Abstract
There have been many recent trials in systemic sclerosis (SSc) that have explored treatment for skin or lung. Some have been encouraging, but there has also been disappointment reflecting potential limitations of treatment effect of study design. These trials are discussed and reviewed. Studies conducted in SSc are described and discussed with a focus on endpoint selection and trial design as well as potential mechanism of action and treatment effect. Studies have included very encouraging trials of interleukin 6 blockade, immunosuppression, and broad-spectrum tyrosine kinase inhibition. Other trials including recent studies of peroxisome proliferator-activated receptor agonists and specific intracellular signaling inhibitors such as imatinib or anti-transforming growth factor beta blocking strategies have been more disappointing. Trial design is improving, and overall, there are now almost positive trials using agents with great promise, and studies are also providing important biological insight into SSc. It is hoped that ongoing studies will further progress the field and move it toward better treatments for SSc that still represent a major unmet medical need.
Keywords: Systemic sclerosis, clinical trial, research design
Introduction
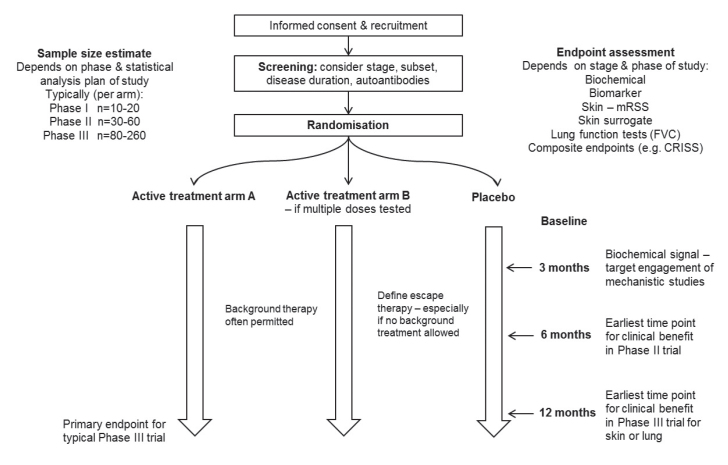
Systemic sclerosis (SSc) is a challenging disease clinically and has proven to be a difficult condition for undertaking clinical trials. This has been a result of the pathobiology of the disease that leads to tissue damage that may be difficult to reverse and also a result of the difficulty of identifying the best trial endpoints. In addition, the condition is heterogeneous and so it can be challenging to identify the most informative patients for a specific therapeutic approach. However, there has been substantial progress, and this has included better study design (1). Some of the principles of scleroderma trial design are included in Figure 1, and there have been several relevant publications that highlight the most appropriate methodology for different types of study depending on the target organ or manifestation. One recent development that may help in trial design is the development of a composite endpoint, the Combined Response Index for Systemic Sclerosis (CRISS), which has been provisionally endorsed by the American College of Rheumatology and is now being used as a primary or secondary endpoint in a number of clinical studies. This may facilitate drug development by giving a signal of clinical benefit that is more relevant to patients and more robust than previous approaches such as the modified Rodnan skin score (mRSS).
Figure 1.
Schematic summarizing overall clinical trial templates for systemic sclerosis.*
*Possible approaches for trial design in systemic sclerosis (SSc). Definitive studies should be placebo-controlled although background therapy may be permitted. Endpoints can include skin (modified Rodnan skin score [mRSS]), lung function (forced vital capacity [FVC]), or composite clinical endpoints such as the Combined Response Index for Systemic Sclerosis (CRISS). Molecular or biomarker endpoints may be considered earlier at 3–6 months linked to blood or skin biopsy studies although these will not assess clinical utility.
Overview of recently completed studies
There have been multiple studies performed in SSc over the past decade, and this is testament to the high unmet medical need and the importance of establishing a robust evidence base for current potential treatment approaches. It also reflects growing interest in potential, new, and more targeted approaches to SSc management. A summary of the most recent trials targeting skin or lung fibrosis is shown in Table 1.
Table 1.
Summary of endpoint data from recent placebo-controlled trials in systemic sclerosis.
| Trial | Total (n) | Duration (weeks) | Drug | mRSS | Δ mRSS | FVC | CRISS | HAQ-DI | Global patient | Global physician |
|---|---|---|---|---|---|---|---|---|---|---|
| FAST (2) | 45 | 52 | Low dose prednisolone with IV cyclophosphamide then oral azathioprine | NA | NA | 0.08 | NA | NA | NA | NA |
| SLS-1 (3) | 158 | 52 | Oral cyclophosphamide | 0.008 | −3.06 | 0.03 | NA | 0.009 | NA | NA |
| BUILD-2 (42) | 163 | 52 | Bosentan | NS | −0.30 | NS | NA | NA | NA | NA |
| AIMSPRO (44) | 20 | 26 | Hyperimmune caprine serum | 0.06 | NA | NA | NA | NS | NS | NS |
| faSScinate (26) | 87 | 48 | SC TCZ | 0.06* | −3.55 | 0.10 | 0.01 | NS | NS | 0.08 |
| focuSSced (27) | 212 | 48 | SC TCZ | 0.10* | −1.73 | 0.002 | 0.02 | NS | NS | NS |
| JBT-101-SSc (41) | 42 | 16 | Lenabasum | 0.09 | −2.60 | NS | 0.04 | 0.03 | 0.10 | 0.02 |
| ASSET (20) | 88 | 52 | Abatacept | NS | −1.75 | NS | 0.03 | 0.005 | NS | 0.03 |
| FASST (31) | 145 | 48 | Lanifibranor | NS | +0.90 | NS | NA | NA | 0.08 | NA |
| RISE-SSc (43) | 121 | 52 | Riociguat | 0.08 | −2.34 | NS | NS | NS | NA | NA |
| SENSCIS (35) | 576 | 52 | Nintedanib | NS | NS | 0.04 | NS | NA | NA | NA |
There was significant reduction in meaningful worsening of mRSS.
mRSS: modified Rodnan skin score; FVC: forced vital capacity; CRISS: Combined Response Index for Systemic Sclerosis; HAQ-DI: Health Assessment Questionnaire Disability Index; FAST: Fibrosing Alveolitis in Scleroderma Trial; IV: intravenous; NA: not applicable; SLS: Scleroderma Lung Study; BUILD-2: Bosentan in Interstitial Lung Disease in Systemic Sclerosis-2; NS: not significant; AIMSPRO: Anti-inflammatory Immuno-Suppressive Product; faSScinate: Safety and Efficacy of Subcutaneous Tocilizumab in Adults with Systemic Sclerosis; SC: subcutaneous; TCZ: tocilizumab; focuSSced: Efficacy and Safety of Tocilizumab in Participants with Systemic Sclerosis; JBT-101-SSc: Safety, Tolerability, Efficacy, and Pharmacokinetics of JBT-101 in Systemic Sclerosis; ASSET: Abatacept Systemic Sclerosis Trial; FASST: For A Systemic Sclerosis Treatment; RISE-SSc: Riociguat in Patients with Early Diffuse Cutaneous Systemic Sclerosis; SENSCIS: Safety and Efficacy of Nintedanib in Systemic Sclerosis.
Conventional immunosuppression
The first studies focused on lung fibrosis and collectively have demonstrated strong evidence for benefit, including the Fibrosing Alveolitis in Scleroderma Trial (FAST) (2) and Scleroderma Lung Study (SLS)-I trials (3) and more recently SLS-II (4) that had a non-inferiority design and demonstrated group level improvement in lung function for both oral cyclophosphamide over 12 months and mycophenolate mofetil (MMF) over 24 months. Other important trials have included the European Scleroderma Observational Study (5) that assessed skin score over 12 months and evidence supportive of potential benefit confirmed that this was modest. This is in line with the impact of conventional immunosuppression on lung fibrosis.
Combined analysis of SLS-I and -II has pointed toward defining minimal clinically important difference at a group level (6), anchored for lung by changes in symptoms, and also confirmed benefit for mRSS albeit over more than one clinical trial (7).
High intensity immunosuppression with autologous hematopoietic stem cell transplantation
Case reports and small series suggested that high dose immunosuppression with hematopoietic stem cell transplantation (HSCT) may be beneficial in some cases of SSc. This led to phase I studies (8) and then a series of phase II trials that compared HSCT after conditioning with monthly intravenous (IV) cyclophosphamide given over 12 months. The three trials completed to date have all suggested benefit especially in terms of long-term survival. The largest study Autologous Stem cell Transplantation International Scleroderma (ASTIS) trial (9) reported a 10% treatment-related mortality that resulted in a greater number of early deaths in the transplant arm but later deaths were much less common. Therefore long term, there was a much better survival than for conventional immunosuppression with IV cyclophosphamide over 12 months. The small American Scleroderma Stem cell versus Immune Suppression Trial (ASSIST) study (10) also favored transplant but the size of the study and the switching of patients to transplant when they progressed on cyclophosphamide limits interpretation. The most recently reported study was Scleroderma: Cyclophosphamide or Transplantation (SCOT) (11). This was a small but rigorous study that used a novel hierarchical composite endpoint to aid interpretability of treatment effect. Using this novel approach, there was a clear signal of benefit, and the treatment-related mortality was lowest at 3% of the three published SSc trials. Together, these studies underpin the routine use of HSCT in poor prognosis cases of SSc, but major challenges in case selection and in standardization of the treatment protocols remain and should be addressed in future clinical research studies.
Targeted immunosuppression
Biological agents have the potential to test therapies and also better understand the molecular and cellular pathogenesis of SSc. The following have been evaluated.
Anti-tumor necrosis factor
The transformative impact of anti-tumor necrosis factor (TNF) treatment for inflammatory arthritis prompted evaluation in many other immune-mediated diseases. For SSc, there was a concern that the crosstalk between proinflammatory and profibrotic cytokines might lead to worsening of fibrotic pathology despite clear evidence for preclinical models that fibrosis could be reduced by TNF blockade. This prompted assessment of use of anti-TNF agents in SSc. There was a review of cases treated within a multicentric international European Scleroderma Trials and Research Group (EUSTAR) register (12) that supported possible benefit and did not point to adverse effects. A small open-label study in diffuse cutaneous SSc (dcSSC) (13) was undertaken using infliximab monotherapy. There was a trend of benefit for skin improvement but no major treatment effect, although in this study there were frequent human anti-chimeric antibody responses that may have attenuated benefit and been a consequence of not co-prescribing other immunosuppression.
Basiliximab
Targeted immunosuppression using an interleukin (IL)-2 receptor antagonist (basiliximab, anti-CD25) was tested in a small study that reported potential benefit and no adverse effects. There was improvement in lung function and skin, but the study design prevented robust interpretation of treatment effect. This approach however has not been pursued (14).
Rituximab
There is strong indirect evidence that the B cell compartment is overactive and potentially pathogenic in SSc. This comes from links to autoantibodies and association of certain reactivities such as Scl-70 with much higher frequency of lung fibrosis and increased mortality. The benefit observed for rituximab (RTX) in other rheumatic diseases prompted testing in SSc, and there have now been several case series and controlled trials that support benefit. There has also been an encouraging retrospective review of the EUSTAR multicenter international cohort that offers some support for benefit for skin. Although the Rituximab Versus Cyclophosphamide in Connective Tissue Disease-Interstitial Lung Disease (RECITAL) clinical trial that includes many SSc cases will provide much needed comparative data between RTX and IV cyclophosphamide, large studies are lacking (15–18).
Abatacept
Another more selective approach to immunomodulation is the use of abatacept, which is a recombinant fusion protein composed of the Fc region of the immunoglobulin G1 fused to the extracellular domain of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). T cell activation depends on two signals. One of those signals is the major histocompatibility complex, combined with the antigen, and the other signal is the CD80 or CD86 molecule. Abatacept binds to the CD80 and CD86 molecule to prevent the second signal, and the T cell cannot be activated. There were initial case reports suggesting benefit for abatacept in SSc and localized scleroderma. A small clinical trial. (19) suggested benefit in dcSSc and led to a larger study, Abatacept Systemic Sclerosis Trial (ASSET), which was a placebo-controlled trial in 88 patients who were without other immunosuppressive treatment (20). This trial was one of the several recent studies that failed to reach its primary endpoint of skin benefit, although a trend of benefits was observed. However, subgroup analysis was convincing and secondary endpoints were reached including the Health Assessment Questionnaire Disability Index, which is generally regarded as an important functional score that correlates with other important outcomes in SSc. In addition, this study has identified molecular markers that may predict clinical response in the skin and thus opens the way to more stratified or personalized medicine approaches in the future. In this way, the study is one of several that failed technically in primary endpoint but almost certainly has meaningful impact and has advanced understanding of trial design, informative endpoints, and case stratification.
Tocilizumab
The rationale for blocking IL-6 in SSc has substantial evidential support that includes seminal studies showing that SSc fibroblasts in culture produce large amounts of IL-6 protein compared with healthy control dermal fibroblasts (21) and studies linking serum levels of IL-6 to poor clinical outcome (22). In addition, there are studies in animals that support the role of IL-6 signaling in fibrosis. The clinical cohort data include a discovery study linking IL-6 levels to risk of progression and poor survival in SSc that was followed by a validation cohort analysis (23), which confirmed that elevated IL-6 in serum predicted poor survival and decline in lung function in SSc cases of lung fibrosis. This was, however, only seen in cases with milder disease, using the Goh et al. (24) staging system, that is, forced vital capacity (FVC) above 70% predicted was associated with progression if IL-6 was elevated. This suggests a potential role in earlier stage lung fibrosis and that other processes or mediators may be involved in later stage or more severe lung fibrosis. A robust study of IL-6 serum levels and skin disease confirmed the plausibility of IL-6 as a mediator in cases of skin disease that are severe and refractory (25). High levels of IL-6 were associated with the failure of skin score to improve over 36 months and with much worse long-term survival.
Two well-conducted clinical trials were built upon the foundation of these data and both have generated interesting and largely confirmatory outcome data. The first study, Safety and Efficacy of Subcutaneous Tocilizumab in Adults with Systemic Sclerosis (faSScinate), was a phase II trial that enrolled 87 patients randomized to tocilizumab (TCZ) or placebo without other background therapy (26). The primary endpoint at 24 weeks showed a trend of benefit for skin that almost reached significance at 48 weeks. There was a remarkable benefit for lung function with much less decline on TCZ than placebo. This was relevant because the study design had really tried to enrich for cases that had high IL-6 by specifying high platelet count or elevated C-reactive protein. Baseline lung function was largely preserved because the cases were early stage and did not contain lung fibrosis. Skin threshold was 15–40 mRSS.
On the basis of the encouraging phase II data, a larger phase III clinical trial, Efficacy and Safety of Tocilizumab in Participants with Systemic Sclerosis (focuSSced) was designed. This followed a similar design but was less rigorous in acute phase requirement and allowed cases with a broader range of skin score (mRSS 10–35). The overall results for focuSSced were disappointing. The trial replicated faSScinate by showing a trend of benefit for mRSS change at 48 weeks despite a much larger sample size. This reflected greater improvement on placebo and perhaps also the much lower skin score on entry that implies milder cases being enrolled that may have a greater likelihood of spontaneous improvement in mRSS over the time frame of the study. This aligns with other trials and perhaps reflects the greater stringency of skin disease in faSScinate. Interestingly, there was a meaningful reduction in skin progression by 20% of mRSS. This reached statistical significance but could not be interpreted as positive as this was a phase III trial with a strict prespecified statistical analysis plan (27).
Of much more interest and significance was the impact of TCZ on lung function in focuSSced. The outstanding treatment effect observed in faSScinate was replicated and exceeded. The phase III study had much more rigorous lung data including baseline and end of treatment computed tomography (CT) scan and centralized and quality-controlled lung function measurement. The subset of cases with lung fibrosis at baseline by visual read of CT were the main driver of the overall cohort impact on lung function, and exploratory analysis of CT quantitation was highly supportive. Thus, the two TCZ studies together provide a very robust evidence base that in active dcSSc, lung function decline can be almost completely prevented at a group level, and this benefit is especially seen in those with lung fibrosis on CT scan at baseline.
Anti-fibrotic therapy
The hallmark pathology of SSc is connective tissue fibrosis. Therefore, it has been of great interest to develop potential anti-fibrotic therapies. In addition, these could have applications well beyond SSc as fibrosis is a major contributor to other health problems, including liver, lung, renal, and cardiac disease. There have been several recent trials in SSc that test putative anti-fibrotic therapy, focusing on skin or lung disease.
Lysophosphatidic acid receptor 1 antagonist
There is strong preclinical evidence that a lysophosphatidic acid receptor 1 (LPA1) antagonist may prevent lung fibrosis (28), and this was tested in a small phase II trial that randomized patients with dcSSc to an oral LPA1 antagonist or placebo. The goal of this study was to assess target engagement and mechanistic impact, and this was fully achieved with the analysis of skin biopsies. The LPA1 fibroblast signature was determined using cultured fibroblasts, and this was very strongly attenuated in the skin of patients receiving the study drug compared with placebo. There was a trend for benefit in skin score, but the study was not designed or powered to test that; thus, further trials will be needed to properly assess the benefit of this approach. An interesting aspect of this phase II trial was that the patients were permitted to continue stable background immunosuppressive treatments. This is important as it suggests that any additional treatment effect may be separate from immunosuppression (29).
Peroxisome proliferator-activated receptor agonist
Preclinical evidence strongly suggests that peroxisome proliferator-activated receptor (PPAR) activity is reduced in fibrotic diseases, including SSc, and this has also been supported in mouse models. Therefore, it was an attractive proposition to use a pan-PPAR agonist to treat SSc (30). A phase II trial, For A Systemic Sclerosis Treatment (FASST) was recently completed but unfortunately was entirely negative. This study allowed background immunosuppression; thus, it is possible that this blunted the ability to demonstrate treatment effect. It had been hoped that a broad PPAR agonist profile (lanifibranor, IVA337) might not only have been more effective but also better tolerated than selective PPAR agonists. Although not yet fully published, the available data do not support this (31).
Tyrosine kinase inhibitors
There has been a longstanding interest in the potential for tyrosine kinase inhibitors (TKI) to be anti-fibrotic. This led to studies of imatinib and nilotinib in SSc that were not positive (32–34) and highlighted the poor tolerability of these agents despite routine use in oncology. However, a less specific TKI, nintedanib, has been shown to be effective in idiopathic lung fibrosis and is licensed for this indication. This prompted a trial in SSc that has recently been published. The primary endpoint was met in this study, and thus, lung function decline was significantly reduced in patients on nintedanib.
The Safety and Efficacy of Nintedanib in Systemic Sclerosis (SENSCIS) trial showed a group level benefit of 41 mL reduction on loss of FVC over 52 weeks (35). Although modest, this reached statistical significance and some patients demonstrated substantially larger treatment effect. Overall, unlike the trials of MMF, there was no improvement in FVC suggesting that this form of therapy might slow the progression of fibrosis rather than reverse established pathology. This contrasts with immunomodulation with MMF or IV cyclophosphamide in the FAST study in the UK and with reports for myeloablative autologous stem cell transplant in the SCOT study.
Anti-transforming growth factor beta therapy
The central role of transforming growth factor beta (TGFβ) as a mediator of tissue fibrosis has emerged from many sources. These include preclinical studies as well as tissue and cell-based analysis of disease tissue, including SSc skin and lung. In vivo mouse models with TGFβ activation, especially targeted to fibroblasts, provide compelling evidence, and the deletion of TGFβ receptor II on fibroblasts renders mice resistant to skin or lung fibrosis. These data supported the rationale for targeting TGFβ as a treatment. Indeed, it has been suggested that until targeting TGFβ is definitely shown to be too unsafe or definitely ineffective, it remains the most logical target in SSc for disease modifying therapy. Interestingly, the fibroblast studies from the phase II TCZ trial faSScinate demonstrated almost complete reversal of the TGFβ-activated phenotype of SSc fibroblasts in explant culture (36). This provides additional mechanistic insight into the role of IL-6 in modulating TGFβ activity as has been suggested in other recent work (37). The first trial of anti-TGFβ tested a relatively low affinity mono-specific antibody, CAT-192 (metelimumab) (38). This was a placebo-controlled trial testing three doses of antibody. There was no clear evidence of benefit and improvement overall in all groups, including placebo. This study was a major disappointment in study design because very early treatment naive dcSSc were recruited and even these cases often improved over 6 months of observation. This provides important insight into the natural history of skin fibrosis and is in contrast to the features of lung fibrosis in this same population of early dcSSc as evidenced by two TCZ trials. The most recent study was more convincing by testing a more potent pan-specific anti-TGFβ (fresolimumab) with impact on molecular markers and skin sclerosis (39). Unfortunately, this was an open-label study and so interpretability is challenging.
Endocannabinoid trials
A novel approach to treating fibrosis is seen in trials of the endocannabinoid agonist lenabasum. This is a lipid mediator that has selectivity for the cannabinoid receptor type 2 that has emerged as a potent modulator of inflammation and appears to promote resolution and have anti-fibrotic potential (40). A small phase II trial in SSc was very promising (41). It assessed treatment over 16 weeks with lenabasum (JBT-101) or placebo and showed striking benefit for the CRISS composite index. However, there are limitations to the interpretations as the trial was much shorter than the design underpinning CRISS validation. A phase III trial is currently underway, and this will confirm whether there is benefit for lenabasum on a range of endpoints, including CRISS and mRSS.
Therapies utilized in pulmonary arterial hypertension
The suspected role of endothelin in the pathophysiology of lung fibrosis led to a 12 month placebo-controlled trial of bosentan, a nonselective endothelin receptor antagonist, in a cohort enriched for patients with active and progressive SSc-related interstitial lung disease (ILD). Unfortunately, there was no significant improvement in the primary endpoint of exercise tolerance and no significant treatment effect for the other secondary end points including time to death and decline in lung function (42).
Riociguat, a soluble guanylate cyclase (sGC) stimulator with vasoactive, anti-proliferative and antifibrotic effects was hypothesized to reduce progression of skin fibrosis in patients with dcSSc. In a placebo-controlled trial of early dcSSc patients, there was a non-significant benefit in mRSS with riociguat but no significant difference between treatment groups in changes in predicted FVC or DLCO (diffusing capacity for carbon monoxide). Analysis at a subgroup level looking only at SSc-ILD patients suggested a potential efficacy signal for riociguat for smaller decline in FVC and DLCO that will need further evaluation (43).
Reverse translation from clinical trials and proof-of-concept studies
One of the consistent messages from recent trials has been that it is now possible to differentiate active drug from placebo, and the use of novel therapeutics is providing powerful insight into possible mechanism of action for therapies and about fundamental aspects of disease biology. Examples of this have been shown above for LPA1 inhibition and anti-TGFβ. Likewise, there were compelling mechanistic data from the phase II TCZ trial.
Another interesting study was a phase II trial evaluating hyperimmune caprine serum (Anti-inflammatory Immuno-Suppressive Product, AIMSPRO) as a novel biological therapy (44). This had benefit for skin in an extended dataset analyzed after 6 months of therapy compared with placebo and parallel serum analysis that highlighted the potential anti-fibrotic effect of alpha-melanocortin stimulating hormone (α-MSH) that was rapidly stimulated by the AIMSPRO (45). Animal and in vitro human experiments have suggested that α-MSH has anti-fibrotic potential (46).
Conclusions and future perspective
In summary, there have been many clinical trials over the past two decades and these are increasingly positive. There is now compelling evidence that both nintedanib and TCZ can prevent the progression of lung fibrosis in SSc, although they may act by distinct mechanisms and at different stages or extent of disease. There are encouraging signals for several trials for skin or for improvement in the CRISS score, and thus, there is a reason to be optimistic that the current portfolio of trials underway will further inform and advance treatments for SSc. Despite this, there have been many failed trials along the way, and it is important to learn from these and to refine and improve study design to start to achieve the therapeutic advances that patients with SSc deserve. In the meantime, there is enormous unmet need from non-lethal aspects of the disease, including gut problems, calcinosis, fatigue, and the physical and facial consequences of SSc. Any progress for skin or lung will just be a first step toward better and more effective treatment for this intransigent disease.
Main Points.
Compelling evidence that both nintedanib and tocilizumab can prevent progression of lung fibrosis in systemic sclerosis (SSc).
Encouraging signals for several trials for skin or for improvement in the Combined Response Index for Systemic Sclerosis score.
Important to learn from trial failures and to refine and improve study design to start to achieve therapeutic advances that patients with SSc deserve.
Enormous unmet need from non-lethal aspects of SSc remains.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.P.D., P.Y., V.H.O.; Supervision - C.P.D., P.Y., V.H.O.; Data Collection and/or Processing - C.P.D., P.Y., V.H.O.; Analysis and/or Interpretation - C.P.D., P.Y., V.H.O.; Literature Search - C.P.D., P.Y., V.H.O.; Writing Manuscript - C.P.D., P.Y., V.H.O.; Critical Review - C.P.D., P.Y., V.H.O.
Conflict of Interest: C.P.D. was supported by personal fees from Roche, Inventiva, GSK, Bayer, Sanofi, CSL Behring, Corbus, Boehringer-Ingelheim, and by grants Inventiva, GSK, CSL Behring during the conduct of the study.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Johnson SR, Khanna D, Allanore Y, Matucci-Cerinic M, Furst DE. Systemic sclerosis trial design moving forward. J Scleroderma Relat Disord. 2016;1:177–80. doi: 10.5301/jsrd.5000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): A randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4:708–19. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrick AL, Pan X, Peytrignet S, Lunt M, Hesselstrand R, Mouthon L, et al. Treatment outcome in early diffuse cutaneous systemic sclerosis: The European Scleroderma Observational Study (ESOS) Ann Rheum Dis. 2017;76:1207–18. doi: 10.1136/annrheumdis-2016-210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kafaja S, Clements PJ, Wilhalme H, Tseng CH, Furst DE, Kim GH, et al. Reliability and minimal clinically important differences of forced vital capacity: Results from the Scleroderma Lung Studies (SLS-I and SLS-II) Am J Respir Crit Care Med. 2018;197:644–52. doi: 10.1164/rccm.201709-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna D, Clements PJ, Volkmann ER, Wilhalme H, Tseng CH, Furst DE, et al. Minimal clinically ımportant differences for the modified rodnan skin score: Results from the Scleroderma Lung Studies (SLS-I and SLS-II) Arthritis Res Ther. 2019;21:23. doi: 10.1186/s13075-019-1809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binks N, Passweg JR, Furst D, McSweeney P, Sullivan K, Besenthal C, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: Procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60:577–84. doi: 10.1136/ard.60.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: A randomized clinical trial. JAMA. 2014;311:2490–8. doi: 10.1001/jama.2014.6368. [DOI] [PubMed] [Google Scholar]
- 10.Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): An open-label, randomised phase 2 trial. Lancet. 2011;378:498–506. doi: 10.1016/S0140-6736(11)60982-3. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med. 2018;378:35–47. doi: 10.1056/NEJMoa1703327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Distler JH, Jordan S, Airo P, Alegre-Sancho JJ, Allanore Y, Balbir Gurman A, et al. Is there a role for TNFα antagonists in the treatment of SSc? EUSTAR expert consensus development using the Delphi technique. Clin Exp Rheumatol. 2011;29:S40–5. [PubMed] [Google Scholar]
- 13.Denton CP, Engelhart M, Tvede N, Wilson H, Khan K, Shiwen X, et al. An open-label pilot study of infliximab therapy in diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2009;68:1433–9. doi: 10.1136/ard.2008.096123. [DOI] [PubMed] [Google Scholar]
- 14.Becker MO, Brückner C, Scherer HU, Wassermann N, Humrich JY, Hanitsch LG, et al. The monoclonal anti-CD25 antibody basiliximab for the treatment of progressive systemic sclerosis: An open-label study. Ann Rheum Dis. 2011;70:1340–1. doi: 10.1136/ard.2010.137935. [DOI] [PubMed] [Google Scholar]
- 15.Elhai M, Boubaya M, Distler O, Smith V, Matucci-Cerinic M, Alegre Sancho JJ, et al. Outcomes of patients with systemic sclerosis treated with rituximab in contemporary practice: A prospective cohort study. Ann Rheum Dis. 2019;78:979–87. doi: 10.1136/annrheumdis-2018-214816. [DOI] [PubMed] [Google Scholar]
- 16.Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T, et al. A multicenter, open-label, comparative study of B-cell depletion therapy with Rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum. 2017;46:625–31. doi: 10.1016/j.semarthrit.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Saunders P, Tsipouri V, Keir GJ, Ashby D, Flather MD, Parfrey H, et al. Rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease (RECITAL): Study protocol for a randomised controlled trial. Trials. 2017;18:275. doi: 10.1186/s13063-017-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melsens K, Vandecasteele E, Deschepper E, Badot V, Blockmans D, Brusselle G, et al. Two years follow-up of an open-label pilot study of treatment with rituximab in patients with early diffuse cutaneous systemic sclerosis. Acta Clin Belg. 2018;73:119–25. doi: 10.1080/17843286.2017.1372244. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty EF, Martyanov V, Fiorentino D, Wood TA, Haddon DJ, Jarrell JA, et al. Gene expression changes reflect clinical response in a placebo-controlled randomized trial of abatacept in patients with diffuse cutaneous systemic sclerosis. Arthritis Res Ther. 2015;17:159. doi: 10.1186/s13075-015-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanna D, Spino C, Johnson S, Chung L, Whitfield M, Denton CP, et al. Abatacept in early diffuse cutaneous systemic sclerosis - results of a phase 2 investigator-initiated, multicenter, double-blind randomized placebo-controlled trial. Arthritis Rheumatol. 2020;72:125–36. doi: 10.1002/art.41055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of pathogenesis in scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol. 1992;19:1207–11. [PubMed] [Google Scholar]
- 22.Muangchant C, Pope JE. The significance of interleukin-6 and C-reactive protein in systemic sclerosis: A systematic literature review. Clin Exp Rheumatol. 2013;31:122–34. [PubMed] [Google Scholar]
- 23.De Lauretis A, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh N, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. 2013;40:435–46. doi: 10.3899/jrheum.120725. [DOI] [PubMed] [Google Scholar]
- 24.Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–54. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 25.Khan K, Xu S, Nihtyanova S, Derrett-Smith E, Abraham D, Denton CP, et al. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann Rheum Dis. 2012;71:1235–42. doi: 10.1136/annrheumdis-2011-200955. [DOI] [PubMed] [Google Scholar]
- 26.Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet. 2016;387:2630–40. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 27.Khanna D, Lin CJF, Goldin J, Kim G, Kuwana M, Allanore Y, et al. OP0245 preservation of lung function observed in a phase 3 randomized controlled trial of tocilizumab for the treatment of early SSc. Ann Rheum Dis. 2019;78:202–3. doi: 10.1136/annrheumdis-2019-eular.2120. [DOI] [Google Scholar]
- 28.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 29.Allanore Y, Distler O, Jagerschmidt A, Illiano S, Ledein L, Boitier E, et al. Lysophosphatidic acid receptor 1 antagonist SAR100842 for patients with diffuse cutaneous systemic sclerosis: A double-blind, randomized, eight-week placebo-controlled study followed by a sixteen-week open-label extension study. Arthritis Rheumatol. 2018;70:1634–43. doi: 10.1002/art.40547. [DOI] [PubMed] [Google Scholar]
- 30.Ruzehaji N, Frantz C, Ponsoye M, Avouac J, Pezet S, Guilbert T, et al. Pan PPAR agonist IVA337 is effective in prevention and treatment of experimental skin fibrosis. Ann Rheum Dis. 2016;75:2175–83. doi: 10.1136/annrheumdis-2015-208029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inventiva. Inventiva announces results from phase IIb clinical trial with lanifibranor in systemic sclerosis. Feb 18, 2019. Available from: URL: http://inventivapharma.com/2019/02/results-from-phase-iib-clinical-trial-with-lanifibranor-in-systemic-sclerosis.
- 32.Pope J, McBain D, Petrlich L, Watson S, Vanderhoek L, de Leon F, et al. Imatinib in active diffuse cutaneous systemic sclerosis: Results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single center. Arthritis Rheum. 2011;63:3547–51. doi: 10.1002/art.30549. [DOI] [PubMed] [Google Scholar]
- 33.Khanna D, Saggar R, Mayes MD, Abtin F, Clements PJ, Maranian P, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum. 2011;63:3540–6. doi: 10.1002/art.30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon JK, Martyanov V, Magro C, Wildman HF, Wood TA, Huang WT, et al. Nilotinib (Tasigna™) in the treatment of early diffuse systemic sclerosis: An open-label, pilot clinical trial. Arthritis Res Ther. 2015;17:213. doi: 10.1186/s13075-015-0721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis-associated ınterstitial lung disease. N Engl J Med. 2019;380:2518–28. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 36.Denton CP, Ong VH, Xu S, Chen-Harris H, Modrusan Z, Lafyatis R, et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: Insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis. 2018;77:1362–71. doi: 10.1136/annrheumdis-2018-213031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty D, Šumová B, Mallano T, Chen CW, Distler A, Bergmann C, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8:1130. doi: 10.1038/s41467-017-01236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: A multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56:323–33. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 39.Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez EG, Selvi E, Balistreri E, Akhmetshina A, Palumbo K, Lorenzini S, et al. Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. Ann Rheum Dis. 2012;71:1545–51. doi: 10.1136/annrheumdis-2011-200314. [DOI] [PubMed] [Google Scholar]
- 41.Spiera R, Hummers L, Chung L, Frech T, Domsic R, Furst D, et al. OP0126 A phase 2 study of safety and efficacy of anabasum (JBT-101) in systemic sclerosis. Ann Rheum Dis. 2017;76:105. doi: 10.1136/annrheumdis-2017-eular.2712. [DOI] [Google Scholar]
- 42.Seibold JR, Denton CP, Furst DE, Guillevin L, Rubin LJ, Wells A, et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis (BUILD-2) Arthritis Rheum. 2010;62:2101–8. doi: 10.1002/art.27466. [DOI] [PubMed] [Google Scholar]
- 43.Distler O, Allanore Y, Denton CP, Kuwana M, Matucci-Cerinic M, Pope JE, et al. OP0183 Efficacy and safety of riociguat in patients with early diffuse cutaneous systemic sclerosis and interstitial lung disease (SSc-ILD): Results from the Phase IIB RISE-SSc Study. Ann Rheum Dis. 2019;78:167. doi: 10.1136/annrheumdis-2019-eular.6889. [DOI] [Google Scholar]
- 44.Quillinan NP, McIntosh D, Vernes J, Haq S, Denton CP. Treatment of diffuse systemic sclerosis with hyperimmune caprine serum (AIMSPRO): A phase II double-blind placebo-controlled trial. Ann Rheum Dis. 2014;73:56–61. doi: 10.1136/annrheumdis-2013-203674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quillinan NP, Clark KE, Youl B, Vernes J, McIntosh D, Haq S, et al. Multiplex serum protein analysis reveals potential mechanisms and markers of response to hyperimmune caprine serum in systemic sclerosis. Arthritis Res Ther. 2017;19:45. doi: 10.1186/s13075-017-1252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokot A, Sindrilaru A, Schiller M, Sunderkötter C, Kerkhoff C, Eckes B, et al. Alpha-melanocyte-stimulating hormone suppresses bleomycin-induced collagen synthesis and reduces tissue fibrosis in a mouse model of scleroderma: Melanocortin peptides as a novel treatment strategy for scleroderma? Arthritis Rheum. 2009;60:592–603. doi: 10.1002/art.24228. [DOI] [PubMed] [Google Scholar]