Abstract
Cardiac involvement in systemic sclerosis (SSc-CI) may be either primary or secondary to pathologic processes in other organs. In contrast to other autoimmune rheumatic diseases, primary SSc-CI preferentially manifests as non-ischemic myocardial fibrosis, with or without myocardial inflammation and minimal involvement of epicardial coronary arteries. Recent developments in cardiovascular (CV) imaging modalities and their increasing availability necessitate the creation of concrete recommendations for use in SSc patients, based on the most recent scientific evidence. Echocardiography offers rapid, effective, multiparametric, and widely available imaging evaluation of SSc patients, owing to its ability to analyze both left and right chambers, as well as pulmonary hemodynamics. However, it is an operator- and acoustic window-dependent modality that cannot perform tissue characterization, which is crucial in these conditions. CV magnetic resonance in SSc patients can accurately evaluate biventricular volumes, ejection fractions, myocardial fibrosis load, and changes suggestive of myocarditis. T2 mapping is the best index of edema indicating acute myocardial inflammation, while late gadolinium enhancement is an index of replacement fibrosis. Extracellular volume fraction (ECV) is an indicator of diffuse myocardial fibrosis only in the absence of significant myocardial inflammation. However, if myocardial inflammation/fibrosis coexist, ECV reflects a combination of the two, but it cannot completely discriminate between them. SSc-CI hangs like the sword of Damocles over physicians managing SSc patients. A constructive partnership between the rheumatologist and the cardiologist is necessary to provide each SSc patient with a comprehensive screening protocol for early detection and treatment of cardiopulmonary pathologic processes.
Keywords: Echocardiography, cardiovasular magnetic resonance, systemic sclerosis, oedema, fibrosis, myocarditis, myositis
Introduction
Systemic sclerosis (SSc) or scleroderma is an autoimmune rheumatic disease, typified by the triad of autoimmune activation, vasculopathy, and progressive fibrosis of the skin and internal organs (1). Although officially labeled a rare disease, recent epidemiologic evidence suggests that SSc affects more than two million patients worldwide, with approximately 300,000 new cases diagnosed each year (1). The systemic component of SSc refers to the involvement of major organs, those most often being the lungs and kidneys. However, SSc may also involve the cardiovascular (CV) system, which currently represents a significant contributor to mortality in this patient group (2).
Clinical manifestations of cardiac involvement in SSc
Cardiac involvement in SSc (SSc-CI) may be either primary or secondary to pathologic processes in other major organs (e.g., pulmonary or renal involvement). In contrast to other autoimmune rheumatic diseases mainly characterized by the occurrence of ischemic CV disease (CVD), primary SSc-CI preferentially manifests as non-ischemic myocardial fibrosis, with or without myocardial inflammation and minimal or no involvement of epicardial coronary arteries (3). SSc patients may thus present with myocarditis, heart failure (systolic or more commonly diastolic dysfunction), conduction abnormalities, and/or pericardial and valvular disease (3).
Mild diastolic dysfunction is present in a significant proportion of SSc patients, whereas restrictive cardiomyopathy leading to severe diastolic dysfunction has been rarely documented (4). Systolic dysfunction is relatively uncommon, usually occurring as a consequence of coronary artery disease or more commonly in the context of myocarditis and/or myocardial fibrosis (5). Cardiac conduction abnormalities including supraventricular/ventricular arrhythmias and/or atrioventricular block may be present in up to a third of SSc patients, and are associated with increased mortality (4). Small or moderate pericardial effusions and rarely pericardial tamponade or constrictive pericarditis have also been reported in SSc patients (6). Valvular disease in the form of valvular leaflet thickening and valve prolapse, as well as non-infectious endocarditis has been rarely documented in SSc patients (7).
Aetiopathogenesis of cardiac involvement in SSc
The hallmark of SSc-CI is myocardial fibrosis with or without the presence of inflammation (4). The most prevalent hypothesis is a vascular mechanism underlying the fibrotic process (8). Microvasculopathy is one of the earliest pathological features of SSc, often preceding but likely contributing to the fibrotic pattern of SSc and underlying the clinical manifestation of Raynaud phenomenon and pulmonary hypertension (PH). Similarly, repeated focal microvascular ischemia and reperfusion is thought to underlie myocardial fibrosis and diastolic dysfunction (8). More recently, the presence of myocardial inflammation in the early stages of the disease has been reported, suggesting that fibrotic processes may be preceded by inflammatory changes (7).
Current criteria according to rheumatologists to diagnose SSc cardiac involvement
Identification of patients necessitating a high index of suspicion
Epidemiologic studies have identified the following risk factors for the development of SSc-CI: (a) diffuse cutaneous SSc, (b) anti-topoisomerase antibody (anti-Scl70) seropositivity, (c) male gender, (d) presence of myositis, (e) interstitial lung disease (ILD), and (f) tendon friction rubs (5). These factors can be employed in clinical practice to maintain a higher index of suspicion in these specific patient subgroups. The presenting symptoms of SSc-CI are broad and typically comprised of dyspnea, peripheral edema, fatigue, chest pain, palpitations, or syncope. Additionally, routine annual echocardiographic monitoring for PH may identify incidental early myocardial changes.
Further evaluation of suspected primary cardiac involvement in SSc patients
If SSc-CI is suspected owing to any of the aforementioned reasons, further assessment is necessary. However, few recommendations are available for guiding clinical practice. The initial UK SSc working group practice guidelines were mainly based on expert opinion and lacked widespread substantiation by scientific evidence (9). Management of traditional cardiovascular risk factors is advocated as per standard practice. Recommendations for the use of electrocardiography (ECG), Holter monitoring, plasma cardiac biomarkers, echocardiography, and CV magnetic resonance (CMR) are also provided. Nevertheless, an integrated, up-to-date, evidence-based approach to diagnostic considerations in SSc-CI is still needed.
Current gaps in evidence and the purpose of this review
Recent technological developments in non-invasive CV imaging modalities as well as their increasing availability necessitate the generation of concrete clinical practice guidelines for their use in the SSc patient population, based on the most recent scientific evidence. This, in turn, may allow the optimization of screening, early identification, risk stratification, and reduction of associated mortality of SSc-CI, as well as the introduction of tailored treatment based on diagnostic findings. The purpose of this narrative review is to present available evidence on non-invasive CV imaging modalities and their use in SSc-CI, as well as implications for current practice and future research.
Echocardiography in systemic sclerosis
Echocardiography offers the advantage of non-invasive, real-time evaluation of cardiac structure and function, together with the ability to estimate pulmonary artery pressure (PAP) and other non-invasive hemodynamic measurements.
PH is defined as resting mean PAP≥25 mm Hg, measured invasively, and is a frequent finding in SSc patients (10). PH may be the result of an isolated pulmonary arteriopathy, but also the consequence of ILD or left ventricular (LV) systolic/diastolic dysfunction (11). Different etiologies are associated with distinct pulmonary and systemic hemodynamics. Then, the task of the clinician is to make the correct diagnosis, to choose specific therapeutic management, and improve patient prognosis, keeping in mind that multiple mechanisms can coexist and overlap. Early diagnosis of PH or cardiac involvement in SSc is pivotal because identification at early stages may confer a better prognosis. However, it should be noted that lung computed tomography is the gold standard evaluation for the assessment of pulmonary pathology, particularly in cases where concomitant cardiac involvement may need to be excluded in patients at high risk of progression.
Standard echocardiography
Resting 2D echocardiography (2DE) in SSc patients allows the indirect assessment of systolic PAP (PAPs) and a thorough evaluation of cardiac morphology and function (11) (Table 1). PAPs measurement is based on the peak tricuspid regurgitation velocity (TRV) and right atrial pressure (RAP) estimation, which, in turn, is derived from the diameter of the inferior vena cava and its respiratory variation. Because of the inaccuracy of RAP estimation, guidelines recommend using peak TRV as the primary variable for assigning the echocardiographic probability of PH (10), taking into account that underestimation [e.g., severe tricuspid regurgitation or right ventricular (RV) systolic dysfunction] or overestimation (e.g., errors in adjusting Doppler gain) may also occur (12). Other echocardiographic variables may be considered for confirming a suspicion of PH, such as an enlarged RV and right atrium with dilated pulmonary artery diameter, RV outflow Doppler acceleration time <105 ms with a mid-systolic notching, or a systolic flattening of the interventricular septum due to pressure overload imposed on the RV (10).
Table 1.
Summary for performing a comprehensive transthoracic echocardiographic examination in patients with systemic sclerosis.
| Technique | Parameter | Advantages | Limitations |
|---|---|---|---|
| 2D linear measurements |
|
|
|
| 2D volumes |
|
|
|
| 3D volumes and mass |
|
|
|
| Speckle-tracking |
|
|
|
|
| |||
| RV/RA size and function | |||
| Technique | Parameter | Advantages | Limitations |
|
| |||
| 2D linear measurements |
|
|
|
| Tissue Doppler Imaging |
|
|
|
| 2D areas |
|
|
|
| 3D volumes |
|
|
|
| Speckle-tracking |
|
|
|
|
| |||
| Hemodynamics | |||
| Technique | Parameter | Advantages | Limitations |
|
| |||
| LV diastolic function |
|
|
|
| Pulmonary circulation |
|
|
|
|
|
|
|
|
|||
PVR=10 · TRV/RVOTVTI
LV: left ventricle; LA: left; EDD: end-diastolic diameter; ESD: end-systolic diameter; IVS: interventricular septum; ILW: infero-lateral wall; LVMI: left ventricular mass index; RWT: relative wall thickness; LAVI: left atrial volume index; EF: ejection fraction; GLS: global longitudinal strain; RVOT: right ventricle outflow tract; RVD1: right ventricle basal diameter; RVD2: right ventricle mid diameter; RVD3: right ventricle longitudinal diameter; TAPSE: tricuspid annular plane systolic excursion; RAVI: right atrial volume index; DT: deceleration time; PAPs: systolic pulmonary artery pressure; VTI: velocity-time integral; PVR: pulmonary vascular resistance.
Echocardiographic LV evaluation is well-established and reliable, as extensively described in international recommendations (12), but a comprehensive assessment of RV structure and function in SSc is advisable. RV measurements are challenging, owing to the complex geometry of this chamber. A 2DE RV-focused apical four-chamber view obtained with either lateral or medial transducer orientation is advisable for reducing inter-reader variability (Figure 1). Increased basal and midlevel diameters indicate RV dilatation; likewise, the right atrium can be assessed from the same view, measuring the area or volume (13). RV free-wall thickness in the subcostal view should also be evaluated, because the RV develops concentric hypertrophy in response to pressure overload during the adaptation phase that precedes heart failure (13). RV systolic function can be roughly assessed using the longitudinal excursion of the tricuspid annular plane during systole (TAPSE). TAPSE has the advantage of being simple, independent of RV preload, and related to stroke volume (12). Tissue-Doppler-Imaging-derived S′-wave velocity of tricuspid lateral annular velocity is another reliable and reproducible parameter for expressing RV systolic function (14), even though it is angle-dependent. However, both TAPSE and S′-wave do not represent global RV performance (12). Fractional area change during the cardiac cycle can offer a more comprehensive assessment of RV systolic function, but neglects the contribution of the RV outflow tract to overall systolic function and has low inter-observer reproducibility. 3D-echocardiography (3DE) derived volumetric measurements are more robust and accurate compared with 2D linear dimension delineation in assessing cardiac structure and function (12), making 3DE the preferred approach. Nevertheless, a thorough echocardiographic evaluation, although highly advised, may sometimes not be possible to perform owing to patient constraints, lack of expertise, or lack of appropriate analytic software. In this case, a core set of basic parameters that can guide the clinician to refer patients to more specialized centers should at least include the parameters presented in Figure 2.
Figure 1.
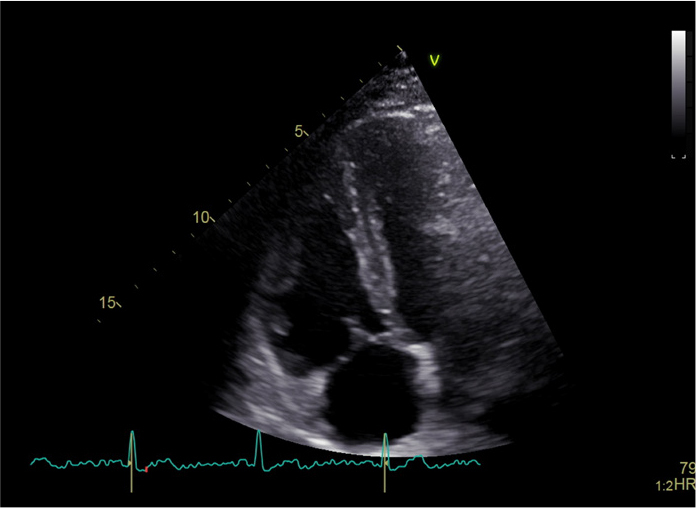
Echocardiographic assessment of right ventricle (RV) from an RV-focused apical four-chamber view in a healthy subject.
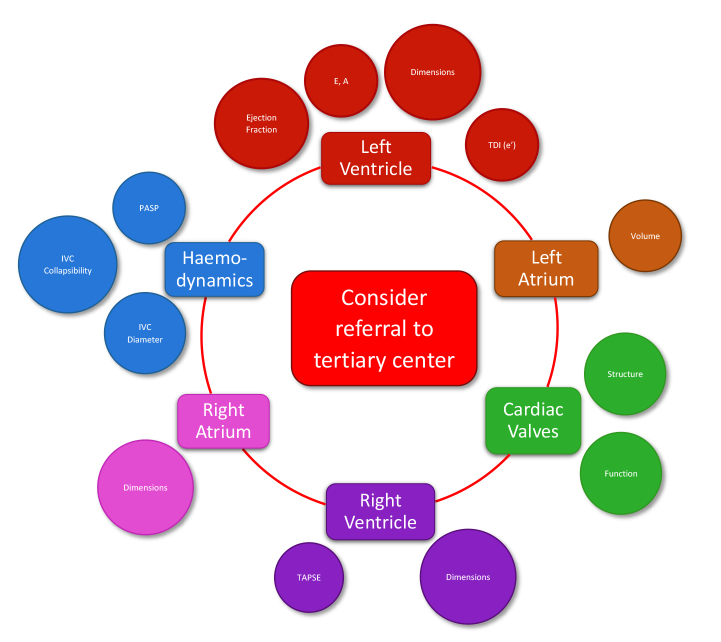
Figure 2.
Core echocardiographic parameters that should be considered in all cases before referral to a tertiary scleroderma center. TDI tissue Doppler imaging, PASP pulmonary artery systolic pressure, IVC inferior vena cava, TAPSE tricuspid annular plane systolic excursion.
Finally, the addition of lung ultrasound to conventional transthoracic 2DE is relatively time-saving and has incremental value in identifying the etiology of PH (11). Indeed, the presence of multiple, diffuse, bilateral B-lines with a regular pleural line and a gravity-related distribution can corroborate the diagnosis of decompensated left heart failure, as a sign of pulmonary congestion (15). In contrast, a normal LV scan with multiple, diffuse B-lines and an irregular pleural line are highly suggestive for ILD (16).
Exercise echocardiography
The study of pulmonary hemodynamics during exercise is fascinating and challenging at the same time. Right heart catheterization is the gold standard for assessing PH at rest and during exercise, but exercise echocardiography represents a non-invasive tool with evolving clinical applications (11). Exercise PH can be defined as the presence of resting mean PAP<25 mm Hg and mean PAP>30 mm Hg during exercise, with pulmonary vascular resistance (PVR) greater than 3 Wood units (10). It represents the result of early pulmonary vascular disease, left heart disease, lung disease, or a combination of these conditions. As all these mechanisms may evolve and/or coexist in SSc, exercise echocardiography could be useful in evaluating pulmonary hemodynamics as well as the state of the left and right heart chambers.
It is essential to consider that exercise PH in SSc patients is dependent not only on PVR but also on LV filling pressure (17). Echocardiography may help identify the relative hemodynamic contribution of each parameter during exercise to better understand the main determinants of increased PAP, unveiling early signs of LV or RV dysfunction (18). However, even though exercise PH has a high prevalence in SSc patients, only a small proportion go on to develop PH during follow-up (19). Additionally, PH does not occur over time in SSc patients without exercise PH (20). So far, there is a lack of robust clinical evidence on targeted medical therapy for exercise PH, as recognized by the latest European Guidelines (10). Larger studies are needed to test the value of exercise echocardiography in assessing the pathologic background of altered pulmonary hemodynamics in SSc and determining its role in detecting patients with a very low probability of developing PH.
New frontiers: 3DE and STE
3DE overcomes the limitations of conventional 2DE, providing an accurate reconstruction of LV and RV volumes. Dedicated single-beat or multi-beat 3D acquisition software is available for evaluating the left and right cardiac chambers without geometric assumptions; specific training in image post-processing and analysis is required for their operation. Although 3DE tends to underestimate cardiac volumes in comparison with CMR, the gold standard for right heart evaluation (21), the correlation between 3DE and CMR is significantly higher than that between 2DE and CMR (22).
Speckle tracking echocardiography (STE) provides a useful assessment of both LV and RV global and regional systolic function (23). 2DE-STE is influenced by image quality and frame rate, but is independent of the angle of insonation. Peak LV global longitudinal strain (GLS) reflects the deformation of all LV segments and represents the most robust and reproducible STE-derived parameter in various cardiac diseases, including SSc (23). In the context of the RV, GLS usually refers to the RV free wall segments alone (Figure 3); peak RV-GLS provides incremental prognostic value in PH (24).
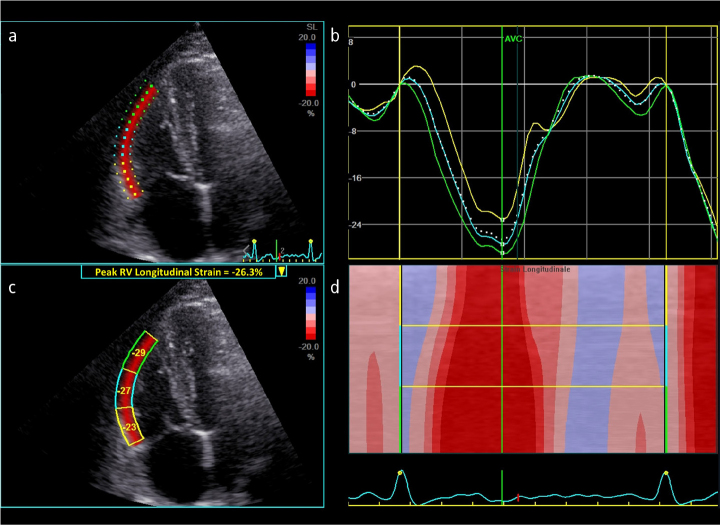
Figure 3. a–d.
(a) Speckle-tracking echocardiography for longitudinal strain analysis of the right ventricle (RV) free-wall from an RV-focused apical four-chamber view in a healthy subject. (b) The different colors of the curves represent different myocardial segments, while the white dotted curve represents the peak RV longitudinal strain of the free-wall during the cardiac cycle. (c) Regional and global peak RV free-wall longitudinal strain. (d) Anatomical M-mode color-coded display of segmental strain variations during the cardiac cycle.
To conclude, echocardiography offers rapid, effective, multiparametric, and widely available imaging evaluation for the study of SSc patients, owing to its ability to analyze both left and right chambers, as well as pulmonary hemodynamics. A constructive partnership between the rheumatologist and the cardiologist is necessary to give each patient with SSc a comprehensive screening protocol for early detection of both heart and pulmonary involvement.
CMR in SSc
CMR is a non-invasive imaging modality that does not employ ionizing radiation and is of great importance for the assessment of CVD. Specifically, for SSc patients, an integrated CMR examination should include the following:
Measurement of ventricular volumes and ejection fractions
CMR is the current diagnostic gold standard for the assessment of cardiac volumes and ejection fractions of both ventricles and specifically of the RV, which is of special interest in SSc. RV assessment is not always adequately performed when using 2DE, whereas 3DE seems more promising; there are still diagnostic dilemmas in cases with RV dilatation (21). CMR measurements of volumes and ejection fractions are more accurate and reproducible compared with other imaging modalities. Although other modalities correlate well with CMR-derived measurements, the absolute cut-off values are different and not interchangeable between modalities. Therefore, it is absolutely necessary that the same patients be followed with the same modality over time. CMR is ideal for the serial evaluation of individual patients with respect to changes in RV and LV volumes, ejection fractions, LV mass, and wall motion, owing to its high reproducibility and operator independence (21). This, in turn, permits the early detection of subclinical deterioration or the evaluation of medication effectiveness in clinical trials with SSc patients (21).
Evaluation of inflammation
CMR excels at evaluating inflammation, owing to its ability to characterize myocardial tissues. In order to perform CMR, a strong magnetic field is used to polarize hydrogen atoms in the body so that their magnetic moments (vectors perpendicular to their angular momentum) align along the same axis, either parallel or anti-parallel to the magnetic field. A radiofrequency photon pulse is then emitted, which provides additional energy for the protons to transition from the low-energy state (parallel) to the high-energy state (anti-parallel). As the protons start to return to the lower energy state, they re-emit the energy they absorbed in the form of photons, which are used by a detector to digitally reconstruct an image. Protons have two ways of returning to their original position, the so-called longitudinal relaxation and transverse relaxation. The average amount of time required for longitudinal relaxation (T1) and transverse relaxation (T2) differs according to the composition of each tissue and can thus be used to characterize myocardial tissues essentially on the basis of their proton content (25).
CMR is the only non-invasive imaging modality capable of detecting myocardial inflammation, before functional changes start to occur (e.g., in LV and RV volumes and ejection fractions). Combined with the use of a paramagnetic gadolinium-based contrast agent, CMR provides a fundamental contribution to the diagnosis of myocarditis using three types of images: T2-weighted (T2-W) images, early T1-weighted (T1-W) images (early gadolinium enhancement [EGE]), and delayed enhanced images (late gadolinium enhancement [LGE]) (the former taken after 1 min and the latter after 10–20 min following the injection of paramagnetic contrast agent).
According to Lake Louise criteria for the diagnosis of myocarditis, the myocardial to skeletal muscle T2-signal ratio, as well as EGE and LGE imaging should be evaluated before reaching a diagnostic conclusion. The examination is considered as positive for myocarditis, if 2/3 of the examined indices are positive (26). These criteria have been successfully used for acute myocarditis detection, with sensitivities and specificities ranging from 53% to 92% and 57% to 95%, respectively. However, lower values were seen when endomyocardial biopsies (EMBs) were used as reference standard instead of clinical/angiographic findings (26). In an EMB-based study, Lurz et al. (27) differentiated the diagnostic performance of the criteria for acute and chronic myocarditis. In acute myocarditis, sensitivity and specificity were 76% and 54%, respectively. However, they were considerably less accurate in chronic myocarditis, yielding a sensitivity and specificity of 63% and 40%, respectively, for the diagnosis of EMB-documented chronic myocarditis (27). In a study on EMB-documented acute and chronic myocarditis, T2-mapping alone yielded an area under the receiver-operator characteristics curve (AUC) of 0.81 and 0.77 for the detection of acute and chronic myocarditis, respectively, whereas the criteria yielded an AUC of 0.56 and 0.53, respectively (28). These findings raise concerns about the diagnostic value of the Lake Louise criteria, particularly in patients with chronic myocarditis.
More recently introduced T1-based indices reflect myocardial disease involving both the myocytes and interstitium, whereas the extracellular volume fraction (ECV) is a direct gadolinium contrast-based measurement of extracellular space size, reflecting the presence of interstitial pathology. A recent study documented that ECV and native T1 mapping performed at least equally well compared with established CMR-indices such as the Lake Louise criteria (29). In another study, the application of Lake Louise criteria revealed silent myocarditis in SSc patients without cardiac symptoms and had no correlation with blood inflammatory indices, cardiac troponin, or disease characteristics (30). In this study, CMR was proven a promising tool for diagnosing and monitoring the response to immunomodulatory treatment in SSc patients with silent myocarditis (30).
Pathophysiology behind the CMR assessment of inflammation-fibrosis
The increase in water content of myocardial tissues, owing to myocardial edema occurring during the inflammatory process, is the main cause of longer T2 relaxation times. Paramagnetic contrast agents are not used for T2-based sequences, as they only affect T1 relaxation time. There are three ways to assess edema using T2-W sequences.
The visual assessment: T2-W sequences are very sensitive to water increase. Therefore, there is a bright area in the myocardium using T2-W images because of increased water and myocardial edema. This is a qualitative approach and can be potentially misleading, if there are motion/respiratory artifacts (31).
T2 signal ratio of myocardium to skeletal muscle: This is a semi-quantitative approach. A T2 signal ratio of >1.9 has been used as an indicator of myocardial edema in Lake Louise criteria (31).
T2 mapping: This is a pure quantitative approach. Normal myocardial T2-mapping values, acquired using steady-state free precession imaging, have been reported to be 52.18±3.4 ms using a 1.5T magnetic field (32) and 51.6±3 ms using a 3T magnetic field (33).
EGE
EGE describes the phenomenon of regional vasodilatation, increased blood flow, and capillary leakage usually occurring in inflammatory processes. These phenomena lead to increased paramagnetic contrast retention in the early washout period, resulting in prolongation of T1 relaxation times (34). Analysis of EGE images is commonly performed using the global relative enhancement (gRE), which is calculated as myocardial SI divided by skeletal muscle SI. Most studies use a gRE value of 4.0 as the threshold between healthy and abnormal myocardium (35).
The sensitivity, specificity, and diagnostic accuracy of EGE for diagnosing acute myocarditis are 66%, 70%, and 67%, respectively, with a wide range of diagnostic performances reported for both myocardial signal enhancement and gRE analysis techniques. Interestingly, Bohnen et al. (36) found no statistical difference in gRE between heart failure patients with histologically confirmed inflammation and those without. gRE values significantly decrease between acute and convalescent phases of myocarditis (37). EGE may also be an index of area at risk of both reversible and irreversible cardiac lesions post-acute myocardial infarction (38).
LGE
CMR can detect and quantify replacement-type myocardial fibrosis, due to irreversible myocardial damage (viability study). The preferred imaging time for replacement fibrosis detection is between 10 and 20 min after paramagnetic contrast agent administration, when the contrast agent concentrations in the different tissue compartments have reached a steady state. This method is referred to in the literature as LGE CMR and is the diagnostic gold standard for the assessment of replacement-type fibrosis in vivo (Figure 4) (39). CMR can detect fibrosis in as little as 1 cm3 of tissue, thus having significantly higher spatial resolution than other in vivo methods of evaluation, such as echocardiography and nuclear imaging modalities. CMR also has excellent agreement with histologic findings in both animal and human studies (39). However, SSc may be accompanied by diffuse interstitial-type myocardial fibrosis next to the replacement-type identified by LGE (39). Diffuse interstitial fibrosis takes place in the interstitial space between myocardial cells and cannot be detected by LGE. Therefore, novel CMR indices have been developed that are sensitive for the expansion of the extracellular space (presented in the following section).
Figure 4.
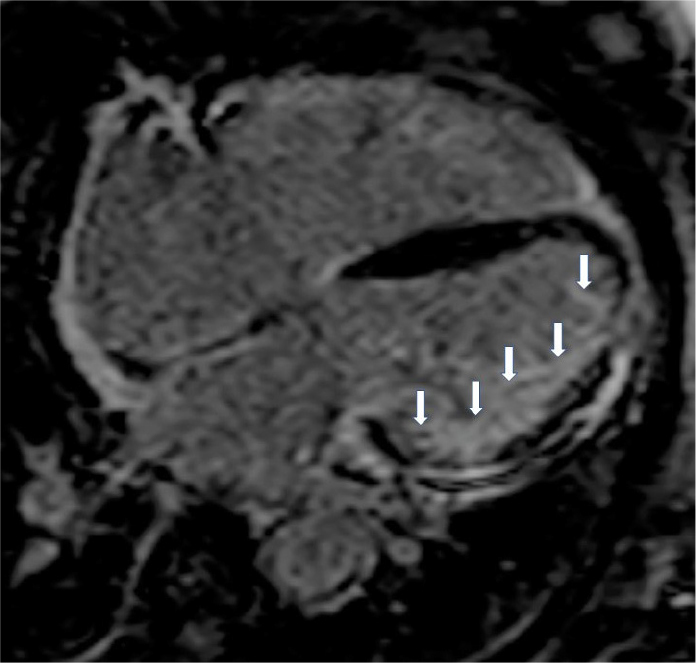
Four chamber inversion recovery image showing diffuse subendocardial fibrosis from an SSc patient with rapid cardiac deterioration.
New CMR indices
Native T1 mapping
Native T1 is a promising method for the detection of myocardial abnormalities without the need for administration of paramagnetic contrast agents. Normal myocardial native T1 values, acquired using the modified look-locker inversion recovery magnetic resonance (MR) method, have been reported to be 930±21 ms and 1052±23 ms at a magnetic field strength of 1.5 T and 3 T, respectively (40). The native T1 value of the myocardium is also dependent on age and sex with men and older subjects having slightly higher values than women and younger subjects (41). Pathologic processes can alter either the water composition or the molecular environment of the myocardium and consequently alter T1 relaxation time in diffuse interstitial fibrosis, edema, and inflammation (42, 43).
Extracellular volume fraction values
The myocardium can be divided into its cellular and extracellular or interstitial components. The extracellular space contains interstitial free fluid, collagen fibrils, and glycoproteins (44). The interstitium is a dynamic environment of vital significance for normal cardiac function (44, 45). Extracellular space expansion is an important factor in ventricular remodeling and a potential therapeutic target. Myocardial fibrosis, a common pathologic finding of heart diseases and a major independent predictor of adverse cardiac events, is the major cause of extracellular space expansion (44). Other pathologic processes such as edema and inflammation may also cause extracellular space expansion (45). Until recently, EMB was the only available method for the quantification of diffuse fibrosis. T1 mapping sequences have enabled the non-invasive quantitative estimation of myocardial interstitial remodeling. The myocardial ECV is derived on the basis of native T1 mapping values and T1 mapping values after injection of paramagnetic contrast agent corrected for the hematocrit level, and its clinical utility has been demonstrated by several studies (43).
CMR issues in the evaluation of SSc patients
Specifically in SSc, several difficulties may be encountered in the evaluation of myocardial inflammation/fibrosis:
Replacement-type myocardial fibrosis constitutes the “trademark” of SSc and thus cannot be considered as an acute myocarditis index. Distinguishing between edema and fibrosis in SSc patients is not possible even when employing T1 mapping (46). In addition, there is a great variation in LGE signal intensity, although the significance of this variation remains unknown. A signal intensity above 5 standard deviations (SD) of the normal myocardium should be considered as full intensity LGE and a grayscale analysis of intermediate-signal intensity LGE should be performed for cases with ≥2SD but <5SD of the normal myocardium (47). The presence and extent of LGE is also linked to ventricular arrhythmias in SSc patients (48).
In parallel with replacement fibrosis, identified by LGE in SSc, diffuse interstitial fibrosis can be also detected. The latter remains unidentified by LGE and only the new CMR indices including T1 mapping and extracellular volume index (ECV) are able to assess it (46). However, recent findings comparing ECV with EMB in inflammatory cardiomyopathies proved that the concept of ECV as an index of diffuse myocardial fibrosis is correct only in the absence of significant myocardial inflammation. Taking into account that various degrees of myocardial inflammation and fibrosis may coexist in various diseases including SSc, the measured ECV will reflect a sum of different pathologies occurring in the extracellular volume, but will not inform exclusively about the extent of diffuse fibrosis (47, 49).
CMR maybe not always be in agreement with EMB. However, considerations including the exact timing of CMR and/or EMB play an important role in this case. Furthermore, CMR is more suited to identification of acute inflammatory processes and may underestimate chronic inflammatory lesions (35).
Conclusion
Non-invasive CV imaging in the form of echocardiography and CMR are of paramount importance for the detection of CVD in SSc. We recommend a thorough baseline echo and, if available, a CMR evaluation at diagnosis. Serial echocardiographic evaluations are also strongly recommended, possibly every 6–12 months, according to the overall clinical picture. Patients with any change in echocardiographic parameters during follow-up or with any significant increase in myocardial necrosis biomarkers (cardiac troponins) should be immediately evaluated with CMR, and administration of relevant cardiac and anti-rheumatic medication should be promptly contemplated. CMR can be also used as a screening tool in order to avoid unnecessary coronary angiography in patients presenting with chest pain, ECG changes, and/or elevated cardiac biomarker levels.
To conclude, the indications to refer SSc patients for CMR include:
ECG and/or echocardiographic abnormalities necessitating further evaluation.
Typical or atypical cardiac symptoms not otherwise clearly explained.
A significant increase in myocardial stress/injury biomarkers.
Any mismatch between clinical symptoms and echocardiographic findings or biomarker values.
Evaluation/change of cardiac and anti-rheumatic medication.
Most of these indications are unfortunately not supported by solid published data yet, and up-to-date essentially reflect routine clinical experience. There is a strong need of a cooperative effort from the scientific community to provide robust evidence for the use of advanced cardiac imaging to support the management of these patients, whose natural history and specific needs in presence of SSc-CI are so different from the more common and known cardiac conditions.
Main Points.
The CMR examination in SSc should include:
Biventricular volume and ejection fraction evaluation.
Detection of autoimmune myocardial inflammation, because such patients should be promptly treated with immunosuppressive medication. Recently, the Journal of the American College of Cardiology Scientific Expert Panel provided consensus recommendations for an update of the Lake Louise Criteria that include options to use parametric mapping techniques. The authors proposed that CMR provides strong evidence for myocardial inflammation, with increasing specificity, if the CMR scan demonstrates the combination of myocardial edema with other CMR markers of inflammatory myocardial injury. This is based on at least one T1-based criterion (increased myocardial T1, extracellular volume, or LGE) with at least one T2-based criterion (global or regional increase in myocardial T2 relaxation time or an increased signal intensity in T2-weighted CMR images). Although having both a positive T2-based marker and a T1-based marker will increase specificity for diagnosing acute myocardial inflammation, having only one (i.e., T2-based OR T1-based) marker may still support a diagnosis of acute myocardial inflammation in an appropriate clinical scenario, but with less specificity.
T2 mapping is the best index of edema indicating acute myocardial inflammatory reaction.
ECV is an indicator of diffuse myocardial fibrosis only in the absence of significant myocardial inflammation. However, if myocardial inflammation and fibrosis coexist, ECV reflects a combination of inflammation and fibrosis, but does not provide information exclusively about the extent of diffuse fibrosis.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.I.M., M.B., G.M.M., B.D., N.R.P., L.G.; Literature Search - S.I.M., M.B., G.M.M., B.D., N.R.P., L.G.; Writing Manuscript - S.I.M., M.B., G.M.M., B.D., N.R.P., L.G.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Barnes J, Mayes MD. Epidemiology of systemic sclerosis: Incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol. 2012;24:165–70. doi: 10.1097/BOR.0b013e32834ff2e8. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 3.Rangarajan V, Matiasz R, Freed BH. Cardiac complications of systemic sclerosis and management: Recent progress. Curr Opin Rheumatol. 2017;29:574–84. doi: 10.1097/BOR.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 4.Maione S, Cuomo G, Giunta A, Tanturri De Horatio L, La Montagna G, Manguso F, et al. Echocardiographic alterations in systemic sclerosis: A longitudinal study. Semin Arthritis Rheum. 2005;34:721–7. doi: 10.1016/j.semarthrit.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010;69:218–21. doi: 10.1136/ard.2008.103382. [DOI] [PubMed] [Google Scholar]
- 6.Thompson AE, Pope JE. A study of the frequency of pericardial and pleural effusions in scleroderma. Br J Rheumatol. 1998;37:1320–3. doi: 10.1093/rheumatology/37.12.1320. [DOI] [PubMed] [Google Scholar]
- 7.Dinser R, Frerix M, Meier FMP, Klingel K, Rolf A. Endocardial and myocardial involvement in systemic sclerosis - is there a relevant inflammatory component? Jt Bone Spine. 2013;80:320–3. doi: 10.1016/j.jbspin.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Mavrogeni S, Bratis K, van Wijk K, Stavropoulos E, Hautemann D, Reiber JHC, et al. Myocardial perfusion-fibrosis pattern in systemic sclerosis assessed by cardiac magnetic resonance. Int J Cardiol. 2012;159:e56–8. doi: 10.1016/j.ijcard.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Bissell LA, Anderson M, Burgess M, Chakravarty K, Coghlan G, Dumitru RB, et al. Consensus best practice pathway of the UK Systemic Sclerosis Study group: Management of cardiac disease in systemic sclerosis. Rheumatology (Oxford) 2017;56:912–21. doi: 10.1093/rheumatology/kew488. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 11.Gargani L, Voilliot D, D’Alto M, Agoston G, Moreo A, Serra W, et al. Pulmonary circulation on the crossroads between the left and right heart in systemic sclerosis: A clinical challenge for cardiologists and rheumatologists. Heart Fail Clin. 2018;14:271–81. doi: 10.1016/j.hfc.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara F, Gargani L, Ruohonen S, Vriz O, Scalese M, Russo V, et al. Reference values and correlates of right atrial volume in healthy adults by two-dimensional echocardiography. Echocardiography. 2018;35:1097–107. doi: 10.1111/echo.14015. [DOI] [PubMed] [Google Scholar]
- 14.Innelli P, Esposito R, Olibet M, Nistri S, Galderisi M. The impact of ageing on right ventricular longitudinal function in healthy subjects: A pulsed tissue Doppler study. Eur J Echocardiogr. 2009;10:491–8. doi: 10.1093/ejechocard/jen313. [DOI] [PubMed] [Google Scholar]
- 15.Gargani L, Volpicelli G. How i do it: Lung ultrasound. Cardiovasc Ultrasound. 2014;12:25. doi: 10.1186/1476-7120-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang YK, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res Ther. 2017;19:206. doi: 10.1186/s13075-017-1409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huez S, Roufosse F, Vachiéry JL, Pavelescu A, Derumeaux G, Wautrecht JC, et al. Isolated right ventricular dysfunction in systemic sclerosis: Latent pulmonary hypertension? Eur Respir J. 2007;30:928–36. doi: 10.1183/09031936.00025607. [DOI] [PubMed] [Google Scholar]
- 18.Gargani L, Pignone A, Agoston G, Moreo A, Capati E, Badano LP, et al. Clinical and echocardiographic correlations of exercise-induced pulmonary hypertension in systemic sclerosis: A multicenter study. Am Heart J. 2013;165:200–7. doi: 10.1016/j.ahj.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Callejas-Rubio JL, Moreno-Escobar E, De La Fuente PM, López Pérez L, Rios Fernández R, Sánchez-Cano D, et al. Prevalence of exercise pulmonary arterial hypertension in scleroderma. J Rheumatol. 2008;35:1812–6. [PubMed] [Google Scholar]
- 20.Voilliot D, Magne J, Dulgheru R, Kou S, Henri C, Caballero L, et al. Prediction of new onset of resting pulmonary arterial hypertension in systemic sclerosis. Arch Cardiovasc Dis. 2016;109:268–77. doi: 10.1016/j.acvd.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Crean A, Ballard G, Maredia N, Greenwood J, Thomson J. 3D echo systematically underestimates right ventricular volume compared to cardiac magnetic resonance in a population with adult congenital heart disease. J Cardiovasc Magn Reson. 2010;12:P18. doi: 10.1186/1532-429X-12-S1-P18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Der Zwaan HB, Geleijnse ML, McGhie JS, Boersma E, Helbing WA, Meijboom FJ, et al. Right ventricular quantification in clinical practice: Two-dimensional vs. three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12:656–64. doi: 10.1093/ejechocard/jer107. [DOI] [PubMed] [Google Scholar]
- 23.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 24.Haeck MLA, Scherptong RWC, Marsan NA, Holman ER, Schalij MJ, Bax JJ, et al. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:628–36. doi: 10.1161/CIRCIMAGING.111.971465. [DOI] [PubMed] [Google Scholar]
- 25.Mavrogeni S, Markousis-Mavrogenis G, Koutsogeorgopoulou L, Kolovou G. Cardiovascular magnetic resonance imaging: Clinical implications in the evaluation of connective tissue diseases. J Inflamm Res. 2017;10:55–61. doi: 10.2147/JIR.S115508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito A, Francone M, Faletti R, Centonze M, Cademartiri F, Carbone I, et al. Lights and shadows of cardiac magnetic resonance imaging in acute myocarditis. Insights Imaging. 2016;7:99–110. doi: 10.1007/s13244-015-0444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: The myoracer-trial. J Am Coll Cardiol. 2016;67:1800–11. doi: 10.1016/j.jacc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Corona-Villalobos CP, Kiani AN, Eng J, Kamel IR, Zimmerman SL, et al. Myocardial T2 mapping by cardiovascular magnetic resonance reveals subclinical myocardial inflammation in patients with systemic lupus erythematosus. Int J Cardiovasc Imaging. 2015;31:389–97. doi: 10.1007/s10554-014-0560-3. [DOI] [PubMed] [Google Scholar]
- 29.Nadjiri J, Hendrich E, Will A, Pankalla C, Shehu N, Martinoff S, et al. Performance of native and contrast enhanced T1 mapping to detect myocardial damage in patients with suspected myocarditis: A head to head comparison of different CMR-techniques. J Cardiovasc Magn Reson. 2015;17:O86. doi: 10.1186/1532-429X-17-S1-O86. [DOI] [PubMed] [Google Scholar]
- 30.Mavrogeni S, Koutsogeorgopoulou L, Karabela G, Stavropoulos E, Katsifis G, Raftakis J, et al. Silent myocarditis in systemic sclerosis detected by cardiovascular magnetic resonance using Lake Louise criteria. BMC Cardiovasc Disord. 2017;17:187. doi: 10.1186/s12872-017-0619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giri S, Shah S, Xue H, Chung YC, Pennell ML, Guehring J, et al. Myocardial T2 mapping with respiratory navigator and automatic nonrigid motion correction. Magn Reson Med. 2012;68:1570–8. doi: 10.1002/mrm.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA, Wassmuth R, Greiser A, Schwenke C, et al. Myocardial T1 and T2 mapping at 3 T: Reference values, influencing factors and implications. J Cardiovasc Magn Reson. 2013;15:53. doi: 10.1186/1532-429X-15-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: Comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 35.Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging. 2012;5:513–24. doi: 10.1016/j.jcmg.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, et al. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging. 2015;8:e003073. doi: 10.1161/CIRCIMAGING.114.003073. [DOI] [PubMed] [Google Scholar]
- 37.Mavrogeni S, Spargias C, Bratis C, Kolovou G, Markussis V, Papadopoulou E, et al. Myocarditis as a precipitating factor for heart failure: Evaluation and 1-year follow-up using cardiovascular magnetic resonance and endomyocardial biopsy. Eur J Heart Fail. 2011;13:830–7. doi: 10.1093/eurjhf/hfr052. [DOI] [PubMed] [Google Scholar]
- 38.Hammer-Hansen S, Leung SW, Hsu LY, Wilson JR, Taylor J, Greve AM, et al. Early gadolinium enhancement for determination of area at risk: A preclinical validation study. JACC Cardiovasc Imaging. 2017;10:130–9. doi: 10.1016/j.jcmg.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S, et al. Cardiovascular magnetic resonance in rheumatology: Current status and recommendations for use. Int J Cardiol. 2016;217:135–48. doi: 10.1016/j.ijcard.2016.04.158. [DOI] [PubMed] [Google Scholar]
- 40.Dabir D, Child N, Kalra A, Rogers T, Gebker R, Jabbour A, et al. Reference values for healthy human myocardium using a T1 mapping methodology: Results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:69. doi: 10.1186/s12968-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piechnik SK, Ferreira VM, Lewandowski AJ, Ntusi NA, Banerjee R, Holloway C, et al. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson. 2013;15:13. doi: 10.1186/1532-429X-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–7. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mavrogeni S, Apostolou D, Argyriou P, Velitsista S, Papa L, Efentakis S, et al. T1 and T2 mapping in cardiology: “Mapping the obscure object of desire”. Cardiology. 2017;138:207–17. doi: 10.1159/000478901. [DOI] [PubMed] [Google Scholar]
- 44.Spinale FG, Frangogiannis NG, Hinz B, Holmes JW, Kassiri Z, Lindsey ML. Crossing into the next frontier of cardiac extracellular matrix research. Circ Res. 2016;119:1040–5. doi: 10.1161/CIRCRESAHA.116.309916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dongaonkar RM, Stewart RH, Geissler HJ, Laine GA. Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc Res. 2010;87:331–9. doi: 10.1093/cvr/cvq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mavrogeni SI, Schwitter J, Gargani L, Pepe A, Monti L, Allanore Y, et al. Cardiovascular magnetic resonance in systemic sclerosis: “Pearls and pitfalls”. Semin Arthritis Rheum. 2017;47:79–85. doi: 10.1016/j.semarthrit.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Krumm P, Mueller KAL, Klingel K, Kramer U, Horger MS, Zitzelsberger T, et al. Cardiovascular magnetic resonance patterns of biopsy proven cardiac involvement in systemic sclerosis. J Cardiovasc Magn Reson. 2016;18:70. doi: 10.1186/s12968-016-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mavrogeni S, Gargani L, Pepe A, Monti L, Markousis-Mavrogenis G, De Santis M, et al. Cardiac magnetic resonance predicts ventricular arrhythmias in scleroderma: The Scleroderma Arrhythmia Clinical Utility Study (SAnCtUS) Rheumatology (Oxford) 2019 Nov 25; doi: 10.1093/rheumatology/kez494. doi: 10.1093/rheumatology/kez494. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lurz JA, Luecke C, Lang D, Besler C, Rommel KP, Klingel K, et al. CMR-derived extracellular volume fraction as a marker for myocardial fibrosis: The importance of coexisting myocardial inflammation. JACC Cardiovasc Imaging. 2018;11:38–45. doi: 10.1016/j.jcmg.2017.01.025. [DOI] [PubMed] [Google Scholar]