Abstract
This study was conducted to investigate the effects of dietary sodium butyrate (SB) supplementation on growth performance, liver function, antioxidant capacity, carcass characteristics, and meat quality in broilers under hot climatic conditions. A total of 288 one-day-old Arbor Acres broilers were randomly allocated to 4 dietary treatments as follow: CON, control diet without SB; T1, control diet with 300 mg/kg SB; T2, control diet with 600 mg/kg SB; and T3, control diet with 1,200 mg/kg SB. Each treatment had 6 replication pens and 12 broilers per pen. The results indicated that the BW on day 35; ADG from day 1 to 21, day 22 to 35, and day 1 to 35; and ADFI from day 22 to 35 linearly (P < 0.05) increased with SB supplementation. Interestingly, alanine aminotransferase and aspartate aminotransferase content in serum were linearly (P < 0.05) decreased by SB supplementation. There was linear (P < 0.05) improvement in activity of superoxide dismutase and catalase in the liver, whereas the content of malondialdehyde was linearly (P < 0.05) decreased with the inclusion of SB. Increasing SB level linearly (P < 0.05) increased CP composition and decreased drip loss percentage on day 1 and 3 of breast muscle. Furthermore, there was linear (P < 0.05) improvement in activity of superoxide dismutase, glutathione peroxidase, and catalase, whereas the content of malondialdehyde showed decreasing trend (P < 0.10) with the inclusion of SB in breast muscle. In conclusion, SB can be used as an effective feed additive to improve growth performance, liver function, and meat quality of broilers under hot climatic conditions.
Key words: sodium butyrate, growth performance, anti-oxidant capacity, meat quality, broiler
Introduction
Despite the development in the design of animal farming house facilities and cooling technologies, animal health and production are still severely affected by high ambient temperature. Modern poultry genotypes seem to be particularly sensitive to heat stress because of higher metabolic activity (Settar et al., 1999; Deeb and Cahaner, 2002). It has been indicated that heat stress resulted in annual economic loss to the US poultry production industry of $128 to $165 million (St-Pierre et al., 2003). Heat stress has become a considerable challenge in the poultry industry because poultry production and welfare are adversely affected by heat stress (Lara and Rostagno, 2013). Heat conditions caused changes in mortality (Quinteiro-Filho et al., 2010), growth performance (Sohail et al., 2012), metabolism (Mujahid et al., 2005), immune response (Niu et al., 2009; Bozkurt et al., 2012; Deng et al., 2012), and meat quality (Zhang et al., 2017) and disturb the balance between the production of reactive oxygen species and the antioxidant defense system (Feng et al., 2008; Liu et al., 2013). Proper nutritional strategy is a method to alleviate the detrimental effects on poultry health and production induced by heat stress. Abdel-Wareth et al. (2019) demonstrated that dietary symbiotic improved growth performance and meat quality of broilers in hot climatic regions. Hartanto et al. (2019) indicated that dietary nutmeg oil could inhibit lipid oxidation and maintain the liver function of broilers under heat stress.
A common nutritional strategy to improve poultry production while decreasing mortality is through antibiotics as a growth promoter. The use of antibiotics was questioned as a public concern owing to antimicrobial resistance and residues. Butyrate is known as a safe alternative to antibiotics, received wide attention in the poultry industry. Butyrate is odorous and unstable; however, sodium butyrate (SB) was used as a replacement in the poultry production owing to its stable and nonodorous properties. It has been indicated that SB plays an important role as an energy source to gastrointestinal epithelial cells and has antimicrobial, anti-inflammatory, and antioxidant properties (Guilloteau et al., 2010; Zhang et al., 2011a,b; Liu et al., 2014; Song et al., 2017). Recent studies also indicated that SB could alleviate the detrimental effects of lipopolysaccharide or corticosterone-challenged broilers by improving growth performance, antioxidant capacity, and meat quality (Zhang et al., 2011a,b). Muscle meat quality is related to antioxidant capacity (Zhang et al., 2017). Former studies have reported that heat stress results in decreasing antioxidant enzyme activity in serum (Liu et al., 2013) and breast muscle (Lu et al., 2017) of broilers. Therefore, the negative effects of heat stress on meat quality may be associated with the change of antioxidant capacity. However, no practical animal study was conducted to evaluate the effect of dietary SB supplementation on meat quality and muscle antioxidant status of broilers under heat stress. Hence, we hypothesize that dietary SB may improve meat quality through decreasing oxidative stress under heat stress. Therefore, the purpose of this study was to evaluate the effect of dietary SB supplementation on muscle antioxidant status and meat quality of broilers under hot climatic conditions.
Materials and methods
Experiment Design and Dietary Treatments
A total of 288 1-day-old female Arbor Acres broilers were randomly allocated to 4 dietary treatments with 6 replication pens per treatment and 12 broilers per replication pen. The treatments were CON, control diet without SB; T1, control diet with 300 mg/kg SB; T2, control diet with 600 mg/kg SB; and T3, control diet with 1,200 mg/kg SB. The diet was provided in a mashed form and was formulated to meet or exceed the nutritional requirements of broilers during the starter (day 1–21) and grower (day 22–35) phases, as per the NRC (1994) recommendation (Table 1). The SB product was provided by a commercial company (Beijing Shengtaiyuan Biotechnology Co., Ltd., China) and contained 54% SB protected by a physical and chemical matrix of buffer salts.
Table 1.
Ingredient composition and nutrient content of diets.
| Item | Starter (day 1–21) | Grower (day 22–35) |
|---|---|---|
| Ingredients, % | ||
| Corn | 54.57 | 62.44 |
| Soybean meal, 48% CP | 29.95 | 25.58 |
| Corn gluten meal, 60% CP | 5.90 | 3.30 |
| Soybean oil | 5.50 | 4.89 |
| Tricalcium phosphate | 2.46 | 2.29 |
| Limestone | 0.89 | 0.75 |
| Salt | 0.20 | 0.20 |
| DL-Met, 88% | 0.07 | 0.07 |
| L-Lys·HCl (78.4%) | 0.06 | 0.08 |
| Vitamin premix1 | 0.20 | 0.20 |
| Mineral premix2 | 0.20 | 0.20 |
| Calculated composition | ||
| ME, MJ/kg | 12.95 | 12.74 |
| CP, % | 21.89 | 18.90 |
| Ca, % | 1.05 | 0.96 |
| Lys, % | 1.12 | 1.01 |
| Met + Lys, % | 0.90 | 0.86 |
| Available P, % | 0.81 | 0.73 |
| Analyzed composition, % | ||
| CP | 21.12 | 20.02 |
| Ca | 1.03 | 0.95 |
| Met + Lys | 0.89 | 0.87 |
| Available P | 0.44 | 0.42 |
Provided per kilogram of complete diet: 12,8000 IU vitamin A, 1,600 IU vitamin D3, 60 IU vitamin E, 1.6 mg vitamin K3, 0.12 mg biotin, 50 mg choline, 1.2 mg folic acid, 32 mg Nicotinic acid, 16 mg pantothenic acid, 4.8 mg riboflavin, 2.4 mg thiamine (B1), 3.2 mg vitamin B6, and 0.03 mg vitamin B12.
Provided per kilogram of diet: Mg, 79 mg as manganese oxide; Zn, 60 mg as zinc oxide; Cu,100 mg as copper sulfate; Fe, 120 mg as iron sulfate; I, 0.96 mg as potassium iodine; Co, 0.16 mg as cobalt sulfate and Se,0.24 mg as sodium selenite.
Experimental Conditions
Broiler management procedures in this study were approved by the Animal Care and Use Committee of Guangdong Ocean University. Broilers of each replication were assigned in battery pens (124 cm length × 64 cm width × 40 cm height). Artificial light was provided 24 h per day by fluorescent lights and had free access to feed and tap water. The ambient temperature and relative humidity were recorded daily and are shown in Table 2. The average minimum and maximum temperature during the experimental period were 28.80°C and 36.47°C (32.53°C ± 1.63°C) and relative humidity were 56.67 and 85.00% (73.77 ± 7.00%), respectively. The temperature and relative humidity were recorded at 08:00, 12:00, and 18:00 daily, and average temperature and relative humidity were calculated.
Table 2.
The ambient temperature and relative humidity during experimental period.
| Item | Temperature, °C |
Humidity, % |
||||||
|---|---|---|---|---|---|---|---|---|
| 08:00 | 12:00 | 18:00 | Average | 08:00 | 12:00 | 18:00 | Average | |
| Day 1 | 28.80 | 32.70 | 29.10 | 30.20 | 98.0 0 | 65.00 | 82.00 | 81.67 |
| Day 2 | 30.80 | 30.90 | 31.00 | 30.90 | 83.00 | 85.00 | 78.00 | 82.00 |
| Day 3 | 31.10 | 33.70 | 31.70 | 32.17 | 81.00 | 68.00 | 79.00 | 76.00 |
| Day 4 | 31.20 | 34.50 | 31.20 | 32.30 | 79.00 | 64.00 | 81.00 | 74.67 |
| Day 5 | 31.40 | 34.70 | 33.20 | 33.10 | 77.00 | 63.00 | 62.00 | 67.33 |
| Day 6 | 30.00 | 35.20 | 33.80 | 33.00 | 82.00 | 58.00 | 56.00 | 65.33 |
| Day 7 | 30.80 | 36.70 | 33.10 | 33.53 | 82.00 | 52.00 | 63.00 | 65.67 |
| Day 8 | 31.60 | 36.80 | 35.10 | 34.50 | 77.00 | 58.00 | 56.00 | 63.67 |
| Day 9 | 32.10 | 35.10 | 32.90 | 33.37 | 83.00 | 65.00 | 76.00 | 74.67 |
| Day 10 | 30.30 | 31.70 | 31.70 | 31.23 | 85.00 | 66.00 | 72.00 | 74.33 |
| Day 11 | 38.70 | 29.20 | 30.80 | 32.90 | 88.00 | 80.00 | 81.00 | 83.00 |
| Day 12 | 28.20 | 32.10 | 31.70 | 30.67 | 84.00 | 77.00 | 72.00 | 77.67 |
| Day 13 | 30.30 | 32.50 | 30.80 | 31.20 | 88.00 | 74.00 | 81.00 | 81.00 |
| Day 14 | 29.20 | 34.40 | 29.20 | 30.93 | 91.00 | 67.00 | 80.00 | 79.33 |
| Day 15 | 30.30 | 34.20 | 30.20 | 31.57 | 84.00 | 69.00 | 85.00 | 79.33 |
| Day 16 | 31.30 | 33.10 | 31.10 | 31.83 | 80.00 | 67.00 | 81.00 | 76.00 |
| Day 17 | 29.90 | 36.30 | 34.10 | 33.43 | 89.00 | 62.00 | 62.00 | 71.00 |
| Day 18 | 32.10 | 36.60 | 34.10 | 34.27 | 81.00 | 61.00 | 66.00 | 69.33 |
| Day 19 | 32.40 | 36.80 | 32.50 | 33.90 | 79.00 | 61.00 | 77.00 | 72.33 |
| Day 20 | 32.70 | 38.50 | 32.20 | 34.47 | 70.00 | 56.00 | 74.00 | 66.67 |
| Day 21 | 29.60 | 37.60 | 32.20 | 33.13 | 84.0 0 | 54.00 | 74.00 | 70.67 |
| Day 22 | 29.80 | 34.40 | 30.50 | 31.57 | 83.00 | 68.00 | 84.00 | 78.33 |
| Day 23 | 33.10 | 37.10 | 33.30 | 34.50 | 77.00 | 58.00 | 71.00 | 68.67 |
| Day 24 | 30.10 | 37.20 | 36.20 | 34.50 | 84.00 | 56.00 | 59.00 | 66.33 |
| Day 25 | 34.40 | 37.00 | 38.00 | 36.47 | 69.00 | 51.00 | 50.00 | 56.67 |
| Day 26 | 30.50 | 38.00 | 36.20 | 34.90 | 79.00 | 52.00 | 59.00 | 63.33 |
| Day 27 | 31.20 | 25.40 | 38.00 | 31.53 | 80.00 | 99.00 | 50.00 | 76.33 |
| Day 28 | 29.60 | 34.90 | 33.80 | 32.77 | 77.00 | 66.00 | 67.00 | 70.00 |
| Day 29 | 33.50 | 35.50 | 31.80 | 33.60 | 71.00 | 56.00 | 72.00 | 66.33 |
| Day 30 | 28.20 | 29.40 | 28.80 | 28.80 | 87.00 | 86.00 | 82.00 | 85.00 |
| Day 31 | 32.40 | 33.10 | 30.70 | 32.07 | 76.00 | 71.00 | 78.00 | 75.00 |
| Day 32 | 38.70 | 29.20 | 30.80 | 32.90 | 88.00 | 80.00 | 81.00 | 83.00 |
| Day 33 | 29.20 | 32.10 | 31.70 | 31.00 | 84.00 | 77.00 | 72.00 | 77.67 |
| Day 34 | 30.30 | 32.50 | 30.80 | 31.20 | 88.00 | 74.00 | 81.00 | 81.00 |
| Day 35 | 29.20 | 32.40 | 29.20 | 30.27 | 91.00 | 77.0 0 | 80.00 | 82.67 |
Performance Parameters
The BW was recorded on pen basis on day 1, 21, and 35. Feed consumption was recorded on pen basis every week to calculate ADFI and feed conversion ratio (FCR).
Serum Biochemical Parameters
On day 35, after 12 h fasting, 6 broilers per treatment (1 broiler from each replication pen) were randomly selected. The broilers were individually weighed, and then, blood samples were collected from the brachial vein into nonheparinized tubes and centrifuged at 3,000 × g for 10 min at 4°C to obtain serum. The serum samples stored at −20°C until analysis. The analysis of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was in accordance with the method described by Senanayake et al. (2015). The activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) and the content of malondialdehyde (MDA) in serum were measured with corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as per the manufacturer's instructions.
Carcass Traits and Relative Organ Weight
After blood sample collection, the broilers were killed by cervical dislocation and exsanguination. The carcass weight was calculated by removing the feathers and blood, and the eviscerated weight was calculated by removing the head, feet, abdominal fat (fat surrounding the cloaca and gizzard), and all of the viscera except the lungs and kidneys. The dressing and eviscerated yield were expressed as the percentage of live BW. Breast and leg muscle and abdominal fat were excised and weighed to calculate the muscle and abdominal fat yield based on eviscerated weight, respectively. The left part of breast muscle was used to analyze meat quality, and the right part was immediately frozen and stored at −20°C for further analysis.
Breast Muscle Chemical Composition and Meat Quality
Breast samples were analyzed for DM, CP, crude fat, and ash in accordance with Association of Analytical Chemists methods (AOAC, 1995). Briefly, about 5 g of breast muscle (fresh weight) was weighted to determine the moisture and crude fat, about 0.2 g (DM basis) of sample was weighted to determine CP content, and about 2.5 g (DM basis) of sample was weighted to determine ash content.
The breast muscle pH was measured duplicate at a depth of 2.5 cm below the surface using a pH meter at 45 min (pH45 min) and 24 h (pH24 h) postmortem (Fisher Scientific, Pittsburgh, PA). Meat color was measured on 2 points of breast muscle at 45 min postmortem, the lightness, redness, and yellowness were measured using a Model CR-410 Chroma meter (Konica Minolta Sensing Inc., Osaka, Japan). Cooking loss was measured as described previously by Sullivan et al. (2007). For cooking loss determination, approximately 4 g sample was weighed, placed in a plastic bag, and cooked to an internal temperature of 75°C using a water bath; then, the cooked samples were allowed to cool for 30 min, blotted dry, and weighed. Cooking loss values were calculated based on the following equation, cooking loss (%) = (raw weight-cooked weight)/raw weight × 100. The drip loss from 0 to 1, 3, 5, 7 D was assessed from the muscle packaged in a transparent polythene bag and stored at 4°C for 1, 3, 5, and 7 D, after which the excess moisture was wiped out, and the breast samples were weighed (on day 1, 3, 5, 7); drip loss was measured using approximately 4 g of meat sample as per the plastic bag method described by Honikel (1998).
Antioxidant Enzyme Activity and Lipid Peroxidation in the Liver and Breast Muscle
For antioxidant enzyme activity and MDA content measurement, about 1 g frozen liver or breast muscle sample was homogenized in 9 mL of ice-cooled 0.86% sodium chloride solution and centrifuged at 3,000 × g for 10 min at 4°C. The supernatant was collected for further analysis. The protein concentration of the supernatant was determined by the Bradford method using BSA as the standard. The activity of SOD, GSH-Px, and CAT and the content of MDA were measured with corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as per the manufacturer's instruction.
Statistical Analysis
The pen was used as the experiment unit, and all data were analyzed with SAS 2003 (v. 9.1; SAS Institute Inc., Cary, NC) using the mixed procedure. All data were analyzed using 1-way ANOVA followed by Duncan's multiple range test to analyze differences among treatments. Orthogonal polynomial contrasts were used to study the linear and quadratic effects of dietary SB level. Differences were considered significant at P < 0.05, and P < 0.10 was considered as a trend.
Results
Performance Parameters
The effects of SB on growth performance under hot climatic conditions are shown in Table 3. On day 1, no significant differences were observed on BW among the treatments; however, on day 21 and 35, there was a linear (P < 0.05) increase in BW associated with the inclusion of SB in the diet. From day 1 to 21, day 22 to 35, and day 1 to 35, there were linear (P < 0.05) improvements in ADG associated with the inclusion of SB. There was a linear increase in ADFI from day 22 to 35 with the inclusion of SB. No significant differences were observed in FCR among treatments.
Table 3.
Effects of sodium butyrate on growth performance of broilers.
| Item | Sodium butyrate level1, mg/kg |
SE |
P-value |
||||
|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | Linear | Quadratic | ||
| BW (g) | |||||||
| Day 1 | 32.83 | 31.67 | 31.83 | 31.67 | 0.85 | 0.3900 | 0.5621 |
| Day 21 | 709.57a | 711.79a | 725.58a,b | 674.71b | 5.19 | 0.0009 | <0.0001 |
| Day 35 | 1449.67c | 1508.00b | 1549.67a | 1458.67c | 5.50 | 0.0112 | <0.0001 |
| ADG (g) | |||||||
| Day 1–21 | 32.22b | 32.39a,b | 33.04a | 30.62c | 0.25 | 0.0013 | <0.0001 |
| Day 22–35 | 52.87a | 56.87b | 58.86c | 56.00d | 0.06 | <0.0001 | <0.0001 |
| Day 1–35 | 40.48c | 42.18b | 43.37a | 40.77c | 0.16 | 0.0082 | <0.0001 |
| ADFI (g) | |||||||
| Day 1–21 | 51.39a | 50.69a | 50.03a,b | 48.81b | 0.56 | 0.0031 | 0.6458 |
| Day 22–35 | 106.44b | 111.84a,b | 112.37a,b | 115.29a | 2.04 | 0.0076 | 0.5495 |
| Day 1–35 | 73.41 | 75.15 | 74.97 | 75.40 | 0.91 | 0.1690 | 0.4808 |
| FCR | |||||||
| Day 1–21 | 1.60 | 1.57 | 1.51 | 1.59 | 0.02 | 0.4840 | 0.0063 |
| Day 22–35 | 2.01 | 1.97 | 1.91 | 2.06 | 0.04 | 0.6503 | 0.0173 |
| Day 1–35 | 1.81 | 1.78 | 1.73 | 1.85 | 0.07 | 0.5825 | 0.0030 |
a,b,cWithin the same row with different superscripts differ (P < 0.05).
Abbreviation: FCR, feed conversion ration.
Sodium butyrate level: CON, control diet without sodium butyrate; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Antioxidant Enzyme Activity and Lipid Peroxidation of Serum
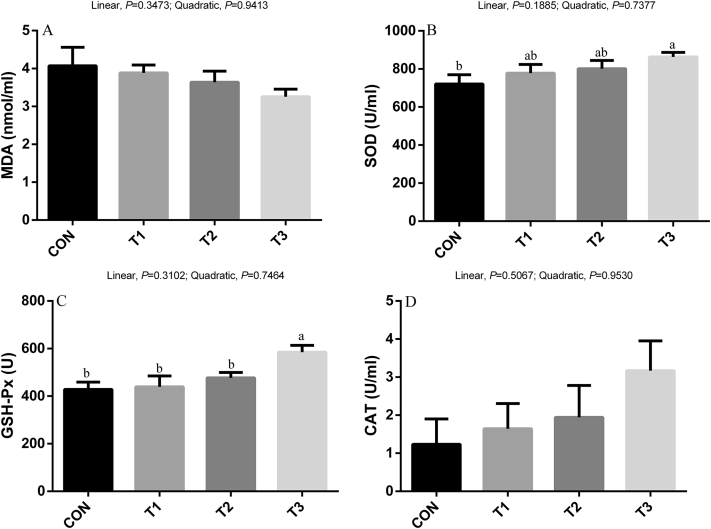
No significant differences were observed in MDA content and the activity of CAT in serum among treatments (Figure 1). The activity of SOD and GSH-Px was higher (P < 0.05) in broilers fed the T3 diet than in the CON group.
Figure 1.
Effects of sodium butyrate on antioxidant indices and lipid peroxidation in serum of broilers. Values are mean ± SE. The values having different superscript letters are different (P < 0.05). a,bMeans with different superscripts differ (P < 0.05). Abbreviations: CAT, catalase; CON, control diet without sodium butyrate; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Liver Function
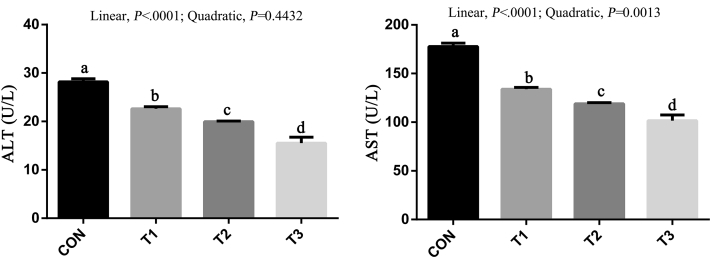
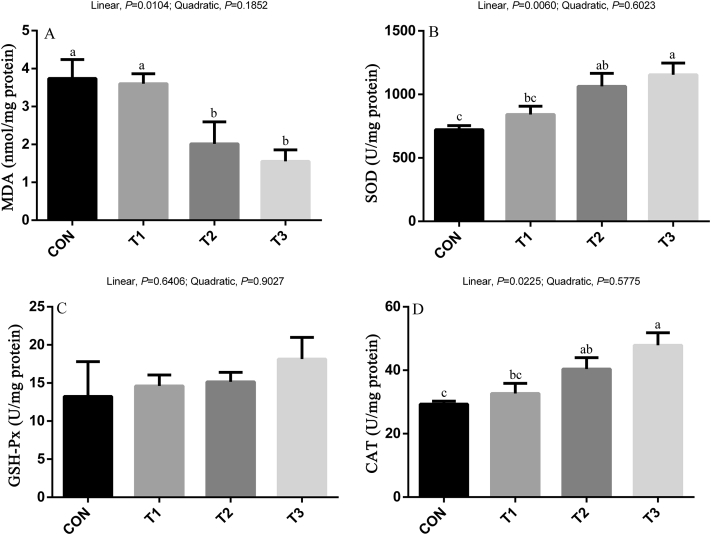
Dietary SB supplementation significantly decreased ALT and AST levels compared with the CON group; there was a linear (P < 0.05) decrease in ALT and AST levels with the inclusion of SB (Figure 2). No differences were observed in the activity of GSH-Px in the liver among treatments (Figure 3). There was a linear (P < 0.05) improvement in activity of SOD and CAT in the liver with the inclusion of SB, whereas the content of MDA shown a linear (P < 0.05) decrease with the inclusion of SB.
Figure 2.
Effects of sodium butyrate on alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum of broilers. Values are mean ± SE. The values having different superscript letters are different (P < 0.05). a,b,c,dMeans with different superscripts differ (P < 0.05). Abbreviations: CON, control diet without sodium butyrate; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Figure 3.
Effects of sodium butyrate on antioxidant indices and lipid peroxidation in the liver of broilers. Values are mean ± SE. The values having different superscript letters are different (P < 0.05). a,b,cMeans with different superscripts differ (P < 0.05). Abbreviations: CAT, catalase; CON, control diet without sodium butyrate; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Carcass Traits, Breast Muscle Chemical Composition, and Meat Quality
No significant differences were observed in dressing, eviscerated, breast muscle, leg muscle, and abdominal fat yield (Table 4). There was a linear (P < 0.05) improvement in the breast muscle CP composition associated with the inclusion of SB, but no significant differences were observed in moisture, ether extract, or ash composition (Table 5). No significant differences were observed in pH45 min or breast muscle color (lightness, redness, and yellowness) among treatments (Table 6). Cooking loss and drip loss on day 5 and 7 shown a linear decreasing trend (P < 0.10), and drip loss on day 1 and 3 shown a linear decrease associated with the inclusion of SB (P < 0.05).
Table 4.
Effects of sodium butyrate on carcass traits of broilers.
| Item | Sodium butyrate level1, mg/kg |
SE |
P-value |
||||
|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | Linear | Quadratic | ||
| Carcass traits2, % | |||||||
| Dressing | 91.10 | 90.37 | 90.25 | 91.47 | 0.71 | 0.7668 | 0.1884 |
| Eviscerated | 75.78 | 74.68 | 73.82 | 74.33 | 0.75 | 0.1359 | 0.2953 |
| Breast muscle | 20.94 | 20.04 | 20.44 | 21.91 | 1.22 | 0.5508 | 0.3432 |
| Leg muscle | 23.23 | 23.17 | 23.04 | 23.89 | 0.85 | 0.6331 | 0.5988 |
| Abdominal fat | 2.27 | 2.56 | 1.89 | 2.31 | 0.30 | 0.6872 | 0.8347 |
Sodium butyrate level: CON, control diet without sodium butyrate; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Dressing, abdominal fat, and eviscerated yield percentages were calculated by dividing these traits by final live weight after fasting. The percentages of breast muscle and thigh muscle were calculated as a percentage of eviscerated carcass weight.
Table 5.
Effects of sodium butyrate on the chemical composition of breast muscle.
| Item, % | Sodium butyrate level1, mg/kg |
SE |
P-value |
||||
|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | Linear | Quadratic | ||
| Moisture | 71.47 | 71.63 | 72.18 | 73.25 | 1.14 | 0.2615 | 0.6947 |
| CP | 25.59a | 26.86a,b | 26.23a,b | 27.16b | 0.43 | 0.0446 | 0.6938 |
| Ether extract | 4.84 | 4.64 | 4.70 | 4.68 | 0.51 | 0.8591 | 0.8656 |
| Ash | 5.32 | 5.09 | 5.27 | 5.15 | 0.24 | 0.7473 | 0.8224 |
a,bWithin the same row with different superscripts differ (P < 0.05).
Sodium butyrate level: CON, control diet without sodium butyrate; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Table 6.
Effects of sodium butyrate on meat quality of broilers.
| Item | Sodium butyrate level1, mg/kg |
SE |
P-value |
||||
|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | Linear | Quadratic | ||
| pH45min | 6.73 | 6.79 | 6.88 | 6.71 | 0.05 | 0.9015 | 0.0414 |
| pH24h | 6.56b | 6.70a | 6.68a | 6.60a,b | 0.04 | 0.4836 | 0.0089 |
| Color indexes, 45 min postmortem | |||||||
| Lightness (L∗) | 52.42 | 49.45 | 51.24 | 50.30 | 0.97 | 0.3047 | 0.3065 |
| Redness (a∗) | 10.26 | 10.35 | 10.29 | 10.53 | 0.14 | 0.2230 | 0.6022 |
| Yellowness (b∗) | 8.81 | 8.82 | 8.94 | 8.92 | 0.15 | 0.5062 | 0.9366 |
| Cooking loss, % | 25.77 | 24.74 | 19.75 | 19.55 | 0.03 | 0.0897 | 0.8912 |
| Drip loss, % | |||||||
| Day 1 | 3.70a | 2.56a,b | 2.44a,b | 1.37b | 0.01 | 0.0077 | 0.9451 |
| Day 3 | 6.09a | 4.42a,b | 4.58a,b | 3.48b | 0.01 | 0.0165 | 0.6704 |
| Day 5 | 7.74 | 6.04 | 6.26 | 5.47 | 0.01 | 0.0677 | 0.5581 |
| Day 7 | 9.01 | 7.71 | 7.79 | 6.92 | 0.01 | 0.0905 | 0.7841 |
a,bWithin the same row with different superscripts differ (P < 0.05).
Sodium butyrate level: CON, control diet without sodium butyrate; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Breast Muscle AntiOxidant Status
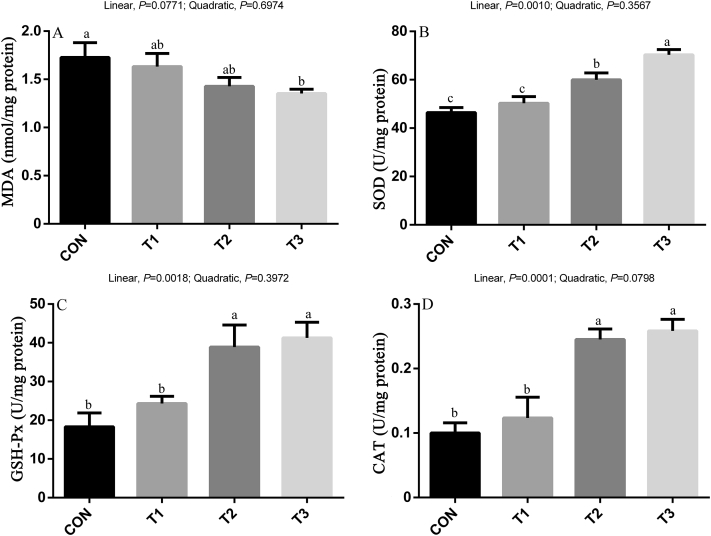
The antioxidant status of breast muscle is shown in Figure 4. There was a linear (P < 0.05) improvement in the activity of SOD, GSH-Px, and CAT with the inclusion of SB, whereas the content of MDA showed a decreasing trend (P < 0.10) with the inclusion of SB.
Figure 4.
Effects of sodium butyrate on antioxidant indices and lipid peroxidation in breast muscle of broilers. Values are mean ± SE. The values having different superscript letters are different (P < 0.05). a,b,cMeans with different superscripts differ (P < 0.05). Abbreviations: CAT, catalase; CON, control diet without sodium butyrate; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; T1, sodium butyrate at 300 mg/kg; T2, sodium butyrate at 600 mg/kg; T3, sodium butyrate at 1,200 mg/kg.
Discussion
Poultry species are more susceptible to heat stress owing to limited heat dissipation capacity associated with lacking sweat glands and covering firm feathers and high body temperature (Yahav et al., 2004). As previously reported, exposing broilers to high temperature leads to behavioral, physiological and immunologic responses, which impose detrimental effects on growth performance and feed conversion efficiency (Sahin et al., 2010). The results of present study showed that dietary SB had beneficial effects on BW, ADG, and ADFI when supplemented at levels of 300 mg/kg or 600 mg/kg; these results were in agreement with those of previous studies, which indicated that dietary butyric acid or its sodium salt had positive effects on performance by increasing feed intake, weight gain, and feed conversion efficiency (Wu et al., 2016; Sikandar et al., 2017). However, negative effects were observed on BW on day 21, as well as ADG and ADFI from day 1 to 21 with a supplemented level at 1,200 mg/kg. This is in agreement with the study by Hu and Guo (2007), who indicated that FCR was significantly increased with SB at the level of 2,000 mg/kg; it has been suggested that overuse of SB had negative effects on performance. Broilers subjected to chronic heat stress had impaired growth performance, reduced feed intake, lower BW, and lower feed conversion efficiency (Niu et al., 2009; Sohail et al., 2012). Besides, dietary SB significantly alleviated the BW loss induced by stress treatment (Zhang et al., 2011a,b; Jiang et al., 2015). However, no beneficial effects on performance were also reported by other studies (Mahdzvi and Torki, 2009; Aghazadeh and TahaYazdi, 2012; Zou et al., 2019). These inconsistent results indicate that SB may play a more effective role on broilers with stress challenge.
Exposure to heat stress may disturb the balance between oxidative stress and antioxidant defense system via increasing lipid peroxidation and depleting antioxidant enzymes (Azad et al., 2010; Yang et al., 2010; Liu et al., 2013), eventually contributing to the increase in nutrient expenditure and oxidative damage (Hall et al., 2010; Sahin et al., 2010). In the present study, SB supplementation increased the activity of SOD and GSH-Px in serum, reduced the content of MDA, and increased the activity of SOD and CAT in the liver, as well as reduced the content of MDA and increased the activity of SOD, GSH-Px, and CAT in breast muscle. It has reported that under thermoneutral temperature, SB supplementation reduced the content of MDA and increased the activity of SOD, CAT, and total antioxidant capacity (Zhang et al., 2011b; Wu et al., 2018). In addition, several studies indicated that SB can alleviate negative effects associated with corticosterone-induced oxidative stress (Zhang et al., 2011a; Jiang et al., 2015). Based on the results of the present study, the improved antioxidant capacity of broilers observed in the SB-supplemented groups suggested that SB-induced effects were independent of the presence of heat stress, although the study on the antioxidant capacity of butyric acid or SB is relative few, especially, in broilers. Hamer et al. (2009) indicated that rectal administration of butyrate enhanced the colonic antioxidant capacity of healthy patients by increasing glutathione content. Sauer et al. (2007) demonstrated that butyrate attenuated H2O2-induced DNA damage and increased CAT activity in human colon cells in vitro. However, the mechanism by how butyrate or its sodium salt attenuates oxidative stress remains unknown. Therefore, the antioxidant property of butyric acid or its sodium salt needs to be further studied.
The liver that plays a major role in controlling glucose storage and flux is known to be the hub of the metabolism. Heat stress is known to induce tissue injury and severe liver damage in ducks (Zeng et al., 2014), and increased serum level of AST demonstrated hepatocyte and liver dysfunction (Tessari et al., 2010). The present study indicated that dietary SB decreased the AST and ALT level; it can be postulated that SB supplementation improved liver function under hot climatic conditions.
It has been reported that heat stress had negative effects on meat chemical composition and quality, decreased the proportion of breast muscle, increased the proportion of thigh muscle and fat deposition, and decreased the protein content (Imik et al., 2012a; Dai et al., 2012; Zhang et al., 2012). However, no significant differences were observed in relative weight of breast muscle, leg muscle, or abdominal fat in the present study but CP composition in breast muscle has been increased with SB supplementation under hot climatic conditions. In addition, dietary SB supplementation improved meat quality by posing a positive effect on pH24 h, cooking loss, and drip loss. Generally, the pH variation in muscle is due to glycogenolysis, heat stress increased glycogen breakdown, and the pH decreased postslaughter, which directly reflect muscle acid content and influence shelf life, water-holding capacity, and protein denaturation (Briskey and Wismer, 1961; Sams, 1999; Ahmed et al., 2016). The drip loss and cooking loss are the important indicators of water-holding capacity and reflect the juiciness of meat (Rasmussen and Andersson, 1996). In the present study, there was a significant decrease in drip loss of breast muscle on day 1 and 3, which is in agreement with the results reported by Zhang et al. (2011a), who indicated that dietary SB decreased 24-h drip loss of corticosterone-challenged broilers. However, no significant differences were observed in cooking loss. Lipid peroxidation is one of the most important factors causing deterioration of meat quality, which leads to flavor and texture problems, alter appearance, and decrease nutritive value (Salih et al., 1987; Fernández et al., 1997). Malondialdehyde is the main end product of lipid peroxidation, and its concentration reflects the lipid peroxidation in muscle (Janero, 1990). In the present study, dietary SB supplementation decreased MDA content in breast muscle, indicating that SB supplementation decreased lipid peroxidation of breast muscle, which may have beneficial effects on meat quality and shelf life. As expected, dietary SB supplementation significantly improved antioxidant status in the breast muscle by increasing the activity of SOD, GSH-Px, and CAT, which was similar to the results reported by Zhang et al. (2011a), who reported that dietary SB alleviated the negative effects of corticosterone-challenged broilers through enhancing CAT activity and decreasing the MDA level. Meat quality has a close relationship with muscle antioxidant status. Numerous studies in broilers indicated that dietary supplementation with antioxidant nutrients, such as vitamin E (Olivo et al., 2001; Hashizawa et al., 2013), ascorbic acid, α-lipoic acid (Imik et al., 2012b), and resveratrol (Zhang et al., 2017), can improve muscle antioxidant status, and finally improve meat quality. The studies on the antioxidant capacity of butyric acid or its sodium salt are limited, especially in breast muscle. Until now, the effects of SB on meat quality are only studied in corticosterone-induced oxidative stress broilers, the mechanism of SB changing breast muscle antioxidant status remains unknown, and the antioxidant property of SB is interesting, which needs to be further studied.
Conclusions
In conclusion, the results of the present study indicate that SB can be used as an effective feed additive to improve growth performance, liver function, and meat quality of broilers under hot climatic conditions. Furthermore, SB addition, particularly at a dose of 1,200 mg/kg alleviated the oxidative stress induced by high ambient temperature in breast muscle and the liver, as a consequence, might improve the meat quality and liver function of broilers.
Acknowledgments
Financial support provided by program for scientific research start-up funds of Guangdong Ocean University (101402/R18005) and Key Platform Project of Innovation strong school Engineering by Department of Education of Guangdong Province (2018302), is gratefully acknowledged.
Conflict of Interest Statement: No potential conflict of interest was reported by the authors.
References
- Abdel-Wareth A.A., Hammad S., Khalaphallah R., Salem W.M., Lohakare J. Synbiotic as eco-friendly feed additive in diets of chickens under hot climatic conditions. Poult. Sci. 2019;98:1–9. doi: 10.3382/ps/pez115. [DOI] [PubMed] [Google Scholar]
- Aghazadeh A., TahaYazdi M. Effect of butyric acid supplementation and whole wheat inclusion on the performance and carcass traits of broilers. S. Afr. J. Anim. Sci. 2012;42:241–248. [Google Scholar]
- Ahmed S.T., Mun H.S., Islam M.M., Ko S.Y., Yang C.J. Effects of dietary natural and fermented herb combination on growth performance, carcass traits and meat quality in grower-finisher pigs. Meat Sci. 2016;122:7–15. doi: 10.1016/j.meatsci.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Azad M., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Phys. A. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Küçükyilmaz K., Catli A., Çınar M., Bintaş E., Çöven F. Performance, egg quality, and immune response of laying hens fed diets supplemented with mannan-oligosaccharide or an essential oil mixture under moderate and hot environmental conditions. Poult. Sci. 2012;91:1379–1386. doi: 10.3382/ps.2011-02023. [DOI] [PubMed] [Google Scholar]
- Briskey E., Wismer P. Biochemistry of Pork Muscle Structure. 1. Rate of anaerobic glycolysis and temperature change versus the apparent structure of muscle tissue. J. Food Sci. 1961;26:297–305. [Google Scholar]
- Chemists, A. O. A. AOAC; Washington: 1995. Official Methods of Analysis of AOAC International. [Google Scholar]
- Council, N. R. National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Dai S., Gao F., Xu X., Zhang W., Song S., Zhou G. Effects of dietary glutamine and gamma-aminobutyric acid on meat colour, pH, composition, and water-holding characteristic in broilers under cyclic heat stress. Br. Poult. Sci. 2012;53:471–481. doi: 10.1080/00071668.2012.719148. [DOI] [PubMed] [Google Scholar]
- Deeb N., Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002;81:293–301. doi: 10.1093/ps/81.3.293. [DOI] [PubMed] [Google Scholar]
- Deng W., Dong X., Tong J., Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012;91:575–582. doi: 10.3382/ps.2010-01293. [DOI] [PubMed] [Google Scholar]
- Feng J., Zhang M., Zheng S., Xie P., Ma A. Effects of high temperature on multiple parameters of broilers in vitro and in vivo. Poult. Sci. 2008;87:2133–2139. doi: 10.3382/ps.2007-00358. [DOI] [PubMed] [Google Scholar]
- Fernández J., Pérez-Álvarez J.A., Fernández-López J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;59:345–353. [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- Hall M.E., Blount J.D., Forbes S., Royle N.J. Does oxidative stress mediate the trade-off between growth and self-maintenance in structured families? Funct. Ecol. 2010;24:365–373. [Google Scholar]
- Hamer H.M., Jonkers D.M., Bast A., Vanhoutvin S.A., Fischer M.A., Kodde A., Troost F.J., Venema K., Brummer R.J.M. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009;28:88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hartanto S., Ko H.S., Jee S.H., Kang J.U., Seo J.S., Kang Y.H., Kim H.N., Ohh S.J. Effect of dietary nutmeg oil on heat-stress tolerance-related parameters in Korean native chicken reared under hot temperature. J. Anim. Physiol. N. 2019;103:1160–1167. doi: 10.1111/jpn.13113. [DOI] [PubMed] [Google Scholar]
- Hashizawa Y., Kubota M., Kadowaki M., Fujimura S. Effect of dietary vitamin E on broiler meat qualities, color, water-holding capacity and shear force value, under heat stress conditions. Anim. Sci. J. 2013;84:732–736. doi: 10.1111/asj.12079. [DOI] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat. Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Hu Z., Guo Y. Effects of dietary sodium butyrate supplementation on the intestinal morphological structure, absorptive function and gut flora in chickens. Anim. Feed. Sci. Tech. 2007;132:240–249. [Google Scholar]
- Imik H., Atasever M.A., Urcar S., Ozlu H., Gumus R., Atasever M. Meat quality of heat stress exposed broilers and effect of protein and vitamin E. Br. Poult. Sci. 2012;53:689–698. doi: 10.1080/00071668.2012.736609. [DOI] [PubMed] [Google Scholar]
- Imik H., Ozlu H., Gumus R., Atasever M.A., Urcar S., Atasever M. Effects of ascorbic acid and α-lipoic acid on performance and meat quality of broilers subjected to heat stress. Br. Poult. Sci. 2012;53:800–808. doi: 10.1080/00071668.2012.740615. [DOI] [PubMed] [Google Scholar]
- Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Bio. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhang W., Gao F., Zhou G. Micro-encapsulated sodium butyrate attenuates oxidative stress induced by corticosterone exposure and modulates apoptosis in intestinal mucosa of broiler chickens. Anim. Prod. Sci. 2015;55:587–594. [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., He J., Xie H., Yang Y., Li J., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2013;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Liu W., Yang Y., Zhang J., Gatlin D.M., Ringø E., Zhou Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014;112:15–29. doi: 10.1017/S0007114514000610. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agr. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Mahdzvi R., Torki M. Study on usage period of dietary protected butyic acid on performance carcass characteristics, serum metabolite levels and humoral immune response of broiler chickens. J. Anim. Vet. Adv. 2009;8:1702–1709. [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Niu Z., Liu F., Yan Q., Li W. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Olivo R., Scares A.L., Ida E.I., Shimokomaki M. Dietary vitamin E inhibits poultry PSE and improves meat functional properties. J. Food Biochem. 2001;25:271–283. [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sakai M., Sá L.R. M.d., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Rasmussen A., Andersson M. Proceedings of the 42nd International Congress of Meat Science and Technology. Elsevier Applied Science; Lillehammer, Norway: 1996. New method for determination of drip loss in pork muscles. [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Ali S., Sahin N., Hayirli A. Epigallocatechin-3-gallate prevents lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails. Poult. Sci. 2010;89:2251–2258. doi: 10.3382/ps.2010-00749. [DOI] [PubMed] [Google Scholar]
- Salih A., Smith D., Price J., Dawson L. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987;66:1483–1488. doi: 10.3382/ps.0661483. [DOI] [PubMed] [Google Scholar]
- Sams A. Looking for solutions pale meat, poor yield. Broiler Industry. 1999;62:26–30. [Google Scholar]
- Sauer J., Richter K.K., Pool-Zobel B.L. Physiological concentrations of butyrate favorably modulate genes of oxidative and metabolic stress in primary human colon cells. J. Nutr. Bioche. 2007;18:736–745. doi: 10.1016/j.jnutbio.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Senanayake S., Ranasinghe J., Waduge R., Nizanantha K., Alexander P. Changes in the serum enzyme levels and liver lesions of broiler birds reared under different management conditions. Trop.Agri. Res. 2015;26:584–595. [Google Scholar]
- Settar P., Yalcin S., Turkmut L., Ozkan S., Cahanar A. Season by genotype interaction related to broiler growth rate and heat tolerance. Poult. Sci. 1999;78:1353–1358. doi: 10.1093/ps/78.10.1353. [DOI] [PubMed] [Google Scholar]
- Sikandar A., Zaneb H., Younus M., Masood S., Aslam A., Khattak F., Ashraf S., Yousaf M.S., Rehman H. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-austral. J. Anim. 2017;30:690–699. doi: 10.5713/ajas.16.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail M., Hume M., Byrd J., Nisbet D., Ijaz A., Sohail A., Shabbir M., Rehman H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012;91:2235–2240. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- Song B., Li H., Wu Y., Zhen W., Wang Z., Xia Z., Guo Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim. Feed. Sci. Tech. 2017;232:6–15. [Google Scholar]
- St-Pierre N., Cobanov B., Schnitkey G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003;86:E52–E77. [Google Scholar]
- Sullivan Z.M., Honeyman M.S., Gibson L.R., Prusa K.J. Effects of triticale-based diets on finishing pig performance and pork quality in deep-bedded hoop barns. Meat. Sci. 2007;76:428–437. doi: 10.1016/j.meatsci.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Tessari E.N., Kobashigawa E., Cardoso A.L.S., Ledoux D.R., Rottinghaus G.E., Oliveira C.A. Effects of aflatoxin B1 and fumonisin B1 on blood biochemical parameters in broilers. Toxins. 2010;2:453–460. doi: 10.3390/toxins2040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Xiao Z., An W., Dong Y., Zhang B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS One. 2018;13:e0197762. doi: 10.1371/journal.pone.0197762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou Y., Lu C., Ahmad H., Zhang H., He J., Zhang L., Wang T. Influence of butyrate loaded clinoptilolite dietary supplementation on growth performance, development of intestine and antioxidant capacity in broiler chickens. PLoS One. 2016;11:e0154410. doi: 10.1371/journal.pone.0154410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S., Straschnow A., Luger D., Shinder D., Tanny J., Cohen S. Ventilation, sensible heat loss, broiler energy, and water balance under harsh environmental conditions. Poult. Sci. 2004;83:253–258. doi: 10.1093/ps/83.2.253. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Phys. C. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Zeng T., Li J.J., Wang D.Q., Li G.Q., Wang G.L., Lu L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell. Stress Chaperon. 2014;19:895–901. doi: 10.1007/s12192-014-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhao X., Wang L., Yang L., Chen X., Geng Z. Resveratrol beneficially affects meat quality of heat-stressed broilers which is associated with changes in muscle antioxidant status. Anim. Sci. J. 2017;88:1569–1574. doi: 10.1111/asj.12812. [DOI] [PubMed] [Google Scholar]
- Zhang W., Gao F., Zhu Q., Li C., Jiang Y., Dai S., Zhou G. Dietary sodium butyrate alleviates the oxidative stress induced by corticosterone exposure and improves meat quality in broiler chickens. Poult. Sci. 2011;90:2592–2599. doi: 10.3382/ps.2011-01446. [DOI] [PubMed] [Google Scholar]
- Zhang W., Jiang Y., Zhu Q., Gao F., Dai S., Chen J., Zhou G. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011;52:292–301. doi: 10.1080/00071668.2011.578121. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Jia G., Zuo J., Zhang Y., Lei J., Ren L., Feng D. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zou X., Ji J., Qu H., Wang J., Shu D., Wang Y., Liu T., Li Y., Luo amd C. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult. Sci. 2019;98:4449–4456. doi: 10.3382/ps/pez279. [DOI] [PubMed] [Google Scholar]