Abstract
Chimeric antigen receptor (CAR) T cell therapy has garnered significant excitement due to its success for hematological malignancies in clinical studies leading to the US Food and Drug Administration (FDA) approval of three CD19-targeted CAR T cell products. In contrast, the clinical experience with CAR T cell therapy for solid tumors and brain tumors has been less encouraging, with only a few patients achieving complete responses. Clinical and preclinical studies have identified multiple “roadblocks,” including (1) a limited array of targetable antigens and heterogeneous antigen expression, (2) limited T cell fitness and survival before reaching tumor sites, (3) an inability of T cells to efficiently traffic to tumor sites and penetrate physical barriers, and (4) an immunosuppressive tumor microenvironment. Herein, we review these challenges and discuss strategies that investigators have taken to improve the effector function of CAR T cells for the adoptive immunotherapy of solid tumors.
Graphical Abstract

Currently, CAR T cell therapy has limited antitumor activity for solid tumors. In this review, Wagner and colleagues review the clinical experience with CAR T cells for solid tumors, including brain tumors, appraise CAR T cell therapy roadblocks, and discuss strategies that investigators have taken to improve their effector function.
Main Text
Adoptive cell therapies utilizing T cells expressing chimeric antigen receptors (CARs) have been propelled to the forefront of experimental cell therapies due to their clinical success for hematological malignancies targeting a range of antigens, including CD19, CD22, CD30, kappa, and B cell maturation antigen (BCMA).1, 2, 3, 4, 5, 6, 7, 8, 9, 10 The landscape of CAR T cell therapies for hematological malignancies, including successes and challenges, has been the subject of multiple recent reviews.11, 12, 13 We therefore do not discuss these in detail, except for highlighting the “lessons learned” as they relate to targeting solid tumors, including brain tumors. First, CAR T cells can eradicate chemorefractory cancer cells regardless of their underlying oncogenic driver mutations; second, lymphodepleting chemotherapy is at present a sine qua non to enable expansion and persistence of infused CAR T cells; third, inclusion of at least one co-stimulatory signaling domain in the CAR is critical for its success; fourth: antigen loss variants have emerged as one mechanism of therapeutic failure, even for antigens that are highly and homogeneously expressed such as CD19; and fifth, CAR T cells can induce significant side effects, including cytokine release syndrome (CRS) and neurotoxicity.11, 12, 13, 14, 15
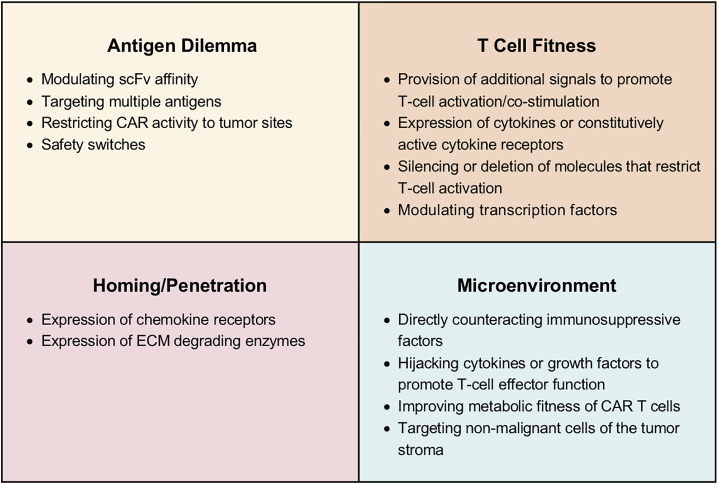
In contrast to CAR T cell therapy for hematological malignancies, CAR T cell therapies for solid tumors, including brain tumors, have shown limited antitumor activity in early phase clinical testing despite targeting a variety of target antigens and tumor types.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 This therapeutic failure is most likely multifactorial and includes (1) CAR design and CAR T cell generation, (2) a limited array of targetable antigens and heterogeneous antigen expression (aka “antigen dilemma”), (3) limited T cell fitness, (4) inefficient homing to and penetration of solid tumors, and (5) the hostile tumor microenvironment (TME) (Figure 1).27, 28, 29, 30 Herein, we review CAR design, the current clinical experience with autologous CAR T cells for solid tumors, including brain tumors, and genetic approaches to overcome the aforementioned roadblocks. Since allogeneic CAR T cells are at present mainly explored for hematological malignancies in early phase clinical studies, we refer the interested reader to recently published reviews.31,32 We also do not review CRS and neurotoxicity since several recent reviews have covered this topic.14,33
Figure 1.
CAR T Cell Immunotherapy “Roadblocks” for Solid Tumors
Top left: antigen dilemma. Heterogeneous antigen expression results in immune escape variants, and antigen expression in normal tissues can lead to on target/off cancer toxicity. Top right: CAR T cell fitness. Expansion, contractions, and persistence of CAR T cells is often limited after infusion. Bottom left: homing/penetration. CAR T cells have a limited ability to traffic to and penetrate solid tumors. Bottom right: microenvironment. The solid tumor microenvironment is hostile, limiting CAR T cell effector function.
Design of CARs
CARs have a modular design that consists of an antigen-binding domain, most commonly a single-chain variable fragment (scFv) derived from a monoclonal antibody (mAb), a hinge and transmembrane domain, and an intracellular signaling domain.27,34, 35, 36, 37, 38 In addition to scFvs, natural ligands of receptors such as cytokines or peptides that bind cell surface molecules are also being explored as antigen-binding domains.22,39,40 While most CARs recognize epitopes of cell surface proteins in a major histocompatibility complex (MHC)-independent manner, scFvs have also been incorporated into CARs that recognize a peptide in the context of a human leukocyte antigen (HLA) molecule.41, 42, 43 While this approach allows CAR T cells to recognize intracellular molecules, it renders target antigen recognition dependent on a particular HLA type, restricting its application to a subset of patients. In addition, CAR T cells become sensitive to decreased HLA expression and defects in the antigen processing pathway, with both pathways used by tumor cells to actively evade immune responses.44
First-generation CARs contained only a T cell activation domain, and the most commonly utilized domain is derived from CD3ζ.37,38 Since optimal T cell activation also relies on co-stimulation, signaling domains from co-stimulatory molecules were included into CARs. Initial studies focused on domains derived from the canonical co-stimulatory molecules CD28 and 41BB.37,38 Since then, signaling domains from a broad range of molecules have been explored, including OX40, CD27, and ICOS.37,45, 46, 47, 48, 49 CD28 and 41BB co-stimulation in the context of CAR T cells has been extensively studied, including detailed phosphoproteomic and single-cell RNA sequencing (RNA-seq) analyses.50, 51, 52 They activate different pathways within T cells, with CD28 signaling promoting glycolytic metabolism and an effector memory phenotype, in contrast to 41BB signaling, which induces oxidative metabolism and a central memory phenotype.53 Depending on the number of co-stimulatory molecules included in the CAR signaling domain, CARs are either designated second (one domain) or third (two domains) generation. In preclinical studies, the benefit of including two co-stimulatory molecules in CARs is model-dependent.54,55 Results of one clinical study, comparing CD28-CAR versus CD28.41BB-CAR T cells targeting CD19, suggest that third-generation CARs endow T cells with a greater ability to expand after infusion in humans.56
Optimizing CAR function remains a challenge since there is an intricate interplay between the functional (antigen recognition and signaling domains) and nonfunctional components (hinge and transmembrane domain) of CARs.57,58 In addition, the location of the epitope within the targeted cell surface molecule determines CAR T cell activity.59 For example, epitopes that are proximal to the plasma membrane induce greater CAR T cell activation than do distal epitopes.57,59,60 Studies have also highlighted that too much CAR T cell activation is detrimental to T cell function. For example, CARs with two of three mutated immunoreceptor tyrosine-based activation motifs (ITAMs) in the CD3ζ chain endow CAR T cells with improved effector function.61 In addition, excessive CD28 co-stimulation has detrimental effects,62 and mutations in the YMXM motif of CD28 that reduce its signaling activity improve T cell function.63 In addition to CAR signaling after activation, baseline (aka tonic) signaling determines CAR T cell activity.64,65 Recently, investigators have also focused on studying the immunological synapse that is formed between CAR T cells and target cells, and they have correlated synapse formation with CAR T cell effectiveness.66,67
Lastly, several studies have highlighted limitations of CARs with standard co-stimulatory molecules, which most likely cannot be overcome by further refining its structure.27,37,38 Foremost, CARs only provide signal 1 (activation) and signal 2 (co-stimulation) of canonical T cell activation and not signal 3 (inflammatory cytokines). While CAR-activated T cells initially produce cytokines, they do not secrete sufficient amounts of cytokines after recursive exposure to antigen-positive target cells, leading to rapid erosion of their effector function.68, 69, 70 This occurs even in the absence of an immunosuppressive TME, highlighting that additional modification of CAR T cells or combinatorial therapies are needed to sustain and/or enhance their effector function. These are discussed in detail in the section Improving CAR T Cell Fitness.
CAR T Cells for Solid Tumors and Brain Tumors—Clinical Experience
CAR T cells have been evaluated in early phase clinical studies for more than a decade, targeting a broad range of tumor antigens, including B7-H3, CAIX, CEACAM5, CD133, CD171, epidermal growth factor receptor (EGFR), EGFRvIII, FRα, GD2, GPC3, HER2, interleukin (IL)-13Rα2, mesothelin, MUC1, prostate-specific membrane antigen (PSMA), ROR1, and vascular endothelial growth factor (VEGF)-R2.19,20,22, 23, 24, 25, 26,71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82 Table 1 lists selected published studies, and Table 2 lists ongoing clinical studies. Early studies focused on first-generation CARs specific for CAIX, CD171, FRα, GD2, or IL-13Rα2.16,20,22,83 Two studies utilized polyclonal, activated CAR T cells (CAIX, FRα);20,84 two studies focused on CAR T cell clones (CD171, IL-13Rα2);75,83,85 and one study compared GD2-CAR T cells with GD2-CAR/Epstein-Barr virus (EBV)-specific T cells.81,82 First-generation CAR T cells did not expand after infusion and had limited antitumor activity, except for the study with GD2-CAR/EBV-specific T cells, in which 3 of 11 patients had a complete response (CR). Despite having no antitumor activity, CAIX-CAR T cells induced “on target/off cancer” toxicity in the form of cholangitis.19,20 In hindsight, limited antitumor activity was not surprising since CARs without co-stimulatory endodomains were grafted onto T cells, and patients did not receive lymphodepleting chemotherapy prior to the adoptive transfer of CAR T cells—two prerequisites of successful CAR T cell therapies for hematological malignancies.1, 2, 3, 4, 5, 6, 7, 8, 9, 10
Table 1.
Selected Published CAR T Cell Clinical Studies for Solid Tumors and Brain Tumors
| Antigen | Diagnosis | Signaling Domain | Vector | T Cell Product | IL-2 after T Cells | Comment | References |
|---|---|---|---|---|---|---|---|
| Intravenous Administration, No Lymphodepleting Chemotherapy | |||||||
| FRα | OVCA | ζ | RV | ATCsa | Y | 14/14 NR | 21 |
| CAIX | RCC | ζ | RV | ATCs | N | 12/12 NR; on target/off cancer toxicity—bile ducts | 19,20 |
| CD171 | NB | ζ | Plasmid | T cell clone | N | 1/6 PR | 83 |
| EGFRvIII | HGG | 4-1BB | LV | ATCs | N | 1/10 SD | 17 |
| GD2 | NB | ζ | RV | ATCs, VSTs | N | 3/11 CR | 81,82 |
| HER2 | sarcoma | CD28ζ | RV | ATCs | N | 4/17 SD | 23 |
| HER2 | HGG | CD28ζ | RV | VSTs | N | 3/17 SD | 26 |
| Mesothelin | PCA | 41BBζ | mRNA | ATCs | N | 2/6 SD | 80 |
| Intravenous Administration after Lymphodepleting Chemotherapy | |||||||
| CD133 | HCC, PCA, CRC | 41BBζ | LV | ATCs | N | 3/23 PR, 14/23 SD | 24 |
| CEACAM5 | CRC, PDCA, stomach, esophagus | ζ | RV | ATCs | Y | 7/14 SD; on target/off cancer toxicity—lung | 25 |
| EGFR | PCA | 41BBζ | LV | ATCs | N | 4/16 PR, 8/16 SD, 2/16 NE | 86 |
| EGFRvIII | HGG | CD28.41BBζ | RV | ATCs | Y | 17/18 NR, 1/18 NE due to TRMb | 16 |
| GD2 | NB | CD28.OX40ζ | RVc | ATCs | N | 5/11 SD | 87 |
| GPC3 | HCC | CD28ζ | LV | ATCs | N | 2/13 PR, 1/13 SD, 4/13 NE | 88 |
| HER2 | CRC | CD28.41BBζ | LV | ATCs | Y | 1/1 TRMb; on target/off cancer toxicity—lung | 18 |
| HER2 | sarcoma | CD28ζ | RV | ATCs | N | 1/10 CR; 3/10 SD | 89,90 |
| Mesothelin | MPM, PCA, OVCA | 41BBζ | LV | ATCs | N | 11/15d SD | 91 |
| PSMA | prostate | ζ | RV | ATCs | Y | 2/5 PR | 71 |
| ROR1 | TNBC, NSCLC | 41BBζ | LV | ATCs | N | 4/6 mixed response; 1/6 SD; 1/6 not reported |
72 |
| VEGF-R2 | metastatic CA | – | RV | ATCs | Y | 1/23 PR | e |
| Intravenous Administration after Allotransplant | |||||||
| GD2 | NB | ζ | RV | VSTs | N | 3/3 PD | f |
| Locoregional Administration | |||||||
| B7-H3 | meningioma | 41BBζ | LV | ATCs | N | 1/1 evidence of ALV | 73 |
| IL-13Rα2 | HGG | ζ | plasmid | T cell clone | N | 1/3 tumor necrosis | 74 |
| IL-13Rα2 | HGG | ζ | plasmid | T cell clonea | N | imaging study; outcome not reported | 75 |
| IL-13Rα2 | HGG | 41BBζ | LV | ATCs | N | 1/1 CR | 22 |
| Mesothelin | MPM, lung CA, breast CA | CD28ζ | RVc | ATCs | N | 2/21g CR, 5/21 PR, 4/21 SD | 76 |
| MUC1 | SVA | 41BBζ or CD28ζ | LVh | ATCs | N | 1/1 tumor necrosis | 92 |
ALV, antigen loss variant; ATC, activated T cell; CA, cancer; CR, complete response; CRC, colorectal cancer; HCC, hepatocellular carcinoma; HGG, high-grade glioma; LV, lentivirus; MPM, malignant pleural mesothelioma; N, no; NB, neuroblastoma; NE, not evaluable; NR, no response; OVCA, ovarian cancer; PCA, pancreatic adenocarcinoma; PR, partial response; RCC, renal cell carcinoma; RV, retrovirus; SD, stable disease; SVA, seminal vesicle adenocarcinoma; VST, virus-specific T cell; Y, yes.
Several patients received allogeneic cell products.
Pulmonary complications.
CAR vector also encoded inducible caspase-9.
6 of 15 patients received lymphodepleting chemotherapy.
ClinicalTrials.gov: NCT01218867.
ClinicalTrials.gov: NCT01460901.
18 of 21 patients received lymphodepleting chemotherapy and 13 of 21 patients received pembrolizumab.
41BBζ CAR vector also encoded IL-12.
Table 2.
Selected Actively Recruiting CAR T Cell Clinical Studies for Solid Tumors and Brain Tumors
| Antigen | Diagnosis | Signaling Domain | Additional Engineering | Vector | T Cell Product | Other Therapy | NCT No.: ClinicalTrials.gov |
|---|---|---|---|---|---|---|---|
| Intravenous Administration, No Lymphodepleting Chemotherapy Listed onClinicalTrials.govSite | |||||||
| GPC3 | HCC | – | TGF-β-CAR, IL-7-CCL19 | – | ATCs | N | NCT03198546 |
| HCC | – | N | – | ATCs | N | NCT04121273 | |
| PSMA | solid tumors | – | N | – | ATCs | N | NCT04429451 |
| Intravenous Administration after Lymphodepleting Chemotherapy | |||||||
| B7-H3 | solid tumors | – | iC9 | LV | ATCs | N | NCT04432649 |
| CD171 | NB | 41BBζ or CD28.41BBζ | N | LV | ATCs | N | NCT02311621 |
| Claudin 18.2 | solid tumors | – | N | – | ATCs | N | NCT03874897 |
| EGFR806 | solid tumors | 41BBζ | CD19-CAR | LV | ATCs | N | NCT03618381 |
| IL-13Rα2 | Melanoma | 41BBζ | N | LV | ATCs | IL-2 | NCT04119024 |
| GD2 | NB | – | iC9, IL-15 | RV | ATCs | N | NCT03721068 |
| solid tumors | – | iC9 | – | ATCs | N | NCT03373097 | |
| solid tumors | – | C7R | RV | ATCs | N | NCT03635632 | |
| DIPG, HGG | – | C7R | RV | ATCs | N | NCT04099797 | |
| NB | – | IL-15 | RV | iNKT | N | NCT03294954 | |
| NB | – | N | – | ATCs | N | NCT02761915 | |
| DIPG | 41BBζ | iC9 | RV | ATCs | N | NCT04196413 | |
| GPC3 | solid tumors | 41BBζ | N | RV | ATCs | N | NCT02932956 |
| HCC | 41BBζ | N | RV | ATCs | N | NCT02905188 | |
| HCC | – | N | – | ATCs | N | NCT03884751 | |
| Mesothelin | solid tumors | – | PD-1 KO | – | ATCs | N | NCT03747965 |
| solid tumors | – | N | LV | ATCs | N | NCT03054298 | |
| MUC-1 | esophageal CA | – | PD-1 KO | – | ATCs | IL-2 | NCT03706326 |
| NSCLC | – | PD-1 KO | – | ATCs | IL-2 | NCT03525782 | |
| breast Cancer | 41BBζ | N | LV | ATCs | N | NCT04020575 | |
| PSCA | prostate CA | 41BBζ | N | LV | ATCs | N | NCT03873805 |
| solid tumors | ζ | iMC | RV | ATCs | N | NCT02744287 | |
| PSMA | solid tumors | – | iC9 | gene editing | ATCs | N | NCT04249947 |
| prostate CA | – | DNR TGFβ | LV | ATCs | N | NCT03089203 | |
| prostate CA | – | DNR TGF-β | LV | ATCs | N | NCT04227275 | |
| Intravenous and Locoregional Administration After Lymphodepleting Chemotherapy | |||||||
| MUC16ecto | solid tumors | 41BBζ | IL-12 | LV | ATCs | N | NCT02498912 |
| Locoregional Administration, No Lymphodepleting Chemotherapy | |||||||
| B7-H3 | CNS tumors | – | N | LV | ATCs | N | NCT04185038 |
| CNS tumors | – | N | RV | ATCs | TMZ | NCT04385173 | |
| CNS tumors | – | N | RV | ATCs | TMZ | NCT04077866 | |
| EGFR806 | CNS tumors | 41BBζ | N | LV | ATCs | N | NCT03618381 |
| EGFR family | SCCHN | CD28ζ | IL-4/IL-2 CCR | RV | ATCs | N | NCT01818323 |
| HER2 | CNS metastases | 41BBζ | N | LV | ATCs | N | NCT03696030 |
| CNS tumors | CD28ζ | N | RV | ATCs | N | NCT02442297 | |
| CNS tumors | 41BBζ | N | LV | ATCs | N | NCT03500991 | |
| IL-13Rα2 | HGG | 41BBζ | N | LV | ATCs | nivo/ipi | NCT04003649 |
| HGG | 41BBζ | N | LV | ATCs | N | NCT02208362 | |
| Locoregional Administration after Lymphodepleting Chemotherapy | |||||||
| FRα | OVCA | 41BBζ | N | LV | ATCs | N | NCT03585764 |
| Mesothelin | pleural disease | CD28ζ | iC9 | – | ATCs | Pembro | NCT02414269 |
ATC, activated T cell; CA, cancer; CCR, chimeric cytokine receptor; C7R, constitutive active IL-7 receptor α; DNR, dominant negative receptor; HCC, hepatocellular carcinoma; HGG, high-grade glioma; iC9, inducible caspase-9; iMC, inducible Myd88.CD40; ipi, ipilimumab; KO, knockout; LV, lentivirus; N, no; NB, neuroblastoma; nivo, nivolumab; OVCA, ovarian cancer; pembro, pembrolizumab; RV, retrovirus; SCCHN, squamous cell carcinoma of the head and neck; Y, yes.
In subsequent studies, CARs were evaluated with co-stimulatory endodomains (CD28, 41BB, CD28/41BB, CD28/OX40) targeting B7-H3, CEACAM5, CD133, CD171, EGFR, EGFRvIII, FRα, GD2, GPC3, HER2, IL-13Rα2, mesothelin, MUC1, PSMA, ROR1, and VEGF-R2.72,86, 87, 88, 89,91,92 Similar to first-generation CAR T cells, adoptive transfer of second- or third-generation CAR T cells or CAR virus-specific T cells did not result in significant T cell expansion in two studies, and variable expansion in one study.23,26,87 Thus, inclusion of a co-stimulatory domain into the CAR by itself is not sufficient to consistently induce systemic T cell expansion. In contrast, lymphodepleting chemotherapy prior to CAR T cell infusion resulted in a significant expansion of CAR T cells irrespective of the utilized co-stimulatory domain and targeted antigen.9,93,94 Despite significant in vivo expansion, antitumor activity remained limited (Table 1). However, single case reports of patients with recurrent refractory solid tumors or brain tumors, who achieved CRs after CAR T cell therapy, have been reported (Table 1).22,90 Clinical studies have also highlighted safety concerns. Two patients died of acute respiratory failure after having received lymphodepleting chemotherapy, a high dose (1 to 6 × 1010) of third-generation CAR T cells, and IL-2.16,18 CAR T cells either recognized an antigen that is expressed (HER2) or not expressed (EGFRvIII) on lung endothelial cells, suggesting that on target/off cancer toxicity as well as unspecific CAR T cell activation can result in fatal respiratory failure. Adoptive transfer of high cell doses (1 × 109 to 5 × 1010) of CEACAM5-CAR T cells in conjunction with IL-2 also resulted in pulmonary toxicities (mild pulmonary edema), which was attributed to CEACAM5 expression on lung epithelium.25
Tumor tissue after CAR T cell infusion has only been studied systematically in one study in which patients with high-grade glioma (HGG) received EGFRvIII-CAR T cells.17 Collectively, the analysis showed that CAR T cells were able to migrate to and proliferate at tumor sites. CAR T cells killed tumor cells as evidenced by the selection of target antigen-negative tumors, and, strikingly, induced a reactive immunosuppressive environment as judged by expression of IDO1 and PD-L1, and an influx of regulatory T cells (Tregs).
Thus, CAR T cells at present rarely induce CRs in patients with solid tumors or brain tumors. In addition, several studies have highlighted their potential to induce on target/off cancer toxicity. In the following sections we review approaches that investigators have taken to improve their efficacy and safety, with special focus on (1) clinical grade CAR T cell production and T cell subsets for CAR T cell therapy, (2) expanding the array of targetable antigens and overcoming heterogeneous antigen expression, (3) CAR T cell fitness, (4) CAR T cell trafficking and tumor penetration, and (5) counteracting the immunosuppressive TME (Figure 2).
Figure 2.
Strategies to Overcome CAR T Cell Immunotherapy Roadblocks for Solid Tumors
Summary of strategies that are discussed in detail in this review.
Improving Clinical-Grade CAR T Cell Production
Preclinical studies have highlighted that the effector function of T cells progressively decreases as T cells differentiate from naive (TN) to stem cell memory (TSCM), central memory (TCM), effector memory (TEM), and terminally differentiated T cells that express the CD45RA effector (TEMRA).95 Transcription factors govern T cell differentiation. For example, TCF7 promotes self-renewal and memory formation, while TOX, BLIMP1, and TBET promote terminal T cell differentiation.96, 97, 98, 99, 100, 101, 102 More recent studies have also highlighted the critical role of epigenetic programs enforced by the de novo DNA methyltransferase 3A (DNMT3A) in determining T cell fate.103, 104, 105 In addition, a recent case report highlighted that disruption of TET2 in CD19-CAR T cells, an enzyme that regulates DNA demethylation, resulted in clonal T cell expansion.106
Despite these insights in T cell biology, most initial clinical studies utilized polyclonal, CD3/CD28-activated T cells that are expanded in the γ-cytokine IL-2. These expansion protocols in general result in significant T cell differentiation paired with inferior effector function in preclinical models. Investigators have explored alternative γ-cytokines to preserve CAR T cell function.107 For example, replacing IL-2 with a combination of IL-7 and IL-15 for the ex vivo expansion of CAR T cells increases the frequency of CD8+CD45RA+CCR7+ stem cell-like CAR T cells with superior antitumor functionality.108 Based on these findings, many clinical-grade CAR T cell production protocols are currently using IL-7 and IL-15. In addition, IL-21 has been shown to prevent differentiation of genetically modified T cells paired with improved effector function in preclinical studies.109,110 Inhibiting or activating pathways in T cells that promote or prevent T cell differentiation are alternative strategies that have been explored. These include activating Wnt/β-catenin signaling pathways or inhibiting mTOR or AKT signaling.111,112
Notwithstanding these advances, there is at present a lack of generally accepted biomarkers that predict efficacy of the infused CAR T cell product. Several studies with CD19-CAR T cells are starting to shed light on this issue. In one study in patients with chronic lymphocytic leukemia (CLL), the presence of CD27+PD-1−CD8+ CD19-CAR T cells expressing high levels of the IL-6 receptor in the T cell product correlated with antitumor activity,113 and in another study in patients with acute lymphocytic leukemia (ALL), the presence of tumor necrosis factor (TNF)-α+CD8+ CD19-CAR T cells, respectively.114 One recent study, using RNA-seq analysis, demonstrated that CD19-CAR T cell clones that expand after infusion are derived from clusters of CAR T cells in the product that express higher levels of genes associated with T cell proliferation and cytolytic activity.115 While this study found no correlation with vector integration sites, another recent report demonstrated that vector integration site distribution in CD19-CAR T cells is linked to clinical outcome.116 Clearly, additional studies are needed to identify biomarkers not only for the CAR T cell product, but also for the targeted tumor.
Besides improving culture conditions for CAR T cell generation, investigators have also focused on the genetic modification step of T cells during CAR T cell production. Most clinical studies so far have utilized CAR T cells that were generated with retroviral or lentiviral vectors with an excellent safety record.117, 118, 119 However, clinical-grade production and release testing of recombinant viral vectors is expensive, and patients require additional monitoring. Therefore, alternative approaches have been developed, including the use of PiggyBac or Sleeping Beauty transposon systems.120,121 More recently, combining gene-editing technologies (e.g., CRISPR-Cas9, TALEN, ZFN, meganucleases) with homology-directed DNA repair (HDR) has enabled the integration of transgenes into specific loci.122, 123, 124 This approach allows for control of transgene expression with endogenous promoters. For example, CD19-CARs have been knocked in to the T cell receptor (TCR) α constant (TRAC) locus using CRISPR-Cas9 gene editing to drive CAR expression from the endogenous TCR α chain promoter. This resulted in consistent CAR expression, reduced baseline signaling, and improved antitumor activity compared to CD19-CAR T cells generated by viral transduction.125 The clinical experience with CAR T cells generated with non-viral methods is so far limited,78 and most ongoing studies are focused on targeting hematological malignancies.
T Cell Subsets for CAR T Cell Therapy
Investigators have explored CAR T cell products with a defined CD8+/CD4+ T cell subset ratio and products that were derived from TSCM, TCM, or T helper (Th17) cells.48,126, 127, 128, 129 These studies have highlighted that T cell subset-derived CAR T cell populations have improved effector function in comparison to polyclonal, CD3/CD28-activated CAR T cells. Importantly, the co-stimulatory domain of the CAR might have to be tailored for a specific T cell subset with one study demonstrating superior antitumor activity when CD4+ T cells, expressing CARs with an ICOS co-stimulatory endodomain, were combined with CD8+ T cells, expressing CARs with a 41BB co-stimulatory endodomain.130
However, the translation of these approaches into the clinic might be limited due to the frequency of some of these T cell subset populations; for example, TSCM cells are very low in the peripheral circulation.131 In addition, sequential cycles of chemotherapy have depleted T cell subsets, including TN cells, in heavily pretreated cancer patients, who are the current CAR T cell study population.132
Selecting virus-specific T cells for CAR T cell generation is an alternative approach to selecting TCM and TEM cells with unknown specificity. Examples include the aforementioned clinical study with GD2-CAR/EBV-specific T cells in which investigators demonstrated improved persistence in comparison to GD2-CAR T cells.81 In addition, a clinical study is ongoing, which evaluates the safety and efficacy of GD2-CAR/varicella zoster virus (VZV)-specific T cells (ClinicalTrials.gov: NCT01953900). Advantages of this approach are not only that CAR/virus-specific T cells might have improved effector function in comparison to their unselected CAR T cell counterparts, but these CAR/virus-specific T cells can be boosted after infusion through their endogenous, virus-specific αβ TCR either by vaccination (e.g., VZV vaccine) or reactivation of the latent virus (e.g., EBV). Lastly CAR-expressing γδ T cells133 and invariant natural killer (iNK)T cells have been successfully explored in preclinical studies,134 and a clinical study with iNK T cells expressing GD2-CARs and IL-15 is in progress (ClinicalTrials.gov: NCT03294954).
CAR T Cell Therapy Targets—The Antigen Dilemma
An “ideal” CAR target should be highly and homogeneously expressed throughout the tumor, across multiple patients, and have minimal to no expression in vital normal tissues. Unfortunately, these characteristics do not apply to most CAR targets that are currently being explored for solid tumors and brain tumors (Table 2). In general, current targets are differentially expressed antigens, which may be present at low levels in normal tissues. The only exceptions are EGFRvIII, a cancer-specific splice variant expressed in HGG, and EGFR806, a conformational EGFR epitope that is also present in HGG.135,136 While neoantigens are a potential solution to this “antigen dilemma,” most of these are patient specific, raising concerns about feasibility. In addition, most neoantigens described so far are derived from intracellular proteins, which are difficult to target with standard CAR T cells.137 Investigators have therefore embarked on discovering novel CAR targets using genomic and proteomic approaches.138, 139, 140 In addition, there is an intense focus on engineering CARs and CAR T cells to increase their specificity for antigens expressed on tumor cells. These include (1) modulating the affinity of the scFv, (2) targeting multiple antigens, and (3) restricting CAR activity to tumor sites. In addition, investigators have developed safety switches that can be activated in the event of adverse events secondary to CAR T cells, including on target/off cancer toxicity.
Modulating scFv Affinity
This approach is only applicable to antigens that are expressed at very high levels such as gene-amplified HER2 in HER2-positive breast cancer. For example, investigators have shown that reducing the scFv affinity of HER2-CARs or EGFR-CARs decreases the risk of on target/off cancer toxicity.141 In addition, in murine models, the incidence of neurotoxicity of GD2-CAR T cells varied according to the affinity of the expressed CAR.142 In addition to modulating scFv affinity, recent studies have also highlighted that the choice of hinge, transmembrane, and/or costimulatory domains of CARs can influence antigen density requirements for CAR T cell activation.58,143,144
Targeting Multiple Antigens
Heterogeneity is a hallmark of cancer and a major roadblock for all targeted cancer therapies, including immunotherapy.145 Heterogeneous antigen expression is one consequence of tumor heterogeneity leading to the outgrowth of tumor cell subpopulations when a single antigen is targeted. Investigators have therefore focused on targeting cancer stem cells (CSCs) or tumor-initiating cells (TICs),146, 147, 148 or multiple antigens to prevent immune escape. In addition, multi-specific CAR T cells have the potential to reduce the risk of on target off/cancer toxicity if they target an array of antigens present in a particular tumor and not in normal tissues. Several approaches are actively being pursued, including expressing CARs with two scFvs. Investigators have either assembled two scFvs in a linear (aka tandem CAR) fashion or by alternating heavy and light chains of the two scFvs (aka loop CAR), similar to the dual-affinity retargeting (DART) bispecific antibody platform.149, 150, 151 Investigators have also expressed two or three different CARs in T cells.152 The clinical experience with T cells expressing bispecific CARs or two CARs is currently limited to CD19/CD22- and CD19/CD20-targeted approaches for leukemia and/or lymphoma. An alternate approach is to design CARs in which the antigen-binding domain is derived from a ligand that binds multiple antigens, and the most relevant example for solid tumors is the T1E peptide that binds to the EGFR family of receptors.40 So-called “universal CARs” have also been developed to target multiple antigens that contain an ectodomain that can be paired with multiple antigen recognition domains. Examples include (1) avidin-CARs/biotin-labeled scFvs, (2) CD16-CAR/mAbs, (3) anti-fluorescein isothiocyanate (FITC)-CARs/FITC-labeled scFvs, (4) coiled-coil CARs (SUPRA CARs), (5) anti-PNE-CARs/PNE-scFvs, and (6) NKG2D-CARs/ULBP2-mAbs.153, 154, 155, 156, 157, 158, 159 Lastly, bispecific T cell engagers (BiTEs) have been expressed in T cells,160,161 and more recently adapted to CAR T cells, opening up the opportunity to target multiple antigens and redirecting bystander T cells to tumor cells.162
In addition to designing CAR T cells to target multiple antigens, strategies are being pursued to engineer T cells to activate bystander T cells to recognize tumor cells (aka induce antigen/epitope spreading). These include the transgenic expression of cytokines (e.g., IL-18 or IL-36γ) or CD40L.163, 164, 165 Likewise, one recent study has demonstrated that engineering T cells to secrete the FLT3 ligand promotes epitope spreading.166
Restricting CAR Activity to Tumor Sites
CAR activity can be restricted to tumor sites by several measures. First, CAR expression can be induced once T cells have migrated to tumor sites with small molecules, such as a doxycycline-inducible expression system.167 Second, CAR T cells can take clues from the TME to express a CAR. These include hypoxia-inducible expression systems,168 as well as synthetic signaling circuits that take advantage of Boolean logic.169, 170, 171 One of the best examples of Boolean logic-gated CARs is the synthetic notch (synNotch) system,169, 170, 171 in which an antigen-specific synNotch receptor induces the expression of a second CAR upon activation. For example, EpCAM- or B7H3-specific synNotch receptors have been used to restrict ROR1-CAR expression to tumor sites, reducing on target/off cancer toxicity in preclinical models.172 Other strategies include engineering T cells to express two CARs with different specificity in which one CAR provides signal 1 and the other signal 2 to limit full CAR T cell activation to tumor sites at which both antigens are present.173,174 With this approach full CAR T cell activation has been restricted to (1) PSMA- and PSCA-positive or (2) MUC1- and HER2-positive tumor cells in preclinical models.173,174 Expressing inhibitory CARs that recognizes an antigen expressed on normal tissues in conjunction with a tumor-specific CAR is another approach that has been evaluated using CD19 as a tumor-specific antigen and PSMA as a model antigen expressed on non-targeted cancer cells.175 Lastly, inactive CARs have been designed (e.g., EGFR)176 that require proteolytic processing into an active form by matrix metalloproteinases (MMPs) within the TME.
Safety Switches
Neither of the aforementioned strategies may end up being sufficient to prevent on target/off cancer toxicities. Investigators have therefore developed inducible safety switches to ablate CAR T cells when the need arises. These safety switches can be conceptually divided into four approaches. The first approach relies on activating a prodrug that is toxic to T cells. The best studied example is the antiviral ganciclovir, which readily kills T cells that are genetically modified with the herpes simplex virus thymidine kinase (HSV-tk).177 The second approach takes advantage of dimerizer drugs to activate a molecule that is transgenically expressed in CAR T cells. Several dimerizer systems have been developed;178 of these, the inducible caspase-9 (iC9) system has been evaluated in humans with encouraging results.179,180 Other promising strategies to increase the safety profile of CAR T cells include splitting CARs into two domains, which need to be linked with a small molecule to be active,181,182 or taking advantage of the small molecule-assisted shut-off (SMAsh) technology.183 The third approach relies on expressing a molecule on the cell surface of T cells that can be targeted with a clinically approved mAb (e.g., CD20 and truncated EGFR).184,185 At present the clinical experience with these approaches is limited. Finally, the last approach relies on exploiting intrinsic vulnerabilities of T cells with a prime example being the tyrosine kinase inhibitor (TKI) dasatinib, which inhibits TCR and CAR signaling through inhibition of Lck.186
Improving CAR T Cell Fitness
As mentioned in the Design of CARs section, even outside the immunosuppressive TME, CAR T cells have a limited ability to produce cytokines, expand, and kill when recursively exposed to tumor cells. T cell exhaustion is a widely used term to describe this T cell response to chronic antigen exposure, and there is significant debate in the field how to best define T cell exhaustion.187 While traditionally cell surface markers, such as PD-1, LAG-3, TIM3, and TIGIT, have been used to define T cell exhaustion, more recent studies have highlighted that expression of transcription factors such as TOX or epigenetic programs might be more suitable to reliably define T cell exhaustion.100, 101, 102, 103,105 To improve the fitness of CAR T cells, investigators have focused on (1) providing additional signals to promote T cell activation/co-stimulation, (2) providing signal 3 by transgenic expression of cytokines or constitutively active cytokine receptors, (3) silencing or deleting molecules that restrict T cell activation, and (4) transgenic expression of transcription factors.
Provision of Additional Signals to Promote T Cell Activation/Co-stimulation
Standard CARs only provide co-stimulation, and not directly signal 3, a necessary signal for T cell expansion. To overcome this limitation, investigators have incorporated the truncated cytoplasmic domain of IL-2Rβ and a STAT3-binding YXXQ motif into CD28.ζ-CARs targeting CD19.188 Other groups have shown that T cells expressing CD28.ζ-CARs that incorporate the Toll/IL-1 receptor domain of Toll-like receptor (TLR)2 improve CAR T cell effector function.189 In both approaches, signals are provided simultaneously, which is in contrast to physiological T cell activation in which signals are provided in a temporospatial fashion. T cells express 41BB upon activation, and studies have demonstrated that expression of the 41BB ligand (41BBL) on the cell surface of CD28.ζ-CAR T cells endows CAR T cells with superior effector function in comparison to incorporating the 41BB signaling domain directly into the CAR.55,190 Other ligands of the TNF superfamily have also been expressed on the cell surface of CAR T cells, including CD40L.165 Lastly, investigators are actively exploring the activation of TLR pathways through inducible co-stimulatory molecules containing signaling domains of MyD88,69,191 the central signaling molecule of TLRs, IL-1β, and IL-18.192
Transgenic Expression of Cytokines or Constitutively Active Cytokine Receptors
Cytokines or Cytokine Receptors That Signal through the JAK/STAT Pathway
Common γ-cytokines (IL-2, IL-7, IL-15, IL-21) and IL--12 and IL23 activate the JAK/STAT signaling pathways in T cells. While common γ-cytokines signal through JAK1/JAK2, and predominantly STAT5, IL-12 and IL-23 signal through JAK2, TYK2, and STAT3 and/or STAT4.193, 194, 195 Transgenic expression of these cytokines, demonstrated in numerous preclinical studies, improves the ability of CAR T cells to expand and/or persist, resulting in improved antitumor activity.196, 197, 198 Investigators have not only explored secretory but also membrane-bound versions of cytokines, which might be advantageous since they have the potential to improve cytokine activity (e.g., IL-15) and also restrict most of the cytokine’s action to the genetically modified cell.199,200 To mitigate systemic side effects of secreted cytokines, investigators have engineered T cells in which cytokine expression is controlled by the nuclear factor of activated T cells (NFAT) promoter. However, while systemic side effects of IL-12 could be mitigated in preclinical studies, one clinical study with NFAT-IL-12-modified tumor-infiltrating lymphocytes (TILs) indicated that the NFAT promoter is insufficient to restrict gene expression to activated T cells.201 Another approach to reduce cytokine secretion of gene-modified CAR T cells is to use a construct in which the cytokine gene is placed 3′ prime of an internal ribosomal entry site (IRES).202 A clinical study with MUC16ecto-CAR/IRES-IL-12 T cells is in progress (ClinicalTrials.gov: NCT02498912). GD2-CAR T cells (ClinicalTrials.gov: NCT03721068) or GD2-CAR iNK T cells (ClinicalTrials.gov: NCT03294954) expressing IL-15 are also currently being evaluated in patients with neuroblastoma. In one of these studies (ClinicalTrials.gov: NCT03721068), CAR/IL-15 T cells are also modified with the iC9 safety switch to allow for the control of potential side effects. Alternative approaches to activate the JAK/STAT pathways are also being pursued, including expression of constitutively active cytokine receptors (e.g., constitutively active IL-7 receptor α [C7R]).68
Cytokines That Signal through MyD88
IL-1β and IL-18 signal through MyD88, and at present only transgenic expression of IL-18 has been explored by several groups of investigators. Consistently, IL-18 improved the effector function of CAR T cells not only in xenograft models but also in syngeneic murine models.163,203,204 However, in some models, administration of CAR/IL-18 T cells was associated with significant toxicities, which could be mitigated in one study by expressing IL-18 under the transcriptional control of the NFAT promoter.204
Silencing or Deletion of Molecules That Restrict T Cell Activation
T cells have developed an intricate system to limit their activation, and investigators have conducted unbiased screens to discover key molecules within this tightly regulated system. While the first screens relied on short hairpin RNA (shRNA) approaches identifying the phosphatase pp2r2d as a negative regulator of αβ TCR T cell activation in tumor models,205 more recent studies have taken advantage of CRISPR-Cas9 gene-editing technology.206 One recently published in vitro screen identified TCEB2, SOCS1, CBLB, and RASA2 as negative regulators of αβ TCR activation.206 An in vivo screen also has highlighted REGNASE1 as a key negative regulator, and REGNASE1 knockout CD19-CAR T cells had improved antitumor activity in a syngeneic leukemia model.207
Modulating Transcription Factors
Transcription factor networks are critical for T cell plasticity, and several recent studies have highlighted their importance. For example, c-Myb (1) is a transcriptional activator of TCF7, a transcription factor critical for memory formation, and (2) represses ZEB2, a transcription factor that promotes T cell differentiation.208 In addition, TOX has emerged as a key transcription factor that enforces T cell exhaustion, and investigators have shown that TOX- and TOX2-deficient murine CAR T cells have improved effector function.100, 101, 102,209 Other studies have highlighted that the nuclear receptor transcription factors NR4A1 (NUR77), NR4A2 (NURR1), and NR4A3 (NOR1) orchestrate transcription programs that promote T cell exhaustion and that deleting all three factors in CAR T cells is necessary for optimal function.210 Lastly, overexpression of c-Jun (transcription factor AP-1) in CAR T cells increases their functional capacities and reduces terminal T cell differentiation, resulting in improved antitumor activity.211
CAR T Cell Trafficking and Tumor Penetration
In hematological malignancies, CAR T cells and their corresponding target malignancies share hematopoietic origins, and thus have a higher propensity to migrate to similar areas such bone marrow or lymph nodes. In contrast, many solid tumors do not attract T cells. These tumors are called cold tumors, and they can be further subdivided into tumors that do not (cold, ignored) or have (cold, excluded) a T cell infiltrate in their periphery.212 T cell migration into tumors is influenced not only by chemokines and adhesion molecules, but also the tumor vasculature and the presence of other immune cells within the tumor.212, 213, 214 Recent studies have highlighted the role of tissue-resident memory T cells for cancer immunotherapies, which express a unique pattern of adhesion molecules and residency markers.215 Engineering approaches have so far mainly focused on taking advantage of chemokines that are secreted by tumor cells for which T cells do not express the corresponding chemokine receptor (e.g., CXCL8 by melanoma or CCL2 by neuroblastoma).216,217 In preclinical models, transgenic expression of chemokine receptors on tumor-specific T cells, including CAR T cells, has been shown to enhance their ability to home to tumor sites, resulting in improved antitumor activity,216,217 and this approach is being evaluated in a clinical study for the adoptive immunotherapy of TILs in melanoma (ClinicalTrials.gov: NCT01740557). Alternatively, combining the infusion of CAR T cells with the local delivery of an oncolytic adenovirus encoding RANTES and IL-15 has improved homing to and persistence of CAR T cells at tumor sites in preclinical models.218
The tumor stroma itself can be extremely dense with various cell types protecting the tumor. Targeting the extracellular matrix (ECM) and degrading these physically protective proteins has also shown promise preclinically. Heparin sulfate proteoglycans (HSPGs) are expressed in the ECM as well as neuronal tissue during development.219,220 Many cancer cells, especially neuroblastoma, express high levels of HSPGs. Expression of heparinase on CAR T cells to degrade HSPGs has demonstrated improved tumor infiltration and antitumor activity in vivo.221 However, the addition of ECM-degrading enzymes to standard chemotherapies has provided mixed results, with some clinical trials showing decreased survival in patients with the combination therapy.222
Counteracting the Immunosuppressive TME
Solid tumor cells are intermixed with suppressive cell populations such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), Tregs, and cancer-associated fibroblasts (CAFs) (Figure 3).223, 224, 225 Tumor cells and/or cells of the TME express a broad range of immune checkpoints, including PD-L1 and ligands for LAG-3, TIM-3, and TIGIT. MDSCs, Tregs, and/or CAFs also secrete immunosuppressive cytokines such as transforming growth factor (TGF)-β and IL-10.226,227 In addition, amino acids that are critical for T cell function such as arginine and glutamine are often depleted within the TME, contributing to limited T cell fitness (Figure 3).228,229 Some of the approaches that counteract the immunosuppressive microenvironment rely on strategies that were discussed in the section Improving CAR T Cell Fitness. For example, transgenic expression of IL-15 in cytotoxic T cells counteracts Treg-mediated suppression.230 Herein, we review additional strategies, including (1) directly counteracting immunosuppressive factors, (2) hijacking cytokines or growth factors to promote T cell effector function, (3) improving T cell metabolism in the TME, and (4) targeting non-malignant cells of the tumor stroma.
Figure 3.
Immunosuppressive Solid Tumor Microenvironment
Scheme of the solid tumor microenvironment that consists of (1) tumor vasculature; (2) stromal cells, including cancer-associated fibroblasts; (3) immunosuppressive cells, including myeloid cells, macrophages, and regulatory T cells (Tregs); (4) suppressive metabolites; (5) inhibitory ligands, including PD-1; and (6) suppressive cytokines. See text for additional details.
Directly Counteracting Immunosuppressive Factors
Several preclinical studies have shown that combining PD-1/PD-L1 blockade with CAR T cell therapy improves antitumor activity.231 In an effort to reduce side effects from systemic checkpoint blockade, CAR T cells were genetically modified to express a PD-1/CD28 switch or a truncated PD-1 receptor that functions as a dominant negative receptor (DNR).70,232 In addition, PD-1 was deleted in CAR T cells by CRISPR-Cas9 gene editing.233, 234, 235 All three approaches resulted in improved effector function of CAR T cells in preclinical models. Thus far the clinical experience with these approaches is limited, and only PD-1 knockout αβTCR T cells have been evaluated clinically.236 Other approaches include silencing CTLA-4 or FAS expression on the cell surface of tumor-specific or CAR T cells.237,238 More recently, investigators demonstrated that expression of a DNR FAS receptor also improved the effector function of adoptively transferred tumor-specific T cells.239
Hijacking Cytokines or Growth Factors to Promote T Cell Effector Function
The TME is rich with cytokines that either inhibit T cell function or for which T cells do not express the corresponding receptors. The inhibitory effects of these cytokines can be negated by expressing DNRs or cytokine switch receptors (CSRs) that convert an inhibitory signal into a growth-promoting signal. Examples of cytokine DNRs include DNR-TGF-β receptors.240 DNR-TGF-β-expressing EBV-specific T cells for EBV+ lymphoma have been evaluated in early phase clinical studies, demonstrating safety and suggesting improved efficacy in comparison to their unmodified counterparts.241 CSRs have been developed to take advantage of IL-4 produced within the TME, including IL-4/IL-2, IL-4/IL-7, and IL-4/IL-21 CSRs.242, 243, 244 In addition, a TGF-β CSR, consisting of a TGF-β-specific scFv and a CD28z signaling domain, has shown promise in preclinical CAR T cell therapy models.245 Of these, the IL-4/IL-2 CSR is currently being evaluated in one early phase clinical study in the context of TE1-CAR T cells (ClinicalTrials.gov: NCT01818323). Lastly, colony-stimulating factor-1 (CSF-1) is an example of a cytokine that is present in the TME and for which T cells do not express the cognate receptor. One preclinical study has shown that expression of CSF-1R in CAR T cells improves their effector function in a CSF-1-dependent manner.246
Improving Metabolic Fitness of CAR T Cells in the TME
The TME is hypoxic and deprived of critical nutrients that are required for T cell proliferation. In addition, the TME (1) is enriched in metabolic end products that are immunosuppressive, for example, R-2-hydroxyglutarate in tumors with isocitrate dehydrogenase 1/2 mutations;247, 248, 249 (2) has increased concentrations of electrolytes, such as potassium, that are immunosuppressive;250 and (3) harbors reactive oxygen species (ROS) that impair T cell function.251,252 Several strategies are being actively explored to improve the metabolic function of adoptively transferred T cells. These include ex vivo loading of tumor-specific T cells with arginine,228 or the genetic modification of CAR T cells with enzymes that are critical for arginine re-synthesis, including argininosuccinate synthase or ornithine transcarbamylase.253 Investigators have also focused on manipulating glutamine metabolism in the TME, and recent studies have highlighted that glutamine antagonists and transient inhibition of glutaminase increase T cell effector function.254,255 To protect CAR T cells from ROS, investigators have genetically modified T cells to secrete catalase. This not only protected T cells from ROS damage but also protected unmodified bystander immune cells.256 Lastly, small molecules such a metformin are being explored to reduce tumor hypoxia.257
Targeting Non-malignant Cells of the Tumor Stroma
CAFs, MDSCs, TAMs, and tumor-associated endothelial cells are present in the tumor stroma, and while not malignant, they promote tumor growth/metastasis and immunosuppression and/or secrete components to the ECM that hinder immune cell penetration. CAFs have been targeted by several groups with fibroblast activation protein (FAP)-CAR T cells. While FAP-CAR T cells had potent antitumor activity in preclinical models, some safety concerns were raised due to FAP expression on stromal cells in healthy tissues; nevertheless, this approach has been translated into the clinic.258, 259, 260, 261 TAMs have been targeted with CD123- or folate receptor β (FRβ)-CAR T cells in preclinical models,262,263 and CSF1R-CARs have been developed, opening up the possibility to target CSF1R-positive TAMs.264 MDSCs express high levels of NKG2D ligands, and in one preclinical model, combining NKG2D-CAR NK cells with GD2-CAR T cells resulted in improved antitumor activity for neuroblastoma.265 Lastly, endothelial cells of the tumor vasculature have been targeted with CARs specific for VEGF-R2, PSMA, or the EDB/EIIIB splice variant of fibronectin in preclinical models.266, 267, 268 Of these approaches, only VEGF-R2 CAR T cells have been successfully evaluated in one clinical study. While the results have not been published, they are available (ClinicalTrials.gov: NCT01218867), and out of 23 infused patients, only 1 patient achieved a partial response.
Combinatorial Therapies
While this review has focused on additional genetic modification to improve the effector function of CAR T cells, numerous studies are underway to combine CAR T cell therapy with other treatment modalities to improve outcomes by creating therapeutic synergies.269,270 These include efforts, as already mentioned in this review, to combine CAR T cell therapy with cytokine administration, checkpoint blockade, oncolytic viruses, radiation, and/or vaccines. Examples for oncolytics/CAR T cell therapy combinations include the administration of oncolytic viruses encoding BiTEs, cytokines, and/or checkpoint inhibitors,271, 272, 273 or oncolytic viruses that encode CAR targets.274,275 In addition, investigators have explored the use of T cells to directly deliver viruses into tumors.276, 277, 278 Checkpoint blockade/CAR T cell therapy combinations are actively being pursued,279 including the aforementioned clinical studies combining mesothelin-CAR T cells with pembrolizumab for the treatment of mesothelioma.76 Furthermore, several studies have highlighted the benefit of combining radiotherapy with the administration of CAR T cells, and early clinical studies for patients with B cell lymphoma have produced encouraging results.280 Recent preclinical studies have also demonstrated the benefit of boosting CAR T cells outside the immunosuppressive TME with amphiphile CAR ligands or RNA-based vaccines.281,282
Other approaches include combining CAR T cells with small molecules that target cancer cells that do not affect CAR T cells, with the best example being combining the BTK inhibitor ibrutinib with CD19-CAR T cell therapies for lymphoma.283 In addition, combining CAR T cells with chemotherapeutic agents that upregulate the expression of the targeted antigens is an attractive option to enhance the antitumor activity of CAR T cells that is actively being pursued.284 Finally, combinational treatment of HER2-CAR T cells with the smac-mimetic birinapant, which promotes apoptosis, significantly enhanced antitumor activity in a TNF-dependent manner.285 Thus, there is a myriad of combinatorial approaches, and selection of an optimal combination will most likely depend on the targeted tumor type and disease status. For example, brain tumors, which often present as local disease or with limited sites of recurrences, might be ideal tumors to combine local radiation or delivery of an oncolytic virus with CAR T cell therapy. In contrast, combining local delivery of agents with CAR T cell therapy for metastatic cancer should only be pursued if treating selected metastases induces an abscopal effect,286 leading to the regression of distant, untreated metastases. Feasibility and costs are also a major consideration; in this regard, repurposing FDA-approved drugs for combinatorial CAR T cell therapy should actively be explored as for other cancer therapeutics.287,288
Conclusions
CAR T cells for solid tumors so far have limited activity in early phase clinical studies, raising concerns of whether CAR T cells can be developed into a curative therapeutic approach in the future. While currently ineffective, clinical and preclinical studies have identified roadblocks, including (1) an antigen dilemma, (2) T cell fitness and survival before reaching the tumor site, (3) inability of T cells to efficiently traffic to tumor sites and penetrate physical barriers, and (4) an immunosuppressive TME. In the last decade, investigators have developed elegant solutions and/or countermeasures in preclinical studies, engineering CAR T cells with significantly improved effector function and/or combining them with other treatment modalities. Thus, we strongly believe that the future of CAR T cells for solid tumors is bright, and they will not become a mere footnote in the history of cancer immunotherapy. A key challenge will be how to prioritize and streamline the clinical evaluation of the developed approaches. In addition, there is a continued need to advance our understanding in basic CAR T cell biology and how cancer cells and resident immune cells interact with adoptively transferred CAR T cells. Lastly, future CAR T cell design might benefit from considering the “ten principles for good design” that were put forward by the eminent industrial designer Dieter Rams:289 good design (1) is innovative, (2) makes a product useful, (3) is aesthetic, (4) makes a product understandable, (5) is unobtrusive, (6) is honest, (7) is long-lasting, (8) is thorough down to the last detail, (9) is environmentally friendly, and (10) involves as little design as possible.
Author Contributions
J.W., E.W., and S.G. wrote the manuscript. All authors (J.W., E.W., C.D., and S.G.) reviewed and edited the manuscript.
Conflicts of Interest
J.W., C.D., and S.G. have patent applications in the fields of T cell and/or gene therapy for cancer. S.G. has a research collaboration with TESSA Therapeutics, is a DSMB member of Immatics, and is on the Scientific Advisory Board of Tidal.
Acknowledgments
The work was supported by National Institutes of Health grant R01NS106379 (to S.G.); the Alex's Lemonade Stand Foundation and the Cure4Cam Foundation (ALSF; Young Investigator Grant; to J.W.); the Alliance for Cancer Gene Therapy (ACGT; to S.G.); and by the American Lebanese Syrian Associated Charities (to C.D. and S.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Figures were created with BioRender.
References
- 1.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner R.A., Finney O., Annesley C., Brakke H., Summers C., Leger K., Bleakley M., Brown C., Mgebroff S., Kelly-Spratt K.S. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos C.A., Grover N.S., Beaven A.W., Lulla P.D., Wu M.F., Ivanova A., Wang T., Shea T.C., Rooney C.M., Dittus C. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J. Clin. Oncol. 2020 doi: 10.1200/JCO.20.01342. Published online July 23, 2020. 10.1200/JCO.20.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos C.A., Savoldo B., Torrano V., Ballard B., Zhang H., Dakhova O., Liu E., Carrum G., Kamble R.T., Gee A.P. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Invest. 2016;126:2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raje N., Berdeja J., Lin Y., Siegel D., Jagannath S., Madduri D., Liedtke M., Rosenblatt J., Maus M.V., Turka A. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer J.N., Somerville R.P.T., Lu T., Yang J.C., Sherry R.M., Feldman S.A., McIntyre L., Bot A., Rossi J., Lam N., Rosenberg S.A. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol. Ther. 2017;25:2245–2253. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holstein S.A., Lunning M.A. CAR T-cell therapy in hematologic malignancies: a voyage in progress. Clin. Pharmacol. Ther. 2020;107:112–122. doi: 10.1002/cpt.1674. [DOI] [PubMed] [Google Scholar]
- 12.Boyiadzis M.M., Dhodapkar M.V., Brentjens R.J., Kochenderfer J.N., Neelapu S.S., Maus M.V., Porter D.L., Maloney D.G., Grupp S.A., Mackall C.L. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J. Immunother. Cancer. 2018;6:137. doi: 10.1186/s40425-018-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majzner R.G., Mackall C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019;25:1341–1355. doi: 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
- 14.Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Locke F.L., Lin Y., Jain N., Daver N., Gulbis A.M., Adkins S. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit “ALL”. Nat. Rev. Clin. Oncol. 2018;15:218. doi: 10.1038/nrclinonc.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D.W., Santomasso B.D., Locke F.L., Ghobadi A., Turtle C.J., Brudno J.N., Maus M.V., Park J.H., Mead E., Pavletic S. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goff S.L., Morgan R.A., Yang J.C., Sherry R.M., Robbins P.F., Restifo N.P., Feldman S.A., Lu Y.C., Lu L., Zheng Z. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J. Immunother. 2019;42:126–135. doi: 10.1097/CJI.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Rourke D.M., Nasrallah M.P., Desai A., Melenhorst J.J., Mansfield K., Morrissette J.J.D., Martinez-Lage M., Brem S., Maloney E., Shen A. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaa0984. eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers C.H., Sleijfer S., Vulto A.G., Kruit W.H., Kliffen M., Debets R., Gratama J.W., Stoter G., Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 20.Lamers C.H., Sleijfer S., van Steenbergen S., van Elzakker P., van Krimpen B., Groot C., Vulto A., den Bakker M., Oosterwijk E., Debets R., Gratama J.W. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kershaw M.H., Westwood J.A., Parker L.L., Wang G., Eshhar Z., Mavroukakis S.A., White D.E., Wunderlich J.R., Canevari S., Rogers-Freezer L. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Chen M., Wu Z., Tong C., Dai H., Guo Y., Liu Y., Huang J., Lv H., Luo C. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. OncoImmunology. 2018;7:e1440169. doi: 10.1080/2162402X.2018.1440169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thistlethwaite F.C., Gilham D.E., Guest R.D., Rothwell D.G., Pillai M., Burt D.J., Byatte A.J., Kirillova N., Valle J.W., Sharma S.K. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 2017;66:1425–1436. doi: 10.1007/s00262-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed N., Brawley V., Hegde M., Bielamowicz K., Kalra M., Landi D., Robertson C., Gray T.L., Diouf O., Wakefield A. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 2017;3:1094–1101. doi: 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Garcia A., Palazon A., Noguera-Ortega E., Powell D.J., Jr., Guedan S. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front. Immunol. 2020;11:1109. doi: 10.3389/fimmu.2020.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidts A., Maus M.V. Making CAR T cells a solid option for solid tumors. Front. Immunol. 2018;9:2593. doi: 10.3389/fimmu.2018.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knochelmann H.M., Smith A.S., Dwyer C.J., Wyatt M.M., Mehrotra S., Paulos C.M. CAR T cells in solid tumors: blueprints for building effective therapies. Front. Immunol. 2018;9:1740. doi: 10.3389/fimmu.2018.01740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Depil S., Duchateau P., Grupp S.A., Mufti G., Poirot L. “Off-the-shelf” allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 2020;19:185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 32.Qasim W. Allogeneic CAR T cell therapies for leukemia. Am. J. Hematol. 2019;94(S1):S50–S54. doi: 10.1002/ajh.25399. [DOI] [PubMed] [Google Scholar]
- 33.Frey N., Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol. Blood Marrow Transplant. 2019;25:e123–e127. doi: 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 34.Kuwana Y., Asakura Y., Utsunomiya N., Nakanishi M., Arata Y., Itoh S., Nagase F., Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 35.Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.June C.H., Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dotti G., Gottschalk S., Savoldo B., Brenner M.K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaffer D.R., Savoldo B., Yi Z., Chow K.K., Kakarla S., Spencer D.M., Dotti G., Wu M.F., Liu H., Kenney S., Gottschalk S. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011;117:4304–4314. doi: 10.1182/blood-2010-04-278218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies D.M., Foster J., Van Der Stegen S.J., Parente-Pereira A.C., Chiapero-Stanke L., Delinassios G.J., Burbridge S.E., Kao V., Liu Z., Bosshard-Carter L. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol. Med. 2012;18:565–576. doi: 10.2119/molmed.2011.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Q., Garber H.R., Lu S., He H., Tallis E., Ding X., Sergeeva A., Wood M.S., Dotti G., Salvado B. A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy. 2016;18:985–994. doi: 10.1016/j.jcyt.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafiq S., Purdon T.J., Daniyan A.F., Koneru M., Dao T., Liu C., Scheinberg D.A., Brentjens R.J. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms tumor 1 antigen. Leukemia. 2017;31:1788–1797. doi: 10.1038/leu.2016.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maus M.V., Plotkin J., Jakka G., Stewart-Jones G., Rivière I., Merghoub T., Wolchok J., Renner C., Sadelain M. An MHC-restricted antibody-based chimeric antigen receptor requires TCR-like affinity to maintain antigen specificity. Mol. Ther. Oncolytics. 2017;3:1–9. doi: 10.1038/mto.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Töpfer K., Kempe S., Müller N., Schmitz M., Bachmann M., Cartellieri M., Schackert G., Temme A. Tumor evasion from T cell surveillance. J. Biomed. Biotechnol. 2011;2011:918471. doi: 10.1155/2011/918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rafiq S., Yeku O.O., Jackson H.J., Purdon T.J., van Leeuwen D.G., Drakes D.J., Song M., Miele M.M., Li Z., Wang P. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018;36:847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hombach A.A., Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int. J. Cancer. 2011;129:2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- 47.Collinson-Pautz M.R., Chang W.C., Lu A., Khalil M., Crisostomo J.W., Lin P.Y., Mahendravada A., Shinners N.P., Brandt M.E., Zhang M. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia. 2019;33:2195–2207. doi: 10.1038/s41375-019-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guedan S., Chen X., Madar A., Carpenito C., McGettigan S.E., Frigault M.J., Lee J., Posey A.D., Jr., Scholler J., Scholler N. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nair S., Wang J.B., Tsao S.T., Liu Y., Zhu W., Slayton W.B., Moreb J.S., Dong L., Chang L.J. Functional improvement of chimeric antigen receptor through intrinsic interleukin-15Rα signaling. Curr. Gene Ther. 2019;19:40–53. doi: 10.2174/1566523218666181116093857. [DOI] [PubMed] [Google Scholar]
- 50.Salter A.I., Ivey R.G., Kennedy J.J., Voillet V., Rajan A., Alderman E.J., Voytovich U.J., Lin C., Sommermeyer D., Liu L. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 2018;11:eaat6753. doi: 10.1126/scisignal.aat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boroughs A.C., Larson R.C., Marjanovic N.D., Gosik K., Castano A.P., Porter C.B.M., Lorrey S.J., Ashenberg O., Jerby L., Hofree M. A distinct transcriptional program in human CAR T cells bearing the 4-1BB signaling domain revealed by scRNA-seq. Mol. Ther. 2020 doi: 10.1016/j.ymthe.2020.07.023. Published online July 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xhangolli I., Dura B., Lee G., Kim D., Xiao Y., Fan R. Single-cell analysis of CAR-T cell activation reveals a mixed TH1/TH2 response independent of differentiation. Genomics Proteomics Bioinformatics. 2019;17:129–139. doi: 10.1016/j.gpb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawalekar O.U., O’ Connor R.S., Fraietta J.A., Guo L., McGettigan S.E., Posey A.D., Jr., Patel P.R., Guedan S., Scholler J., Keith B. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44:712. doi: 10.1016/j.immuni.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Quintarelli C., Orlando D., Boffa I., Guercio M., Polito V.A., Petretto A., Lavarello C., Sinibaldi M., Weber G., Del Bufalo F. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. OncoImmunology. 2018;7:e1433518. doi: 10.1080/2162402X.2018.1433518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Z., Condomines M., van der Stegen S.J.C., Perna F., Kloss C.C., Gunset G., Plotkin J., Sadelain M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramos C.A., Rouce R., Robertson C.S., Reyna A., Narala N., Vyas G., Mehta B., Zhang H., Dakhova O., Carrum G. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol. Ther. 2018;26:2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guest R.D., Hawkins R.E., Kirillova N., Cheadle E.J., Arnold J., O’Neill A., Irlam J., Chester K.A., Kemshead J.T., Shaw D.M. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J. Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 58.Majzner R.G., Rietberg S.P., Sotillo E., Dong R., Vachharajani V.T., Labanieh L., Myklebust J.H., Kadapakkam M., Weber E.W., Tousley A.M. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hudecek M., Lupo-Stanghellini M.T., Kosasih P.L., Sommermeyer D., Jensen M.C., Rader C., Riddell S.R. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.James S.E., Greenberg P.D., Jensen M.C., Lin Y., Wang J., Till B.G., Raubitschek A.A., Forman S.J., Press O.W. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 2008;180:7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feucht J., Sun J., Eyquem J., Ho Y.J., Zhao Z., Leibold J., Dobrin A., Cabriolu A., Hamieh M., Sadelain M. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 2019;25:82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wijewarnasuriya D., Bebernitz C., Lopez A.V., Rafiq S., Brentjens R.J. Excessive costimulation leads to dysfunction of adoptively transferred T cells. Cancer Immunol. Res. 2020;8:732–742. doi: 10.1158/2326-6066.CIR-19-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guedan S., Madar A., Casado-Medrano V., Shaw C., Wing A., Liu F., Young R.M., June C.H., Posey A.D., Jr. Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability. J. Clin. Invest. 2020;130:3087–3097. doi: 10.1172/JCI133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomes-Silva D., Mukherjee M., Srinivasan M., Krenciute G., Dakhova O., Zheng Y., Cabral J.M.S., Rooney C.M., Orange J.S., Brenner M.K., Mamonkin M. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 2017;21:17–26. doi: 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong W., Chen Y., Kang X., Chen Z., Zheng P., Hsu Y.H., Jang J.H., Qin L., Liu H., Dotti G., Liu D. Immunological synapse predicts effectiveness of chimeric antigen receptor cells. Mol. Ther. 2018;26:963–975. doi: 10.1016/j.ymthe.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davenport A.J., Cross R.S., Watson K.A., Liao Y., Shi W., Prince H.M., Beavis P.A., Trapani J.A., Kershaw M.H., Ritchie D.S. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA. 2018;115:E2068–E2076. doi: 10.1073/pnas.1716266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shum T., Omer B., Tashiro H., Kruse R.L., Wagner D.L., Parikh K., Yi Z., Sauer T., Liu D., Parihar R. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 2017;7:1238–1247. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mata M., Gerken C., Nguyen P., Krenciute G., Spencer D.M., Gottschalk S. Inducible activation of MyD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov. 2017;7:1306–1319. doi: 10.1158/2159-8290.CD-17-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cherkassky L., Morello A., Villena-Vargas J., Feng Y., Dimitrov D.S., Jones D.R., Sadelain M., Adusumilli P.S. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Junghans R.P., Ma Q., Rathore R., Gomes E.M., Bais A.J., Lo A.S., Abedi M., Davies R.A., Cabral H.J., Al-Homsi A.S., Cohen S.I. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate. 2016;76:1257–1270. doi: 10.1002/pros.23214. [DOI] [PubMed] [Google Scholar]
- 72.Specht J.M., Lee S., Turtle C., Berger C., Veatch J., Gooley T., Mullane E., Chaney C., Riddell S., Maloney D.G. Phase I study of immunotherapy for advanced ROR1+ malignancies with autologous ROR1-specific chimeric antigen receptor-modified (CAR)-T cells. J. Clin. Oncol. 2018;36(Suppl):TPS79. [Google Scholar]
- 73.Tang X., Liu F., Liu Z., Cao Y., Zhang Z., Wang Y., Huang J., Fan S., Zhao S., Chen Y. Bioactivity and safety of B7-H3-targeted chimeric antigen receptor T cells against anaplastic meningioma. Clin. Transl. Immunology. 2020;9:e1137. doi: 10.1002/cti2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown C.E., Badie B., Barish M.E., Weng L., Ostberg J.R., Chang W.C., Naranjo A., Starr R., Wagner J., Wright C. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keu K.V., Witney T.H., Yaghoubi S., Rosenberg J., Kurien A., Magnusson R., Williams J., Habte F., Wagner J.R., Forman S. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci. Transl. Med. 2017;9:eaag2196. doi: 10.1126/scitranslmed.aag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adusumilli P.S., Zauderer M.G., Rusch V.W., O’Cearbhaill R.E., Zhu A., Ngai D.A., McGee E., Chintala N.K., Messinger J.C., Vincent A. A phase I clinical trial of malignant pleural disease treated with regionally delivered autologous mesothelin-targeted CAR T cells: safety and efficacy. Cancer Res. 2019;79(Suppl):CT026. [Google Scholar]
- 77.Hegde M.,D.C., Zhang H., Mata M., Gerken C., Shree A., Yi Z., Brawley V., Dakhova O., Wu M.-F. Expansion of HER2-CAR T cells after lymphodepletion and clinical responses in patients with advanced sarcoma. J. Clin. Oncol. 2017;35(15 Suppl) 10508–10508. [Google Scholar]
- 78.Kebriaei P., Singh H., Huls M.H., Figliola M.J., Bassett R., Olivares S., Jena B., Dawson M.J., Kumaresan P.R., Su S. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Invest. 2016;126:3363–3376. doi: 10.1172/JCI86721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu A.X., Gold P.J., El-Khoueiry A.B., Abrams T.A., Morikawa H., Ohishi N., Ohtomo T., Philip P.A. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2013;19:920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 80.Beatty G.L., O’Hara M.H., Lacey S.F., Torigian D.A., Nazimuddin F., Chen F., Kulikovskaya I.M., Soulen M.C., McGarvey M., Nelson A.M. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., Huls M.H., Liu E., Gee A.P., Mei Z. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D., Rossig C., Russell H.V., Diouf O., Liu E. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park J.R., Digiusto D.L., Slovak M., Wright C., Naranjo A., Wagner J., Meechoovet H.B., Bautista C., Chang W.C., Ostberg J.R., Jensen M.C. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 84.Ao X., Yang Y., Li W., Tan Y., Guo W., Ao L., He X., Wu X., Xia J., Xu X., Guo J. Anti-αFR CAR-engineered NK-92 cells display potent cytotoxicity against αFR-positive ovarian cancer. J. Immunother. 2019;42:284–296. doi: 10.1097/CJI.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown C.E., Aguilar B., Starr R., Yang X., Chang W.C., Weng L., Chang B., Sarkissian A., Brito A., Sanchez J.F. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol. Ther. 2018;26:31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y., Guo Y., Wu Z., Feng K., Tong C., Wang Y., Dai H., Shi F., Yang Q., Han W. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial. Cytotherapy. 2020;22:P573–P580. doi: 10.1016/j.jcyt.2020.04.088. [DOI] [PubMed] [Google Scholar]
- 87.Heczey A., Louis C.U., Savoldo B., Dakhova O., Durett A., Grilley B., Liu H., Wu M.F., Mei Z., Gee A. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 2017;25:2214–2224. doi: 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi D., Shi Y., Kaseb A.O., Qi X., Zhang Y., Chi J., Lu Q., Gao H., Jiang H., Wang H. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin. Cancer Res. 2020;26:3979–3989. doi: 10.1158/1078-0432.CCR-19-3259. [DOI] [PubMed] [Google Scholar]
- 89.Navai S.A., Derenzo C., Joseph S., Sanber K., Byrd T., Zhang H., Mata M., Gerken C., Shree A., Mathew P.R. Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Res. 2019;79(Suppl) LB-147. [Google Scholar]
- 90.Hegde M., Joseph S.K., Pashankar F., DeRenzo C., Sanber K., Navai S., Byrd T.T., Hicks J., Xu M.L., Gerken C. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 2020;11:3549. doi: 10.1038/s41467-020-17175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haas A.R., Tanyi J.L., O’Hara M.H., Gladney W.L., Lacey S.F., Torigian D.A., Soulen M.C., Tian L., McGarvey M., Nelson A.M. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 2019;27:1919–1929. doi: 10.1016/j.ymthe.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.You F., Jiang L., Zhang B., Lu Q., Zhou Q., Liao X., Wu H., Du K., Zhu Y., Meng H. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci. China Life Sci. 2016;59:386–397. doi: 10.1007/s11427-016-5024-7. [DOI] [PubMed] [Google Scholar]
- 93.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andersch L., Radke J., Klaus A., Schwiebert S., Winkler A., Schumann E., Grunewald L., Zirngibl F., Flemmig C., Jensen M.C. CD171- and GD2-specific CAR-T cells potently target retinoblastoma cells in preclinical in vitro testing. BMC Cancer. 2019;19:895. doi: 10.1186/s12885-019-6131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaech S.M., Wherry E.J., Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 96.Danilo M., Chennupati V., Silva J.G., Siegert S., Held W. Suppression of Tcf1 by inflammatory cytokines facilitates effector CD8 T cell differentiation. Cell Rep. 2018;22:2107–2117. doi: 10.1016/j.celrep.2018.01.072. [DOI] [PubMed] [Google Scholar]
- 97.Kim E.H., Sullivan J.A., Plisch E.H., Tejera M.M., Jatzek A., Choi K.Y., Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J. Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rutishauser R.L., Martins G.A., Kalachikov S., Chandele A., Parish I.A., Meffre E., Jacob J., Calame K., Kaech S.M. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Joshi N.S., Cui W., Chandele A., Lee H.K., Urso D.R., Hagman J., Gapin L., Kaech S.M. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alfei F., Kanev K., Hofmann M., Wu M., Ghoneim H.E., Roelli P., Utzschneider D.T., von Hoesslin M., Cullen J.G., Fan Y. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–269. doi: 10.1038/s41586-019-1326-9. [DOI] [PubMed] [Google Scholar]
- 101.Scott A.C., Dündar F., Zumbo P., Chandran S.S., Klebanoff C.A., Shakiba M., Trivedi P., Menocal L., Appleby H., Camara S. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan O., Giles J.R., McDonald S., Manne S., Ngiow S.F., Patel K.P., Werner M.T., Huang A.C., Alexander K.A., Wu J.E. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghoneim H.E., Fan Y., Moustaki A., Abdelsamed H.A., Dash P., Dogra P., Carter R., Awad W., Neale G., Thomas P.G., Youngblood B. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell. 2017;170:142–157.e19. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Youngblood B., Hale J.S., Kissick H.T., Ahn E., Xu X., Wieland A., Araki K., West E.E., Ghoneim H.E., Fan Y. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552:404–409. doi: 10.1038/nature25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zebley C.C., Gottschalk S., Youngblood B. Rewriting history: epigenetic reprogramming of CD8+ T cell differentiation to enhance immunotherapy. Trends Immunol. 2020;41:665–675. doi: 10.1016/j.it.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fraietta J.A., Nobles C.L., Sammons M.A., Lundh S., Carty S.A., Reich T.J., Cogdill A.P., Morrissette J.J.D., DeNizio J.E., Reddy S. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dwyer C.J., Knochelmann H.M., Smith A.S., Wyatt M.M., Rangel Rivera G.O., Arhontoulis D.C., Bartee E., Li Z., Rubinstein M.P., Paulos C.M. Fueling cancer immunotherapy with common gamma chain cytokines. Front. Immunol. 2019;10:263. doi: 10.3389/fimmu.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu Y., Zhang M., Ramos C.A., Durett A., Liu E., Dakhova O., Liu H., Creighton C.J., Gee A.P., Heslop H.E. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hinrichs C.S., Spolski R., Paulos C.M., Gattinoni L., Kerstann K.W., Palmer D.C., Klebanoff C.A., Rosenberg S.A., Leonard W.J., Restifo N.P. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y., Bleakley M., Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J. Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 111.Muralidharan S., Hanley P.J., Liu E., Chakraborty R., Bollard C., Shpall E., Rooney C., Savoldo B., Rodgers J., Dotti G. Activation of Wnt signaling arrests effector differentiation in human peripheral and cord blood-derived T lymphocytes. J. Immunol. 2011;187:5221–5232. doi: 10.4049/jimmunol.1101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zheng W., O’Hear C.E., Alli R., Basham J.H., Abdelsamed H.A., Palmer L.E., Jones L.L., Youngblood B., Geiger T.L. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia. 2018;32:1157–1167. doi: 10.1038/s41375-017-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fraietta J.A., Lacey S.F., Orlando E.J., Pruteanu-Malinici I., Gohil M., Lundh S., Boesteanu A.C., Wang Y., O’Connor R.S., Hwang W.T. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Finney O.C., Brakke H.M., Rawlings-Rhea S., Hicks R., Doolittle D., Lopez M., Futrell R.B., Orentas R.J., Li D., Gardner R.A., Jensen M.C. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J. Clin. Invest. 2019;129:2123–2132. doi: 10.1172/JCI125423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sheih A., Voillet V., Hanafi L.A., DeBerg H.A., Yajima M., Hawkins R., Gersuk V., Riddell S.R., Maloney D.G., Wohlfahrt M.E. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy. Nat. Commun. 2020;11:219. doi: 10.1038/s41467-019-13880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nobles C.L., Sherrill-Mix S., Everett J.K., Reddy S., Fraietta J.A., Porter D.L., Frey N., Gill S.I., Grupp S.A., Maude S.L. CD19-targeting CAR T cell immunotherapy outcomes correlate with genomic modification by vector integration. J. Clin. Invest. 2020;130:673–685. doi: 10.1172/JCI130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cornetta K., Duffy L., Turtle C.J., Jensen M., Forman S., Binder-Scholl G., Fry T., Chew A., Maloney D.G., June C.H. Absence of replication-competent lentivirus in the clinic: analysis of infused T cell products. Mol. Ther. 2018;26:280–288. doi: 10.1016/j.ymthe.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marcucci K.T., Jadlowsky J.K., Hwang W.T., Suhoski-Davis M., Gonzalez V.E., Kulikovskaya I., Gupta M., Lacey S.F., Plesa G., Chew A. Retroviral and lentiviral safety analysis of gene-modified T cell products and infused HIV and oncology patients. Mol. Ther. 2018;26:269–279. doi: 10.1016/j.ymthe.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bear A.S., Morgan R.A., Cornetta K., June C.H., Binder-Scholl G., Dudley M.E., Feldman S.A., Rosenberg S.A., Shurtleff S.A., Rooney C.M. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol. Ther. 2012;20:246–249. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh H., Huls H., Kebriaei P., Cooper L.J. A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19. Immunol. Rev. 2014;257:181–190. doi: 10.1111/imr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morita D., Nishio N., Saito S., Tanaka M., Kawashima N., Okuno Y., Suzuki S., Matsuda K., Maeda Y., Wilson M.H. Enhanced expression of anti-CD19 chimeric antigen receptor in piggyBac transposon-engineered T cells. Mol. Ther. Methods Clin. Dev. 2017;8:131–140. doi: 10.1016/j.omtm.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bailey S.R., Maus M.V. Gene editing for immune cell therapies. Nat. Biotechnol. 2019;37:1425–1434. doi: 10.1038/s41587-019-0137-8. [DOI] [PubMed] [Google Scholar]
- 123.Roth T.L., Puig-Saus C., Yu R., Shifrut E., Carnevale J., Li P.J., Hiatt J., Saco J., Krystofinski P., Li H. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–409. doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hale M., Lee B., Honaker Y., Leung W.H., Grier A.E., Jacobs H.M., Sommer K., Sahni J., Jackson S.W., Scharenberg A.M. Homology-directed recombination for enhanced engineering of chimeric antigen receptor T cells. Mol. Ther. Methods Clin. Dev. 2017;4:192–203. doi: 10.1016/j.omtm.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bowers J.S., Nelson M.H., Majchrzak K., Bailey S.R., Rohrer B., Kaiser A.D., Atkinson C., Gattinoni L., Paulos C.M. Th17 cells are refractory to senescence and retain robust antitumor activity after long-term ex vivo expansion. JCI Insight. 2017;2:e90772. doi: 10.1172/jci.insight.90772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klebanoff C.A., Gattinoni L., Restifo N.P. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J. Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blaeschke F., Stenger D., Kaeuferle T., Willier S., Lotfi R., Kaiser A.D., Assenmacher M., Döring M., Feucht J., Feuchtinger T. Induction of a central memory and stem cell memory phenotype in functionally active CD4+ and CD8+ CAR T cells produced in an automated good manufacturing practice system for the treatment of CD19+ acute lymphoblastic leukemia. Cancer Immunol. Immunother. 2018;67:1053–1066. doi: 10.1007/s00262-018-2155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schmueck-Henneresse M., Omer B., Shum T., Tashiro H., Mamonkin M., Lapteva N., Sharma S., Rollins L., Dotti G., Reinke P. Comprehensive approach for identifying the T cell subset origin of CD3 and CD28 antibody-activated chimeric antigen receptor-modified T cells. J. Immunol. 2017;199:348–362. doi: 10.4049/jimmunol.1601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guedan S., Posey A.D., Jr., Shaw C., Wing A., Da T., Patel P.R., McGettigan S.E., Casado-Medrano V., Kawalekar O.U., Uribe-Herranz M. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3:e96976. doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Costa Del Amo P., Lahoz-Beneytez J., Boelen L., Ahmed R., Miners K.L., Zhang Y., Roger L., Jones R.E., Marraco S.A.F., Speiser D.E. Human TSCM cell dynamics in vivo are compatible with long-lived immunological memory and stemness. PLoS Biol. 2018;16:e2005523. doi: 10.1371/journal.pbio.2005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Das R.K., Vernau L., Grupp S.A., Barrett D.M. Naïve T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov. 2019;9:492–499. doi: 10.1158/2159-8290.CD-18-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rozenbaum M., Meir A., Aharony Y., Itzhaki O., Schachter J., Bank I., Jacoby E., Besser M.J. Gamma-delta CAR-T cells show CAR-directed and independent activity against leukemia. Front. Immunol. 2020;11:1347. doi: 10.3389/fimmu.2020.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heczey A., Liu D., Tian G., Courtney A.N., Wei J., Marinova E., Gao X., Guo L., Yvon E., Hicks J. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124:2824–2833. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reilly E.B., Phillips A.C., Buchanan F.G., Kingsbury G., Zhang Y., Meulbroek J.A., Cole T.B., DeVries P.J., Falls H.D., Beam C. Characterization of ABT-806, a humanized tumor-specific anti-EGFR monoclonal antibody. Mol. Cancer Ther. 2015;14:1141–1151. doi: 10.1158/1535-7163.MCT-14-0820. [DOI] [PubMed] [Google Scholar]
- 136.Panousis C., Rayzman V.M., Johns T.G., Renner C., Liu Z., Cartwright G., Lee F.T., Wang D., Gan H., Cao D. Engineering and characterisation of chimeric monoclonal antibody 806 (ch806) for targeted immunotherapy of tumours expressing de2-7 EGFR or amplified EGFR. Br. J. Cancer. 2005;92:1069–1077. doi: 10.1038/sj.bjc.6602470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Z., Cao Y.J. Adoptive cell therapy targeting neoantigens: a frontier for cancer research. Front. Immunol. 2020;11:176. doi: 10.3389/fimmu.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abbott R.C., Cross R.S., Jenkins M.R. Finding the keys to the CAR: identifying novel target antigens for T cell redirection immunotherapies. Int. J. Mol. Sci. 2020;21:515. doi: 10.3390/ijms21020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Orentas R.J., Yang J.J., Wen X., Wei J.S., Mackall C.L., Khan J. Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front. Oncol. 2012;2:194. doi: 10.3389/fonc.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bosse K.R., Raman P., Zhu Z., Lane M., Martinez D., Heitzeneder S., Rathi K.S., Kendsersky N.M., Randall M., Donovan L. Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell. 2017;32:295–309.e12. doi: 10.1016/j.ccell.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu X., Jiang S., Fang C., Yang S., Olalere D., Pequignot E.C., Cogdill A.P., Li N., Ramones M., Granda B. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Richman S.A., Milone M.C. Neurotoxicity associated with a high-affinity GD2 CAR-response. Cancer Immunol. Res. 2018;6:496–497. doi: 10.1158/2326-6066.CIR-18-0090. [DOI] [PubMed] [Google Scholar]
- 143.Priceman S.J., Gerdts E.A., Tilakawardane D., Kennewick K.T., Murad J.P., Park A.K., Jeang B., Yamaguchi Y., Yang X., Urak R. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. OncoImmunology. 2017;7:e1380764. doi: 10.1080/2162402X.2017.1380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Watanabe N., Bajgain P., Sukumaran S., Ansari S., Heslop H.E., Rooney C.M., Brenner M.K., Leen A.M., Vera J.F. Fine-tuning the CAR spacer improves T-cell potency. OncoImmunology. 2016;5:e1253656. doi: 10.1080/2162402X.2016.1253656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dagogo-Jack I., Shaw A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 146.Ahmed N., Salsman V.S., Kew Y., Shaffer D., Powell S., Zhang Y.J., Grossman R.G., Heslop H.E., Gottschalk S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin. Cancer Res. 2010;16:474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vora P., Venugopal C., Salim S.K., Tatari N., Bakhshinyan D., Singh M., Seyfrid M., Upreti D., Rentas S., Wong N. The rational development of CD133-targeting immunotherapies for glioblastoma. Cell Stem Cell. 2020;26:832–844.e6. doi: 10.1016/j.stem.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 148.Alhabbab R.Y. Targeting cancer stem cells by genetically engineered chimeric antigen receptor T cells. Front. Genet. 2020;11:312. doi: 10.3389/fgene.2020.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Velasquez M.P., Bonifant C.L., Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131:30–38. doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qin H., Ramakrishna S., Nguyen S., Fountaine T.J., Ponduri A., Stetler-Stevenson M., Yuan C.M., Haso W., Shern J.F., Shah N.N., Fry T.J. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol. Ther. Oncolytics. 2018;11:127–137. doi: 10.1016/j.omto.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hegde M., Mukherjee M., Grada Z., Pignata A., Landi D., Navai S.A., Wakefield A., Fousek K., Bielamowicz K., Chow K.K. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 2019;129:3464. doi: 10.1172/JCI131246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bielamowicz K., Fousek K., Byrd T.T., Samaha H., Mukherjee M., Aware N., Wu M.F., Orange J.S., Sumazin P., Man T.K. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro-oncol. 2018;20:506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Urbanska K., Lanitis E., Poussin M., Lynn R.C., Gavin B.P., Kelderman S., Yu J., Scholler N., Powell D.J., Jr. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–1852. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu K., Liu X., Peng Z., Sun H., Zhang M., Zhang J., Liu S., Hao L., Lu G., Zheng K. Retargeted human avidin-CAR T cells for adoptive immunotherapy of EGFRvIII expressing gliomas and their evaluation via optical imaging. Oncotarget. 2015;6:23735–23747. doi: 10.18632/oncotarget.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lohmueller J.J., Ham J.D., Kvorjak M., Finn O.J. mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. OncoImmunology. 2017;7:e1368604. doi: 10.1080/2162402X.2017.1368604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rataj F., Jacobi S.J., Stoiber S., Asang F., Ogonek J., Tokarew N., Cadilha B.L., van Puijenbroek E., Heise C., Duewell P. High-affinity CD16-polymorphism and Fc-engineered antibodies enable activity of CD16-chimeric antigen receptor-modified T cells for cancer therapy. Br. J. Cancer. 2019;120:79–87. doi: 10.1038/s41416-018-0341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu D., Zhao J., Song Y. Engineering switchable and programmable universal CARs for CAR T therapy. J. Hematol. Oncol. 2019;12:69. doi: 10.1186/s13045-019-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rodgers D.T., Mazagova M., Hampton E.N., Cao Y., Ramadoss N.S., Hardy I.R., Schulman A., Du J., Wang F., Singer O. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl. Acad. Sci. USA. 2016;113:E459–E468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Landgraf K.E., Williams S.R., Steiger D., Gebhart D., Lok S., Martin D.W., Roybal K.T., Kim K.C. convertibleCARs: a chimeric antigen receptor system for flexible control of activity and antigen targeting. Commun. Biol. 2020;3:296. doi: 10.1038/s42003-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Iwahori K., Kakarla S., Velasquez M.P., Yu F., Yi Z., Gerken C., Song X.T., Gottschalk S. Engager T cells: a new class of antigen-specific T cells that redirect bystander T cells. Mol. Ther. 2015;23:171–178. doi: 10.1038/mt.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu X., Barrett D.M., Jiang S., Fang C., Kalos M., Grupp S.A., June C.H., Zhao Y. Improved anti-leukemia activities of adoptively transferred T cells expressing bispecific T-cell engager in mice. Blood Cancer J. 2016;6:e430. doi: 10.1038/bcj.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Choi B.D., Yu X., Castano A.P., Bouffard A.A., Schmidts A., Larson R.C., Bailey S.R., Boroughs A.C., Frigault M.J., Leick M.B. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 163.Avanzi M.P., Yeku O., Li X., Wijewarnasuriya D.P., van Leeuwen D.G., Cheung K., Park H., Purdon T.J., Daniyan A.F., Spitzer M.H., Brentjens R.J. Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep. 2018;23:2130–2141. doi: 10.1016/j.celrep.2018.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li X., Daniyan A.F., Lopez A.V., Purdon T.J., Brentjens R.J. Cytokine IL-36γ improves CAR T-cell functionality and induces endogenous antitumor response. Leukemia. 2020 doi: 10.1038/s41375-020-0874-1. Published online May 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuhn N.F., Purdon T.J., van Leeuwen D.G., Lopez A.V., Curran K.J., Daniyan A.F., Brentjens R.J. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell. 2019;35:473–488.e6. doi: 10.1016/j.ccell.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lai J., Mardiana S., House I.G., Sek K., Henderson M.A., Giuffrida L., Chen A.X.Y., Todd K.L., Petley E.V., Chan J.D. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat. Immunol. 2020;21:914–926. doi: 10.1038/s41590-020-0676-7. [DOI] [PubMed] [Google Scholar]
- 167.Sakemura R., Terakura S., Watanabe K., Julamanee J., Takagi E., Miyao K., Koyama D., Goto T., Hanajiri R., Nishida T. A Tet-On inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration. Cancer Immunol. Res. 2016;4:658–668. doi: 10.1158/2326-6066.CIR-16-0043. [DOI] [PubMed] [Google Scholar]
- 168.Juillerat A., Marechal A., Filhol J.M., Valogne Y., Valton J., Duclert A., Duchateau P., Poirot L. An oxygen sensitive self-decision making engineered CAR T-cell. Sci. Rep. 2017;7:39833. doi: 10.1038/srep39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morsut L., Roybal K.T., Xiong X., Gordley R.M., Coyle S.M., Thomson M., Lim W.A. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164:780–791. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roybal K.T., Rupp L.J., Morsut L., Walker W.J., McNally K.A., Park J.S., Lim W.A. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang Z.J., Yu Z.Y., Cai Y.M., Du R.R., Cai L. Engineering of an enhanced synthetic Notch receptor by reducing ligand-independent activation. Commun. Biol. 2020;3:116. doi: 10.1038/s42003-020-0848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Srivastava S., Salter A.I., Liggitt D., Yechan-Gunja S., Sarvothama M., Cooper K., Smythe K.S., Dudakov J.A., Pierce R.H., Rader C., Riddell S.R. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell. 2019;35:489–503.e8. doi: 10.1016/j.ccell.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilkie S., van Schalkwyk M.C., Hobbs S., Davies D.M., van der Stegen S.J., Pereira A.C., Burbridge S.E., Box C., Eccles S.A., Maher J. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 175.Fedorov V.D., Themeli M., Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Han X., Bryson P.D., Zhao Y., Cinay G.E., Li S., Guo Y., Siriwon N., Wang P. Masked chimeric antigen receptor for tumor-specific activation. Mol. Ther. 2017;25:274–284. doi: 10.1016/j.ymthe.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bonini C., Ferrari G., Verzeletti S., Servida P., Zappone E., Ruggieri L., Ponzoni M., Rossini S., Mavilio F., Traversari C., Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 178.Jones B.S., Lamb L.S., Goldman F., Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front. Pharmacol. 2014;5:254. doi: 10.3389/fphar.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Straathof K.C., Pulè M.A., Yotnda P., Dotti G., Vanin E.F., Brenner M.K., Heslop H.E., Spencer D.M., Rooney C.M. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Giordano-Attianese G., Gainza P., Gray-Gaillard E., Cribioli E., Shui S., Kim S., Kwak M.J., Vollers S., Corria Osorio A.J., Reichenbach P. A computationally designed chimeric antigen receptor provides a small-molecule safety switch for T-cell therapy. Nat. Biotechnol. 2020;38:426–432. doi: 10.1038/s41587-019-0403-9. [DOI] [PubMed] [Google Scholar]
- 182.Zajc C.U., Dobersberger M., Schaffner I., Mlynek G., Pühringer D., Salzer B., Djinović-Carugo K., Steinberger P., De Sousa Linhares A., Yang N.J. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc. Natl. Acad. Sci. USA. 2020;117:14926–14935. doi: 10.1073/pnas.1911154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Juillerat A., Tkach D., Busser B.W., Temburni S., Valton J., Duclert A., Poirot L., Depil S., Duchateau P. Modulation of chimeric antigen receptor surface expression by a small molecule switch. BMC Biotechnol. 2019;19:44. doi: 10.1186/s12896-019-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Griffioen M., van Egmond E.H., Kester M.G., Willemze R., Falkenburg J.H., Heemskerk M.H. Retroviral transfer of human CD20 as a suicide gene for adoptive T-cell therapy. Haematologica. 2009;94:1316–1320. doi: 10.3324/haematol.2008.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang X., Chang W.C., Wong C.W., Colcher D., Sherman M., Ostberg J.R., Forman S.J., Riddell S.R., Jensen M.C. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mestermann K., Giavridis T., Weber J., Rydzek J., Frenz S., Nerreter T., Mades A., Sadelain M., Einsele H., Hudecek M. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 2019;11:eaau5907. doi: 10.1126/scitranslmed.aau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Blank C.U., Haining W.N., Held W., Hogan P.G., Kallies A., Lugli E., Lynn R.C., Philip M., Rao A., Restifo N.P. Defining “T cell exhaustion”. Nat. Rev. Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kagoya Y., Tanaka S., Guo T., Anczurowski M., Wang C.H., Saso K., Butler M.O., Minden M.D., Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lai Y., Weng J., Wei X., Qin L., Lai P., Zhao R., Jiang Z., Li B., Lin S., Wang S. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T cells. Leukemia. 2018;32:801–808. doi: 10.1038/leu.2017.249. [DOI] [PubMed] [Google Scholar]
- 190.Nguyen P., Okeke E., Clay M., Haydar D., Justice J., O’Reilly C., Pruett-Miller S., Papizan J., Moore J., Zhou S. Route of 41BB/41BBL costimulation determines effector function of B7-H3-CAR.CD28ζ T cells. Mol. Ther. Oncolytics. 2020;18:202–214. doi: 10.1016/j.omto.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Foster A.E., Mahendravada A., Shinners N.P., Chang W.C., Crisostomo J., Lu A., Khalil M., Morschl E., Shaw J.L., Saha S. Regulated expansion and survival of chimeric antigen receptor-modified T cells using small molecule-dependent inducible MyD88/CD40. Mol. Ther. 2017;25:2176–2188. doi: 10.1016/j.ymthe.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Deguine J., Barton G.M. MyD88: a central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Horvath C.M. The Jak-STAT pathway stimulated by interferon α or interferon β. Sci. STKE. 2004;2004:tr10. doi: 10.1126/stke.2602004tr10. [DOI] [PubMed] [Google Scholar]
- 194.Majoros A., Platanitis E., Kernbauer-Hölzl E., Rosebrock F., Müller M., Decker T. Canonical and non-canonical aspects of JAK-STAT signaling: lessons from interferons for cytokine responses. Front. Immunol. 2017;8:29. doi: 10.3389/fimmu.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liau N.P.D., Laktyushin A., Lucet I.S., Murphy J.M., Yao S., Whitlock E., Callaghan K., Nicola N.A., Kershaw N.J., Babon J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018;9:1558. doi: 10.1038/s41467-018-04013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ma X., Shou P., Smith C., Chen Y., Du H., Sun C., Porterfield Kren N., Michaud D., Ahn S., Vincent B. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020;38:448–459. doi: 10.1038/s41587-019-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu Y., Di S., Shi B., Zhang H., Wang Y., Wu X., Luo H., Wang H., Li Z., Jiang H. Armored inducible expression of IL-12 enhances antitumor activity of glypican-3-targeted chimeric antigen receptor-engineered T cells in hepatocellular carcinoma. J. Immunol. 2019;203:198–207. doi: 10.4049/jimmunol.1800033. [DOI] [PubMed] [Google Scholar]
- 198.Pegram H.J., Lee J.C., Hayman E.G., Imperato G.H., Tedder T.F., Sadelain M., Brentjens R.J. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hurton L.V., Singh H., Najjar A.M., Switzer K.C., Mi T., Maiti S., Olivares S., Rabinovich B., Huls H., Forget M.A. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. USA. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Krenciute G., Prinzing B.L., Yi Z., Wu M.F., Liu H., Dotti G., Balyasnikova I.V., Gottschalk S. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 2017;5:571–581. doi: 10.1158/2326-6066.CIR-16-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang L., Morgan R.A., Beane J.D., Zheng Z., Dudley M.E., Kassim S.H., Nahvi A.V., Ngo L.T., Sherry R.M., Phan G.Q. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 2015;21:2278–2288. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Renaud-Gabardos E., Hantelys F., Morfoisse F., Chaufour X., Garmy-Susini B., Prats A.C. Internal ribosome entry site-based vectors for combined gene therapy. World J. Exp. Med. 2015;5:11–20. doi: 10.5493/wjem.v5.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hu B., Ren J., Luo Y., Keith B., Young R.M., Scholler J., Zhao Y., June C.H. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 2017;20:3025–3033. doi: 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chmielewski M., Abken H. CAR T cells releasing IL-18 convert to T-Bethigh FoxO1low effectors that exhibit augmented activity against advanced solid tumors. Cell Rep. 2017;21:3205–3219. doi: 10.1016/j.celrep.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 205.Zhou P., Shaffer D.R., Alvarez Arias D.A., Nakazaki Y., Pos W., Torres A.J., Cremasco V., Dougan S.K., Cowley G.S., Elpek K. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shifrut E., Carnevale J., Tobin V., Roth T.L., Woo J.M., Bui C.T., Li P.J., Diolaiti M.E., Ashworth A., Marson A. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell. 2018;175:1958–1971.e15. doi: 10.1016/j.cell.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wei J., Long L., Zheng W., Dhungana Y., Lim S.A., Guy C., Wang Y., Wang Y.D., Qian C., Xu B. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature. 2019;576:471–476. doi: 10.1038/s41586-019-1821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gautam S., Fioravanti J., Zhu W., Le Gall J.B., Brohawn P., Lacey N.E., Hu J., Hocker J.D., Hawk N.V., Kapoor V. The transcription factor c-Myb regulates CD8+ T cell stemness and antitumor immunity. Nat. Immunol. 2019;20:337–349. doi: 10.1038/s41590-018-0311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seo H., Chen J., González-Avalos E., Samaniego-Castruita D., Das A., Wang Y.H., López-Moyado I.F., Georges R.O., Zhang W., Onodera A. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl. Acad. Sci. USA. 2019;116:12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen J., López-Moyado I.F., Seo H., Lio C.J., Hempleman L.J., Sekiya T., Yoshimura A., Scott-Browne J.P., Rao A. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567:530–534. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lynn R.C., Weber E.W., Sotillo E., Gennert D., Xu P., Good Z., Anbunathan H., Lattin J., Jones R., Tieu V. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576:293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.van der Woude L.L., Gorris M.A.J., Halilovic A., Figdor C.G., de Vries I.J.M. Migrating into the tumor: a roadmap for T cells. Trends Cancer. 2017;3:797–808. doi: 10.1016/j.trecan.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 213.Lanitis E., Dangaj D., Irving M., Coukos G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann. Oncol. 2017;28(suppl 12):xii18–xii32. doi: 10.1093/annonc/mdx238. [DOI] [PubMed] [Google Scholar]
- 214.Vilgelm A.E., Richmond A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front. Immunol. 2019;10:333. doi: 10.3389/fimmu.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mami-Chouaib F., Blanc C., Corgnac S., Hans S., Malenica I., Granier C., Tihy I., Tartour E. Resident memory T cells, critical components in tumor immunology. J. Immunother. Cancer. 2018;6:87. doi: 10.1186/s40425-018-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Peng W., Ye Y., Rabinovich B.A., Liu C., Lou Y., Zhang M., Whittington M., Yang Y., Overwijk W.W., Lizée G., Hwu P. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin. Cancer Res. 2010;16:5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Craddock J.A., Lu A., Bear A., Pule M., Brenner M.K., Rooney C.M., Foster A.E. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nishio N., Diaconu I., Liu H., Cerullo V., Caruana I., Hoyos V., Bouchier-Hayes L., Savoldo B., Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Iozzo R.V. Basement membrane proteoglycans: from cellar to ceiling. Nat. Rev. Mol. Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 220.Iozzo R.V., Sanderson R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Caruana I., Savoldo B., Hoyos V., Weber G., Liu H., Kim E.S., Ittmann M.M., Marchetti D., Dotti G. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 2015;21:524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ramanathan R.K., McDonough S.L., Philip P.A., Hingorani S.R., Lacy J., Kortmansky J.S., Thumar J., Chiorean E.G., Shields A.F., Behl D. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol. 2019;37:1062–1069. doi: 10.1200/JCO.18.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fane M., Weeraratna A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer. 2020;20:89–106. doi: 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Duan Q., Zhang H., Zheng J., Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 226.Jarnicki A.G., Lysaght J., Todryk S., Mills K.H. Suppression of antitumor immunity by IL-10 and TGF-β-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J. Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 227.Jiang X., Wang J., Deng X., Xiong F., Ge J., Xiang B., Wu X., Ma J., Zhou M., Li X. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Geiger R., Rieckmann J.C., Wolf T., Basso C., Feng Y., Fuhrer T., Kogadeeva M., Picotti P., Meissner F., Mann M. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167:829–842.e13. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rivadeneira D.B., Delgoffe G.M. Antitumor T-cell reconditioning: improving metabolic fitness for optimal cancer immunotherapy. Clin. Cancer Res. 2018;24:2473–2481. doi: 10.1158/1078-0432.CCR-17-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Perna S.K., De Angelis B., Pagliara D., Hasan S.T., Zhang L., Mahendravada A., Heslop H.E., Brenner M.K., Rooney C.M., Dotti G., Savoldo B. Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin. Cancer Res. 2013;19:106–117. doi: 10.1158/1078-0432.CCR-12-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.John L.B., Devaud C., Duong C.P., Yong C.S., Beavis P.A., Haynes N.M., Chow M.T., Smyth M.J., Kershaw M.H., Darcy P.K. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 232.Liu X., Ranganathan R., Jiang S., Fang C., Sun J., Kim S., Newick K., Lo A., June C.H., Zhao Y., Moon E.K. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rupp L.J., Schumann K., Roybal K.T., Gate R.E., Ye C.J., Lim W.A., Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017;7:737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hu W., Zi Z., Jin Y., Li G., Shao K., Cai Q., Ma X., Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol. Immunother. 2019;68:365–377. doi: 10.1007/s00262-018-2281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nakazawa T., Natsume A., Nishimura F., Morimoto T., Matsuda R., Nakamura M., Yamada S., Nakagawa I., Motoyama Y., Park Y.S. Effect of CRISPR/Cas9-mediated PD-1-disrupted primary human third-generation CAR-T cells targeting EGFRvIII on in vitro human glioblastoma cell growth. Cells. 2020;9:998. doi: 10.3390/cells9040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stadtmauer E.A., Fraietta J.A., Davis M.M., Cohen A.D., Weber K.L., Lancaster E., Mangan P.A., Kulikovskaya I., Gupta M., Chen F. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367:eaba7365. doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Condomines M., Arnason J., Benjamin R., Gunset G., Plotkin J., Sadelain M. Tumor-targeted human t cells expressing CD28-based chimeric antigen receptors circumvent CTLA-4 inhibition. PLoS ONE. 2015;10:e0130518. doi: 10.1371/journal.pone.0130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dotti G., Savoldo B., Pule M., Straathof K.C., Biagi E., Yvon E., Vigouroux S., Brenner M.K., Rooney C.M. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yamamoto T.N., Lee P.H., Vodnala S.K., Gurusamy D., Kishton R.J., Yu Z., Eidizadeh A., Eil R., Fioravanti J., Gattinoni L. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J. Clin. Invest. 2019;129:1551–1565. doi: 10.1172/JCI121491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bollard C.M., Rössig C., Calonge M.J., Huls M.H., Wagner H.J., Massague J., Brenner M.K., Heslop H.E., Rooney C.M. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 241.Bollard C.M., Tripic T., Cruz C.R., Dotti G., Gottschalk S., Torrano V., Dakhova O., Carrum G., Ramos C.A., Liu H. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed hodgkin lymphoma. J. Clin. Oncol. 2018;36:1128–1139. doi: 10.1200/JCO.2017.74.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wilkie S., Burbridge S.E., Chiapero-Stanke L., Pereira A.C., Cleary S., van der Stegen S.J., Spicer J.F., Davies D.M., Maher J. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J. Biol. Chem. 2010;285:25538–25544. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang Y., Jiang H., Luo H., Sun Y., Shi B., Sun R., Li Z. An IL-4/21 Inverted cytokine receptor improving CAR-T cell potency in immunosuppressive solid-tumor microenvironment. Front. Immunol. 2019;10:1691. doi: 10.3389/fimmu.2019.01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Leen A.M., Sukumaran S., Watanabe N., Mohammed S., Keirnan J., Yanagisawa R., Anurathapan U., Rendon D., Heslop H.E., Rooney C.M. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 2014;22:1211–1220. doi: 10.1038/mt.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chang Z.L., Lorenzini M.H., Chen X., Tran U., Bangayan N.J., Chen Y.Y. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 2018;14:317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lo A.S., Taylor J.R., Farzaneh F., Kemeny D.M., Dibb N.J., Maher J. Harnessing the tumour-derived cytokine, CSF-1, to co-stimulate T-cell growth and activation. Mol. Immunol. 2008;45:1276–1287. doi: 10.1016/j.molimm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 247.DeBerardinis R.J. Tumor microenvironment, metabolism, and immunotherapy. N. Engl. J. Med. 2020;382:869–871. doi: 10.1056/NEJMcibr1914890. [DOI] [PubMed] [Google Scholar]
- 248.Huang J., Yu J., Tu L., Huang N., Li H., Luo Y. Isocitrate dehydrogenase mutations in glioma: from basic discovery to therapeutics development. Front. Oncol. 2019;9:506. doi: 10.3389/fonc.2019.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Reiter-Brennan C., Semmler L., Klein A. The effects of 2-hydroxyglutarate on the tumorigenesis of gliomas. Contemp. Oncol. (Pozn.) 2018;22:215–222. doi: 10.5114/wo.2018.82642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vodnala S.K., Eil R., Kishton R.J., Sukumar M., Yamamoto T.N., Ha N.H., Lee P.H., Shin M., Patel S.J., Yu Z. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. 2019;363:eaau0135. doi: 10.1126/science.aau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gelderman K.A., Hultqvist M., Holmberg J., Olofsson P., Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc. Natl. Acad. Sci. USA. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cemerski S., Cantagrel A., Van Meerwijk J.P., Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J. Biol. Chem. 2002;277:19585–19593. doi: 10.1074/jbc.M111451200. [DOI] [PubMed] [Google Scholar]
- 253.Fultang L., Booth S., Yogev O., Martins da Costa B., Tubb V., Panetti S., Stavrou V., Scarpa U., Jankevics A., Lloyd G. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood. 2020;136:1155–1160. doi: 10.1182/blood.2019004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Oh M.H., Sun I.H., Zhao L., Leone R.D., Sun I.M., Xu W., Collins S.L., Tam A.J., Blosser R.L., Patel C.H. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Invest. 2020;130:3865–3884. doi: 10.1172/JCI131859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Leone R.D., Zhao L., Englert J.M., Sun I.M., Oh M.H., Sun I.H., Arwood M.L., Bettencourt I.A., Patel C.H., Wen J. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ligtenberg M.A., Mougiakakos D., Mukhopadhyay M., Witt K., Lladser A., Chmielewski M., Riet T., Abken H., Kiessling R. Coexpressed catalase protects chimeric antigen receptor-redirected T cells as well as bystander cells from oxidative stress-induced loss of antitumor activity. J. Immunol. 2016;196:759–766. doi: 10.4049/jimmunol.1401710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zannella V.E., Dal Pra A., Muaddi H., McKee T.D., Stapleton S., Sykes J., Glicksman R., Chaib S., Zamiara P., Milosevic M. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin. Cancer Res. 2013;19:6741–6750. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- 258.Wang L.C., Lo A., Scholler J., Sun J., Majumdar R.S., Kapoor V., Antzis M., Cotner C.E., Johnson L.A., Durham A.C. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014;2:154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kakarla S., Chow K.K., Mata M., Shaffer D.R., Song X.T., Wu M.F., Liu H., Wang L.L., Rowley D.R., Pfizenmaier K., Gottschalk S. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 2013;21:1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tran E., Chinnasamy D., Yu Z., Morgan R.A., Lee C.C., Restifo N.P., Rosenberg S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Curioni A.B.C., Hiltbrunner S., Bankel L., Gulati P., Weder W., Opitz I., Lauk O., Caviezel C., Knuth A., Munz C. A phase I clinical trial of malignant pleural mesothelioma treated with locally delivered autologous anti-FAP-targeted CAR T-cells. Ann. Oncol. 2019;30(Suppl 5):v501. [Google Scholar]
- 262.Tie Y., Zheng H., He Z., Yang J., Shao B., Liu L., Luo M., Yuan X., Liu Y., Zhang X. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Target. Ther. 2020;5:6. doi: 10.1038/s41392-020-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ruella M., Klichinsky M., Kenderian S.S., Shestova O., Ziober A., Kraft D.O., Feldman M., Wasik M.A., June C.H., Gill S. Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov. 2017;7:1154–1167. doi: 10.1158/2159-8290.CD-16-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang P., Zhao S., Wu C., Li J., Li Z., Wen C., Hu S., An G., Meng H., Zhang X., Yang L. Effects of CSF1R-targeted chimeric antigen receptor-modified NK92MI & T cells on tumor-associated macrophages. Immunotherapy. 2018;10:935–949. doi: 10.2217/imt-2018-0012. [DOI] [PubMed] [Google Scholar]
- 265.Parihar R., Rivas C., Huynh M., Omer B., Lapteva N., Metelitsa L.S., Gottschalk S.M., Rooney C.M. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol. Res. 2019;7:363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chinnasamy D., Yu Z., Kerkar S.P., Zhang L., Morgan R.A., Restifo N.P., Rosenberg S.A. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xie Y.J., Dougan M., Jailkhani N., Ingram J., Fang T., Kummer L., Momin N., Pishesha N., Rickelt S., Hynes R.O., Ploegh H. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl. Acad. Sci. USA. 2019;116:7624–7631. doi: 10.1073/pnas.1817147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Santoro S.P., Kim S., Motz G.T., Alatzoglou D., Li C., Irving M., Powell D.J., Jr., Coukos G. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol. Res. 2015;3:68–84. doi: 10.1158/2326-6066.CIR-14-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xu J., Wang Y., Shi J., Liu J., Li Q., Chen L. Combination therapy: a feasibility strategy for CAR-T cell therapy in the treatment of solid tumors. Oncol. Lett. 2018;16:2063–2070. doi: 10.3892/ol.2018.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Anderson K.G., Stromnes I.M., Greenberg P.D. Obstacles posed by the tumor microenvironment to t cell activity: a case for synergistic therapies. Cancer Cell. 2017;31:311–325. doi: 10.1016/j.ccell.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Porter C.E., Rosewell Shaw A., Jung Y., Yip T., Castro P.D., Sandulache V.C., Sikora A., Gottschalk S., Ittman M.M., Brenner M.K., Suzuki M. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol. Ther. 2020;28:1251–1262. doi: 10.1016/j.ymthe.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wing A., Fajardo C.A., Posey A.D., Jr., Shaw C., Da T., Young R.M., Alemany R., June C.H., Guedan S. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol. Res. 2018;6:605–616. doi: 10.1158/2326-6066.CIR-17-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S., Brenner M.K., Suzuki M. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol. Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Park A.K., Fong Y., Kim S.I., Yang J., Murad J.P., Lu J., Jeang B., Chang W.C., Chen N.G., Thomas S.H. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020;12:eaaz1863. doi: 10.1126/scitranslmed.aaz1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Aalipour A., Le Boeuf F., Tang M., Murty S., Simonetta F., Lozano A.X., Shaffer T.M., Bell J.C., Gambhir S.S. Viral delivery of CAR targets to solid tumors enables effective cell therapy. Mol. Ther. Oncolytics. 2020;17:232–240. doi: 10.1016/j.omto.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Power A.T., Bell J.C. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol. Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- 277.Cole C., Qiao J., Kottke T., Diaz R.M., Ahmed A., Sanchez-Perez L., Brunn G., Thompson J., Chester J., Vile R.G. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat. Med. 2005;11:1073–1081. doi: 10.1038/nm1297. [DOI] [PubMed] [Google Scholar]
- 278.Donnelly O., Vile R., Pandha H., Harrington K., Melcher A. The hitchhiker’s guide to virotherapy. Oncotarget. 2012;3:735–736. doi: 10.18632/oncotarget.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Grosser R., Cherkassky L., Chintala N., Adusumilli P.S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36:471–482. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Minn I., Rowe S.P., Pomper M.G. Enhancing CAR T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol. 2019;20:e443–e451. doi: 10.1016/S1470-2045(19)30461-9. [DOI] [PubMed] [Google Scholar]
- 281.Ma L., Dichwalkar T., Chang J.Y.H., Cossette B., Garafola D., Zhang A.Q., Fichter M., Wang C., Liang S., Silva M. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365:162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Reinhard K., Rengstl B., Oehm P., Michel K., Billmeier A., Hayduk N., Klein O., Kuna K., Ouchan Y., Wöll S. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367:446–453. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 283.Ruella M., Kenderian S.S., Shestova O., Fraietta J.A., Qayyum S., Zhang Q., Maus M.V., Liu X., Nunez-Cruz S., Klichinsky M. The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T cells (CART19) improves responses against mantle cell lymphoma. Clin. Cancer Res. 2016;22:2684–2696. doi: 10.1158/1078-0432.CCR-15-1527. [DOI] [PubMed] [Google Scholar]
- 284.Ramakrishna S., Highfill S.L., Walsh Z., Nguyen S.M., Lei H., Shern J.F., Qin H., Kraft I.L., Stetler-Stevenson M., Yuan C.M. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin. Cancer Res. 2019;25:5329–5341. doi: 10.1158/1078-0432.CCR-18-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Michie J., Beavis P.A., Freeman A.J., Vervoort S.J., Ramsbottom K.M., Narasimhan V., Lelliott E.J., Lalaoui N., Ramsay R.G., Johnstone R.W. Antagonism of IAPs enhances CAR T-cell efficacy. Cancer Immunol. Res. 2019;7:183–192. doi: 10.1158/2326-6066.CIR-18-0428. [DOI] [PubMed] [Google Scholar]
- 286.Ng J., Dai T. Radiation therapy and the abscopal effect: a concept comes of age. Ann. Transl. Med. 2016;4:118. doi: 10.21037/atm.2016.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhang Z., Zhou L., Xie N., Nice E.C., Zhang T., Cui Y., Huang C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020;5:113. doi: 10.1038/s41392-020-00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mottini C., Napolitano F., Li Z., Gao X., Cardone L. Computer-aided drug repurposing for cancer therapy: approaches and opportunities to challenge anticancer targets. Semin. Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.09.023. Published online September 25, 2019. [DOI] [PubMed] [Google Scholar]
- 289.Rams D. Ten principles for good design. In: De Jong C.W., editor. Ten Principles for Good Design: Dieter Rams. Prestel; 2017. pp. 92–133. [Google Scholar]