and procerus muscles |
| Raposio and Caruana38 | Prospective | 43 Age range: 18–72 y Female: 88.3% |
E 3–5 incisions (length: 1.5 cm) behind the hairline using a specifically modified endoscope Placement of 3 surgical sutures in the superciliary region to lift the frontal skin |
Corrugator, depressor, and procerus muscles section performing one myotomy (full-thickness to reach the subcutaneous tissue) per side parallel and approximately 2 mm medially and laterally to each nerve |
| Kurlander et al39 | Retrospective analysis | 34 Age range: 20–70 y Female: 89.6% |
TP | Corrugator resection |
| Punjabi et al40 | Cross- sectional study | 185 | TP | Corrugator, depressor, and procerus resection + fat from medial compartment of the upper eyelid to fill any defect left by the excised muscles |
| Gatherwright et al41 | Prospective, blinded randomized cohort study |
13 Mean age: 41.8 y Female: 100% |
TP | 4 groups: 1. Myectomy 2. Myectomy + foraminotomy/fasciotomy 3. Myectomy + arterectomy 4. Forminotomy/fasciotomy |
| Filipovic et al42 | Prospective | 22 Mean age: 42 y Female: 68.1% |
E | Complete release of STN and SON by cutting the periosteum at the level of the supraorbital ridge Glabellar muscles were not removed |
| Fallucco et al43 | Cadaveric study | 30 | TP | Transpalpebral bilateral approach |
| Ortiz et al44 | Prospective | 61 | TP | Transpalpebral bilateral approach |
E, endoscopic approach; TP, transpalpebral approach.
With respect to outcome measurements, the most frequently used methods were migraine headache index (MHI)32,33,38,41 and the headache questionnaire.36–39,42
Follow-up period varied from 6 months to 126 months. The majority of the studies defined a successful migraine treatment as migraine attack elimination or at least a 50% reduction of its symptoms.
Eleven studies27–29,31,33–39 reported the success rate as a percent value. Overall, 68.3%–93.3% of patients presented satisfactory results. Specifically, the complete elimination of migraine attacks varied from 28.3% to 59.1%, and the rate of significant improvement (at least a 50% reduction of symptoms) varied from 26.5% to 60.5%.
A double-blind controlled clinical trial30 compared two groups of patients who had undergone actual surgery and sham surgery (placebo), expressing the result as an absolute score using the Migraine Disability Assessment, the Migraine-Specific Quality of Life, and the Medical Outcomes Study 36-Item Short Form Health Survey, where significantly better results were observed in the actual surgery group. Another comparative study32 investigated the difference between performing only glabellar myectomy and performing glabellar myectomy combined with supraorbital foraminotomy. This study used MH severity, frequency, and duration, as well as the MHI and the forehead pain score and reported significantly better results in the group of patients who had undergone a glabellar myectomy combined with supraorbital foraminotomy compared with the group who had only undergone a myectomy (postoperative migraine frequency: 7.8 per month versus 4.1 per month; postoperative migraine severity: 5.6 versus 4.4; MHI: 26.5 versus 11.12; persistent forehead pain: 48.8% versus 25.6%). Punjabi et al40 analyzed the appearance of secondary trigger sites after decompression primary surgery showing that the most frequent unmasked secondary trigger after site I surgery is in site III (20.83%). Another article41 suggested that patients who had undergone arterectomy obtained better outcomes in terms of MHI (51.71 versus 5.55), MFD (18 versus 24), and frequency (12 versus 6.11) compared with patients who had not undergone arterectomy. Moreover, 31% of patients who had not undergone arterectomy needed a second surgery consisting of a revision arterectomy and after the procedure showed a statistically equivalent improvement in MFD (20.75 versus 24, P = 0.178) compared with the patients who had undergone arterectomy as primary surgery. Finally, the most recent study included in the review37 showed a decrease in VAS headache intensity from 8.10 before surgery to 1.09 after surgery (P < 0.001).
Seven of 16 studies mentioned postoperative complications.27–31,39,42 The most common complication reported in 6 studies27–29,31,39,42 was transient paresthesia, followed by pruritus reported in 2 studies,30,39 and eyebrow asymmetry or uneven movement,27,30 frontal muscle paralysis,27 eyelid ptosis39 and hematoma formation reported in one patient.31 Many authors suggest wearing a compressive bandage for 24–48 hours after surgery.
Table 3 reports in detail the outcomes after the surgical treatment of each study (outcome measures method, MH elimination or improvement, patient satisfaction, and postoperative complications).
Table 3.
Outcomes of Surgical Deactivation of Frontal Trigger Site Migraine
| Study | Sample (patients) | Outcomes Measurements | Follow-up (mo) | Results | Complications |
|---|---|---|---|---|---|
| Guyuron et al7 | 39 | – | 46.5 | 79.5% positive response 38.4% → elimination 41% → significant improvement |
–Paresthesia –Eyebrow asymmetry –Frontalis muscle paralysis |
| Dirnberger28 | 60 | % reduction of MH days, drugs, side effects, and severity of MH Patient satisfaction using a scale from 1 to 5 (1 = elimination; 5 = any change) |
6 and 18 | 68.3% positive response 28.3% → elimination 40% →significant improvement 31.7% → minimal or no change |
Paraesthesia, disappeared in all patients within 3–9 months. |
| Bearden and Anderson29 | 12 | Onset, frequency, severity, and duration of MH episodes; headache medications; and botulinum toxin |
6–19 | 92% →improvement | Any |
| Guyuron et al30 | 29 Actual surgery 19 Sham surgery 10 |
–Migraine Disability Assessment –MSQEM –Medical Outcomes Study 36-Item Short Form Health Survey |
12 |
Baseline actual surgery versus sham surgery: –Frequency: 9.8 versus 7.6–Intensity: 5.9 versus 6.1 –Duration: 0.56 versus 1.1–MHI: 24.3 versus 27.5 –MSQEM: 48.8 versus 37.2–Study 36-Item –Short Form Health Survey: 45.4 versus 46.7 1 year postoperative actual surgery versus sham surgery–Frequency: 6.37 (P < 0.001) versus 1.5 (P < 0.18) –Intensity: 2.5 (P = 0.005) versus 2.1 (P = 0.51) –Duration: 0.24 (P = 0.01) versus 0.18 (P = 0.57) –MHI: 15.4 (P = 0.003) versus 12.2 (P = 0.03) –MSQEM: 24 (P = 0.02) versus 0.46 (P = 0.97) –36-Item Short Form Health Survey: 5.9 (P = 0.002) versus 1.5 (P = 0.51) |
–Temporary intense pruritus →11% –Uneven brow movement →5% –Residual corrugator muscle function →5% |
| de Ru et al31 | 10 | Pain severity scoring verbal numerical rating scale (NRS): from 0 (no pain) to 10 (severe pain) |
3–30 | 90% → lowered pain score (from 8.1 to 0.8) 10% → any change |
Numbness in 3 patients Paresthesia and hematoma formation in 1 patient |
| Chepla et al32 | 86 | MH severity, frequency, and duration MHI Forehead pain |
12 |
Glabellar myectomy versus Glabellar myectomy + supraorbital foraminotomy Postoperative migraine frequency: 7.8 versus 4.1 per month Severity: 5.6 versus 4.4 MHI: 26.5 versus 11.1 Persistent forehead pain: 48.8% versus 25.6% |
Not reported |
| Lee et al33 | 132 | MHI (success defined as >50% of reduction) 2 groups: a) preoperative BTA responsive (109 patients) b) preoperative BTA NON responsive (23 patients) |
>12 |
Total: 83.3% → positive response 56.8% → elimination 26.5 → >50% reduction BTA responsive versus BTA NON responsive group: Migraine elimination: 33.7% versus 7.6% >50% reduction: 92.5% versus 69.2% |
Not reported |
| Liu et al34 | 35 | MH frequency, duration and intensity |
12–126 (mean: 34) |
77% → positive response | Not reported |
| Caruana et al35 | 54 Age range: 18–75 y |
36-item short questionnaire (before surgery) 29-item short questionnaire (6 months and 2 years after surgery) |
24 |
6 months (51 patients): 84.3% → positive response 41.2% → elimination 43.1% → significant improvement 2 years (29 patients): 89.6% → positive response 31% → complete elimination 58.6% → significant improvement |
Not reported |
| Caruana et al36 | 16 | Headache questionnaire | – | 81.5% → positive response 31.5% → elimination 50% → significant improvement |
Not reported |
| Polotto et al37 | 43 | Headache questionnaire | 24 | 93.3% → positive response to the surgery: 33.3% → complete elimination 60% → significant improvement |
Not reported |
| Raposio and Caruana38 | 43 | Headache questionnaire | 6 and 24 |
6-month-long follow-up (43 patients): 81.4% → positive response 39.5% → elimination 41.9% → significant improvement 2-year-long follow-up (15 patients): 93.3% →positive response 33.3% → elimination 60% →significant improvement |
Not reported |
| Kurlander et al39 | 34 | Frontal-specific MHI Reduction in migraine days (duration × frequency) |
12 | 88% → positive response 59% → elimination |
Numbness →32.1% Pruritus →8.9% Hypersensitivity →8.9% Eyelid Ptosis →3.6% |
| Punjabi et al40 | 185 | Migraine headache questionnaire | 13 | 17.8% of the cohort reported new postoperative migraines Site I: 20.83% → Site III (septo-nasal) unmasked after surgery |
Not reported |
| Gatherwright et al41 | 13 | Migraine headache severity and duration MHI MFDs |
21.6 (7.6–34.1) | MHI: from 52.6 (3.8–85) to 4.7(0–21.3), P = 0.0001Arterectomy group (9 patients): MHI: from 51.71 to 5.55 Frequency: 12 versus 6.11 Improvement MFDs: from 18 to 24 No arterectomy (4 patients): Improvement MFDs: 13.25 Less than arterectomy group (13.25 versus 24 MFDs): 31% required a site I revision that included an arterectomy. Following revision, both groups had statistically equivalent improvement in MFDs (20.75 versus 24 MFDs) |
Not reported |
| Filipovic et al42 | 22 | Daily headache diary (4 points only) Headache questionnaire |
12–107 (mean: 29.5) | VAS headache intensity from 8.10 to 1.3 at 3 months after surgery and to 1.09 at 12 months after surgery Accompanying headache symptoms (photophobia, phonophobia, nausea, and vomiting) were completely abolished in all patients, except in 1 case |
–Transient paresthesia → 2 patients (3 months duration) –Temporary hair loss above the incision → 1 patient (12 months duration) |
| Fallucco et al43 | 30 | – | – | –Supraorbital foramen → 26.6% of cases –Supraorbital notch → 83.3% of cases –Fascial band → 86% of supraorbital region that contained a notch and classified into 3 types |
– |
| Ortiz et al44 | 61 | – | – | –Supraorbital foramen → 41% of cases –Supraorbital notch → 49% of cases –Supraorbital foramen and notch → 9.3% of cases SON (66%) or STN (29%) Compression → 95% of cases. Nerve edema, flattening, or discoloration → 74% |
– |
BTA, botulinum toxin type A; MFD, migraine-free days; MSQEM, Migraine-Specific Quality of Life.
DISCUSSION
Since the unexpected finding of frontal headache amelioration consequent to glabellar muscle resection and periosteal release performed during brow-lift procedures,27 the field of migraine surgery has rapidly progressed. Our study evidences the positive effects of frontal nerve decompression surgery and underlines how the migraine surgical field is still evolving. In our review, seven studies reported a transpalpebral approach, 6 studies reported an endoscopic approach, and 5 studies reported both. This proportion denotes that there is still no consensus as to what the best approach to treat frontal migraine is. The transpalpebral approach consents the direct visualization and the excision of the glabellar muscles, leaving the periosteal and the fascial structures undamaged. This can be considered as an extension of an upper eyelid blepharoplasty. The anatomical findings that emerged from the two studies included in the review43,44 reported a relatively high prevalence of supraorbital foramen, supraorbital notch, and fascial band encasing the supraorbital neurovascular bundle, which may suggest that a transpalpebral approach allows for better visualization and treatment of nerve compression. Conversely, some authors argue that the endoscopic technique, at the same time allowing a complete periosteal release on the orbital ridge and a wide glabellar muscles dissection, should be considered as the best choice whenever anatomically possible. The endoscopic approach is not recommended for patients with a forehead length greater than 8 cm or patients presenting with a protruding forehead.45 A retrospective study34 included in our review reported significantly higher success rates in cases of endoscopic approach than in the transpalpebral option. As aforementioned, among the described endoscopic techniques, surgical access varies from 1 single incision of 1.5 cm to 5 incisions, all of which are positioned behind the hairline. The minimally invasive technique described by Raposio,36–38,46–48 in addition to the single midline incision, requires the use of a modified endoscope and the placement of 3 surgical sutures bilaterally in the superciliary region to have a better visualization of the anatomical structures lifting the frontal skin.
Regardless of the approach, another noticeable difference among the studies is related to surgical procedures. All the authors agree that glabellar muscles group excision and SON and STN preservation are mandatory. The necessity to perform an arterectomy, a fasciotomy, and a foraminotomy is still a matter of debate. Gatherwright,41 in a prospective blinded randomized cohort study, demonstrated the role of arterectomy in frontal migraine surgery showing that patients who undergo arterectomy obtain better outcomes. Moreover, the study reported that in about 30% of cases, patients who had not undergone arterectomy needed a revision consisting of an arterectomy; after which, a statistically equivalent improvement was achieved when compared with patients who had undergone arterectomy as the primary surgery. Another retrospective comparative study32 investigated the role of supraorbital foraminotomy proving a reduction of migraine frequency, migraine severity, MHI, and forehead pain in patients who had undergone foraminotomy. This clinical finding is supported by recent radiological and anatomical evidence49 showing that SON and especially supraorbital foramen contribute significantly to MH symptoms. This radiological study suggests that an analysis of all available face or perinasal sinus CT images could be helpful in preoperative planning, possibly including foraminotomy and fasciotomy.
An important criticism in frontal migraine management is the lack of consensus among clinicians regarding the methods to measure surgical outcomes. In our review the most frequently used methods were MHI7,28,34,36 and the headache questionnaire.36–38,40,42 Quality of life documentation before and after a migraine surgery is exiguous, mirroring the literature regarding trigger site decompression surgery. In our opinion, to improve the migraine surgery effect reporting, future investigations should spotlight what is the most complete evaluation method to universalize outcome measurements.
Patient follow-up period varied from 6 to 126 months; results were considered as stable three months after surgery by most of the authors. Overall, 68.3%–93.3% of patients presented satisfactory results. Complete migraine elimination rate ranged from 28.3% to 59% and significant improvement (>50% of reduction) rate varied from 26.5% to 60%. The wide range can be justified by the fact that different surgical techniques were performed. One double-blind controlled clinical trial30 compared actual surgery and sham surgery (placebo), showing significantly better results in the actual surgery group. Consensus about why some patients remain refractory to frontal migraine surgery has yet to be reached. The frontal migraine crisis pathogenesis remains unclear and additional clinical and anatomical studies have to be accomplished to improve surgical outcomes.
Undeniably, some authors are not convinced that decompression surgery represents an effective treatment for headaches50,51 and the neural entrapment theory is still a matter of debate. Certainly, the lack of clarity regarding patient selection criteria, the scarcity of controlled studies, the lack of consistent outcome measures, and the relative brevity of follow-ups represent weak points that can lead to prejudiced results. However, recent studies have described intraoperative findings of SON and STN compression, nerve edema, flattening, or discoloration, thus demonstrating the concreteness and the anatomical-clinical correlation of the neural entrapment theory. In our opinion, standardization of patient selection and outcome measures after decompression surgery are the most critical points needed to convince neurologists of the effectiveness of this type of treatment in selected patients. In fact, the MHI represents a non-validated instrument that may increase the possibility of obtaining positive results.49 A constructive and open discussion between surgeon and neurologist would surely improve the management of these patients and allow for the building of an integrated therapeutic algorithm to better evaluate the postoperative results.
Postoperative complications were relatively rare and a few were reported. The most common complication was a transient paresthesia, followed by pruritus, eyebrow asymmetry or uneven movement, frontal muscle paralysis, eyelid ptosis, and hematoma formation.
CONCLUSIONS
Our systematic review of the literature suggests that frontal trigger site nerve decompression may be an effective strategy to treat migraine refractory patients, allowing for the resolution or at least a significant improvement of symptoms in a considerable percentage of patients. However, the poor quality of the included studies, the scarcity of controlled trials, the lack of consistent outcome measures, and the multitude of varied surgical techniques do not permit the conclusion of efficacy with respect to frontal migraine surgical treatment. Certainly, higher level studies need to be conducted to confirm the effectiveness of this treatment. Moreover, why some patients are still unresponsive to surgical treatment is still a matter of discussion.
Nowadays, there is not a standard surgical technique. Prospective studies to compare excision or blunt dissection of the glabellar muscles, periosteum release, vessel coagulation and foraminotomy would be helpful to reach a better understanding as to what is the best surgical strategy to treat these patients.
Footnotes
Published online 15 October 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Lipton RB, Bigal ME, Diamond M, et al. ; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 2.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin. 2009;27:321–334. [DOI] [PubMed] [Google Scholar]
- 3.Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: Results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52:1456–1470. [DOI] [PubMed] [Google Scholar]
- 4.Irimia P, Palma JA, Fernandez-Torron R, et al. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Amico D, Leone M, Grazzi L, et al. When should “chronic migraine” patients be considered “refractory” to pharmacological prophylaxis? Neurol Sci. 2008;29 Suppl 1:S55–S58. [DOI] [PubMed] [Google Scholar]
- 6.Kung TA, Guyuron B, Cederna PS. Migraine surgery: A plastic surgery solution for refractory migraine headache. Plast Reconstr Surg. 2011;127:181–189. [DOI] [PubMed] [Google Scholar]
- 7.Guyuron B, Varghai A, Michelow BJ, et al. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106:429–34.; discussion 435. [DOI] [PubMed] [Google Scholar]
- 8.Guyuron B, Tucker T, Davis J. Surgical treatment of migraine headaches. Plast Reconstruct Surg. 2003;112164S–170S. [DOI] [PubMed] [Google Scholar]
- 9.Guyuron B, Kriegler JS, Davis J. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Sur. 2005;1151–9. [PubMed] [Google Scholar]
- 10.Poggi JT, Grizzell BE, Helmer SD. Confirmation of surgical decompression to relieve migraine headaches. Plast Reconstr Surg. 2008;122:115–22.; discussion 123. [DOI] [PubMed] [Google Scholar]
- 11.Faber C, Garcia RM, Davis J, et al. A socioeconomic analysis of surgical treatment of migraine headaches. Plast Reconstruct Surg. 2012;129:871–877. [DOI] [PubMed] [Google Scholar]
- 12.Behin F, Behin B, Behin D, et al. Surgical management of contact point headaches. Headache. 2005;45:204–210. [DOI] [PubMed] [Google Scholar]
- 13.Guyuron B, Kriegler JS, Davis J, et al. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg. 2011;127:603–608. [DOI] [PubMed] [Google Scholar]
- 14.Raposio E, Bertozzi N. Trigger site inactivation for the surgical therapy of occipital migraine and tension-type headache: Our experience and review of the literature. Plast Reconstr Surg Glob Open. 2019;7:e2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raposio E, Bertozzi N, Bordin C, et al. Surgical therapy of headaches: minimally invasive approaches. In: Clinical Advances in Head & Neck Surgery. 2018:Las Vegas, USA: Openaccess eBooks; 1–23. [Google Scholar]
- 16.Raposio E, Bertozzi N, Bordin C, et al. Turker H, ed. Surgical therapy of migraine and tension-type headache. In: Current Perspectives on Less-known Aspects of Headache. 2017London, U.K.: InTech; [Google Scholar]
- 17.Raposio E, Caruana G. Tips for the surgical treatment of occipital nerve-triggered headaches. Eur J Plast Surg. 2017;40:177–182. [Google Scholar]
- 18.Bertozzi N, Simonacci F, Lago G, et al. Surgical therapy of temporal triggered migraine headache. Plast Reconstr Surg Glob Open. 2018;6:e1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seyed Forootan NS, Lee M, Guyuron B. Migraine headache trigger site prevalence analysis of 2590 sites in 1010 patients. J Plast Reconstr Aesthet Surg. 2017;70:152–158. [DOI] [PubMed] [Google Scholar]
- 20.Olla D, Sawyer J, Sommer N, et al. Migraine treatment. Clin Plast Surg. 2020;47:295–303. [DOI] [PubMed] [Google Scholar]
- 21.Miller TA, Rudkin G, Honig M, et al. Lateral subcutaneous brow lift and interbrow muscle resection: Clinical experience and anatomic studies. Plast Reconstr Surg. 2000;105:1120–7; discussion 1128. [DOI] [PubMed] [Google Scholar]
- 22.Knize DM. A study of the supraorbital nerve. Plast. Reconstr.Surg. 1995;96:564.. [DOI] [PubMed] [Google Scholar]
- 23.Cuzalina AL, Holmes JD. A simple and reliable landmark for identification of the supraorbital nerve in surgery of the forehead: an in vivo anatomical study. J Oral Maxillofac Surg. 2005;63:25–27. [DOI] [PubMed] [Google Scholar]
- 24.Janis JE, Ghavami A, Lemmon JA, et al. The anatomy of the corrugator supercilii muscle: Part II. Supraorbital nerve branching patterns. Plast Reconstr Surg. 2008;121:233–240. [DOI] [PubMed] [Google Scholar]
- 25.Janis JE, Hatef DA, Hagan R, et al. Anatomy of the supratrochlear nerve: Implications for the surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;131:743–750. [DOI] [PubMed] [Google Scholar]
- 26.Guyuron B, Nahabet E, Khansa I, et al. The current means for detection of migraine headache trigger sites. Plast Reconstr Surg. 2015;136:860–867. [DOI] [PubMed] [Google Scholar]
- 27.Liu MT, Armijo BS, Guyuron B. A comparison of outcome of surgical treatment of migraine headaches using a constellation of symptoms versus botulinum toxin type A to identify the trigger sites. Plast Reconstr Surg. 2012;129:413–419. [DOI] [PubMed] [Google Scholar]
- 28.Dirnberger F, Becker K. Surgical treatment of migraine headaches by corrugator muscle resection. Plast Reconstr Surg. 2004;114:652–7.; discussion 658. [DOI] [PubMed] [Google Scholar]
- 29.Bearden WH, Anderson RL. Corrugator superciliaris muscle excision for tension and migraine headaches. Ophthalmic Plast Reconstr Surg. 2005;21:418–422. [DOI] [PubMed] [Google Scholar]
- 30.Guyuron B, Reed D, Kriegler JS, et al. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009;124:461–468. [DOI] [PubMed] [Google Scholar]
- 31.de Ru JA, Schellekens PP, Lohuis PJ. Corrugator supercilii transection for headache emanating from the frontal region: A clinical evaluation of ten patients. J Neural Transm (Vienna). 2011;118:1571, 157–4. [DOI] [PubMed] [Google Scholar]
- 32.Chepla KJ, Oh E, Guyuron B. Clinical outcomes following supraorbital foraminotomy for treatment of frontal migraine headache. Plast Reconstr Surg. 2012;129:656e–662e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M, Monson MA, Liu MT, et al. Positive botulinum toxin type a response is a prognosticator for migraine surgery success. Plast Reconstr Surg. 2013;131:751–757. [DOI] [PubMed] [Google Scholar]
- 34.Liu MT, Chim H, Guyuron B. Outcome comparison of endoscopic and transpalpebral decompression for treatment of frontal migraine headaches. Plast Reconstr Surg. 2012;129:1113–1119. [DOI] [PubMed] [Google Scholar]
- 35.Caruana G, Grignaffini E, Raposio E. Endoscopic forehead muscle resection for nerve decompression: A modified procedure. Plast Reconstr Surg Glob Open. 2015;3:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caruana G, Bertozzi N, Boschi E, et al. Endoscopic forehead surgery for migraine therapy personal technique. Ann Ital Chir. 2014;85:583–586. [PubMed] [Google Scholar]
- 37.Polotto S, Simonacci F, Grignaffini E, et al. Surgical treatment of frontal and occipital migraines: A comparison of results. Plast Reconstr Surg Glob Open. 2016;4:e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raposio E, Caruana G. Frontal endoscopic myotomies for chronic headache. J Craniofac Surg. 2015;26:e201, e20–3. [DOI] [PubMed] [Google Scholar]
- 39.Kurlander DE, Ascha M, Sattar A, et al. In-depth review of symptoms, triggers, and surgical deactivation of frontal migraine headaches (Site I). Plast Reconstr Surg. 2016;138:681–688. [DOI] [PubMed] [Google Scholar]
- 40.Punjabi A, Brown M, Guyuron B. Emergence of secondary trigger sites after primary migraine surgery. Plast Reconstr Surg. 2016;137:712e–716e. [DOI] [PubMed] [Google Scholar]
- 41.Gatherwright JR, Wu-Fienberg Y, Guyuron B. The importance of surgical maneuvers during treatment of frontal migraines (site I): A prospective, randomized cohort study evaluating foraminotomy/fasciotomy, myectomy, and arterectomy. J Plast Reconstr Aesthet Surg. 2018;71:478–483. [DOI] [PubMed] [Google Scholar]
- 42.Filipovic B, de Ru JA, Hakim S, et al. Treatment of frontal secondary headache attributed to supratrochlear and supraorbital nerve entrapment with oral medication or botulinum toxin type A vs Endoscopic Decompression surgery. JAMA Facial Plast Surg. 2018;20:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fallucco M, Janis JE, Hagan RR. The anatomical morphology of the supraorbital notch: Clinical relevance to the surgical treatment of migraine headaches. Plast Reconstr Surg. 2012;130:1227–1233. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz R, Gfrerer L, Hansdorfer MA, et al. Migraine surgery at the frontal trigger site: An analysis of intraoperative anatomy. Plast Reconstr Surg. 2020;145:523–530. [DOI] [PubMed] [Google Scholar]
- 45.Janis JE, Barker JC, Javadi C, et al. A review of current evidence in the surgical treatment of migraine headaches. Plast Reconstr Surg. 2014;134(4 Suppl 2):131S–141S.. [DOI] [PubMed] [Google Scholar]
- 46.Raposio E. Atlas of Surgical Therapy for Migraine and Tension-Type Headache. 2019New York: Springer; [Google Scholar]
- 47.Raposio E. Atlas of Endoscopic Plastic Surgery. 2015New York: Springer; [Google Scholar]
- 48.Simonacci F, Lago G, Raposio E. Endoscopic Plastic Surgery. In: Advances of Plastic and Reconstructive Surgery. 2019:Irving, Tex.: Austin Publishing Group; 42–74. [Google Scholar]
- 49.Pourtaheri N, Guyuron B. Computerized tomographic evaluation of supraorbital notches and foramen in patients with frontal migraine headaches and correlation with clinical symptoms. J Plast Reconstr Aesthet Surg. 2018;71:840–846. [DOI] [PubMed] [Google Scholar]
- 50.Mathew PG. A critical evaluation of migraine trigger site deactivation surgery. Headache. 2014;54:142–52.. [DOI] [PubMed] [Google Scholar]
- 51.McGeeney BE. Migraine trigger site surgery is all placebo. Headache. 2015;55:1461–1463. [DOI] [PubMed] [Google Scholar]


