Background:
Migraine headache in the occipital region is characterized by a recurrent pain of moderate to severe intensity. However, the diagnosis can be difficult because of the multitude of symptoms overlapping with similar disorders and a pathophysiology that is not well-understood. For this reason, the medical management is often complex and ineffective.
Methods:
A literature search according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines was conducted to evaluate the surgical treatment of occipital migraines. Inclusion criteria were: English language, diagnosis of migraine, occipital neuralgia, or tension headache in compliance with the classification of the International Headache Society, follow-up at minimum 3 months, and adult age. The treatment had to consist of peripheral occipital nerve surgery.
Results:
323 records were identified after duplicates were removed, 30 full text articles were assessed for eligibility, and 9 records were selected for inclusion. A total of 1046 patients were included in the review. General positive response after surgery (>50% reduction in occipital migraine headaches) ranged from 80.0% to 94.9%. However, many differences in the selection of patients, target of decompression surgery, and measurement outcome were described.
Conclusion:
Despite the decennial proven effectiveness and safeness of surgical therapy for chronic occipital migraine headaches, more significant proof is needed to definitively confirm its use as a standard therapy.
INTRODUCTION
Migraine headache (MH) has always been commonly described as a complex, inherited disorder of brain function characterized by the tendency to lose control of brain inputs projected from nociceptive durovascular afferents to the thalamus and cortex. However, controversy over the origin of the pain is a hot topic: the origin of the neuronal mechanisms underlying the primary condition is in fact still unknown.1 Recently, new anatomical data on pain nerve fiber course through the skull,2 expression of pro inflammatory genes in the periosteum of affected patients,3 pathologic changes in peripheral compressed nerves,4 and effective extracranial tissues therapeutic approaches5 have focused attention on possible extracranial pathophysiologies in activating MH.6 Despite many clinical guidelines still not including surgery among the primary treatments for MH,7–9 countless international groups of researchers have highlighted that extracranial trigger site surgery is associated with a predictable positive outcome with a low rate of complication for appropriately selected patients.10–14 At first, supraorbital and suprathroclear nerves were identified as the first trigger site (site I: frontal) exposed to compression exerted by the corrugator supercilii muscle.15–17 Subsequent studies have described additional main peripheral triggers such as temporal (site II: zygomatic–temporal branch of the trigeminal nerve), nasal (site III: trigeminal end branches), and occipital (site IV: great occipital nerve).18 Surgical management of MH has gained popularity because of the high percentage of non-responders to standard pharmacologic therapies19 or abuse thereof.20 In 2018, the Executive Committee of the American Society of Plastic Surgeon stated the safety and efficacy of peripheral nerve/trigger site surgery for refractory chronic MH due to 20 years of peer-reviewed published evidence in high-impact-factor journals.21 Strong data support, as a potential trigger of migraine, nerve compression and/or irritation in the course through or near head and neck muscles or fasciae, arteries and bony canals.22,23
With respect to the occipital site, the involvement of the occipital nerves (the greater occipital nerve (GON); the lesser occipital nerve (LON); the third occipital nerve (TON)) could apply to all the aforementioned types of trigger points: arising from branches of the second and third cervical nerves (coming from the cervical spine), GON, LON, and TON run toward the occipital region crossing or passing through muscles and fascial planes such as inferior obliquus capitis, semispinalis capitis, and trapezius.24–26 A close relationship with the occipital artery (OA) and its minor branches is also well documented.27 The diagnosis of occipital migraine can be difficult owing to the overlap with other disorders and a pathophysiology that is not well-understood.28 It is characterized by recurrent headaches of moderate to severe intensity localized to the occipital region, with the occasional irradiation to the neck and face. Occipital MH treatment has long been focused on GON compression by the semispinal capitis muscle26 and the obliquus capitis29 during forceful, flexion-extension movements of the neck or in cases of trauma. However, the pulsating nature of pain during occipital migraine reinforced the idea of neurovascular etiology for the disease.30–33 Pulsatile distension of terminal branches of external carotid artery can determine traction and pressure stimuli to local terminal branches of the occipital nerve, resulting in a pulsating headache. Afterward, it can determine a chronic antalgic contraction of the surrounding muscles of the head and neck that can overcome the original vascular pain and determined chronic headache.23
Owing to the complexity of this anatomical region, many surgical options are described in the literature for different anatomical targets. This review aims to compare different approaches to peripheral release of sensory occipital nerve entrapment for the treatment of occipital MH in relation to outcome and complications to clarify whether there is a specific surgical approach that is more effective than others.
MATERIALS AND METHODS
Search Criteria
A thorough literature search was conducted in March 2020 across the following databases: PubMed MEDLINE, Scopus, and Cochrane Library. No date limits were set. The search terms used were “surgical treatment AND occipital migraine,”
“surgical treatment AND occipital headache,” “GON block AND occipital migraine,” “surgical treatment AND occipital nerve decompression,” “occipital migraine AND surgery,” “occipital headache AND surgery,” and “occipital nerve AND decompression.” These broad search terms were used to identify all citations reporting the outcomes of occipital headaches surgical therapy. Results were analyzed and double references were excluded. Two different authors independently examined the titles and abstracts of citations and generated a list of articles for review. Additional articles were included reviewing reference list of relevant abstract. This study was conducted according to PRISMA guidelines for systematic reviews.
Selection Criteria
Inclusion and exclusion criteria were defined before searching to avoid selection bias.
Inclusion criteria
Adult human subject
English language
Diagnosis of migraine headache, chronic migraine headache, occipital neuralgia, or tension headache according to the International Headache Society
Outcome data with a follow-up of at least 6 months
Peripheral occipital nerves surgery
Primary data from prospective/retrospective observational studies and RCTs
Exclusion criteria
Studies about radiosurgery, cryosurgery, and botulinum toxin injection without surgery
Technique or case report articles
Studies with fewer than 10 total patients
All the selected studies were then evaluated based on their methodological quality using the University of Oxford Centre for Evidence-Based Medicine’s levels of Evidence.
RESULTS
A total of 176 citations from PubMed, 146 from Scopus, and 41 citations from Chocrane Library were initially identified. After title and abstract review, analyzed by three different reviewers, 30 records were considered relevant. Full text examination excluded further 19 articles. Only 9 articles of the initial research, published from 2009 to 2019, fulfilled inclusion criteria and were included in the systematic review (Fig. 1).
Fig. 1.
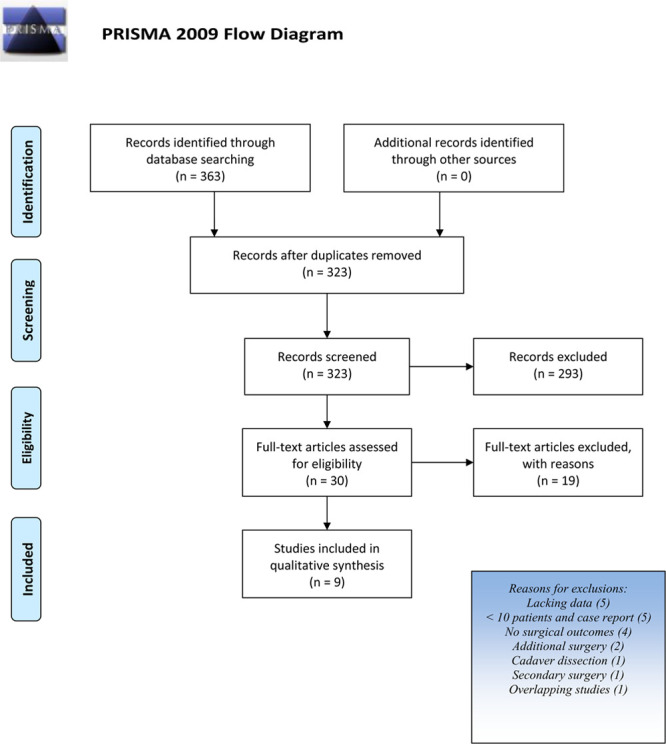
PRISMA flow diagram.
Among the 9 selected studies (Tables 1 and 2), 7 were retrospective studies (4 case-control34–37; 3 case series38–40), 1 was a blinded randomized controlled clinical trial,41 and 1 a prospective cohort study.42 A total of 1135 patients were included in studies on occipital nerve decompression with different surgical techniques. The sample size of each study ranged from 11 to 476 patients. Demographic characteristics of the population taken into account were sex for all the selected studies except 3,37,40,41 with a prevalence of females (range from 39.5% to 87.6%) except for Li et al,39 where more males were present. Patient age was reported as mean or as a range.
Table 1.
Studies Included in Qualitative Synthesis
| Study/Ref No. | Year | Type | Patient Selection | Study Groups | Sample (pts) | Outcomes Measurements | Follow-up (mo) | Limitations |
|---|---|---|---|---|---|---|---|---|
| Chmielewski et al34 | 2013 | Retrospective case–controlled study. | All patients who underwent occipital migraine headache surgery performed by the senior author (B.G.) for a span of 10 years (January 1, 2001 to December 31, 2010) were reviewed. | (1) n = 55 (38 bilateral, 17 unilateral); occipital artery resection (bipolar cautery) group: if the patient’s occipital artery or its branches were found in proximity to the greater occipital nerve (2) n = 115; control group: if the patient’s occipital arteries were not touched. Further subdivision into patients with continuous daily occipital headache and those who had an episodic form (potential indicator for occipital neuritis). |
n = 170 (21 men, 12.4%; 149 women, 87.6%) | Migraine Headache Questionnaire before and 12 mo after surgery: frequency (number of migraine headaches per month), duration (in days), intensity (scale of 1–10, with 10 being the most severe), and location of migraine headache pain. | Follow-up ranged from 12 to 87 mo, with a mean follow-up of 18 mo in the occipital artery resection group and 22 mo in the control group (P = 0.097). | There was a different distribution of the 2 procedures performed throughout the 10-year span: the majority of control procedures were performed in the earlier years, the occipital artery resection procedures had the opposite distribution. Change in the pattern of practice by the senior author over the years and surgery on patients, with a higher frequency and intensity and longer duration. Concomitant migraine surgery sites. The mean preoperative occipital migraine headache frequency was significantly higher in the occipital artery resection group compared with the control group. The occipital artery resection group mean preoperative duration was significantly lower than that of the control group. |
| Lineberry et al35 | 2015 | Retrospective case–controlled study. | Charts were reviewed for all patients who had undergone migraine surgery performed by the senior author (B.G.) from 2000 to 2010. Study inclusion criteria included migraine site IV decompression surgery and record of triamcinolone acetonide injection. |
(1) n = 282; triamcinolone acetonide group; (2) n = 194; control group. |
n = 476 (60 men, 12.6%; 416 women, 87.4%) | Migraine-specific information (preoperative and postoperative). Migraine Headache Questionnaires (preoperative and postoperative). Data points included patient age, sex, and triamcinolone acetonide injection; and migraine headache surgery site, frequency (migraines per month), intensity (based on a visual analog scale from 1 to 10, with 10 being the most severe), duration (in days), location, and characterization. |
At least 1 year. | The timeframe of patients analyzed: This study is a consecutive retrospective study beginning with the year 2000. Slight modifications in surgical technique used by the senior author over this period may have contributed to the success of the triamcinolone acetonide group. Second, the study represents a single surgeon’s experience; therefore, external validity may be limited. Third, the excessive scarring around the greater occipital nerve noted on re-exploration may represent inadequate decompression at the initial operation. |
| Lee et al36 | 2013 | Retrospective case–controlled study. | Charts for all patients who underwent migraine surgery by the senior author (B.G.) from 2000 to 2010 were reviewed. | (1) n = 111; TON avulsion (53 unilateral, 58 bilateral). (2) n = 118; no TON avulsion because no TON encountered. |
n = 229 (29 men, 12.7%; 200 women, 87.3%). | Preoperative and postoperative Migraine Headache Questionnaires. Data obtained included age, sex, MH surgery site, MH frequency (number of migraines per month), intensity (based on a visual analog scale from 1 to 10, with 10 being the most severe), duration (in days), location, and characterization. |
Minimum 6 mo | Not described. |
| Raposio and Bertozzi37 | 2019 | Retrospective case–controlled study. | Patients eligible to undergo migraine deactivation surgery had to be diagnosed by a board-certified neurologist with migraine without aura with >15 d/mo of headache, lasting for >6 mo, or chronic tension-type headache with >15 d/mo of headache, lasting for >6 mo, or new daily persistent headache attacks with >15 d/mo of headache, lasting for >6 mo. Patients with cluster headache, episodic tension-type headache, and major psychiatric disease, secondary migraine/headache as a consequence of other organic pathologies were excluded. |
(1) n =56; OA ligation in the site of close connection with GON; (2) n =22; GON and LON conservative neurolysis (muscolofascial decompression). |
n = 78; 58 bilateral, 20 unilateral. | Data from questionnaires completed before and after surgery. Daily headache diary. MH questionnaires assessing MH parameters preoperatively and following surgery to assess changes in MH. |
Follow-up of 21 mo (range: 12–67 mo) | Not described. |
| Ducic et al38 | 2009 | Restrospective case series. | A retrospective chart review was conducted of 206 consecutive patients presenting to the senior author (I.D.) with occipital neuralgia between February 2005 and June 2007, undergoing surgical treatment for occipital neuralgia. All patients had a workup performed by a neurologist before treatment to rule out other causes. No patients were treated surgically unless they had had symptoms for 6 mo or longer. |
Not applicable. | n = 206 (38 men, 18.4%; 168 women, 81.6%). Average age: 45 ± 2.9 y. 171 bilateral, 35 unilateral. |
Visual analog scale, Migraine headache index [days/months × intensity (0–10) × duration (fraction of 24 h)] (preoperative and postoperative). % of postoperative pain relief. Therapeutic success was defined as a reduction of pain by at least 50%. |
Minimum follow-up was 12 mo. | Not described. |
| Li et al39 | 2011 | Retrospective case series. | Patients with classic symptoms of greater occipital neuralgia (diagnostic criteria for ICHD-II diagnosis) were included when the headache rapidly resolved after infiltration of 1% Lidocaine near the tender area of the nerve trunk. | Not applicable. | n = 76 (46 men, 60.5%; 30 women, 39.5%). Age (y) 58 ± 9; 63 unilateral, 13 bilateral. |
Visual analog scale (VAS) before and after surgery. | Mean follow-up of 20 mo (range: 7–52 mo). | Not described. |
| Afifi et al40 | 2019 | Retrospective case series. | All patients undergoing occipital nerve decompression. | Not applicable. | n = 71; 66% of patients (n = 47) underwent LON surgery as well. | Migraine Headache Index (MHI) Mean Headache Impact Test (HIT-6). |
Thirty-two patients (30 bilateral and 2 unilateral) had >6 mo of follow-up with complete records for evaluation of their outcomes. | Not described. |
| Guyuron et al41 | 2009 | Single blind, randomized control trial. | Patients with frequent moderate to severe migraine headaches triggered from a single or predominant site. Diagnosis of migraine headache was confirmed using the International Classification of Headache Disorders II criteria. Positive response (>50% improvement) to botulinum toxin injection and recurrence of migraine headache after disappearance of its effect. |
(1) n = 7; sham surgery. (2) n = 11; control group (actual surgery). |
n = 18 (considering only occipital site). | Questionnaires before treatment: Medical Outcomes Study 36-Item Short Form Health Survey, Migraine-Specific Quality of Life, Migraine Disability Assessment (preoperative and 1 year postoperative). All patients maintained a daily headache diary and completed migraine headache questionnaires assessing the frequency (number of headaches per month), intensity (visual analog scale, 1–10), and duration (days) of their headaches on a monthly basis. |
Follow-up 1 year. | Not described. |
| Jose et al42 | 2018 | Prospective cohort study. | Occipital neuralgia was diagnosed by a neurologist after ruling out any intracranial cause of headache, using computed tomograms. All patients reported relief of symptoms following diagnostic occipital nerve blocks. Patients who were refractory to medical management were only enrolled. | Not applicable | n = 11 (2 men, 18.2%; 9 women, 81.8%). | Preoperative recording of pain history, pain episodes per month, pain severity, age at onset, symptoms, health status, medication history, and previous treatments. Postoperative (after at least 3 mo) recording of the degree of reduction of pain with regard to severity and frequency and surgical site problems. Comparison of headache frequency (as episodes per month) and pain severity pre- and post-surgery using a 10-point Visual Analog Scale. |
Patients were followed up to 1 year post-surgery. The mean follow-up period was 12.4 ± 51.29 mo, with no loss of follow-up. |
Lesser and third occipital nerves were not addressed. Learning curve may have influenced results. |
Table 2.
Studies Included in Qualitative Synthesis
| Study/Ref No. | Year | Surgical Strategy | Results | Complications |
|---|---|---|---|---|
| Chmielewski et al34 | 2013 | General anesthesia, prone position, midline occipital incision Removal of a small portion of the semispinalis capitis muscle between the midline and the nerve Releasing of the fascia overlying the nerve till to the subcutaneous plane Shielding of the nerve with a subcutaneous flap |
There was no significant difference between sex, mean age, follow-up, and concomitant surgery sites between the 2 groups Preoperative variables: Frequency, MH/mo (OAR, 19.3 ± 8.4 versus control, 14.6 ± 9.4; P = 0.002) Duration, days (OAR, 0.71 ± 0.72 versus control: 1.24 ± 1.42; P = 0.011) Intensity, analog scale (0–10) (OAR, 8.0 ± 2.9 versus control, 8.2 ± 1.9; P = 0.682) Postoperative variables: Frequency, MH/mo (OAR, 9.9 ± 9.8 versus control: 5.1 ± 7.6; P = 0.001) Duration, days (OAR, 0.44 ± 0.73 versus control, 0.42 ± 0.91; P = 0.888) Intensity, analog scale (0–10) (OAR, 4.7 ± 3.1 versus control, 4.1 ± 3.7; P = 0.307 Occipital artery resection patients, (n = 55): n = 44 (80.0%) success (>50% reduction) n = 21 (38.2%) elimination of occipital migraine headache Control patients (n = 115): n = 105 (91.3%) success n = 74 (64.3%) elimination of occipital migraine headache The control group had significantly higher success (P = 0.047) and elimination rates (P = 0.002) compared with the occipital artery resection group Comparison of sides in unilateral arterectomy patients: of the 17 patients who underwent bilateral greater occipital nerve decompression but unilateral arterectomy, 15 experienced equal relief on both sides. Both of the 2 remaining patients who experienced asymmetrical relief after surgery experienced a slightly greater reduction in migraine frequency on the non arterectomy side There was no significant difference between the success rates (P = 0.357) and elimination rates (P = 0.675) of patients with daily continuous occipital migraine headache in the 2 groups |
Not described |
| Lineberry et al35 | 2015 | Local anesthesia (1% lidocaine with 1:100,000 epinephrine), prone position with the neck flexed, a 4-cm vertical midline incision Incision of the trapezius fascia 0.5 cm to the right of the midline and dissection of an approximately 2-cm full-thickness length of muscle medial to the nerve. Removal of a small amount of trapezius fascia or muscle overlying the GON laterally Dissection/removal of any fascial bands remained above the nerve Removal of any arteries in the vicinity of the nerve In the triamcinolone acetonide group: 0.3 mL of triamcinolone acetonide is injected along the entire course of the GON, with a small amount injected into the nerve perineurium Elevation of an approximately 2 × 2 cm subcutaneous flap under the nerve on either side |
A significant reduction was found in the frequency of migraine headaches (−9.8 vs −8.0; P = 0.03) and the migraine headache index (−92.9 vs −65.2; P = 0.0065). There was no significant reduction in migraine headache duration (−0.50 vs −0.70; P = 0.10) or severity (−3.50 versus −3.80; P = 0.38) | Not described |
| Lee et al36 | 2013 | 4-cm midline raphe incision in hair-bearing caudal occipital regionIncision of the trapezius fascia about 0.5 cm lateral to the midlineAvulsion of the TON if encountered (allowed to retract into the proximal portion of the semispinalis capitis muscle)Dissection of the GON from surrounding muscle and fascial bands until the subcutaneous planeRemoval of 2-cm-long segment of the semispinalis capitis muscle between the nerve and the midline rapheLigation of the occipital artery when entangled with the nerve Elevation of a laterally based subcutaneous flap to separate the remaining muscle and nerve |
No statistical difference between the 2 groups in preoperative MH severity (TON R 8.0 versus TON NR 8.3; P = 0.35), MH frequency (TON R 18.1 versus TON NR 16.1; P = 0.09), or MH duration (TON R 0.9 versus TON NR 1.06; P = 0.44) No difference in complete overall MH elimination (TON R 26% versus TON NR 29%; P = 0.45) or overall MH surgery success (TON R 80% versus TON NR 81% group; P = 0.82) between the 2 groups No statistical difference between patients with bilateral or unilateral TON removal in preoperative MH severity, frequency, or duration No statistical difference in Site IV–specific MH elimination (unilateral 55% versus bilateral 60%; P = 0.73), overall MH elimination (unilateral 22.6% versus bilateral 29.3%; P = 0.24), or overall migraine surgery success (unilateral 75.5% versus bilateral 84.5%; P = 0.43) |
Neuroma formation after TON removal did not reach clinical significance |
| Raposio and Bertozzi37 | 2019 | Local assisted anesthesia (40 mL of diluted carbocaine 1% + 40-mL NaCl 0.9%, and 20-mL sodium bicarbonate 8.4%), patient prone, no trichotomy, horizontal occipital scalp incisions of 5 cm in length along the superior nuchal line, at the location of arterial signal detected preoperatively by the handheld DopplerDissection of occipital, trapezius, splenius capitis, and semispinalis capitis muscles to identify the GON and vascular bundle (OA)(1) In case of dilated (or frankly aneurysmatic) OA in close connection with the GON: ligation of the vessel without any other surgical maneuvers(2) In the remaining cases: execution of a conservative neurolysis of the GON and LON with undermining of occipital, trapezius, splenius capitis, and semispinalis capitis muscles along the nerves course until their emergence into the subcutaneous tissue | 94.9% positive response (86.8% complete; 8.1% significant improvement); 5.1% no relief Group underwent OA ligation: 95.5% positive response (90% complete; 5.5% significant improvement); 4.5% no relief Group not underwent OA ligation: 91% positive response (76% complete; 15% significant improvement); 9% no relief All the patients without improvement of the symptoms after OA ligation (4.5%) who suffered from unilateral occipital migraine had complete relief after contralateral secondary surgery Fourteen patients (8.3%) experienced secondary trigger point emergence following primary migraine surgery. Among these, 12 patients had 2 trigger points (10 occipital and frontal, 2 occipital and temporal), whereas 2 patients had all 3 trigger points |
No concerning side effects were reported |
| Ducic et al38 | 2009 | General anesthesia, patient prone, a central horizontal 5- to 6-cm incision approximately 3 cm below the occipital protuberance Exposition of the trapezius and vertical incision of its fascia where 1–3 mm of vertically oriented muscle fibers are present Resection of the small branch of the dorsal occipital nerve if identified Identification of the greater occipital nerve, emerging from the semispinalis capitis muscle. Removal of a little piece of semispinalis and releasing of obliquus capitis fibers overlying the GON Realizing of the trapezial fascial tunnel, any lymphatic structures, occipital artery and vein crossing the GON (dissected free and ligated) If unilateral lesser occipital nerve excision is performed concurrently, a 3-cm incision is made at a separate site lateral to the first incision If bilateral greater occipital nerve decompression and lesser occipital nerve excision are performed, 2 separate incisions are made |
n = 190 (92 %) GON neurolysis alone; n = 12 (6%) GON and LON excision; n = 4 (2 %) LON excision aloneAverage preoperative visual analog scale score was 7.9 ± 1.4 (range: 4–10). Postoperative score was 1.9 ± 1.8 (range, 0–8), a reduction of 6 (76%) (P < 0.0001)Average preoperative migraine headache index was 287 ± 14.9. Postoperative migraine headache index was 24 ± 11.8 (P < 0.0001)n = 166/206 (80.5%) >50% relief of pain, n = 72/166 (43.4%) complete relief, n = 40/206 (19.5%) <50% relief |
n = 2 incisional cellulitis resolved with oral antibiotics. |
| Li et al39 | 2011 | Local anesthesia with monitoring, lateral position, direct skin incision approachMusculofascial decompression at the aponeurosis/tendon of the trapezius muscle. Sometimes, dissection of parts of the muscles (inferior capitis oblique, semispinalis, trapezius)Dissection of swollen lymphnodes and malformed vascular branches twining the great occipital nerve or its branches | n = 68 (76.4%) complete pain relief, n = 5 (6.6%) significant relief without medical treatment. n = 3 (3.9%) recurrence: 1 (1.3%) repeat nerve decompression 6 mo after and 2 (2.6%) experienced recurrence 7 and 13 mo after surgical decompression, respectively |
Hypoesthesia of the innervated area of the great occipital nerve gradually recovered within 1–6 mo after surgery No postsurgical complication besides hypoesthesia |
| Afifi et al40 | 2019 | A horizontal incision (2.5-cm caudal to the external occipital protuberance), for bilateral cases, from the posterior edge of 1 sternocleidomastoid muscle to the other Raising of a deep fat, medially attached, rectangular flap off of the trapezius fascia The trapezius fascia and muscle are then divided vertically just lateral to the base of the flap Identification and decompression of the GON Identification of the LON and execution of a neurectomy or decompression according to the size of the nerve The fat flap is then used to wrap the GON |
Average migraine headache index was 191 preoperatively and 55 postoperatively (P = 0.004), with a mean improvement of 70% 92% of patients experienced at least a 50% reduction in migraine headache index. Migraine frequency, intensity, and duration improved by a mean of 44.25 % (P = 0.0008), 51% (P = 0.01), and 58.4% (P = 0.1), respectively Mean Headache Impact Test (HIT-6) score improved from 67 preoperatively to 57 postoperatively (P < 0.0001) |
One case of wound infection, no cases of seroma or alopecia |
| Guyuron et al41 | 2009 | Under general anesthesia, patient in prone position, 4-cm incision in the midline occipital area(1) Mere exposure of the nerve with the muscle left intact(2) Removal of a segment of the semispinalis capitis muscle medial to the GON (1 × 2.5 cm). Subcutaneous flap interposition to avoid impingement of the nerve | Compared with the sham group, the actual surgery group demonstrated statistically significant improvements in all validated migraine headache measurements at 1 yearImprovement at 12 mo Treatment versus Sham: Frequency, MH/month [8.7 ± 6.1 (<0.001); 5.7 ± 5.6 (0.04)]; Intensity, [4.2 ± 3.4 (<0.001); 1.3 ± 3.2 (0.45)]; Duration, [0.54 ± 0.55 (0.009); 3.37 ± 7.7 (0.34)]; Migraine headache index, [37.1 ± 48.1 (0.03); 8.5 ± 15.1 (0.18)]; MIDAS, [1.5 ± 1.5 (0.01); 0.86 ± 1.7 (0.22)]; MSQEM, [56.0 ± 51.0 (0.005); 18.1 ± 33.2 (0.20)]; MSQPRE, [−24.5 ± 26.9 (0.013); −7.1 ± 19.8 (0.39)]; MSQRES, [−29.2 ± 26.9 (0.005); −11.4 ± 9.1 (0.02)]; SFPH, [−2.1 ± 5.6 (0.24); −8.7 ± 8.6 (0.4)] |
All patients reported some degree of paresthesia in the immediate postoperative period. No neuromas were observed One patient reported some neck stiffness 1 year postoperatively in treatment group No adverse events were observed in the sham surgery group |
| Jose et al42 | 2018 | T-shaped incision was made 1 cm below the occipital protuberanceRemoval of a small medial piece of semispinalis capitis muscle abutting the greater occipital nerveReleasing of the muscle in the trapezial fascia as the nerve runs through it toward the occiput. If the occipital artery was found impinging on the nerve at the supero-lateral end it was dissected and ligatedTen patients underwent unilateral nerve decompression while 1 required bilateral surgeryNo LON decompression | Mean pain episodes reported by the patients before surgery were 17.1 ± 5.63 episodes per month. This reduced to 4.1 ± 3.51 episodes per month (P < 0.0036) postsurgery. The mean intensity of pain also reduced from a preoperative 7.18 ± 1.33 to a postoperative of 1.73 ± 1.95 (P < 0.0033)Postoperative questionnaire: n = 3 (27.3%) complete elimination of pain n = 6 (54.5%) significant relief of their symptoms (positive outcome: 81.8%) N = 2 (18.2%) no significant improvement |
Six patients reported temporary surgical site paraesthesia. No other complications were noted |
Patient Selection
Patients were selected among those who had undergone occipital decompression surgery in a definite timeframe in all retrospective studies. Many differences were present; in particular, Li et al39 included patients after positive nerve block response while Raposio and Bertozzi37 and Ducic et al38 included patients with at least 6 months of symptoms. Guyuron et al41 selected patients with frequent moderate-to-severe migraine headaches triggered from a single or predominant site with previous positive response to botulinum toxin injection. Jose et al42 selected patients with occipital neuralgia (ON) diagnosis who were refractory to medical management. Diagnosis by a board-certified neurologist was described in 4 of 9 studies.37,38,41,42
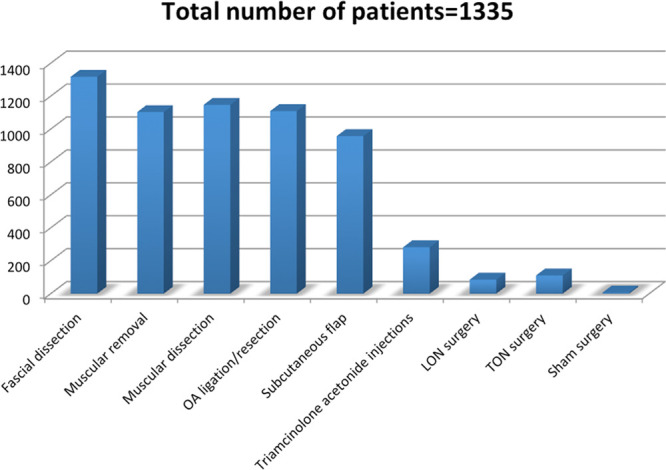
Surgical Treatment
Regarding surgical approach, 5 types of surgical incision are mentioned: a 4-cm vertical midline occipital incision34–36,41 was the most common, while horizontal incisions differed in length and in position between studies,37,38,40,42 a T-shaped incision was described in 1 study42 and no information was specified by Li et al.39 Among surgical techniques, musculofascial decompression through accurate dissection of semispinalis capitis, trapezius, and obliquus capitis was performed in all studies. Removal of small portions of the semispinalis capitis and/or trapezius34–36,38,41,42 and ligation/resection of arteries in the vicinity of the GON34–39,42 were described in most of the studies. A subcutaneous flap to shield the GON from surrounding structures was often described.34–36,39–41 In particular, Afifi et al40 described a deep fat flap with its base attached medially to the deeper tissues over the nuchal ligament, called “W” flap in bilateral cases, as being used to cover the nerve at the site of the resected semispinalis muscle (compression points 2 and 3) and/or used more distally at the crossing of the nerve over the nuchal ridge (points 4–6). While decompression of GON is always described, LON and/or TON are considered in 4 of 9 studies. In particular, Lee et al36 described the avulsion of the TON when encountered in 1 of the two patient study groups, while LON neurolysis or neurectomy was mentioned by Raposio and Bertozzi,37 Ducic et al,38 and Afifi et al.40 Realizing of lymphatic structures surrounding the nerve38 and dissection of swollen lymph nodes intertwining the nerves39 were infrequently mentioned. Lineberry et al35 added the injection of corticosteroids along the entire course and into the perineurium of the greater occipital nerve to migraine decompression surgery in the treatment group (Fig. 2).
Fig. 2.
Surgical techniques.
Outcomes Measurement
There is no corresponding methodology between studies to measure surgical outcome. The most frequently used methods were the migraine headache questionnaire,34–37,41,42 the percentage of postoperatively pain relief,34,36–39,42 and the migraine headache index (MHI).38,40 Only Guyuron et al41 used questionnaires (Medical Outcomes Study 36-Item Short Form Health Survey, Migraine-Specific Quality of Life Questionnaire, Migraine Disability Assessment Questionnaire) to assess general health, quality of life, and grade of disability, while Afifi et al40 assessed the headache’s impact on patients with the Mean Headache Impact Test (HIT-6). Follow-up was at least 6 months in each study, with the maximum of 87 months reported by Chmielewski et al.34
Outcome Data
A successful treatment was defined as migraine attack elimination or at least 50% reduction by the majority of the studies.34,36–38,42 General positive response after surgery (>50% reduction in occipital migraine headaches) ranged from 80.0%34,36 to 94.9%.37 In particular, the elimination rate varied from 26.0%36 to 90.0%.37 The blinded randomized controlled clinical trial41 compared 2 groups of patients who had undergone actual surgery with those who had had sham surgery (just exposure of the nerve with the semispinalis capitis muscle left intact), showing significantly better results in the actual surgery group (despite some grade of improvement also in the sham surgery group). Among retrospective comparative studies, 1 study35 investigated the difference between standard musculofascial decompression whether or not followed by the injection of corticosteroids along the entire course and into the perineurium of the greater occipital nerve,. The results showed a significant reduction in frequency of migraine headache (−9.8 versus −8.0; P = 0.03) and, consequently, in migraine headache index (MHI) (−92.9 versus −65.2; P = 0.0065). Another study36 compared avulsion of the TON if encountered versus no avulsion with no statistical significant difference between the two groups. Among studies that compared the ligation/cauterization or not of the OA in case of a close relationship between the artery and the nerve,34,37 the results were completely discordant. In both studies, the control group consisted of patients who had undergone standard musculofascial decompression of the nerve, while the study group contained patients who had undergone standard musculofascial decompression plus artery resection in the study by Chmielewski et al34 or patients who had only undergone OA ligation/cauterization in the Raposio and Bertozzi37 study. Summarizing, the control group of the first study had significantly higher success (P = 0.047) and elimination rates (P = 0.002) compared with the OA resection group. However, preoperative non-homogeneity of severity of migraine attacks (frequency and duration of migraine) between groups was admitted to have occurred by the authors. In the second study, rate of percentage of positive response and elimination were higher in the study group (no statistical analysis was done).
Adverse Events
Patients with bilateral or unilateral surgery were also compared in two retrospective studies34,36: no statistically significant difference was found. Raposio and Bertozzi37 analyzed the appearance of secondary trigger sites after decompression primary surgery, showing that 8.3% of patients (n = 14) experienced secondary trigger point emergence following primary migraine surgery (12 patients had 2 trigger points, whereas 2 patients had all 3 trigger points).
Three of 9 studies did not deal with postoperative complications. Intense itching, incisional cellulitis, wound infection, neck stiffness but, most of all, some degree of paresthesia in the immediate postoperative period are the complications that, in almost all cases, resolved without sequelae. Neuroma formation was reported as absent by Guyuron et al41 only, while Lee et al36 state that there was no difference between the two groups in symptomatic neuroma formation without specifying the number of neuromas detected in the two groups (TON removed versus TON not removed).
DISCUSSION
Since extracranial mechanisms in headache generation have gained popularity, many studies have tried to provide evidence of anatomic connection between the intracranial and extracranial spaces43,44 to explain how a peripheral trigger in the head or neck can result in activation of intracranial meningeal nociceptors.45 Genetic predisposition, moreover, has been proposed as the possible cause of an extracranial inflammatory disease due to the imbalance in expression of inflammatory genes in the occipital periosteum.3 These theories, which partially explain the ineffectiveness of centrally acting medical therapies, are supported by all those treatments directed to act peripherally such as nerve blocks,46 steroids injections at trigger points,47 and botulinum toxin injections.48 However, the theory of peripheral nerve compression is not free from unresolved dilemmas.49 The extracranial course of the occipital nerves is, in fact, characterized by several areas of possible entrapment. The anatomy of GON is widely described in the literature: once out of the C1–C2 intervertebral space, GON may be compressed between semispinalis and inferior oblique capitis muscles, passing through the semispinal capitis muscle or in correspondence of the trapezium muscle and its aponeurotic band toward the occipital crest; moreover, at this level, a close relationship between GON and OA is often present.50 Although GON musculofascial decompression is a treatment utilized by all the analyzed studies, the same cannot be said for LON and TON, as they are rarely mentioned. Research initially began to find a reason why patients were unresponsive or partially responding to surgery.23,51 LON arises from C2 and/or C3 spinal nerves52 with an exit point along the posterior border of the sternocleidomastoid muscle,53 which does not seem to be a point of compression, whereas an intimate relationship between LON and OA (both as single interaction and as intertwinement) as well as with fascial bands are often present. A target zone for surgical release of LON has therefore been identified by Lee et al.54 The TON is the dorsal ramus of C3, its exit point is located closer to the midline than the previous two, but cadaver studies described a greater variability.45 This characteristic is confirmed by the study done by Lee et al36 even if the role of this nerve in the origin of pain is not clarified. Likewise, the management of occipital vessels is not homogeneous. The involvement of the OA as a cause of compression is sometimes not considered or even indicated as detrimental to the outcome.
A thorough understanding of the anatomy and the potential compression sites of occipital nerves seems essential to obtain a successful decompression treatment. Unfortunately, no adequate imaging techniques are able to investigate all sites of possible compression. Muscle-tendon ultrasound has proved useful in identifying certain segments of GON55 but accurate medical history and physical examination seem to be the main method of identifying trigger sites.56 In our review, some methodologies used to guide subsequent surgical decompression procedures to limit therapeutic failure are mentioned. Nerve block is used in 2 studies. Despite its usefulness, especially in providing for effective decompression nerve surgical treatment,57 its utilization is strictly ligated to the presence of a headache migraine attack during the visit and the identification of the entrapment is often not precise.58 Botulinum toxin does not present this limit. However, despite its efficacy in identifying musculofascial compression of the greater occipital nerve,59 it cannot identify trigger sites related to OA26 that are instead easily detected by a Doppler probe, especially if corroborated by patient self-identification of the trigger points.31,60 Failure to identify all trigger sites could be the cause of the incomplete response in many cases.61 If all sites of possible nerve compression are not carefully assessed preoperatively and/or managed during surgery, the real effectiveness of surgical therapy may not be determined. Recently, specific criteria for their detection in selected patients were summarized by Guyuron et al46: citing that cooperation between neurologists and surgeons is necessary to improve MH management, while patient collaboration in describing symptoms and identifying the headache start site, as well as several diagnostic tools, are fundamental in planning surgery.
Despite a variety of surgical techniques and some limitations underlined in the studies, success in occipital decompression surgery is high, surpassing 90% in several studies. Long-term effects described cannot be the result of a placebo effect.54 However, other randomized clinical trials are necessary to definitively confirm this claim. Currently, only one clinical trial is ongoing, with the aim to compare surgical intervention with continued medical management in post-traumatic occipital headaches.62
A large body of evidence suggests that occipital migraines can be treated by suppressing irritation of peripheral nerves through surgical decompression with a very low appearance of postoperative complications. For these reasons, peripheral nerve trigger surgery for the treatment of resistant chronic MH in selected patients should be considered as a therapeutic option by all the involved specialists, as opposed to remaining anchored to standard treatment schemes that are not sufficiently effective in the management of a disease where the pathophysiology has not yet been clarified.
LIMITATIONS
The retrospective nature of most of the selected studies is one of the main limitations of this review. Moreover, as underlined by the authors themselves, data collection was often carried out over a long period of time (as much as 10 years). This leads to consider the presence of a slight modification in the surgical technique even if performed by the same surgeon. Another aspect to take into consideration concerns the methods of assigning patients to groups in case-control studies, which is closely linked to different anatomical characteristics between groups of patients. Differences in outcomes could therefore be related to the causes of compression rather than to surgical techniques.
CONCLUSIONS
Occipital MH surgery has proved its effectiveness over the years. However, a widely shared surgical approach does not yet seem to be identified and it is not possible to reach substantial conclusions as to which is the best surgical approach. Greater standardization in patient selection, constant use of preoperative and postoperative evaluation methods, and the design of randomized multicenter prospective clinical trials would solidify the extremely positive results described worldwide.
Footnotes
Published online 14 October 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schueler M, Neuhuber WL, De Col R, et al. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache. 2014;54:996–1009. [DOI] [PubMed] [Google Scholar]
- 3.Perry CJ, Blake P, Buettner C, et al. Upregulation of inflammatory gene transcripts in periosteum of chronic migraineurs: implications for extracranial origin of headache. Ann Neurol. 2016;79:1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyuron B, Yohannes E, Miller R, et al. Electron microscopic and proteomic comparison of terminal branches of the trigeminal nerve in patients with and without migraine headaches. Plast Reconstr Surg. 2014;134:796e–805e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein R, Dodick D, Silberstein S. Migraine prophylaxis with botulinum toxin A is associated with perception of headache. Toxicon. 2009;54:624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein R, Blake P, Schain A, et al. Extracranial origin of headache. Curr Opin Neurol. 2017;30:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670–679. [PMC free article] [PubMed] [Google Scholar]
- 8.Agostoni EC, Barbanti P, Calabresi P, et al. ; Italian chronic migraine group. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;20:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiner TJ, Jensen R, Katsarava Z, et al. Aids to management of headache disorders in primary care (2nd edition): on behalf of the European Headache Federation and Lifting the Burden: The global campaign against headache. J Headache Pain. 2019;20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gfrerer L, Austen WG, Jr, Janis JE. Migraine surgery. Plast Reconstr Surg Glob Open. 2019;7:e2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducic I, Felder JM, III, Fantus SA. A systematic review of peripheral nerve interventional treatments for chronic headaches. Ann Plast Surg. 2014;72:439–445. [DOI] [PubMed] [Google Scholar]
- 12.Janis JE, Barker JC, Javadi C, et al. A review of current evidence in the surgical treatment of migraine headaches. Plast Reconstr Surg. 2014;134:131S–141S. [DOI] [PubMed] [Google Scholar]
- 13.Caruana G, Bertozzi N, Boschi E, et al. Endoscopic forehead surgery for migraine therapy personal technique. Ann Ital Chir. 2014;85:583–586. [PubMed] [Google Scholar]
- 14.Simonacci F, Lago G, Raposio E. Endoscopic plastic surgery. In: Advances of Plastic and Reconstructive Surgery. 2019:Irving, Tex.: Austin Publishing Group; 42–74. [Google Scholar]
- 15.Guyuron B, Varghai A, Michelow BJ, et al. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106:429–434; discussion 435. [DOI] [PubMed] [Google Scholar]
- 16.Caruana G, Grignaffini E, Raposio E. Endoscopic forehead muscle resection for nerve decompression: a modified procedure. Plast Reconstr Surg Glob Open. 2015;3:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raposio E, Caruana G. Frontal endoscopic myotomies for chronic headache. J Craniofac Surg. 2015;26:e201–e203. [DOI] [PubMed] [Google Scholar]
- 18.Raposio E, Bertozzi N, Bordin C, et al. Turker H, ed. Surgical therapy of migraine and tension-type headaches. In: Current Perspectives on Less-known Aspects of Headache. 2017:London, U.K.: InTech; 93–114. [Google Scholar]
- 19.Dodick DW. Triptan nonresponder studies: implications for clinical practice. Headache. 2005;45:156–162. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbussche N, Laterza D, Lisicki M, et al. Medication-overuse headache: a widely recognized entity amidst ongoing debate. J Headache Pain. 2018;19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ASPS Executive Committee. Policy statement: migraine headache surgery. Available at https://www.plasticsurgery.org/Documents/Health-Policy/Positions/ASPS-Statement_Migraine-Headache-Surgery.pdf. Accessed March 22, 2020.
- 22.Raposio E. Atlas of Endoscopic Plastic Surgery. 2015New York, N.Y.: Springer; [Google Scholar]
- 23.Dash KS, Janis JE, Guyuron B. The lesser and third occipital nerves and migraine headaches. Plast Reconstr Surg. 2005;115:1752–1758; discussion 1759. [DOI] [PubMed] [Google Scholar]
- 24.Ascha M, Kurlander DE, Sattar A, et al. In-depth review of symptoms, triggers, and treatment of occipital migraine headaches (Site IV). Plast Reconstr Surg. 2017;139:1333e–1342e. [DOI] [PubMed] [Google Scholar]
- 25.Mosser SW, Guyuron B, Janis JE, et al. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004;113:693–697; discussion 698. [DOI] [PubMed] [Google Scholar]
- 26.Janis JE, Hatef DA, Ducic I, et al. The anatomy of the greater occipital nerve: Part II. Compression point topography. Plast Reconstr Surg. 2010;126:1563–1572. [DOI] [PubMed] [Google Scholar]
- 27.Raposio E. Atlas of Surgical Therapy for Migraine and Tension-type Headache. 2019New York, N.Y.: Springer; [Google Scholar]
- 28.Yi X, Cook AJ, Hamill-Ruth RJ, et al. Cervicogenic headache in patients with presumed migraine: missed diagnosis or misdiagnosis? J Pain. 2005;6:700–703. [DOI] [PubMed] [Google Scholar]
- 29.Scherer SS, Schiraldi L, Sapino G, et al. The greater occipital nerve and obliquus capitis inferior muscle: anatomical interactions and implications for occipital pain syndromes. Plast Reconstr Surg. 2019;144:730–736. [DOI] [PubMed] [Google Scholar]
- 30.Polotto S, Simonacci F, Grignaffini E, et al. Surgical treatment of frontal and occipital migraines: a comparison of results. Plast Reconstr Surg Glob Open. 2016;4:e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raposio E, Bertozzi N, Bordin C, et al. Sakkary M, ed.: Surgical therapy of headaches: minimally invasive approaches. In Clinical Advances in Head & Neck Surgery. 2018:Las Vegas, Nev.: Openaccess eBooks; 1–23. [Google Scholar]
- 32.Shimizu S, Oka H, Osawa S, et al. Can proximity of the occipital artery to the greater occipital nerve act as a cause of idiopathic greater occipital neuralgia? An anatomical and histological evaluation of the artery-nerve relationship. Plast Reconstr Surg. 2007;119:2029–2034; discussion 2035. [DOI] [PubMed] [Google Scholar]
- 33.Shevel E. The extracranial vascular theory of migraine—a great story confirmed by the facts. Headache. 2011;51:409–417. [DOI] [PubMed] [Google Scholar]
- 34.Chmielewski L, Liu MT, Guyuron B. The role of occipital artery resection in the surgical treatment of occipital migraine headaches. Plast Reconstr Surg. 2013;131:351e–356e. [DOI] [PubMed] [Google Scholar]
- 35.Lineberry K, Lee M, Monson A, et al. Intraoperative corticosteroid injections in migraine surgery: efficacy in preventing refractory symptoms. Plast Reconstr Surg. 2015;135:393e–396e. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Lineberry K, Reed D, et al. The role of the third occipital nerve in surgical treatment of occipital migraine headaches. J Plast Reconstr Aesthet Surg. 2013;66:1335–1339. [DOI] [PubMed] [Google Scholar]
- 37.Raposio E, Bertozzi N. Trigger site inactivation for the surgical therapy of occipital migraine and tension-type headache: our experience and review of the literature. Plast Reconstr Surg Glob Open. 2019;7:e2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ducic I, Hartmann EC, Larson EE. Indications and outcomes for surgical treatment of patients with chronic migraine headaches caused by occipital neuralgia. Plast Reconstr Surg. 2009;123:1453–1461. [DOI] [PubMed] [Google Scholar]
- 39.Li F, Ma Y, Zou J, et al. Micro-surgical decompression for greater occipital neuralgia. Turk Neurosurg. 2012;22:427–429. [DOI] [PubMed] [Google Scholar]
- 40.Afifi AM, Carbullido MK, Israel JS, et al. Alternative approach for occipital headache surgery: the use of a transverse incision and “W” flaps. Plast Reconstr Surg Glob Open. 2019;7:e2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guyuron B, Reed D, Kriegler JS, et al. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009;124:461–468. [DOI] [PubMed] [Google Scholar]
- 42.Jose A, Nagori SA, Chattopadhyay PK, et al. Greater occipital nerve decompression for occipital neuralgia. J Craniofac Surg. 2018;29:e518–e521. [DOI] [PubMed] [Google Scholar]
- 43.Kosaras B, Jakubowski M, Kainz V, et al. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515:331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schueler M, Messlinger K, Dux M, et al. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622–1631. [DOI] [PubMed] [Google Scholar]
- 45.Blake P, Burstein R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain. 2019;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guyuron B, Nahabet E, Khansa I, et al. The current means for detection of migraine headache trigger sites. Plast Reconstr Surg. 2015;136:860–867. [DOI] [PubMed] [Google Scholar]
- 47.Szperka CL, Gelfand AA, Hershey AD. Patterns of use of peripheral nerve blocks and trigger point injections for pediatric headache: results of a survey of the American Headache Society pediatric and adolescent section. Headache. 2016;56:1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitcup SM, Turkel CC, DeGryse RE, et al. Development of onabotulinumtoxinA for chronic migraine. Ann N Y Acad Sci. 2014;1329:67–80. [DOI] [PubMed] [Google Scholar]
- 49.Gfrerer L, Raposio E, Ortiz R, et al. Surgical treatment of migraine headache: back to the future. Plast Reconstr Surg. 2018;142:1036–1045. [DOI] [PubMed] [Google Scholar]
- 50.Raposio E, Caruana G. Tips for the surgical treatment of occipital nerve-triggered headaches. Eur J Plast Surg. 2017;40:177–182. [Google Scholar]
- 51.Khavanin N, Carl HM, Yang R, et al. Surgical “Safe Zone”: rapid anatomical identification of the lesser occipital nerve. J Reconstr Microsurg. 2019;35:341–345. [DOI] [PubMed] [Google Scholar]
- 52.Tubbs RS, Salter EG, Wellons JC, et al. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007;20:235–238. [DOI] [PubMed] [Google Scholar]
- 53.Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg. 2009;123:859–863; discussion 864. [DOI] [PubMed] [Google Scholar]
- 54.Lee M, Brown M, Chepla K, et al. An anatomical study of the lesser occipital nerve and its potential compression points: implications for surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;132:1551–1556. [DOI] [PubMed] [Google Scholar]
- 55.Narouze S, Souzdalnitski D. Occipital nerve entrapment within the semispinalis capitis muscle diagnosed with ultrasound. Cephalalgia. 2013;33:1358–1359. [DOI] [PubMed] [Google Scholar]
- 56.Caviggioli F, Giannasi S, Vinci V, et al. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg. 2011;128:564e–565e; author reply 565e. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H, Yang X, Lin Y, et al. The efficacy of greater occipital nerve block for the treatment of migraine: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;165:129–133. [DOI] [PubMed] [Google Scholar]
- 58.Bovim G, Bonamico L, Fredriksen TA, et al. Topographic variations in the peripheral course of the greater occipital nerve. Autopsy study with clinical correlations. Spine (Phila Pa 1976). 1991;16:475–478. [DOI] [PubMed] [Google Scholar]
- 59.Lee M, Monson MA, Liu MT, et al. Positive botulinum toxin type a response is a prognosticator for migraine surgery success. Plast Reconstr Surg. 2013;131:751–757. [DOI] [PubMed] [Google Scholar]
- 60.Liu MT, Armijo BS, Guyuron B. A comparison of outcome of surgical treatment of migraine headaches using a constellation of symptoms versus botulinum toxin type A to identify the trigger sites. Plast Reconstr Surg. 2012;129:413–419. [DOI] [PubMed] [Google Scholar]
- 61.Punjabi A, Brown M, Guyuron B. Emergence of secondary trigger sites after primary migraine surgery. Plast Reconstr Surg. 2016;137:712e–716e. [DOI] [PubMed] [Google Scholar]
- 62.Post-traumatic Occipital Neuralgia—Surgical Versus Medical Management. Cochrane Central Register of Controlled Trials. Available at https://clinicaltrials.gov/show/NCT03253523. Accessed April 25, 2020.


