Abstract
CD4+Foxp3+ regulatory T (Treg) cells are crucial for maintaining homeostasis and preventing autoimmune diseases. Nonetheless, we and others have previously reported that natural Treg cells are unstable and dysfunctional in the inflamed environment with a high-salt diet, limiting the Treg function in disease control. In this study, we made an innovative observation showing a high degree of heterogeneity within the Treg pool. We identified that CD126, interleukin (IL)-6 receptor alpha chain, contributed to Treg cell instability. Using a series of in vitro and in vivo experimental approaches, we demonstrated that CD126Lo/− Treg cells presented greater function and were more stable than CD126Hi nTreg cells, even in the presence of IL-6 and inflammation. Blockade of programmed death-1 (PD-1) interrupted CD126Lo/− nTreg cell stability. Additionally, CD126Lo/− Treg cells can treat colitis and established collagen-induced arthritis, while the CD126Hi cell population failed to do this. Moreover, we noted that CD126 expression of Treg cells had a positive correlation to rheumatoid arthritis (RA) severity and the stability of Treg cells. Our results strongly suggest that the manipulation of CD126Lo/− nTreg cells could be a novel strategy for the treatment of autoimmune diseases and for other conditions associated with a deficit of Treg cells.
Graphical Abstract

nTreg cells are unstable and dysfunctional in the inflamed condition. Zheng and colleagues identified a new Treg subset, CD126Low/− Treg, which is more stable and presents better function even in the inflamed condition; high expression of PD-1 on the CD126Low/− Treg cell may be responsible for this.
Introduction
Regulatory T (Treg) cells are one of the most important elements in suppressing immune responses.1,2 Foxp3 deficiency in mice results in fatal autoimmune diseases that can be reversed by the adoptive transfer of Foxp3+ cells.3 Abnormal frequency and function of Treg cells were identified in many patients with autoimmune diseases.4, 5, 6, 7 Adoptive transfer of natural CD4+CD25+ Foxp3+ Treg (nTreg) cells effectively prevents some autoimmune diseases but fails to treat the established diseases in several animal models.8,9 The instability of Foxp3+ Treg cells under inflammatory or disease conditions is still contentious,10 as the origin of this Foxp3-unstable population still remains unclear. It has been reported that Treg cells isolated from the central nervous system (CNS) of experimental autoimmune encephalomyelitis (EAE) are resistant to interleukin (IL)-6-driven IL-17A production,11 while Treg cells from the inflamed joints of collagen-induced arthritis (CIA) are unstable and contribute to the pathogenesis of CIA.12 Several studies have reported that nTreg cells can be trans-differentiated into IL-17A-producing cells and lose function in the presence of pro-inflammatory cytokines.13,14 Cytokines such as IL-6, IL-4, and IL-21 have been shown to be able to disrupt Foxp3 expression and reprogram them to the effector T cell phenotype.13,15
The importance of IL-6 in regulating the balance of Th17 and Treg cells has been well established.16 IL-6 and some other inflammatory cytokines stimulate Stat3 signaling and disrupt Foxp3 expression toward IL-17A expression17 and can also reduce the Treg cell function to inhibit T cell proliferation in vitro.18 CD126 is the membrane-bound alpha-receptor of IL-6 and interacts with gp130 to form the IL-6 signaling complex. IL-6 can bind to the soluble CD126 to initiate IL-6 signaling.19 The findings that IL-6 is an important inflammatory cytokine and that IL-6 binds to its receptor (CD126) to exert a functional role where nTregs express CD126 support the hypothesis that the CD126+ Treg population could be unstable under the inflammatory condition.
In this study, we have developed in vitro and in vivo experimental models to address this issue. Accordingly, subsets of CD126Hi and CD126Lo/− Treg cells—which are, respectively, Foxp3+(GFP+) cells with high CD126 expression (i.e., the fluorescence intensity is above the highest value of control immunoglobulin G [IgG]) and with low CD126 expression (i.e., the fluorescence intensity is below the mean fluorescence intensity (MFI) of control IgG)—were sorted and stimulated with anti-CD3 and CD28 in the presence or absence of IL-6. Both colitis and CIA models were used to test the stability and suppressive function of these two subsets under the disease condition in vivo, and the correlation between CD126 expression of Treg cells and the severity of CIA and rheumatoid arthritis (RA) patients were also analyzed. Our results strongly suggest that CD126 expression in Treg cells is an ideal phenotypic marker for RA progression and that the CD126Lo/− Treg population is a more superior Treg cell subset. Additionally, programmed death-1 (PD-1) signaling contributes to Treg cell stability and functionality by limiting CD126 regulation. With the data taken together, we conclude that the manipulation of CD126Lo/− Treg cells has more effective promise for cell-therapy-based strategies in the prevention of, and in treating patients with, autoimmune and inflammatory diseases, while the function and stability of human CD126Lo/− Treg cells still needs further study.
Results
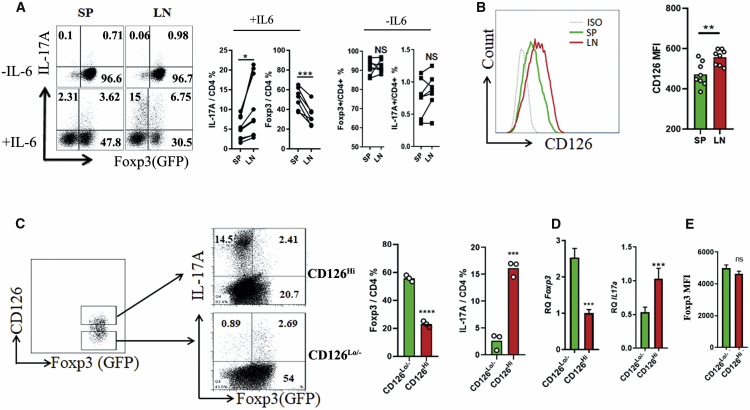
CD126 Expression Is Correlated with the Different Stability of Treg Cells from Spleen (SP) and Lymph Node (LN)
We and others have previously reported that IL-6 induces nTreg cell trans-differentiation to Th17 and Th1 cells in both mice and humans.13,14,20 To investigate and compare the stability of endogenous Treg cells, we isolated CD4+ Foxp3(GFP)+ Treg cells from SP and LN by sorting CD4+CD25+GFP+ and then co-cultured them with antigen-presenting cells (APCs) in the presence of anti-CD3/28 with or without IL-6 for 4 days. As previously described, some of Treg cells in both SP and LN stimulated with IL-6 trans-differentiated to Th17 cells and lost Foxp3 expression, and Treg cells stimulated without IL-6 maintained Foxp3 expression (Figure 1A). However, Treg cells isolated from SP are more stable than those isolated from LN (Figure 1A).
Figure 1.
CD126 Expression Is Correlated with the Stability of nTreg Cells from SP and LN
(A) nTreg cells were isolated from spleen (SP) and lymph node (LN) and then stimulated with anti-CD3/28 and APCs in the presence or absence of IL-6 for 4 days. Cells were harvested for IL-17A staining and analyzed by flow cytometry (left). 7 independent experiments were conducted, and quantitative analyses are shown (right). (B) Total cells isolated from SP or LN were analyzed for CD126 expression (n = 8). (C) CD126Hi and CD126Lo/- nTreg cells were sorted from lymphocytes as indicated and then stimulated with anti-CD3/28 and APCs in the presence of IL-6 for 4 days. Cells were harvested and analyzed by flow cytometry (left), 3 independent experiments were conducted, and quantitative analyses were conducted (right). (D) Foxp3 and IL-17A mRNA expression were detected by real-time PCR on day 2 (48 h) of cell culture; 3 independent experiments were conducted. (E) The Foxp3 MFI of these two subsets were detected. Data are presented as the means ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001, by Student’s t test.
CD126 is an IL-6 receptor and plays an important role in IL-6 signaling. To correct the conversion ability of nTreg-to-CD126 expression, we first analyzed the expression of CD126 in Treg cells from SP and LN, and our results revealed that CD126 expression by Treg cells is significantly higher in LN than in SP (Figure 1B). These results suggest that CD126 expression may correlate with the stability of endogenous Treg cells. To further study the effect of CD126 on the stability of Treg cells, CD126Hi and CD126Lo/− Treg cells were sorted from LN and stimulated in the presence of APCs, anti-CD3/28, and IL-6 for 4 days. Compared with CD126Lo/− Treg cells, IL-6 stimulation greatly downregulated Foxp3 expression and promoted IL-17A expression in CD126Hi Treg cells, while CD126Lo/− Treg cells were resistant to Th17 cell conversion (Figure 1C). Consistent with this, Foxp3 mRNA expression was significantly downregulated, and IL-17A mRNA expression was significantly upregulated under IL-6 stimulation (Figure 1D). We further compared the Foxp3 MFI between CD126Hi and CD126Lo/− Treg cell subsets and found that the Foxp3 MFI of CD126Lo/− Treg cells is slightly higher than that of CD126Lo/− Treg cells, but there is no significant difference between these two Treg cell subsets (Figure 1E).
CD126Lo/− Treg Cells Present a Better Suppressive Function In Vitro and Colitis Model in Vivo
The suppressive function of Treg cells is important for the potential therapeutic benefit.1 A standard in vitro co-culture suppression assay was used to study the suppressive function of these two subpopulations in the presence or absence of IL-6. Splenic-enriched T cells from normal mice were prepared and labeled with carboxyfluorescein succinimidyl ester (CFSE). Then, they were co-cultured with mitomycin-treated APCs in the presence of soluble anti-CD3 antibody with or without the addition of inflammatory cytokine IL-6, CD126Hi, or CD126Lo/− Treg cells to the culture systems at the ratio of 1:2 to enriched T cells, and then CD8+ T cell proliferation was determined by the CFSE dilution after 3 days. Interestingly, CD126Lo/− Treg cells presented a stronger suppressive activity compared to CD126Hi Treg cells, even in the presence of IL-6 (Figure 2A). Furthermore, T cell receptor (TCR) activation led to reduced CD126 expression on Treg cells, and memory Treg cells present better suppressive function than naive Treg cells. To study whether the different function of these two subsets is due to a difference in naive and memory Treg cells, we first analyzed the proportion of naive and memory Treg cells in these two subsets and found that CD126Lo/− Treg cells include a higher percentage of memory Treg cells and a lower percentage of naive Treg cells than CD126Hi Treg cells (Figure S1A). Then we isolated CD126HiCD44+CD62L− (memory Treg), CD126Lo/−CD44-CD62L+(naive Treg), CD126Hi naive, and CD126Lo/− memory Treg cells to test the function of these four subsets. These Treg cells were added to the culture systems at the ratio of 1:1 to enriched T cells. These results indicated that CD126Lo/− Treg cells present better function in both memory Treg and naive Treg cell subsets (Figure S1B).
Figure 2.
CD126Lo/− nTreg Cells Present a Better Suppressive Function In Vitro and Colitis Model
(A) Enriched T cells were labeled with CFSE and stimulated with anti-CD3/28 and APCs in the presence or absence of IL-6; then, sorted CD126Hi and CD126Lo/− Treg cells were added to the culture system separately at the ratio of 1:2 to enriched T cells. CFSE dilution of CD8 T cells was detected by flow cytometry after a 3-day cell culture (left panel); right panel shows the quantitative data; five independent experiments were conducted. (B and C) CD4+CD62L+ naive T cells were isolated from Thy1.1 mice and co-transferred with sorted CD126Hi or CD126Lo/− Treg cells, which were isolated from Thy1.2 mice to Rag1 knockout (KO) mice. Weight was recorded approximately every 4 days (B). After 30 days of cell transfer, mice were sacrificed, and colon was subjected to H&E staining (C); top: the representative HE staining; bottom: the quantitative data. The p values were calculated by one-way ANOVA with Tukey’s post hoc test. (D and E) SP, draining LN, and colon lamina propria (cLP) cells were stained with IL-17A and IFN-γ, which were detected by flow cytometry. The representative flow data gated on Thy1.1+ are indicated on the left, and the quantitative data are indicated on the right (D); the representative flow data gated on Thy1.2+ are indicated on the left, and the quantitative data are indicated on the right (E). The p values were calculated by Student’s t test; three independent experiments were conducted, and each group included 5 mice. Data are presented as means ± SEM. ∗p < 0.05; ∗∗p < 0.01.
To further investigate the phenotypic stability and therapeutic effects of these two subsets on colitis in vivo, we intraperitoneally (i.p.) injected Thy1.1 naive CD4+ CD62L+ cells into Rag1−/− mice to induce colitis as described previously.21 Simultaneously, we sorted CD126Hi and CD126Lo/− Foxp3+ T cells from Thy1.2 Foxp3 GFP reporter mice and adoptively co-transferred these two subsets into the colitis mice. We noted that both subsets significantly prevented weight loss from T cell-induced colitis; however, infusion of CD126Lo/− Foxp3+ cells to colitis mice dramatically reduced weight loss (Figure 2B). In contrast, the colitis mice had significantly swollen colons, evident mucosal ulceration, loss of normal crypt structure, significant inflammatory cell infiltration, and edema (Figure 2C). Although both Treg cell populations reduced the manifestations of colitis, CD126Lo/− Treg cells displayed an effect that was much superior to that of CD126Hi Treg cells on alleviating these typical features in colitis. Moreover, we observed that the transfer of CD126Lo/− but not CD126Hi Treg cells clearly reduced Th17 cell production (Figure 2D). To examine the stability of Foxp3, we analyzed the Treg population gated on Thy1.2 that helps to distinguish the Treg cells from Thy1.1 effector cells; to this end, we demonstrated that CD126Hi Treg cells mostly lost Foxp3 expression in the SP (4.8%), mesenteric lymph node (mLN) (19%), and lamina propria (LP) (18%) and expressed increased IL-17A expression under colitis conditions. Nonetheless, CD126Lo/− Treg cells maintained higher Foxp3 expression and expressed much less IL-17A expression. These results suggest that the transferred CD126Hi Treg cells have less effect on inhibition of IL-17A production and colitis manifestations due to their instability (Figure 2E).
Characterization of These Two Subsets in Arthritic Mice
We further used a CIA model to document the stability of CD126Hi and CD126Lo/− Treg cell subsets in vivo. The instability of Foxp3 may contribute to the pathogenesis of autoimmune arthritic disease in this model and in patients with RA.12 We first analyzed the expression of IL-6 receptor (CD126) in the Foxp3+ cells between CIA and healthy mice. Interestingly, CD126 expression on Foxp3+ cells was much higher in mice with CIA (56 days after immunization with collagen II (CII)/CFA [complete Freund’s adjuvant]) than in healthy mice (Figure 3A). When these cells were stimulated with anti-CD3/CD28 in the presence of APCs and IL-6, they were less stable compared to cells isolated from healthy mice (Figure 3B).
Figure 3.
CD126 Expression on Treg Cells Is Positively Correlated with the Treg Phenotype and the Severity of CIA
(A) CD126 expression by Foxp3+ Treg cells from SP and LN stained at 56 days of CIA. Left: the representative flow data; right: the statistical data (n = 8). (B) CD4+Foxp3 (GFP)+ cells were sorted from normal and CIA model mice and then stimulated with anti-CD3/CD28 and APCs in the presence of IL-6 for 4 days. (C and D) Total lymphocytes were isolated from normal and CIA mice and then were stained with Treg phenotype markers and analyzed by using tSNE (C); positive cells for each marker were analyzed by using tSNE (D). (E) Correlation analysis between CD126 expression and disease clinical score (n = 24). ∗p < 0.05; ∗∗p < 0.01.
We also determined whether Foxp3+ Treg cells from CIA mice have a lower Treg-cell-suppressive molecular signature. Flow cytometry was performed to compare the suppressive functional molecules of CD126Hi and CD126Lo/− Treg cell populations in mice with CIA. It was unexpected that there were no significant changes in suppressive molecular profiles between CIA and healthy mice. We next applied a t-Distributed Stochastic Neighbor Embedding (tSNE) for dimensionality reduction to relate independently Treg cells from both CIA and healthy mice. As shown in Figure 3C, Treg cells from both CIA and normal mice generally partitioned into two main areas; in the top area, there was almost no CD126 expression. We then gated on each cell cluster that was positive for a suppressive molecular signature and found that cytotoxic T lymphocyte-associated protein 4-positive (CTLA-4+), inducible T cell costimulator-positive (ICOS+), and Tigit+ cell clusters were almost exclusively in the top area (Figure 3D). These results indicate that CD126Lo/− Treg cells represent a totally different subset that expresses a more suppressive molecular signature.
We also observed an evident correlation between CD126 expression in Treg cells and the severity of CIA. We further demonstrated that the severity of CIA was positively correlated with the CD126 expression of Foxp3+ cells, regardless of whether they came from SP or from LN (Figure 3E). These results indicate that the instability and dysfunction of Foxp3+ Treg in autoimmune arthritic mice is probably mostly attributed to the higher expression of CD126.
PD-1 Plays an Important Role in Maintaining CD126Lo/− Treg Stability
To further understand underlying mechanisms whereby CD126Lo/− Treg cells are more stable than CD126Hi Treg cells, we sorted these two subsets and subjected them to RNA-sequencing (RNA-seq) analysis. As shown in Figure 4A, several immune suppressor genes were higher in CD126Lo/− Treg cells than in CD126Hi Treg cells, including PD-1 (44% higher), CD103 (also known as ITGAE; 40% higher), IL-10 (96% higher), and T cell immunoreceptor with Ig and ITIM domains (TIGIT; 63% higher). We hypothesize that these genes are responsible for the superior biological features of CD126Lo/− Treg cells. Flow-cytometric analysis indicated that PD-1, CTLA-4, ICOS, CD103, IL-10, and TIGIT all were significantly higher in CD126Lo/− Treg cells compared to the CD126Hi cell compartment (Figure 4B; Figure S2).
Figure 4.
PD-1 Maintains CD126 Low Treg Cell Stability via Inhibiting Akt-mTOR Signaling and Stat3-Dependent Pathway
(A) CD126Hi and CD126Lo/– nTreg cells were subjected to RNA-seq. (B) PD-1 in CD126Lo/− nTreg cells is higher than in CD126Hi (n = 5). (C) Blocking PD-1 reverses CD126Lo/− stability, which is dependent on Stat3 signaling. Left: the representative flow data are from five independent experiments; right: quantitative data. The p values were calculated by one-way ANOVA with Tukey’s post hoc test. (D) Sorted CD126Hi and CD126Lo/− Treg cell populations were stimulated with anti-CD3/28 and APCs in the presence of IL-6 for 48 h, and then cells were harvested and stained with pS6, p-AKT, and Pten. Top: representative flow data; bottom: quantitative data. (E) After 48 h of IL-6 stimulation, cells were harvested for qRT-PCR for HIf1a mRNA expression. (F and G) Cells were harvested to stain p-Stat3 and p-STAT1 and subjected to flow cytometry (F) and ImageStream (G) (40×); representative data are indicated in separate panels. ∗p < 0.05; ∗∗p < 0.01, by Student’s t test; ns, not significant.
The role of PD-1 in the phenotype of Treg cells is still controversial. PD-1 knockout or PD-1 blockade can enhance the suppressive function of Treg cells in vitro and in vivo,22,23 while some other groups found that enhancing PD-1 signaling is important for Treg cell function and long-term Foxp3 expression.24 These findings led us to hypothesize that higher PD-1 expression on CD126Lo/− Treg cells may be important for its stability. To test this hypothesis, we sorted and stimulated CD126Lo/− Treg cells in the presence of anti-PD-1 or control IgG for 4 days and then measured its conversion. Flow data revealed that blockade of the PD-1 signal downregulated its stability and promoted the cells to trans-differentiate into IL-17A-expressing cells (Figure 4C). PD-1 has been shown to inhibit Akt-mTOR (mammalian target of rapamycin) signaling,24 so we investigated the Akt and mTOR phosphorylation of these two subsets in the presence of IL-6 after a 48-h cell culture. We found that CD126Lo/− Treg cells presented lower expression of phosphorylated AKT and S6 ribosomal protein (p-AKT and pS6, respectively) (MFI of p-AKT and mTOR was significantly downregulated; Figure 4D). pS6 is a downstream target of mTOR signaling and can reflect the sustained activation of Akt-mTOR signaling.25,26 Moreover, real-time PCR results also show that CD126Lo/− Treg cells presented lower Hif-1a expression than CD126Hi Treg cells under IL-6 stimulation (Figure 4E). Hif-1a is also a key regulator of mTOR signaling and plays a critical role in the balance of Th17 and Treg cells.26 The mTOR signaling pathway has been shown to be involved in the plasticity in Treg cells.
Moreover, we further analyzed the response of these two subsets to IL-6 by measuring the phosphorylation of Stat1/3 (pStat1/3) via flow cytometry and ImageStream. Our data demonstrated that CD126Lo/− showed lower STAT3 and STAT1 phosphorylation (Figures 4F and 4G). Moreover, the effects of blocking PD-1 can also be revised by a Stat3 inhibitor (Figure 4C). We further evaluated p-Stat3 expression under anti-PD-1 treatment after a 48-h cell culture, and the results showed that blocking PD-1 increased p-Stat3 expression (Figure S3). These results suggest that higher PD-1 expression can be involved in the stability of CD126Lo/− Treg cells via AKT-mTOR and STAT3/1 signaling.
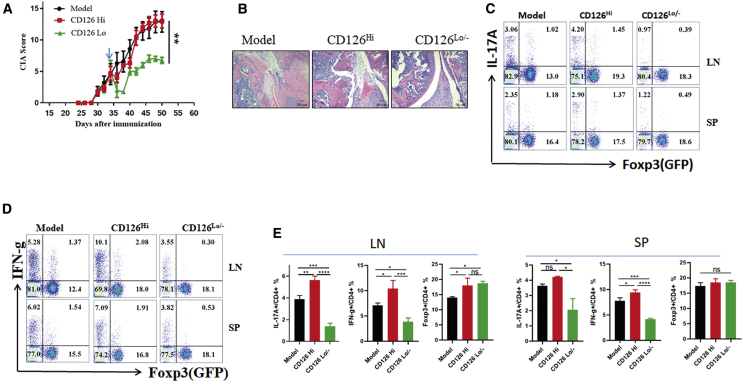
Significant Suppression of the Development of Established Autoimmune Arthritis in Mice Treated with CD126Lo/− nTreg Cells but Not in Mice Treated with CD126Hi nTreg Cells
Previous reports have shown that nTreg cells are effective for protecting CIA progression but fail to treat established CIA because nTreg cells lose Foxp3 expression and trans-differentiate to IL-17A-expressing cells, with loss of suppressive function.9 To compare the therapeutic effects of CD126Hi and CD126Lo/− Foxp3 cells in established CIA, 3 million cells from each of these two subsets were transferred separately to CIA mice with evident clinical scores, which are present about 30 days after collagen immunization. We found that CD126Hi Treg cells did not significantly decrease disease severity, joint damage, bone erosion, or inflammatory cell infiltration (Figures 5A and 5B). Conversely, injections of CD126Lo/− Treg cells significantly prevent the progression of established CIA and also protect from bone erosion. CD126Lo/− Treg cell injection significantly reduced the percentage of cells secreting the pro-inflammatory cytokines interferon (IFN)-γ and IL-17A in the draining LN in CIA mice (Figures 5C–5E), and CD126Lo/−-treated mice produced consistently lower percentages of Th1 and Th17 cells. In addition, CD126Hi and CD126Lo/−-treated mice showed no difference in Foxp3+ cells in SP or in LNs. Our results indicate that CD126Lo/− Treg cells may be a superior Treg population, and use of this Treg cell subset may provide an ideal therapeutic approach to treat autoimmune arthritis, colitis, and other autoimmune diseases.
Figure 5.
Significant Suppression of the Development of Established Autoimmune Arthritis in Mice Treated with CD126Lo/- nTreg Cells but Not in Mice Treated with CD126Hi nTreg Cells
(A) Sorted CD126Hi and CD126Lo/− nTreg cells were intravenously (i.v.) injected into mice with established CIA 35 days after CII immunization as indicated, and the clinical score was recorded every 3 days (n = 5). The p value was calculated by two-way ANOVA. (B) CIA mice were sacrificed 50 days after CII immunization, and joints were subjected to H&E staining (100×; scale bars, 50 μm). (C–E) SP and LN cells were stained with Foxp3 and IL-17A (C) or Foxp3 and IFN-g (D). The representative flow data are from three independent experiments (C and D), and the quantitative data (E) are indicated. The p values were calculated by one-way ANOVA with Tukey’s post hoc test. ∗p < 0.05; ∗∗p < 0.01; ns, not significant. Error bars denote means ± SEM.
CD126 Expression of Treg Cells Is Correlated with Disease Severity in Patients with RA
To study the clinical relevance of CD126 expression in Treg cells with the severity of RA patients, we analyzed CD126 expression in Treg cells from peripheral blood mononuclear cells (PBMCs) in 17 patients with RA and age- and gender-matched healthy donors. CD4+CD25+CD127Low/− is widely accepted as the phenotype of Treg cells in humans.27 In line with the CIA data, CD126 expression of Treg cells was also significantly higher in RA patients compared with healthy donors (Figure 6A). When these cells were sorted and stimulated with anti-CD3/CD28 with APCs in the presence of IL-1β and IL-6, Treg cells from RA patients lost more Foxp3 expression and resulted in higher IL-17 expression than Treg cells isolated from healthy subjects (Figure 6B). Moreover, the frequency of CD126Hi Treg cells had a positive correlation with disease activity in patients with RA (Figure 6C). To determine the suppressive function of Treg cells from healthy and RA donors under inflammatory conditions, enriched T cells from healthy donors were prepared and labeled with CFSE. Then they were co-cultured with mitomycin-treated APCs in the presence of OKT3 with the addition of the inflammatory cytokines IL-6 and IL-1β; Treg cells sorted from healthy and RA patients were added to the culture systems at the ratio of 1:1 to enriched T cells; and then, CD8+ T cell proliferation was determined by the CFSE dilution after 3 days. Our results demonstrated that Treg cells from healthy donors exerted better suppressive function than Treg cells from RA patients under IL-6 and IL-1β treatment (Figure 6D).
Figure 6.
CD126 Expression of Treg Cells Is Correlated with Disease Severity in Patients with RA
(A) CD126 expression in CD4+CD25+CD127Low/− Treg cells of stained PBMCs from healthy and RA patients. Left: the representative flow data; right: the statistical data (healthy, n = 8; RA, n = 17). (B) Sorted Treg cells from RA patients and healthy donors were stimulated with anti-CD3/28 and APCs in the presence of IL-1β and IL-6 for 4 days, and cells were harvested and stained with IL-17A and Foxp3. The representative flow data are from five independent experiments (left) and the quantitative data (right) are indicated. (C) Correlative analysis between CD126 expression by Treg cells and DAS-28 of RA patients (n = 17). (D) Functional analysis of Treg cells from healthy donors and RA patients under IL-6 and IL-1β-conditions. The p values were calculated by one-way ANOVA with Tukey’s post hoc test. ∗∗p < 0.01; ∗∗∗∗p < 0. 0001. Data are presented as means ± SEM.
Discussion
This study defines CD126Lo/− Treg cells as a superior Treg cell subset, which is more stable and suppressive in normal and inflammatory conditions. Compared with CD126Hi Treg cells, CD126Lo/− Treg cells display more power to inhibit disease progression and reduce tissue damage when applied to treat autoimmune colitis. nTreg cells have been previously reported to be able to prevent autoimmune arthritis, although they are less effective in treating established arthritis.28 We now provide a new finding that adoptive transfer of CD126Lo/− Treg cells actually can treat established arthritis via inhibiting pathogenic Th17 cells, suggesting a very useful potential Treg application in treating autoimmune and inflammatory diseases.
Treg cells are essential in the maintenance of immune homeostasis, and manipulation of Treg cells has been considered a potential strategy to treat autoimmune disease, even though Treg cells are less stable in the presence of an inflammatory condition. Maintaining Treg cell stability or identifying a stable Treg cell subset has become an attractive approach to improvement of Treg cell therapy.29 IL-6 has an essential role in the pathogenesis of RA.30 This cytokine promotes the function of multiple cells, including B cells, T cells, macrophages, neutrophils, synoviocytes, and osteoclasts,31 as well as in facilitating the progress of RA. IL-6 initiates cellular action by binding to CD126, followed by recruitment of gp130, and the activating IL-6R complex will phosphorylate STAT3 and activate IL-6 target genes.32 Blocking IL-6 or IL-6R can inhibit the progress of CIA by increasing the ratio of Foxp3+ to Th17 cells and now is an accepted approach for treating RA patients. Similarly, blockade of tumor necrosis factor alpha (TNF-α) also increased the ratio of Treg cells to Th17 cells and helped to recover the suppressive function of Treg cells.33 These reports suggest that the rebalance of Th17 and Treg cells are important for RA remission. Our study provides new insight into how blocking IL-6 signaling regulates the stability and function of Foxp3+ T cells. Indeed, we and others have previously reported that IL-6 converts Treg cells to IL-17-expressing cells that reduce Treg cell suppressive function.13,14
The comparative analysis of gene expression profiles between CD126Hi and CD126Lo/− Treg cells identified a series of overexpressed genes in CD126Lo/− Treg cells, providing additional evidence to support why CD126Lo/− Treg cells are a superior Treg population. Indeed, some immune-suppressive markers, including CTLA-4, CD103, TIGIT, ICOS, IL-10, and PD-1, are overexpressed in CD126Lo/− Treg cells. These molecules are involved in the development and function of Treg cells.29,34, 35, 36, 37 It should be noted that IL-10 signaling is a critical regulator in mucosal immune response, as mice with a deficiency of IL-10 gene develop enterocolitis.38 Analyses of colitis mice treated with Treg-cell-specific ablation of a conditional IL-10 allele showed that Treg-cell-deprived IL-10 does not limit the systemic immune response, while it is essential for keeping immune responses in check at environmental interfaces such as the colon.39 Moreover, IL-10 signaling in Treg cells is essential for suppression of Th17 cell-mediated inflammatory responses.40 Compared with CD126Hi Treg cells, higher expression of IL-10 in CD126Lo/− Treg cells may be responsible for the superior suppressive function in treating the T cell-induced colitis model.
PD-1 overexpression on CD126Lo/− is somewhat complicated. Previous studies have documented that PD-1 deficiency or blockade of Treg cells results in increased suppressive function in vitro and better protection in autoimmune disease;22,23 on the other hand, PD-L1 binding to PD-1 during Treg cell induction in vitro enhanced the Treg generation and is critical for long-term Foxp3 expression.24 We have revealed a new role that PD-1 plays in maintaining Foxp3 stability in a Treg cell subset. This was demonstrated by the PD-1 blockade disrupting CD126Lo/− Treg cell stability, and this can also be reversed by a STAT3 inhibitor. Thus, we propose that PD-1 signaling inhibits IL-6 signaling to help maintain Treg cell stability. It is worth noting that CD126 expression can be induced by IL-6 via mTOR signaling41 and that PD-1 inhibits mTOR signaling. The interaction between PD-1 and PD-L1 or PD-L2 transduces a signal that inhibits T cell cytokine production and cytolytic function.42 Blockade of PD-1 may enhance some cytokine production from a tiny population of non-Treg cells, and these cytokines may provide help to suppress the function and stability of Treg cells. Our observations support a model that blocking PD-1 may enhance CD126 expression by mTOR signaling and then induce STAT3 signaling.
Notably, we found that CD126 expression in Treg cells is correlated with the severity of CIA. Moreover, this correlation can be expanded to patients with RA. CD126 expression of T cells in autoimmune disease is still controversial. A previous animal study showed that CNS CD4+ T cells were lacking expression of CD126;11 on the other hand, it has been reported that CD126 expression on CD4+ T cells was increased and that the enhanced IL-6-CD126 signaling was responsible for the resistance of effector T cells to suppression by Treg cells.43 Our results clearly show that CD126 expression in Treg cells is higher in patients with RA compared to that in healthy subjects. The level of CD126 in Treg cells is also correlated with the DAS28 in patients with RA. These results suggest that CD126 expression by Treg cells may be used as a biomarker for RA disease activity. Previous studies by Wicker et al. indicated that human CD126Hi TIGIT− Treg cells had an IL-17-producing profile,44,45 and these cells were important to inhibit mucosal immune responses.46 This suggests a need for caution when applying CD126Lo/− Treg cells to treat mucosal immune-related diseases. Our data demonstrated a significant role of CD126 in the stability and suppressive function of Treg cells. We also made an innovative observation that the PD-1 signal pathway contributes to the stability of CD126Lo/− Treg cell population. More importantly, we propose that the CD126Lo/− Treg cells are a superior Treg population and that use of this Treg cell subset may be useful to treat many autoimmune diseases including RA, colitis, and others.
Materials and Methods
Mice
Thy1.2 C57BL/6 Foxp3GFP, Thy1.1 C57BL/6 Foxp3GFP, and DBA1 Foxp3GFP reporter mice were bred at Penn State Health Milton S.Hershey Medical Center, The Ohio State University, and Sun Yat-sen University. All mice were maintained and handled under SPF (specific-pathogen-free) conditions according to the Insitutional Animal Care and Use Committee (IACUC) and facilities guidelines. The IACUCs gave approval in each of the respective institutes.
Human Subjects
Peripheral blood samples (5 mL) were collected from healthy and RA donors who met the 1987 American Rheumatism Association criteria or the 2010 ACR/EULAR Classification criteria for RA. 150 mL peripheral blood was obtained for suppression assays. PBMCs were isolated by using Human Lymphocyte Separation Medium (Sigma). The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the Penn State Health Milton S. Hershey Medical Center (Study 00001317), The Ohio State University College of Medicine (2018H0590), and the Third Affiliated Hospital of Sun Yat-Sen University (2018-02-411-01). Signed informed consent was obtained from each participant before entering this study.
Cell Purification and Flow Cytometry
Cells were stained with the following directly conjugated antibodies. Some antibodies were purchased from BioLegend: Percp-cy5.5-CD4 (GK1.5), BV510-PD-1 (29F.1A12), BV605-CTLA4 (UC10-4139), BV785-CD103 (2E7), APC-CD126(D7715A7), APC-IFN-g(XMG1.2), PE-IL-17A(TC11-18H10.1), PE/BV650-Tigit(1G9), PE-ICOS(15F9), Alexa Fluor 647-pSTAT3(13A3-1), PE-p-STAT1(A15158B), and Biotin-CD25(PC65.1). Some antibodies were purchased from BD Biosciences: PE-pAKT(pS473) and PE-Pten(A2B1). PE-pS6 (pS240/244) was purchased from Cell Signaling Technology. Flow-cytometric analysis was performed using a BD FACSCelesta flow cytometer (BD Biosciences), a BD LSRFortessa, and FlowJo software. For cell isolation, enriched T cells from SP and LNs were isolated using nylon wool, and the non-T cells were used as APCs. nTregs were separated by CD25+ positive selection by autoMACS, and CD126Hi and CD126 Lo/− of the Treg cells were further sorted from CD25+ cells using a BD FACSAria II cell sorter. For intracellular cytokine staining, the cells were stimulated with PMA( phorbol myristate acetate) and ionomycin for 5 h and brefeldin A for 4 h. The cells then were stained to assess surface markers, followed by further fixation, permeabilization, and staining to examine Foxp3, IL-17A, and IFN-γ expression.
Amnis ImageStream
For ImageStream experiments to visualize p-STAT1 and p-STAT3 expression in signal CD4+ T cells, cells were similarly stained as described earlier, using flow cytometry. Approximately 5 × 103 cells were acquired by Amnis ImageStream (Merck), and the data were processed using Ideas v.4.0 software (Merck).
Treg Cell Stability Assay In Vitro
For murine Treg cell in vitro stability assay, different subsets of Treg cells were sorted as described earlier and stimulated with soluble anti-CD3/28 in the presence of APCs with rmIL-6 for 4 days as described previously.14,47
Quantitative Real-Time PCR
RNA was extracted from the harvested cells using TRIzol Reagent according to the manufacturer’s instructions. cDNA was synthesized using the RT Master Mix and amplified by quantitative RT-PCR in the ABI 7900 Prism system. The data were analyzed using the relative gene expression method and normalized to HPRT threshold cycle (Ct) values in the samples. Each sample was measured in triplicate. The primer sequences used for the PCR amplification were as follows:
HPRT forward: 5′-GGTGGTCTCCTCTGACTTCAACA-3′;
HPRT reverse: 5′-GTTGCTGTAGCCAAATTCGTTGT-3′;
Foxp3 forward: 5′-CCCATCCCCAGGAGTCTTG-3′;
Foxp3 reverse: 5′-ACCATGACTAGGGGCACTGTA-3′;
IL-17a forward: 5′-TTTAACTCCCTTGGCGCAAAA-3′;
IL-17a reverse: 5′-CTTTCCCTCCGCATTGACAC-3′;
Hif-1a forward: 5′-GTCCCAGCTACGAAGTTACAGC-3′; and
Hif-1a reverse: 5′-CAGTGCAGGATACACAAGGTTT-3′.
Suppressive Assay In Vitro
To examine the suppressive activity of CD126Hi and CD126Lo/−, enriched T cells were stained with CFSE at 2 μM and stimulated with soluble anti-CD3 (0.025 μg/mL) in the presence of APCs with or without IL-6, and then these two Treg cell subsets were added to the culture system at the ratio of 1:2 enriched T cells. After a 3-day cell culture, the suppressive function was assessed by the proliferative levels of CFSE-CD8+ cells, which were evaluated by the rates and intensity of CFSE dilution measured with flow cytometry as previously described.48
T Cell-Induced Colitis Model and Treg Cell Stability In Vivo
The T cell-induced colitis model was carried out as described previously.49 Briefly, 0.6 million CD4+CD62L+ naive T cells isolated from Thy1.1 Foxp3GFP mice were i.p. injected into Rag1−/− mice, and at the same time, CD126Hi and CD126Lo/− Treg cells were sorted from Thy1.2 Foxp3GFP and transferred into Rag1−/− mice, and weight loss was monitored. The stability of these Treg cell subsets was dynamically monitored as previously described.21 The mice were sacrificed at about 4 weeks after cell transfer.
CIA
CIA was induced in DBA1 Foxp3GFP reporter mice according to standard protocol.9,50 CD126Hi and CD126Lo/− nTreg cells were sorted from DBA1 Foxp3GFP mice—the purity of Foxp3 was more than 97%—and these two subsets were adoptively transferred to mice with established CIA at 28 days after CII-CFA immunization. The severity of CIA in mice was evaluated by clinical score as previously described.51
RNA-Seq
Total RNA was extracted from harvest cells using TRIzol Reagent. RNA quality assessment, cDNA library construction, and Illumina sequencing were completed by Beijing Novogene Bioinformatics Technology. Briefly, RNA degradation and contamination were verified using 1% agarose gel, and its quality was assessed using a NanoPhotometer spectrophotometer and the RNA Nano 6000 Assay Kit of the Agilent 2100 Bioanalyzer system. RNA-seq libraries were generated using the NEBNext Ultra RNA Library Prep Kit following the manufacturer’s recommendations. PCR products were purified (with the AMPure XP system), and library quality was assessed on the Agilent 2100 Bioanalyzer system. The library preparations were sequenced on the Illumina HiSeq 2000 platform, and 125-bp/150-bp paired-end reads were generated.
Statistics
Statistical analysis was performed using GraphPad Prism (GraphPad Software). Data were analyzed by Student’s t test in the case of two groups and one-way ANOVA analysis with Tukey’s post hoc test for three or more groups. The correlation between two groups was evaluated by linear correlation. Data are presented, if not indicated elsewhere, as mean ± SEM. A value of p <0.05 was considered to be statistically significant (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, not significant).
Author Contributions
Conceived and designed the experiments: S.G.Z. Performed the experiments: Y.C., Z.X., R.L., J.W., A.X., and N.N. Provided reagents/materials/analysis tools: R.W. Processed and analyzed the data: Y.C., B.L., R.W., W.H., and M.J. Wrote and revised the manuscript: Y.C., N.O., and S.G.Z.
Conflict of Interests
The authors declare no competing interests.
Acknowledgments
We acknowledge the NIAMS (R01 AR059103, STAR Award, and R61 AR073409 to S.G.Z.), NSFC (81671611 and 81871224 to R.L.), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S252 to N.N.) for funding.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.07.020.
Supplemental Information
References
- 1.Lan Q., Fan H., Quesniaux V., Ryffel B., Liu Z., Zheng S.G. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J. Mol. Cell Biol. 2012;4:22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz D.A., Zheng S.G., Gray J.D. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Huter E.N., Punkosdy G.A., Glass D.D., Cheng L.I., Ward J.M., Shevach E.M. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur. J. Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadkarni S., Mauri C., Ehrenstein M.R. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J. Exp. Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma J., Yu J., Tao X., Cai L., Wang J., Zheng S.G. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin. Rheumatol. 2010;29:1251–1258. doi: 10.1007/s10067-010-1510-7. [DOI] [PubMed] [Google Scholar]
- 6.Li N., Wei W., Yin F., Chen M., Ma T.R., Wu Q., Zhou J.R., Zheng S.G., Han J. The abnormal expression of CCR4 and CCR6 on Tregs in rheumatoid arthritis. Int. J. Clin. Exp. Med. 2015;8:15043–15053. [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M., Liu Y., Mo B., Xue Y., Ye C., Jiang Y., Bi X., Liu M., Wu Y., Wang J. Helios but not CD226, TIGIT and Foxp3 is a Potential Marker for CD4+ Treg Cells in Patients with Rheumatoid Arthritis. Cell. Physiol. Biochem. 2019;52:1178–1192. doi: 10.33594/000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S.Y., Hsu W.T., Chen Y.L., Chien C.H., Chiang B.L. Lymphocyte-activation gene 3(+) (LAG3(+)) forkhead box protein 3(-) (FOXP3(-)) regulatory T cells induced by B cells alleviates joint inflammation in collagen-induced arthritis. J. Autoimmun. 2016;68:75–85. doi: 10.1016/j.jaut.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Kong N., Lan Q., Chen M., Wang J., Shi W., Horwitz D.A., Quesniaux V., Ryffel B., Liu Z., Brand D. Antigen-specific transforming growth factor β-induced Treg cells, but not natural Treg cells, ameliorate autoimmune arthritis in mice by shifting the Th17/Treg cell balance from Th17 predominance to Treg cell predominance. Arthritis Rheum. 2012;64:2548–2558. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyao T., Floess S., Setoguchi R., Luche H., Fehling H.J., Waldmann H., Huehn J., Hori S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor R.A., Floess S., Huehn J., Jones S.A., Anderton S.M. Foxp3+ Treg cells in the inflamed CNS are insensitive to IL-6-driven IL-17 production. Eur. J. Immunol. 2012;42:1174–1179. doi: 10.1002/eji.201142216. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu N., Okamoto K., Sawa S., Nakashima T., Oh-hora M., Kodama T., Tanaka S., Bluestone J.A., Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 13.Xu L., Kitani A., Fuss I., Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 14.Zheng S.G., Wang J., Horwitz D.A. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X., Bailey-Bucktrout S., Jeker L.T., Bluestone J.A. Plasticity of CD4(+) FoxP3(+) T cells. Curr. Opin. Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y., Zheng S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016;7:604. doi: 10.3389/fimmu.2016.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Goodman W.A., Levine A.D., Massari J.V., Sugiyama H., McCormick T.S., Cooper K.D. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf J., Rose-John S., Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Ren X., Boriero D., Chaiswing L., Bondada S., St Clair D.K., Butterfield D.A. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:1088–1097. doi: 10.1016/j.bbadis.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W., Xu Z., Zheng Y., Wang J., Qian W., Olsen N., Brand D., Lin J., Zheng S.G. A protocol to develop T helper and Treg cells in vivo. Cell. Mol. Immunol. 2017;14:1013–1016. doi: 10.1038/cmi.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B., Chikuma S., Hori S., Fagarasan S., Honjo T. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc. Natl. Acad. Sci. USA. 2016;113:8490–8495. doi: 10.1073/pnas.1608873113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamada T., Togashi Y., Tay C., Ha D., Sasaki A., Nakamura Y., Sato E., Fukuoka S., Tada Y., Tanaka A. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suto T., Karonitsch T. The immunobiology of mTOR in autoimmunity. J. Autoimmun. 2020;110:102373. doi: 10.1016/j.jaut.2019.102373. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Colello J., Jarjour W., Zheng S.G. Cellular Metabolic Regulation in the Differentiation and Function of Regulatory T Cells. Cells. 2019;8:188. doi: 10.3390/cells8020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W., Putnam A.L., Xu-Yu Z., Szot G.L., Lee M.R., Zhu S., Gottlieb P.A., Kapranov P., Gingeras T.R., Fazekas de St Groth B. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong N., Lan Q., Su W., Chen M., Wang J., Yang Z., Park R., Dagliyan G., Conti P.S., Brand D. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann. Rheum. Dis. 2012;71:1567–1572. doi: 10.1136/annrheumdis-2011-201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christoffersson G., von Herrath M. Regulatory Immune Mechanisms beyond Regulatory T Cells. Trends Immunol. 2019;40:482–491. doi: 10.1016/j.it.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Samson M., Audia S., Janikashvili N., Ciudad M., Trad M., Fraszczak J., Ornetti P., Maillefert J.F., Miossec P., Bonnotte B. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–2503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 31.Alonzi T., Fattori E., Lazzaro D., Costa P., Probert L., Kollias G., De Benedetti F., Poli V., Ciliberto G. Interleukin 6 is required for the development of collagen-induced arthritis. J. Exp. Med. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minami M., Inoue M., Wei S., Takeda K., Matsumoto M., Kishimoto T., Akira S. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc. Natl. Acad. Sci. USA. 1996;93:3963–3966. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie H., Zheng Y., Li R., Guo T.B., He D., Fang L., Liu X., Xiao L., Chen X., Wan B. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 34.Oderup C., Cederbom L., Makowska A., Cilio C.M., Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paust S., Lu L., McCarty N., Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc. Natl. Acad. Sci. USA. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joller N., Lozano E., Burkett P.R., Patel B., Xiao S., Zhu C., Xia J., Tan T.G., Sefik E., Yajnik V. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herman A.E., Freeman G.J., Mathis D., Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J. Exp. Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 39.Rubtsov Y.P., Rasmussen J.P., Chi E.Y., Fontenot J., Castelli L., Ye X., Treuting P., Siewe L., Roers A., Henderson W.R., Jr. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhry A., Samstein R.M., Treuting P., Liang Y., Pils M.C., Heinrich J.M., Jack R.S., Wunderlich F.T., Brüning J.C., Müller W., Rudensky A.Y. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbers C., Kuck F., Aparicio-Siegmund S., Konzak K., Kessenbrock M., Sommerfeld A., Häussinger D., Lang P.A., Brenner D., Mak T.W. Cellular senescence or EGFR signaling induces Interleukin 6 (IL-6) receptor expression controlled by mammalian target of rapamycin (mTOR) Cell Cycle. 2013;12:3421–3432. doi: 10.4161/cc.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saunders P.A., Hendrycks V.R., Lidinsky W.A., Woods M.L. PD-L2:PD-1 involvement in T cell proliferation, cytokine production, and integrin-mediated adhesion. Eur. J. Immunol. 2005;35:3561–3569. doi: 10.1002/eji.200526347. [DOI] [PubMed] [Google Scholar]
- 43.Schneider A., Long S.A., Cerosaletti K., Ni C.T., Samuels P., Kita M., Buckner J.H. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive T(regs) involves IL-6-mediated signaling. Sci. Transl. Med. 2013;5:170ra15. doi: 10.1126/scitranslmed.3004970. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira R.C., Rainbow D.B., Rubio García A., Pekalski M.L., Porter L., Oliveira J.J., Waldron-Lynch F., Wicker L.S., Todd J.A. Human IL-6RhiTIGIT- CD4+CD127lowCD25+ T cells display potent in vitro suppressive capacity and a distinct Th17 profile. Clin. Immunol. 2017;179:25–39. doi: 10.1016/j.clim.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira R.C., Rainbow D.B., Rubio García A., Pekalski M.L., Porter L., Oliveira J.J., Waldron-Lynch F., Wicker L.S., Todd J.A. In-depth immunophenotyping data of IL-6R on the human peripheral regulatory T cell (Treg) compartment. Data Brief. 2017;12:676–691. doi: 10.1016/j.dib.2017.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song X., Sun X., Oh S.F., Wu M., Zhang Y., Zheng W., Geva-Zatorsky N., Jupp R., Mathis D., Benoist C., Kasper D.L. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature. 2020;577:410–415. doi: 10.1038/s41586-019-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo Y., Xue Y., Wang J., Dang J., Fang Q., Huang G., Olsen N., Zheng S.G. Negligible Effect of Sodium Chloride on the Development and Function of TGF-beta-Induced CD4(+) Foxp3(+) Regulatory T Cells. Cell Rep. 2019;26:1869–1879.e3. doi: 10.1016/j.celrep.2019.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu A., Liu Y., Chen W., Wang J., Xue Y., Huang F., Rong L., Lin J., Liu D., Yan M. TGF-β-Induced Regulatory T Cells Directly Suppress B Cell Responses through a Noncytotoxic Mechanism. J. Immunol. 2016;196:3631–3641. doi: 10.4049/jimmunol.1501740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S., Xie C., Chen Y., Wang J., Chen X., Lu Z., June R.R., Zheng S.G. Differential roles of TNFα-TNFR1 and TNFα-TNFR2 in the differentiation and function of CD4+Foxp3+ induced Treg cells in vitro and in vivo periphery in autoimmune diseases. Cell Death Dis. 2019;10:27. doi: 10.1038/s41419-018-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen M., Su W., Lin X., Guo Z., Wang J., Zhang Q., Brand D., Ryffel B., Huang J., Liu Z. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L., Wang J., Li J., Li T., Chen Y., June R.R., Zheng S.G. 1,25-Dihydroxyvitamin D3 Ameliorates Collagen-Induced Arthritis via Suppression of Th17 Cells Through miR-124 Mediated Inhibition of IL-6 Signaling. Front. Immunol. 2019;10:178. doi: 10.3389/fimmu.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.