Abstract
System xc− cystine/glutamate antiporter, composed of a light-chain subunit (xCT, SLC7A11) and a heavy-chain subunit (CD98hc, SLC3A2), is mainly responsible for the cellular uptake of cystine in exchange for intracellular glutamate. In recent years, the xCT molecule has been found to play an important role in tumor growth, progression, metastasis, and multidrug resistance in various types of cancer. Interestingly, xCT also exhibits an essential function in regulating tumor-associated ferroptosis. Despite significant progress in targeting the system xc− transporter in cancer treatment, the underlying mechanisms still remain elusive. It is also unclear why solid tumors are more sensitive to xCT inhibitors such as sulfasalazine, as compared to hematological malignancies. This review mainly focuses on the role of xCT cystine/glutamate transporter in regard to tumor growth, chemoresistance, tumor-selective ferroptosis, and the mechanisms regulating xCT gene expression. The potential therapeutic implications of targeting the system xc− and its combination with chemotherapeutic agents or immunotherapy to suppress tumor growth and overcome drug resistance are also discussed.
Keywords: system xc−, xCT, ROS, ferroptosis
Graphical Abstract
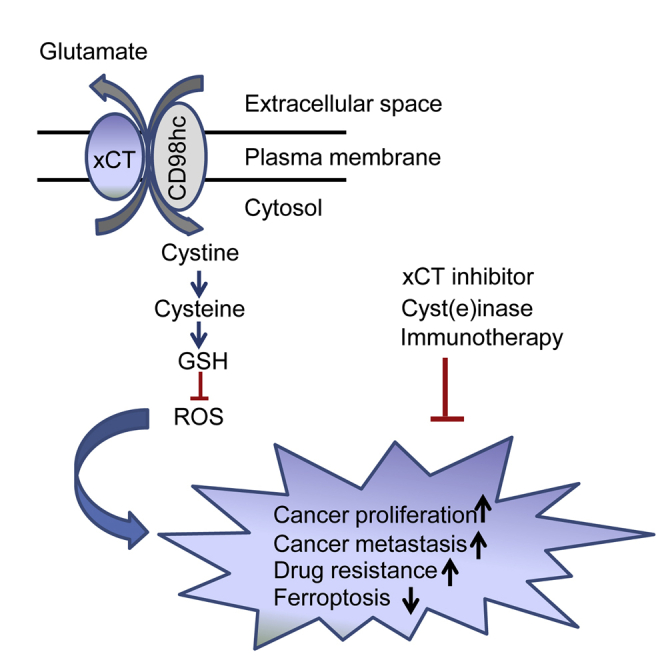
In this review, Liu and colleagues discussed the critical role of xCT cystine/glutamate transporter in regard to redox signaling and cancer metabolism, the molecular mechanisms regulating xCT expression, and the potential therapeutic implications of targeting this transporter for efficacious cancer treatment.
Main Text
During the past two decades, accumulating findings have suggested an increasingly important role of the cystine/glutamate transporter system xc− in cell growth, proliferation, metastasis, and multidrug resistance in multiple cancer types.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 System xc− is a cystine/glutamate exchange transporter composed of a light-chain subunit (xCT, also known as SLC7A11) and a heavy-chain subunit (CD98hc, also known as SLC3A2).28,29 Due to the chemically unstable property of cysteine in the extracellular environment, cells mainly import its oxidized form, cystine (chemically stable), from the microenvironment. Cystine is then reduced to cysteine intracellularly for the synthesis of glutathione (GSH),7 which is the most abundant intracellular antioxidant consisting of three amino acids: cysteine, glutamate, and glycine.30 Zhang et al.7 reported that primary chronic lymphocytic leukemia (CLL) cells have a limited ability to transport cystine for GSH synthesis due to their low expression of xCT, the active subunit of system xc−. Alternatively, the stromal cells in the tumor microenvironment can effectively import cystine via the plasma membrane transporter system xc− and secrete the reduced form of the compound, cysteine, to support the need of CLL cells for GSH synthesis.7 Therefore, xCT plays a pivotal role in intracellular cysteine balance and GSH biosynthesis.
The GSH metabolic pathway has been considered as a potential anticancer target in multiple types of cancer, and GSH depletion induced by loss of xCT contributes to suppression of cell growth and induction of apoptosis in tumor cells.5,7,10,15,31, 32, 33 Recent studies have shed new light on the function of GSH as the reducing power for lipid peroxides, which are responsible for iron-catalyzed, lipid peroxide-dependent cell death, known as ferroptosis,8 in cancer cells.13,14,34 Thus, depletion of intracellular GSH could result in cytotoxicity of tumor cells in different forms of cell death such as apoptosis and ferroptosis. GSH is used as a reducing agent to suppress ferroptosis, which is a consequence of accumulation of lipid reactive oxygen species (ROS) in several types of cancer. Alternatively, xCT has been found to be upregulated in numerous cancers. Thus, its overexpression has been associated with poor prognosis in several types of cancer, and disruption of xCT leads to suppression of tumor growth.34, 35, 36, 37
Significant anti-cancer effects have been observed by blocking the xCT-GSH axis in various tumors (Table 1). However, the molecular and biochemical characteristics of individual tumors that determine the specific anti-cancer effect through targeting xCT still remain to be elucidated. Additionally, a thorough understanding of the underlying mechanisms of xCT regulation would be helpful to develop effective therapeutic strategies based on targeting the xCT-GSH axis in different types of cancer. Herein, we review the effects of targeting xCT in various cancers and the underlying mechanisms. We also discuss the promising anticancer drugs with the potential to eliminate cancer cells through GSH depletion-mediated apoptosis or ferroptosis, and the combinations of xCT inhibitors or cyst(e)inase with other anti-cancer therapeutics, such as immune checkpoint blockade, to synergistically eliminate cancer cells.
Table 1.
Summary of the Key Findings Regarding the Important Role of the xCT Molecule in Various Aspects of Cancer
| Content | Aspect of Cancer | References | Year |
|---|---|---|---|
| Ferroptosis induced in PDAC through cysteine depletion | ferroptosis | Badgley et al.14 | 2020 |
| SLC7A11 overexpression sensitizes cancer cells to GLUT inhibition | chemosensitivity | Liu et al.38 | 2020 |
| ARID1A maintains GSH homeostasis by enhancing xCT transcription | apoptosis | Ogiwara et al.39 | 2019 |
| INFγ released from CD8+ T cells inhibits xCT and promotes ferroptosis | ferroptosis | Wang et al.11 | 2019 |
| Radiotherapy and immunotherapy promote ferroptosis via synergistic repression of xCT | ferroptosis | Lang et al.40 | 2019 |
| Systemic depletion of cyst(e)ine with cyst(e)inase suppresses tumor growth in multiple cancer | tumor growth | Cramer et al.10 | 2017 |
| CD8+ T cells abrogate chemoresistance by altering GSH/cystine metabolism | chemoresistance | Wang et al.9 | 2016 |
| By repressing SLC7A11 transcription, p53 activation reduces cystine uptake and promotes ferroptosis | ferroptosis | Jiang et al.41 | 2015 |
| Glutamine sensitivity analysis identifies xCT as a therapeutic target in triple-negative breast tumor | tumor growth | Timmerman et al.42 | 2013 |
| Stromal control of cystine metabolism promotes CLL cell survival | apoptosis | Zhang et al.7 | 2012 |
| CD44 variant regulates redox status by stabilizing xCT and promotes tumor growth | tumor growth | Ishimoto et al.6 | 2011 |
Role of the xCT Subunit in Cancer Growth and Progression
l-cystine and l-cysteine, collectively known as cyst(e)ine, are non-essential amino acids that can be generated from methionine in the body. Interestingly, human lymphoid cells and leukemic cells are unable to synthesize cyst(e)ine from methionine, rendering them critically dependent on the uptake of the amino acid from their microenvironment.43,44 Therefore, extracellular cystine uptake is required for tumor growth and progression in some lymphoid and leukemia cells. Two decades ago, Gout et al.15 showed that sulfasalazine, a clinically approved anti-inflammatory drug, acted as a potent inhibitor of xCT and effectively inhibited lymphoma growth without apparent side effects. This finding for the first time demonstrated that the xCT molecule might be a novel target for sulfasalazine-like drugs, with potential for clinical applications in the treatment of lymphoma and other malignancies that are dependent on extracellular cyst(e)ine. Subsequently, it was found that in Kaposi’s sarcoma-associated herpes virus/HIV-associated lymphoma, targeting xCT induces cancer cell apoptosis and tumor regression.37 As a putative cancer stem-like cell marker, CD44v was found to interact and stabilize xCT on the plasma membrane and enhance the intracellular level of GSH to promote tumor growth.6 In another study, glutamine sensitivity analysis using 46 independent breast cancer-derived cell lines identified a subset of triple-negative breast cancer cells that are glutamine auxotrophs, and xCT mediated environmental cystine acquisition, which is indirectly supported by glutamine in the microenvironment. In addition, xCT inhibition by sulfasalazine was shown to decrease tumor growth through GSH depletion.42 Similarly, inhibition of xCT also disrupted glioma, melanoma, and prostate cancer cell growth, and it decreased cell proliferation and tumor progression in non-small-cell lung cancer, suggesting a critical role of xCT in solid tumor growth as well.24,35,45, 46, 47, 48, 49
Notably, xCT is essential for oncogenic RAS transformation by enhancing GSH synthesis via xCT upregulation,20 and suppression of xCT was shown to cause synthetic lethality in KRAS mutant lung adenocarcinoma,50 further highlighting xCT as a potential vulnerability for therapeutic targeting in RAS-driven tumors. High basal ROS levels in RAS-driven tumors render tumors heavily reliant on the antioxidant GSH for proliferation and survival, which might partially explain the anticancer effect of targeting the xCT/GSH axis in treating such RAS-driven tumors.
Generally, cancer cells exhibit a higher intracellular level of ROS than non-malignant cells because of genetic alterations and abnormal growth. Therefore, the maintenance of intracellular GSH as the ROS buffering mechanism is essential for the survival and proliferation of cancer cells.51, 52, 53, 54, 55 Alternatively, a high basal level of ROS can render cancer cells highly vulnerable to agents that induce further ROS stress.31,54 For instance, our previous work found that phenethyl isothiocyanate (PEITC), a natural compound found in many consumable cruciferous vegetables, could selectively kill cancer cells through a ROS-mediated mechanism.31,32 Later on, through a chemical engineering approach, Stone and Georgiou’s group, in a collaboration with our group, demonstrated that the systemic administration of an engineered and pharmacologically optimized human cyst(e)inase could suppress the growth of multiple cancers, including prostate carcinoma, breast cancer, and CLL, through the systemic depletion of l-cyst(e)ine and intracellular GSH.10 In detail, cyst(e)inase simultaneously affects both the GSH and thioredoxin (TXN) antioxidant pathways, leading to reduced tumor growth with no apparent side effects in several types of cancer, suggesting that cyst(e)inase is a promising anticancer molecule in a wide range of malignancies. Theoretically, blockade of cystine uptake by xCT inhibition, systemic depletion of cyst(e)ine by the engineered cyst(e)inase, or a combination of these agents with ROS inducers, such as PEITC, may provide effective therapeutic options for cancer treatment. These strategies warrant further investigation to confirm their therapeutic efficacy and evaluate the potential side effects associated with xCT abrogation.
Role of the xCT Molecule in Tumor Invasion, Metastasis, and Prognosis
It has been shown that high xCT expression could be a predictor of disease recurrence in patients with colorectal cancer, and multivariate analyses reveal that elevated xCT expression was correlated with tumor invasion and lymph node metastasis.56 In addition, the overexpression of xCT and/or CD44 has been associated with tumor recurrence and poor survival of patients with oral squamous cell carcinoma.57 Similarly, overexpression of xCT was identified as an indicator of unfavorable prognosis in liver carcinoma.58 In glioma cells, xCT-mediated glutamate release could promote glioma cell invasion, which could be blocked by the xCT inhibitors such as sulfasalazine and (S)-4-carboxyphenylglycine.16 Moreover, in patients with glioblastomas and hepatocellular carcinoma, xCT overexpression was shown to correlate with tumor invasion and shorter overall survival.59,60 Thus, xCT plays an important role in tumor progression and is indicative of poor prognosis in multiple types of cancer.
The role of xCT in tumor metastasis was reported in an early study by Chen et al.,4 who showed that xCT could be a potential target for blocking cancer metastasis. In their in vitro study, they found that RNAi-mediated knockdown of xCT expression enhanced homotypic cell-cell adhesion and reduced cell-extracellular matrix adhesion, accompanied by an upregulated membrane association of caveolin-1 and β-catenin. The xCT inhibitor sulfasalazine suppressed cell invasion of KYSE150, a cell line of esophageal squamous cell carcinoma (ESCC), likely through ROS-induced p38 mitogen-activated protein kinase (MAPK) activation. In vivo, sulfasalazine significantly inhibited tumor metastasis in nude mice bearing ESCC, suggesting xCT inhibition as a potential therapy for treating tumor metastasis.
These findings together suggest that xCT plays an important role in tumor invasion, metastasis, and poor prognosis in multiple malignancies, especially in solid tumors, likely due to the metabolic change associated increased uptake of cystine by the overexpressed xCT. In comparison to the high expression level of xCT in solid tumors, xCT is downregulated in certain hematologic malignancies such as CLL, which heavily depend on the uptake of cysteine from the extracellular microenvironment.7 As such, depletion of cyst(e)ine using novel agents such as cyst(e)inase might be efficient against hematologic malignancies. The different expression patterns suggest that disruption of xCT in solid tumors could be effective to eliminate the cancer cells, whereas systemic depletion of cyst(e)ine would be effective in both solid tumor and leukemia.10
Although it has been known for some time that xCT may promote tumor growth and metastasis, the underlying mechanisms largely remain unknown. For example, is xCT involved in the metastasis-related epithelial-mesenchymal transition process? How does xCT contribute to tumor relapse after initial treatment? These important questions remain to be answered in further mechanistic studies.
Role of xCT Expression in Drug Resistance
One of the major challenges in cancer treatment is the development of drug resistance. It has been reported that xCT plays a role in GSH-mediated drug resistance.1,2,61, 62, 63, 64, 65, 66, 67, 68, 69 An early study showed that disruption of xCT in lung cancer cells (A549, HOP-62) or ovarian cancer cells (SK-OV-3) inhibited the potency of amino acid analogs such as l-alanosine, but enhanced the potency of geldanamycin.2 These observations suggest that xCT might mediate the cellular uptake of l-alanosine but confer resistance to geldanamycin, likely by supplying cystine for the synthesis of GSH. Interestingly, xCT promotes resistance to geldanamycin but not to its analog 17-allylamino-17-demethoxygeldanamycin in A549 and HepG2 cells (human liver carcinoma cells).3
A previous study showed that CD44v could interact and stabilize xCT on the plasma membrane and thus facilitate cystine uptake into tumor cells.6 Inhibition of xCT selectively induces apoptosis in CD44v-expressing tumor cells that are intrinsically resistant to epidermal growth factor receptor (EGFR)-targeted therapy in head and neck squamous cell carcinoma.70 As expected, abrogation of xCT sensitizes the CD44v-expressing tumor cells to EGFR-targeted therapy.70 Mechanistically, CD44v-mediated xCT upregulation to supply cysteine for GSH synthesis would promote drug resistance in these tumor cells. As such, these tumor cells are vulnerable to xCT inhibition. Consistently, CD44v expression has been shown to induce 5-fluorouracil resistance in gastric cancer, which could also be abolished by inhibiting xCT.62
It is well known that glioblastomas are aggressive brain tumors associated with recurrence after radiotherapy. However, the xCT inhibitor sulfasalazine and radiation synergistically increased glioma cell death, which could be reversed by the antioxidant N-acetyl-l-cysteine (NAC).71 This observation suggests that the xCT-GSH axis might be a potential target in the treatment of glioma. In addition, xCT inhibition could overcome drug resistance to cannabidiol and temozolomide in glioblastoma.72,73 Sulfasalazine sensitizes colorectal cancer and tongue squamous cell carcinoma (TSCC) to cisplatin through interruption of GSH synthesis,68,74 and it alleviates drug resistance of CD133+ hepatocellular carcinoma cells to chemoradiation or irradiation therapy.75 Furthermore, xCT contributes to drug resistance of pancreatic cancer, as elevated xCT expression in pancreatic cancer tissues has been associated with gemcitabine resistance.76 It has been recently reported that xCT overexpression promotes the pentose phosphate pathway (PPP) to maintain the cellular reduced nicotinamide adenine dinucleotide phosphate (NADPH) pool and affect cancer cells to glucose transporter inhibition.38 This study demonstrated the crosstalk between xCT and the PPP pathway in regulating glucose limitation-induced cell death, and it revealed the metabolic characteristics in xCThigh cancer cells.
In a phase 1 study, the xCT inhibitor salazosulfapyridine was shown to be safe in combination with cisplatin-pemetrexed for advanced non-small-cell lung cancer.77 However, another phase 1 study showed that one of seven patients with CD44v+ gastric cancer refractory to cisplatin experienced dose-limiting toxicity, and four patients required dose interruption or reduction of sulfasalazine.78 Thus, careful dose modification to avoid toxicity seems very important in future studies.
In the tumor microenvironment, ovarian cancer cells receive stromal support from fibroblast-derived GSH and cysteine to confer chemoresistance. Effector T cell-derived interferon (IFN)γ could abrogate such resistance by upregulation of gamma-glutamyl-transferase and suppression of the xCT transporter in stromal fibroblasts.9 This study suggested a potential combination of chemotherapy and immunotherapy to overcome drug resistance in ovarian cancer. Indeed, a combination of tumor cell xCT deletion with the immunotherapeutic agent anti-CTLA4 dramatically increased the therapeutic efficacy in colon cancer.17 Taken together, xCT inhibition can overcome drug resistance in numerous cancers through the interruption of GSH synthesis, thereby suggesting a potential therapeutic value of xCT inhibitors in overcoming drug resistance in cancer treatment.
Role of the xCT Molecule in Tumor-Selective Ferroptosis
Ferroptosis is a form of non-apoptotic cell death involving the catastrophic accumulation of lipid peroxidation and ROS via iron catalysis.8 In 2012, Dixon et al.8 hypothesized that RAS-selective lethal (RSL) small molecules such as erastin could activate a unique iron-dependent form of cell death termed ferroptosis, which is different from other types of regulated cell death such as apoptosis and necrosis. Since then, the molecular regulation of ferroptosis has been explored in various model systems. According to the biochemical characteristics of ferroptosis, this type of cell death would be suppressed by iron chelators and/or lipophilic antioxidants. Several molecules have been found to regulate ferroptosis. Constitutive activity of xCT suppresses ferroptosis in many types of cells.8,79 It is noteworthy that ferroptosis induced by xCT inhibition could be suppressed by β-mercaptoethanol, which facilitates the reduction of extracellular cystine to cysteine, thus bypassing the system xc−.79 In addition, it was found that ferroptosis is induced by RNAi-mediated knockdown of GSH peroxidase 4 (GPX4), a GPX isoform that protects cellular membranes from lipid peroxidation.34 Importantly, note that the protective effect of GPX4 could be inactivated by the depletion of GSH.34
Certain tumor suppressor genes are involved in regulation of xCT expression. Recent studies found that p53 activation could reduce cystine uptake and promote ferroptosis by repressing xCT transcription, leading to a decrease of GSH and thus impairing GPX4 activity.41,80 These studies revealed a new mode of tumor suppression by p53 based on its ability to affect cystine metabolism and redox response, leading to ferroptosis. Interestingly, it was later reported that prolonged wild-type p53 stabilization and induction of its downstream target CDKN1A (p21) could delay the induction of ferroptosis in human HT-1080 fibrosarcoma cells.81 It was speculated that p21 upregulation and xCT downregulation were the two branches of a p53-coordinated response that were regulated by different p53 modifications such as acetylation or phosphorylation.81 This possibility, however, still remains to be tested. Basal p53 expression was also shown to limit erastin-induced ferroptosis by blocking dipeptidyl peptidase 4 (DPP4) activity in colorectal cancer cells.82 In that study, loss of p53 was found to attenuate the nuclear accumulation of DPP4 and thus promoted plasma membrane-associated DPP4-dependent lipid peroxidation. Altogether, p53 may promote or attenuate ferroptosis through distinct downstream pathways, and further investigations are needed to understand the specific underlying mechanisms in different cell types.
Another tumor suppressor, BRCA1-associated protein 1 (BAP1), which functions as a deubiquitinase (DUB), was also found to regulate cancer cell ferroptosis via epigenetically repressing xCT.83 Mutations of BAP1 in human cancers abolish its ability to promote ferroptosis. Mechanistically, BAP1 overexpression in UMRC6 cells (a BAP1-deficient renal cancer cell line) led to the suppression of xCT expression, a decrease in GSH levels, and an increase in the promotion of ferroptosis. In contrast, a BAP1 mutant deficient in DUB activity was unable to repress xCT expression or promote ferroptosis. These observations together suggest an important role of BAP1 DUB function in regulation of xCT and ferroptosis.
Interestingly, targeting ferroptosis-associated metabolism in tumors could improve the efficacy of cancer immunotherapy.11 IFNγ released from CD8+ T cells could downregulate the expression of two subunits of system xc− and consequently promote ferroptosis. This study also showed that cyst(e)inase in combination with immune checkpoint blockade synergistically enhanced T cell-mediated anti-tumor immunity and induced ferroptosis in melanoma and human fibrosarcoma cells. It was recently shown that pancreatic ductal adenocarcinoma (PDAC) critically depends on system xc− for cystine import.14 GSH and coenzyme A synthesized from cysteine can downregulate ferroptosis, while xCT deletion can induce ferroptosis and inhibit PDAC growth. Importantly, cyst(e)inase treatment, which exhibited a high therapeutic efficacy in multiple cancers, also induced tumor-selective ferroptosis in mice bearing pancreatic tumors.14 In addition, photodynamic therapy could promote ferroptosis as a mechanism for its anticancer action.84 Moreover, the combination of radiotherapy and immunotherapy could promote ferroptosis via synergistic suppression of xCT.40 These findings together suggest that inhibition of the xCT-GSH axis may be a promising therapeutic approach to eliminating cancer cells through induction of ferroptosis, and combination of immunotherapy and radiotherapy could synergistically promote ferroptosis via inhibition of xCT.
Regulation of the xCT Molecule
Since xCT plays a critical role in various respects of cancer cell biology and affects cell fate, a thorough understanding of the mechanisms that regulate xCT expression is very important for developing effective strategies to target xCT for potential treatment of cancer. It has been shown that certain metabolites and oxidative stress can induce changes in xCT expression.83,85 Activation of xCT transcription by either nuclear factor erythroid 2-related factor (NRF2) or activating transcription factor 4 (ATF4) is well correlated with their abilities in modulating ferroptosis and tumor growth.23,86, 87, 88 NRF2 is an intracellular transcription factor that regulates the expression of many molecules such as intracellular redox-balancing proteins and enzymes involved in GSH synthesis. Habib et al.88 reported that the expression of xCT is regulated by NRF2 in human breast cancer cells, a finding consistent with other studies that showed the association between NRF2 and xCT.89, 90, 91 Mechanistically, overexpression of NRF2 promotes xCT expression by upregulating xCT promoter activity, while overexpression of the Kelch-like ECH-associated protein 1 (Keap1) leads to a downregulation of the promoter activity, leading to low xCT expression in MCF-7 cells. It is known that both NRF2 and ATF4 are activated as the downstream consequence of protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling that coordinates the convergence of oxidative stress and endoplasmic reticulum stress. It was found that ATF4-dependent tumor-promoting effects are mediated by its transcriptional target gene xCT.23 Importantly, ATF4-induced proliferation in gliomas can be attenuated by pharmacological inhibition or genetic abrogation of xCT, and ATF4-induced angiogenesis can be diminished by ferroptosis inducers.23 These observations suggest that ATF4 could be a potential target to inhibit tumor growth and suppress angiogenesis through induction of ferroptosis.
Other transcription factors are also involved in the regulation of xCT. It is known that the highly conserved mammalian STAT (signal transducer and activator of transcription) family members, STAT3 and STAT5, are often activated in various cancers.92 Both STAT3 and STAT5 can bind to the promoter region of the xCT gene and transcriptionally activate xCT in human breast cancer cells.93
An early study by Yang and Yee34 showed that insulin-like growth factors (IGF) could regulate cystine uptake and cellular redox status by activating xCT transcription in estrogen receptor-positive (ER+) breast cancer cells. In this case, IGF receptor substrate-1 (IRS-1) and phosphatidylinositol 3-kinase (PI3K) signaling are required for IGF-induced xCT mRNA upregulation. Notably, they also found that the xCT inhibitor sulfasalazine could synergize the inhibitors of type I IGF receptor to enhance the therapeutic effect.34
Besides being regulated by certain transcription factors and growth factors, xCT could also be regulated by certain tumor suppressors. As noted above, the tumor suppressor p53 transcriptionally represses xCT expression to regulate ferroptosis.41 As p53 is frequently mutated or inactivated in many cancers, Liu et al.94 found that mutant p53 could bind to NRF2 and impairs the function of NRF2 in maintaining xCT expression, resulting in a vulnerability of p53 mutant cancer cells to inhibition of the xCT-GSH axis.95 Similar to p53, ARID1A, which encodes a component of the SWI/SNF chromatin-remodeling complex, is frequently mutated in various tumors.96, 97, 98 Ogiwara et al.39 found that ARID1A, together with other SWI/SNF complex subunits and NRF2, forms a complex on the promoter of the xCT gene to regulate its transcription and thus affect cellular GSH homeostasis. Due to a low level of xCT, ARID1A-deficient cancer cells are particularly vulnerable to depletion of GSH. Recently, Zhang et al.83 reported that the tumor suppressor BAP1 normally functions to repress xCT expression partly by deubiquitinating H2Aub on the xCT gene promoter, and thereby inhibits cystine uptake and promotes ferroptosis. This study revealed a link between metabolic regulation of ferroptosis and tumor suppression.
Post-translational mechanisms of xCT regulation have also been reported. Gu et al.99 showed that the mammalian target of rapamycin complex 2 (mTORC2) is a binding partner of xCT, and it could inhibit xCT activity through phosphorylation on serine 26 of xCT. It was shown that both mTORC2 and AKT are equally required for xCT phosphorylation,22 likely due to a positive feedback loop between AKT and mTORC2.100 Interestingly, effector T cell-derived INFγ and HDAC inhibitors could inhibit xCT expression, leading to an increase of ROS in ovarian cancer and melanoma.11,101 Other post-translational regulators of xCT include EGFR, CD44v, and the deubiquitylase OTUB1, which could stabilize xCT protein and control the intracellular GSH level.6,102,103 Similar to the interaction between CD44v and xCT, the intracellular domain of EGFR interacts with xCT and stabilizes it on the plasma membrane to facilitate cystine uptake in glioma cells. Targeted inhibition of xCT in glioma could suppress the EGFR-dependent tumor growth and invasiveness.103 OTUB1, which is overexpressed in human cancers, was found to directly interact with and stabilize xCT. Interestingly, CD44 expression suppressed ferroptosis in cancer cells, partly by promoting OTUB1-xCT interaction.102 Although OTUB1 has also been implicated in the DNA damage response,104 it is unclear whether such a DNA damage response has any link with xCT stability and cysteine metabolism.
As illustrated in Figure 1, there are different mechanisms that regulate xCT at the transcriptional and post-translational levels involving many regulatory molecules. These regulatory pathways provide potential targets to suppress xCT expression for therapeutic purposes.
Figure 1.
Cartoon Illustration of the xCT Regulators
Conclusions
In summary, xCT plays a major role in regulation of cellular redox status and cell fate via its key function in uptake of cystine for synthesis of GSH to counter ROS stress and suppress ferroptosis. The overexpression of xCT observed in many cancers suggests that system xc− is highly important for cancer cell survival and tumor development. This critical role is further evidenced by the findings that inhibition of xCT results in a significant anticancer effect in various types of cancer, including lymphoma, leukemia, glioma, prostate cancer, melanoma, lung, ovarian, breast cancer, and pancreatic cancer. As such, xCT could be a potential therapeutic target for cancer treatment.
To develop effective therapeutic strategies based on targeting xCT, several important questions should be carefully considered. (1) Will inhibition of xCT preferentially impact cancer cells or be equally toxic to cancer and normal cells? Since many cancer cells have high intrinsic oxidative stress, they are likely more dependent on the GSH antioxidant system to maintain redox balance and thus more vulnerable to abrogation of xCT. (2) Will the complex tumor microenvironment compromise the anticancer efficacy of targeting xCT in vivo? Due to the complex interaction between cancer cells and stromal cells in cystine→cysteine→GSH metabolism (Figure 2), it is indeed necessary to evaluate the impact of stromal cells on the anticancer effect of xCT inhibition. As shown in Figure 2, system xc− transporter mediates cyst(e)ine exchange between stromal cells and cancer cells. From a therapeutic perspective, the suppression of xCT expression by T cell-derived IFNγ or depletion of the extracellular cyst(e)ine by cyst(e)inase could block the stroma-cancer exchange and induce ROS accumulation, leading to lipid peroxidation and ferroptosis. Certain cancer cells such as lymphoid leukemic cells are unable to synthesize cyst(e)ine and thus highly depend on the uptake of extracellular cysteine to maintain the intracellular GSH level. The high basal ROS levels in the cancer cells render them vulnerable to further oxidative stress and thereby sensitivity to xCT inhibition. Elevated expression of xCT in many solid tumor cells would facilitate GSH synthesis and thus provide them with a mechanism of drug resistance. In this case, the use of xCT inhibitors or cyst(e)inase to deplete cyst(e)ine would be a strategy to overcome such drug resistance. Furthermore, the combination xCT inhibitors or cyst(e)inase with either immunotherapy or radiotherapy would further promote ferroptosis and enhance anticancer activity. (3) Since inhibition of xCT causes metabolic changes and induces ROS stress in cancer cells, it is important to consider the possibility of cancer cell adaptation to these stresses, potentially leading to the emergence of a more malignant phenotype. This possibility would pose a further challenge in cancer treatment and should be investigated in future studies.
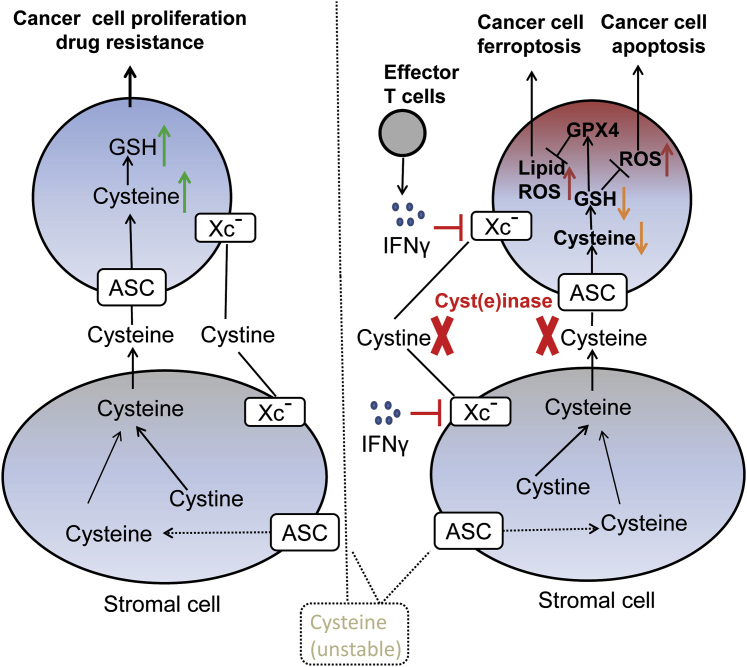
Figure 2.
Cartoon Illustration of xc− Transporter-Mediated Cystine/Cysteine Exchange between Stromal Cells and Cancer Cells
Left: stroma cells provide cyst(e)ine to cancer cells through system alanine-serine-cysteine (ASC) or xc− transporters for cancer cell proliferation and drug resistance. Right: suppression of xCT expression by T cell-derived IFNγ or depletion of the extracellular cyst(e)ine by cyst(e)inase will block the stroma-cancer exchange and induce ROS/lipid ROS accumulation in cancer cells and subsequent apoptosis/ferroptosis.
Author Contributions
J.L. drafted the manuscript; X.X. and P.H. provided critical revisions to the draft. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported in part by a research grant from Sun Yat-sen University Cancer Center. The authors would like to thank Dr. Seeruttun Sharvesh Raj for language editing.
Contributor Information
Jinyun Liu, Email: liujinyun@sysucc.org.cn.
Peng Huang, Email: huangpeng@sysucc.org.cn.
References
- 1.Okuno S., Sato H., Kuriyama-Matsumura K., Tamba M., Wang H., Sohda S., Hamada H., Yoshikawa H., Kondo T., Bannai S. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br. J. Cancer. 2003;88:951–956. doi: 10.1038/sj.bjc.6600786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y., Dai Z., Barbacioru C., Sadée W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005;65:7446–7454. doi: 10.1158/0008-5472.CAN-04-4267. [DOI] [PubMed] [Google Scholar]
- 3.Liu R., Blower P.E., Pham A.N., Fang J., Dai Z., Wise C., Green B., Teitel C.H., Ning B., Ling W. Cystine-glutamate transporter SLC7A11 mediates resistance to geldanamycin but not to 17-(allylamino)-17-demethoxygeldanamycin. Mol. Pharmacol. 2007;72:1637–1646. doi: 10.1124/mol.107.039644. [DOI] [PubMed] [Google Scholar]
- 4.Chen R.S., Song Y.M., Zhou Z.Y., Tong T., Li Y., Fu M., Guo X.L., Dong L.J., He X., Qiao H.X. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/β-catenin pathway. Oncogene. 2009;28:599–609. doi: 10.1038/onc.2008.414. [DOI] [PubMed] [Google Scholar]
- 5.Lo M., Wang Y.Z., Gout P.W. The xc− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J. Cell. Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 6.Ishimoto T., Nagano O., Yae T., Tamada M., Motohara T., Oshima H., Oshima M., Ikeda T., Asaba R., Yagi H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W., Trachootham D., Liu J., Chen G., Pelicano H., Garcia-Prieto C., Lu W., Burger J.A., Croce C.M., Plunkett W. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W., Kryczek I., Dostál L., Lin H., Tan L., Zhao L., Lu F., Wei S., Maj T., Peng D. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell. 2016;165:1092–1105. doi: 10.1016/j.cell.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramer S.L., Saha A., Liu J., Tadi S., Tiziani S., Yan W., Triplett K., Lamb C., Alters S.E., Rowlinson S. Systemic depletion of l-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017;23:120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W., Green M., Choi J.E., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris I.S., Endress J.E., Coloff J.L., Selfors L.M., McBrayer S.K., Rosenbluth J.M., Takahashi N., Dhakal S., Koduri V., Oser M.G. Deubiquitinases maintain protein homeostasis and survival of cancer cells upon glutathione depletion. Cell Metab. 2019;29:1166–1181.e6. doi: 10.1016/j.cmet.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daher B., Parks S.K., Durivault J., Cormerais Y., Baidarjad H., Tambutte E., Pouysségur J., Vučetić M. Genetic ablation of the cystine transporter xCT in PDAC cells inhibits mTORC1, growth, survival, and tumor formation via nutrient and oxidative stresses. Cancer Res. 2019;79:3877–3890. doi: 10.1158/0008-5472.CAN-18-3855. [DOI] [PubMed] [Google Scholar]
- 14.Badgley M.A., Kremer D.M., Maurer H.C., DelGiorno K.E., Lee H.J., Purohit V., Sagalovskiy I.R., Ma A., Kapilian J., Firl C.E.M. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gout P.W., Buckley A.R., Simms C.R., Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 16.Lyons S.A., Chung W.J., Weaver A.K., Ogunrinu T., Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67:9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arensman M.D., Yang X.S., Leahy D.M., Toral-Barza L., Mileski M., Rosfjord E.C., Wang F., Deng S., Myers J.S., Abraham R.T., Eng C.H. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc. Natl. Acad. Sci. USA. 2019;116:9533–9542. doi: 10.1073/pnas.1814932116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabeyama A., Kurita A., Asano K., Miyake Y., Yasuda T., Miura I., Nishitai G., Arakawa S., Shimizu S., Wakana S. xCT deficiency accelerates chemically induced tumorigenesis. Proc. Natl. Acad. Sci. USA. 2010;107:6436–6441. doi: 10.1073/pnas.0912827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X.X., Li X.J., Zhang B., Liang Y.J., Zhou C.X., Cao D.X., He M., Chen G.Q., He J.R., Zhao Q. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett. 2011;585:1363–1367. doi: 10.1016/j.febslet.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Lim J.K.M., Delaidelli A., Minaker S.W., Zhang H.F., Colovic M., Yang H., Negri G.L., von Karstedt S., Lockwood W.W., Schaffer P. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc. Natl. Acad. Sci. USA. 2019;116:9433–9442. doi: 10.1073/pnas.1821323116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato M., Kusumi R., Hamashima S., Kobayashi S., Sasaki S., Komiyama Y., Izumikawa T., Conrad M., Bannai S., Sato H. The ferroptosis inducer erastin irreversibly inhibits system xc− and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 2018;8:968. doi: 10.1038/s41598-018-19213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lien E.C., Ghisolfi L., Geck R.C., Asara J.M., Toker A. Oncogenic PI3K promotes methionine dependency in breast cancer cells through the cystine-glutamate antiporter xCT. Sci. Signal. 2017;10:10. doi: 10.1126/scisignal.aao6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D., Fan Z., Rauh M., Buchfelder M., Eyupoglu I.Y., Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593–5608. doi: 10.1038/onc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin S.S., Jeong B.S., Wall B.A., Li J., Shan N.L., Wen Y., Goydos J.S., Chen S. Participation of xCT in melanoma cell proliferation in vitro and tumorigenesis in vivo. Oncogenesis. 2018;7:86. doi: 10.1038/s41389-018-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goji T., Takahara K., Negishi M., Katoh H. Cystine uptake through the cystine/glutamate antiporter xCT triggers glioblastoma cell death under glucose deprivation. J. Biol. Chem. 2017;292:19721–19732. doi: 10.1074/jbc.M117.814392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okazaki F., Matsunaga N., Hamamura K., Suzuki K., Nakao T., Okazaki H., Kutsukake M., Fukumori S., Tsuji Y., To H. Administering xct inhibitors based on circadian clock improves antitumor effects. Cancer Res. 2017;77:6603–6613. doi: 10.1158/0008-5472.CAN-17-0720. [DOI] [PubMed] [Google Scholar]
- 27.Koppula P., Zhang Y., Zhuang L., Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. (Lond.) 2018;38:12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato H., Tamba M., Ishii T., Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 29.Sato H., Tamba M., Kuriyama-Matsumura K., Okuno S., Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc- Antioxid. Redox Signal. 2000;2:665–671. doi: 10.1089/ars.2000.2.4-665. [DOI] [PubMed] [Google Scholar]
- 30.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Trachootham D., Zhang H., Zhang W., Feng L., Du M., Zhou Y., Chen Z., Pelicano H., Plunkett W., Wierda W.G. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P.J., Achanta G., Arlinghaus R.B., Liu J., Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Franco R., Panayiotidis M.I., Cidlowski J.A. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J. Biol. Chem. 2007;282:30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., Yee D. IGF-I regulates redox status in breast cancer cells by activating the amino acid transport molecule xC−. Cancer Res. 2014;74:2295–2305. doi: 10.1158/0008-5472.CAN-13-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji X., Qian J., Rahman S.M.J., Siska P.J., Zou Y., Harris B.K., Hoeksema M.D., Trenary I.A., Heidi C., Eisenberg R. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene. 2018;37:5007–5019. doi: 10.1038/s41388-018-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo W., Zhao Y., Zhang Z., Tan N., Zhao F., Ge C., Liang L., Jia D., Chen T., Yao M. Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer Lett. 2011;312:55–61. doi: 10.1016/j.canlet.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Dai L., Cao Y., Chen Y., Parsons C., Qin Z. Targeting xCT, a cystine-glutamate transporter induces apoptosis and tumor regression for KSHV/HIV-associated lymphoma. J. Hematol. Oncol. 2014;7:30. doi: 10.1186/1756-8722-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Olszewski K., Zhang Y., Lim E.W., Shi J., Zhang X., Zhang J., Lee H., Koppula P., Lei G. Cystine transporter regulation of pentose phosphate pathway dependency and disulfide stress exposes a targetable metabolic vulnerability in cancer. Nat. Cell Biol. 2020;22:476–486. doi: 10.1038/s41556-020-0496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogiwara H., Takahashi K., Sasaki M., Kuroda T., Yoshida H., Watanabe R., Maruyama A., Makinoshima H., Chiwaki F., Sasaki H. Targeting the vulnerability of glutathione metabolism in ARID1A-deficient cancers. Cancer Cell. 2019;35:177–190.e8. doi: 10.1016/j.ccell.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Lang X., Green M.D., Wang W., Yu J., Choi J.E., Jiang L., Liao P., Zhou J., Zhang Q., Dow A. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang L., Kon N., Li T., Wang S.J., Su T., Hibshoosh H., Baer R., Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timmerman L.A., Holton T., Yuneva M., Louie R.J., Padró M., Daemen A., Hu M., Chan D.A., Ethier S.P., van ’t Veer L.J. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24:450–465. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iglehart J.K., York R.M., Modest A.P., Lazarus H., Livingston D.M. Cystine requirement of continuous human lymphoid cell lines of normal and leukemic origin. J. Biol. Chem. 1977;252:7184–7191. [PubMed] [Google Scholar]
- 44.Gout P.W., Kang Y.J., Buckley D.J., Bruchovsky N., Buckley A.R. Increased cystine uptake capability associated with malignant progression of Nb2 lymphoma cells. Leukemia. 1997;11:1329–1337. doi: 10.1038/sj.leu.2400739. [DOI] [PubMed] [Google Scholar]
- 45.Doxsee D.W., Gout P.W., Kurita T., Lo M., Buckley A.R., Wang Y., Xue H., Karp C.M., Cutz J.C., Cunha G.R., Wang Y.Z. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate. 2007;67:162–171. doi: 10.1002/pros.20508. [DOI] [PubMed] [Google Scholar]
- 46.Chung W.J., Lyons S.A., Nelson G.M., Hamza H., Gladson C.L., Gillespie G.Y., Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 2005;25:7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung W.J., Sontheimer H. Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-κB. J. Neurochem. 2009;110:182–193. doi: 10.1111/j.1471-4159.2009.06129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi S., Kuwata K., Sugimoto T., Igarashi K., Osaki M., Okada F., Fujii J., Bannai S., Sato H. Enhanced expression of cystine/glutamate transporter in the lung caused by the oxidative-stress-inducing agent paraquat. Free Radic. Biol. Med. 2012;53:2197–2203. doi: 10.1016/j.freeradbiomed.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 49.Guan J., Lo M., Dockery P., Mahon S., Karp C.M., Buckley A.R., Lam S., Gout P.W., Wang Y.Z. The xc− cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother. Pharmacol. 2009;64:463–472. doi: 10.1007/s00280-008-0894-4. [DOI] [PubMed] [Google Scholar]
- 50.Hu K., Li K., Lv J., Feng J., Chen J., Wu H., Cheng F., Jiang W., Wang J., Pei H. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J. Clin. Invest. 2020;130:1752–1766. doi: 10.1172/JCI124049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 52.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 53.Harris I.S., Treloar A.E., Inoue S., Sasaki M., Gorrini C., Lee K.C., Yung K.Y., Brenner D., Knobbe-Thomsen C.B., Cox M.A. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Carew J.S., Zhou Y., Albitar M., Carew J.D., Keating M.J., Huang P. Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia. 2003;17:1437–1447. doi: 10.1038/sj.leu.2403043. [DOI] [PubMed] [Google Scholar]
- 55.Oltra A.M., Carbonell F., Tormos C., Iradi A., Sáez G.T. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free Radic. Biol. Med. 2001;30:1286–1292. doi: 10.1016/s0891-5849(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 56.Sugano K., Maeda K., Ohtani H., Nagahara H., Shibutani M., Hirakawa K. Expression of xCT as a predictor of disease recurrence in patients with colorectal cancer. Anticancer Res. 2015;35:677–682. [PubMed] [Google Scholar]
- 57.Lee J.R., Roh J.L., Lee S.M., Park Y., Cho K.J., Choi S.H., Nam S.Y., Kim S.Y. Overexpression of cysteine-glutamate transporter and CD44 for prediction of recurrence and survival in patients with oral cavity squamous cell carcinoma. Head Neck. 2018;40:2340–2346. doi: 10.1002/hed.25331. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L., Huang Y., Ling J., Zhuo W., Yu Z., Luo Y., Zhu Y. Overexpression of SLC7A11: a novel oncogene and an indicator of unfavorable prognosis for liver carcinoma. Future Oncol. 2018;14:927–936. doi: 10.2217/fon-2017-0540. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi S., Wada K., Toyooka T., Shinomiya N., Shimazaki H., Nakanishi K., Nagatani K., Otani N., Osada H., Uozumi Y. Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastomas. Neurosurgery. 2013;72:33–41. doi: 10.1227/NEU.0b013e318276b2de. discussion 41. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita H., Okabe H., Beppu T., Chikamoto A., Hayashi H., Imai K., Mima K., Nakagawa S., Ishimoto T., Miyake K. Cystine/glutamic acid transporter is a novel marker for predicting poor survival in patients with hepatocellular carcinoma. Oncol. Rep. 2013;29:685–689. doi: 10.3892/or.2012.2162. [DOI] [PubMed] [Google Scholar]
- 61.Narang V.S., Pauletti G.M., Gout P.W., Buckley D.J., Buckley A.R. Sulfasalazine-induced reduction of glutathione levels in breast cancer cells: enhancement of growth-inhibitory activity of doxorubicin. Chemotherapy. 2007;53:210–217. doi: 10.1159/000100812. [DOI] [PubMed] [Google Scholar]
- 62.Miyoshi S., Tsugawa H., Matsuzaki J., Hirata K., Mori H., Saya H., Kanai T., Suzuki H. Inhibiting xCT improves 5-fluorouracil resistance of gastric cancer induced by CD44 variant 9 expression. Anticancer Res. 2018;38:6163–6170. doi: 10.21873/anticanres.12969. [DOI] [PubMed] [Google Scholar]
- 63.Horibe S., Kawauchi S., Tanahashi T., Sasaki N., Mizuno S., Rikitake Y. CD44v-dependent upregulation of xCT is involved in the acquisition of cisplatin-resistance in human lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2018;507:426–432. doi: 10.1016/j.bbrc.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 64.Wang S.F., Wung C.H., Chen M.S., Chen C.F., Yin P.H., Yeh T.S., Chang Y.L., Chou Y.C., Hung H.H., Lee H.C. Activated integrated stress response induced by salubrinal promotes cisplatin resistance in human gastric cancer cells via enhanced xCT expression and glutathione biosynthesis. Int. J. Mol. Sci. 2018;19:3389. doi: 10.3390/ijms19113389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wada F., Koga H., Akiba J., Niizeki T., Iwamoto H., Ikezono Y., Nakamura T., Abe M., Masuda A., Sakaue T. High expression of CD44v9 and xCT in chemoresistant hepatocellular carcinoma: potential targets by sulfasalazine. Cancer Sci. 2018;109:2801–2810. doi: 10.1111/cas.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogihara K., Kikuchi E., Okazaki S., Hagiwara M., Takeda T., Matsumoto K., Kosaka T., Mikami S., Saya H., Oya M. Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 2019;110:1431–1441. doi: 10.1111/cas.13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F., Yang Y. Retraction. Breast Cancer Res. Treat. 2015;151:479. doi: 10.1007/s10549-015-3399-y. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P., Wang W., Wei Z., Xu L.I., Yang X., Du Y. xCT expression modulates cisplatin resistance in Tca8113 tongue carcinoma cells. Oncol. Lett. 2016;12:307–314. doi: 10.3892/ol.2016.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bekeschus S., Eisenmann S., Sagwal S.K., Bodnar Y., Moritz J., Poschkamp B., Stoffels I., Emmert S., Madesh M., Weltmann K.D. xCT (SLC7A11) expression confers intrinsic resistance to physical plasma treatment in tumor cells. Redox Biol. 2020;30:101423. doi: 10.1016/j.redox.2019.101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshikawa M., Tsuchihashi K., Ishimoto T., Yae T., Motohara T., Sugihara E., Onishi N., Masuko T., Yoshizawa K., Kawashiri S. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855–1866. doi: 10.1158/0008-5472.CAN-12-3609-T. [DOI] [PubMed] [Google Scholar]
- 71.Sleire L., Skeie B.S., Netland I.A., Førde H.E., Dodoo E., Selheim F., Leiss L., Heggdal J.I., Pedersen P.H., Wang J., Enger P.Ø. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc−, leading to glutathione depletion. Oncogene. 2015;34:5951–5959. doi: 10.1038/onc.2015.60. [DOI] [PubMed] [Google Scholar]
- 72.Singer E., Judkins J., Salomonis N., Matlaf L., Soteropoulos P., McAllister S., Soroceanu L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. doi: 10.1038/cddis.2014.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Polewski M.D., Reveron-Thornton R.F., Cherryholmes G.A., Marinov G.K., Cassady K., Aboody K.S. Increased expression of system xc− in glioblastoma confers an altered metabolic state and temozolomide resistance. Mol. Cancer Res. 2016;14:1229–1242. doi: 10.1158/1541-7786.MCR-16-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma M.Z., Chen G., Wang P., Lu W.H., Zhu C.F., Song M., Yang J., Wen S., Xu R.H., Hu Y., Huang P. Xc− inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH-dependent mechanism. Cancer Lett. 2015;368:88–96. doi: 10.1016/j.canlet.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 75.Song Y., Jang J., Shin T.H., Bae S.M., Kim J.S., Kim K.M., Myung S.J., Choi E.K., Seo H.R. Sulfasalazine attenuates evading anticancer response of CD133-positive hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2017;36:38. doi: 10.1186/s13046-017-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo M., Ling V., Wang Y.Z., Gout P.W. The xc− cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br. J. Cancer. 2008;99:464–472. doi: 10.1038/sj.bjc.6604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otsubo K., Nosaki K., Imamura C.K., Ogata H., Fujita A., Sakata S., Hirai F., Toyokawa G., Iwama E., Harada T. Phase I study of salazosulfapyridine in combination with cisplatin and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci. 2017;108:1843–1849. doi: 10.1111/cas.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shitara K., Doi T., Nagano O., Fukutani M., Hasegawa H., Nomura S., Sato A., Kuwata T., Asai K., Einaga Y. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407) Gastric Cancer. 2017;20:1004–1009. doi: 10.1007/s10120-017-0720-y. [DOI] [PubMed] [Google Scholar]
- 79.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang L., Hickman J.H., Wang S.J., Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14:2881–2885. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tarangelo A., Magtanong L., Bieging-Rolett K.T., Li Y., Ye J., Attardi L.D., Dixon S.J. p53 Suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 2018;22:569–575. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie Y., Zhu S., Song X., Sun X., Fan Y., Liu J., Zhong M., Yuan H., Zhang L., Billiar T.R. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Shi J., Liu X., Feng L., Gong Z., Koppula P., Sirohi K., Li X., Wei Y., Lee H. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018;20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu T., Shi L., Yu C., Dong Y., Qiu F., Shen L., Qian Q., Zhou G., Zhu X. Ferroptosis promotes photodynamic therapy: supramolecular photosensitizer-inducer nanodrug for enhanced cancer treatment. Theranostics. 2019;9:3293–3307. doi: 10.7150/thno.32867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koppula P., Zhang Y., Shi J., Li W., Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 2017;292:14240–14249. doi: 10.1074/jbc.M117.798405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ye P., Mimura J., Okada T., Sato H., Liu T., Maruyama A., Ohyama C., Itoh K. Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol. Cell. Biol. 2014;34:3421–3434. doi: 10.1128/MCB.00221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan Z., Wirth A.K., Chen D., Wruck C.J., Rauh M., Buchfelder M., Savaskan N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6:e371. doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Habib E., Linher-Melville K., Lin H.X., Singh G. Expression of xCT and activity of system xc− are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33–42. doi: 10.1016/j.redox.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiang W., Cahill J.M., Liu J., Kuang X., Liu N., Scofield V.L., Voorhees J.R., Reid A.J., Yan M., Lynn W.S., Wong P.K. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J. Virol. 2004;78:11926–11938. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nandar W., Neely E.B., Unger E., Connor J.R. A mutation in the HFE gene is associated with altered brain iron profiles and increased oxidative stress in mice. Biochim. Biophys. Acta. 2013;1832:729–741. doi: 10.1016/j.bbadis.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 91.Seib T.M., Patel S.A., Bridges R.J. Regulation of the system xC− cystine/glutamate exchanger by intracellular glutathione levels in rat astrocyte primary cultures. Glia. 2011;59:1387–1401. doi: 10.1002/glia.21176. [DOI] [PubMed] [Google Scholar]
- 92.Buettner R., Mora L.B., Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 93.Linher-Melville K., Haftchenary S., Gunning P., Singh G. Signal transducer and activator of transcription 3 and 5 regulate system Xc- and redox balance in human breast cancer cells. Mol. Cell. Biochem. 2015;405:205–221. doi: 10.1007/s11010-015-2412-4. [DOI] [PubMed] [Google Scholar]
- 94.Liu D.S., Duong C.P., Haupt S., Montgomery K.G., House C.M., Azar W.J., Pearson H.B., Fisher O.M., Read M., Guerra G.R. Inhibiting the system xC−/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat. Commun. 2017;8:14844. doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clemons N.J., Liu D.S., Duong C.P., Phillips W.A. Inhibiting system xC− and glutathione biosynthesis—a potential Achilles’ heel in mutant-p53 cancers. Mol. Cell. Oncol. 2017;4:e1344757. doi: 10.1080/23723556.2017.1344757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding L., Bailey M.H., Porta-Pardo E., Thorsson V., Colaprico A., Bertrand D., Gibbs D.L., Weerasinghe A., Huang K.-l., Tokheim C. Perspective on oncogenic processes at the end of the beginning of cancer genomics. Cell. 2018;173:305–320.e10. doi: 10.1016/j.cell.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones S., Wang T.L., Shih IeM., Mao T.L., Nakayama K., Roden R., Glas R., Slamon D., Diaz L.A., Jr., Vogelstein B. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R., Meyerson M., Gabriel S.B., Lander E.S., Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gu Y., Albuquerque C.P., Braas D., Zhang W., Villa G.R., Bi J., Ikegami S., Masui K., Gini B., Yang H. mTORC2 regulates amino acid metabolism in cancer by phosphorylation of the cystine-glutamate antiporter xCT. Mol. Cell. 2017;67:128–138.e7. doi: 10.1016/j.molcel.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang G., Murashige D.S., Humphrey S.J., James D.E. A positive feedback loop between Akt and mTORC2 via SIN1 phosphorylation. Cell Rep. 2015;12:937–943. doi: 10.1016/j.celrep.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 101.Wang L., Leite de Oliveira R., Huijberts S., Bosdriesz E., Pencheva N., Brunen D., Bosma A., Song J.-Y., Zevenhoven J., Los-de Vries G.T. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell. 2018;173:1413–1425.e14. doi: 10.1016/j.cell.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 102.Liu T., Jiang L., Tavana O., Gu W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79:1913–1924. doi: 10.1158/0008-5472.CAN-18-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsuchihashi K., Okazaki S., Ohmura M., Ishikawa M., Sampetrean O., Onishi N., Wakimoto H., Yoshikawa M., Seishima R., Iwasaki Y. The EGF receptor promotes the malignant potential of glioma by regulating amino acid transport system xc(−) Cancer Res. 2016;76:2954–2963. doi: 10.1158/0008-5472.CAN-15-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakada S., Tai I., Panier S., Al-Hakim A., Iemura S., Juang Y.C., O’Donnell L., Kumakubo A., Munro M., Sicheri F. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature. 2010;466:941–946. doi: 10.1038/nature09297. [DOI] [PubMed] [Google Scholar]