Abstract
Providing green light during incubation has been shown to accelerate the embryo development and shorten the hatching time in broilers. Few studies have concentrated on the exact effects on layer breeders in the aspects of hatching and posthatch performance. In this study, 4 strains of layer breeder eggs, namely White Leghorn, Rhode Island Red, Columbia Rock, and Barred Rock were used to assess the effects of monochromatic green light during embryogenesis on hatching performance, chick quality, and pubertal growth. Each strain of 600 eggs was incubated under photoperiods of either 12 h of light and 12 h of darkness (12L:12D, light group) or 0 h of light and 24 h of darkness (0L:24D, dark group) for 18 D, with 2 replicates for each treatment. The results showed hatch time, time reaching 90% hatch, and average hatch time were significantly shorter among the 4 strains in the light group (P < 0.01). In addition, hatch window and peak hatching period were not extended by the green light stimulation (P > 0.05). There was no significant difference in hatchability of fertile eggs, chick weight/egg weight, or chick quality among the 4-strain eggs between the light group and dark group (P > 0.05). There was no difference (P > 0.05) in posthatch BW between different light treatments of the 3 strains (White Leghorn, Columbia Rock, and Barred Rock), whereas the BW of Rhode Island Red was higher in light group than that of the dark group at 8 to 12 wk of age (P < 0.05) and the difference disappeared from week 14. The results demonstrate that 12L:12D monochromatic green light stimulation during embryogenesis shortens the hatching time with no negative effects on hatching and posthatch performance. These effects were consistent among the 4 layer strains.
Key words: incubation, green light, hatching time, hatch window, posthatch growth
Introduction
Light is obviously an important environmental stimulus during the whole life of avian well-being. Production performance and welfare of poultry can be improved by making full use of the light environment. Many research studies have been carried out to develop optimal lighting programs at different posthatch stages for both layers and broilers (Buys et al., 1998; Sun et al., 2017a,b; Shi et al., 2019). In contrast to this, the lighting procedures required for the hatching period are still undetermined because of lacking research supports.
There are some studies investigated the impact of light in the incubator environment. Providing green light of 1,340 to 1,730 lx during incubation from 5 to 15 D has been shown to increase embryo growth (Shafey and Al-Mohsen, 2002). Continuous monochromatic green during embryogenesis accelerated posthatch growth and pectoral muscle growth in broilers (Zhang et al., 2012). There are also other reports showing that light exposure during incubation could affect hatchability (Walter and Voitle, 1972; Garwood et al., 1973; Shafey and Al-Mohsen, 2002; Shafey et al., 2004a) and posthatch performance on broilers (Özkan et al., 2012a; Zhang et al., 2012; Huth and Archer, 2015), layers (Huth and Archer, 2015), and quails (Farghly and Mahrose, 2012). In addition, some studies have found a phenomenon that light stimulation during incubation shortened the hatching time and accelerated embryonic development on layers (Garwood et al., 1973; Bohren and Siegel, 1975), broilers (Shafey and Al-Mohsen, 2002), and turkeys (Ghatpande et al., 1995; Shafey et al., 2004a). Rozenboim et al. (2003) argued that conventional lighting source may emit additional heat into the incubators, making it unclear whether the accelerated embryonic development was caused by additional heat or by light. This seems to be solved with the advent of light-emitting diode (LED), which rarely emitted ambient heat (Huth and Archer, 2015; Sabuncuoglu et al., 2018). Exposing eggs to far-red (670 nm) LED once per day from 0 to 20 D of incubation resulted in chicks pipping (breaking the shell) 2.92 h earlier and duration time between pip and hatch 2.91 h shorter on the domestic chickens (Yeager et al., 2005). Monochromatic green LED lights during the first 18 D of incubation shortened hatching time by 3.4 h in broilers (Tong et al., 2018). The 2 literatures may provide clues to prove that the subsistent effect of light on the shortened hatching time by using LED lights. In addition, different LED monochromatic lights are available for the comparison of their effects on hatching performance (Huth and Archer, 2015). Studies showed that chicken embryos are the most sensitive to light of 550 to 560 nm (green) (Rogers et al., 1998) in the respects of embryonic growth and development, as well as posthatch growth (Halevy et al., 2006; Rozenboim et al., 2013). Therefore, green light may provide a potential application in commercial incubation process. Although many research studies have been carried out to explore the lighting effects during embryogenesis, there are relatively fewer studies on layers. Especially, the concerns on the possible negative effects including lower hatchability and chick quality, wider hatching period, and poor posthatch growth of chicks remain unfocused.
The objective of the present study was therefore to determine whether the shortened hatching time as observed in previous studies in broilers would occur in layers and whether the effects are consistent in different strains by using monochromatic green LED light and to identify the potential negative effects on hatching and posthatch performance.
Materials and methods
Ethics Statement
This study was performed in accordance with local ethical guidelines and met the requirements of the Animal Care and Use Committee (No. IAS2020-14) of Institute of Animal Science of Chinese Academy of Agricultural Sciences.
Experiment Design
Eggs were obtained from White Leghorn (WL), Rhode Island Red (RIR), Columbia Rock (CR), and Barred Rock (BR) hens of 50 wk of age. The 4 pure lines chickens, which were obtained from the University of Guelph, were kept in the experimental farm of Institute of Animal Science of Chinese Academy of Agricultural Sciences. Eggs were collected in 5 D and stored no longer than 7 D at 15°C ± 1°C and 70 to 75% of RH. Fertile eggs of average weight ±3 g of each strain (62.5 ± 3.0 g for WL, 61.5 ± 3.0 g for RIR, 64.10 ± 3.0 g for CR, and 63.00 ± 3.0 g for BR) were selected. Four incubators (NK-hatching; Beili Incubation Equipment Co., Ltd., Sichuan, China) were used, and their front windows and incubator room windows were blacked out with shade cloth to prevent light intrusion. Two incubators were operated at the traditional dark condition (dark group), and another 2 were outfitted with monochromatic green LED strips (Nodark Biolight Technology Co., Ltd., Wuxi, China) fixed on racks of each floor, allowing all eggs receiving uniform light intensity (light group). A total of 600 eggs of each strain were randomly set to the 2 groups. With 2 incubators (replicates) per group, there were a total of 150 eggs per replicate. Monochromatic green lights (520–525 nm) with a schedule of 12L:12D and light intensity of 200 lx were provided for the first 18 D of incubation in the light group.
Incubation
All incubators were calibrated using a standard thermometer and hygrometer before incubation. The temperature and humidity were monitored every 2 h during the whole incubation period. The incubation was maintained at a temperature of 37.8°C ± 0.1°C and a RH around 60% until day 18. From day 19, eggs were transferred to hatching baskets, and a temperature of 37.2°C ± 0.10°C and RH of 70% was set for the hatcher.
Hatching Time
All eggs were candled on day 10 after incubation. Unfertilized eggs or early deaths were removed, and those with evidence of a living embryo were remained in the incubators until their transfer to the hatching baskets on day 18. All incubators were stopped at 512 h after incubation. To monitor the hatching process, the number of hatched chicks was counted every 2 h from 468 h to 512 h after incubation. Hatch time, time reaching 90% hatch, average hatch time, hatch window, and peak hatching period were recorded for each replicate. Hatch time was defined as the hatching time that 100% hatch of the batch. Time reaching 90% hatch was defined as the hatching time that 90% hatch of the batch. Average hatch time was defined as the total hatching time of all chicks/the total number of chicks. Hatch window was calculated by subtracting the hatching time of the last chick from that of the first chick (Careghi et al., 2005; Zhong et al., 2018). The peak hatching period was defined as the duration time that 30 to 70% hatch of the batch (Zhong et al., 2018).
Hatching Performance and Chick Quality Assessment
While counting the number of hatched chicks every 2 h, every chick was weighed and examined macroscopically for chick quality assessment within 4 to 6 h after their hatch following the method described by Tona et al. (2003). In brief, chicks were scored for their activity, appearance (plumage, eyes, and legs), and navel area (cicatrization, retracted yolk, remaining membrane, and yolk) within a total score of 100. After quality assessment (every 4–6 h), chicks were moved to the corresponding hatching basket marked with its replicate and strain to separate from others inside the incubator until the end of the hatching. The ratio of chick weight/egg weight was calculated using the average weight of each replicate. Hatchability of fertile egg, early death (E0–E10), and late death (E11–E21) were calculated accordingly.
Posthatch Growth
After sexing, 30 pullets per replicate, making a total of 60 birds per treatment of each strain, were randomly selected and reared to monitor their posthatch growth. On arrival at the confined and environmentally controlled houses, chicks from each replicate of 4 strains were placed in 2-story cages on the same side of the house as per the random-block design. From 1 to 8 wk, chicks were housed in cages (75 cm × 70 cm × 40 cm) with 10 birds per cage. Five birds from each cage per replicate were randomly selected for weighing of BW weekly. From 9 wk, all birds were transferred to a growing room and housed in cages (63 cm × 36 cm × 33 cm) with 2 birds per cage until 18 wk of age. One bird from each cage per replicate were randomly selected for weighing of BW biweekly. They were fed ad libitum with a standard commercial pelleted diet of 2,850 kcal ME and 19% CP for starters (1–8 wk) and 2,800 kcal ME and 15.5% CP for growers (9–18 wk).
Statistical Analysis
The data of hatching time indicators and hatching performance were analyzed using the 2-way ANOVA (SAS 9.2, SAS Institute Inc., Cary, NC) in relation to light treatments and strains of birds. t test was used to analyze the BW of posthatched chicks. For all the indicators, the incubator was taken as the experimental unit, and there were 2 replicates per treatment. The means of data from the 2 replicates were calculated to represent the treatment. Significance of difference was set at P < 0.05.
Results
Hatching Time
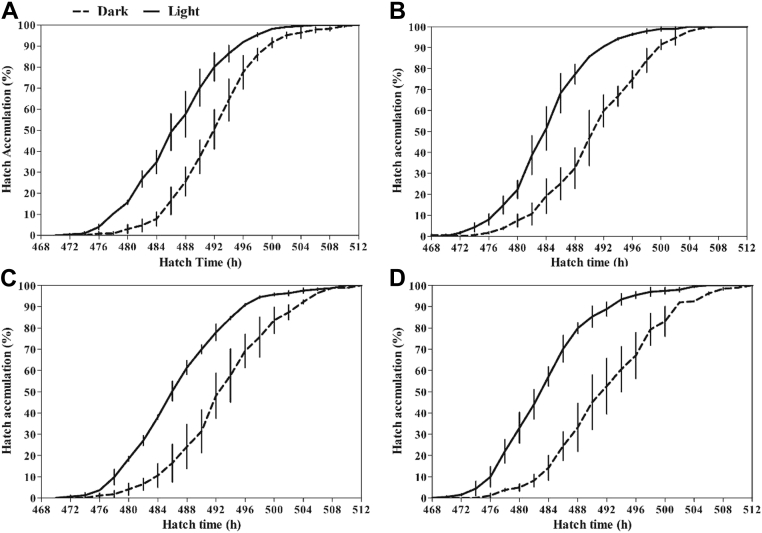
The distribution of hatching time in both the dark and light groups is shown in Figure 1. The light group was found to give the first hatching consistently among the 4 strains. As shown in Table 1, hatch time, time reaching 90% hatch, and average hatch time were shorter in the light group than those in the dark group (P < 0.01). Hatch window and peak hatching period were not prolonged in the light group compared with the dark group (P > 0.05). Strains of birds had a significant effect on hatch time that RIR had the shortest hatch time, followed by WL and BR, and CR was the longest (P < 0.05). There was no significant interaction effect between light treatments and strains of birds (P > 0.05).
Figure 1.
Hatch accumulation of light and dark groups of White Leghorn (A), Rhode Island Red (B), Columbia Rock (C), and Barred Rock (D). Data are presented as mean ± SEM (2 replicates of each group at each incubation time). Abbreviations: dark group, 0L:24D; light group, 12L:12D.
Table 1.
Hatching time related characteristics in relation to light treatments and strains of birds.
| Factor | Level | Hatch time1 (h) | Time reaching 90% hatch2 (h) | Average hatch time3 (h) | Peak hatching period4 (h) | Hatch window5 (h) |
|---|---|---|---|---|---|---|
| Light treatment (L) | Dark | 510.50a | 500.88a | 492.13a | 7.31 | 33.50 |
| Light | 505.50b | 493.56b | 485.40b | 6.56 | 32.75 | |
| SEM | 1.02 | 0.64 | 0.72 | 0.34 | 0.87 | |
| Strains (S) | WL | 508.00b | 497.25 | 490.25 | 6.63 | 32.00 |
| RIR | 504.50c | 496.00 | 488.40 | 6.25 | 31.00 | |
| CR | 511.00a | 499.25 | 487.74 | 7.63 | 35.00 | |
| BR | 508.50b | 496.38 | 488.67 | 7.25 | 34.50 | |
| SEM | 1.65 | 2.21 | 2.16 | 0.46 | 0.95 | |
| P-value | L | <0.01 | <0.01 | <0.01 | 0.07 | 0.46 |
| S | <0.01 | 0.54 | 0.48 | 0.09 | 0.06 | |
| L × S | 0.15 | 0.45 | 0.80 | 0.11 | 0.46 |
Data are the mean of 2 replicates.
a–cWithin columns, values with no common letters are significantly different (P < 0.05).
Abbreviations: BR, Barred Rock; CR, Columbia Rock; RIR, Rhode Islands Red; WL, White Leghorn.
Hatch time was defined as the hatching time that 100% hatch of the batch.
Time reaching 90% hatch was defined as the hatching time that 90% hatch of the batch.
Average hatch time was defined as the total hatching time of all chicks/the total number of chicks.
The peak hatching period was defined as the duration time that 30 to 70% hatch of the batch.
Hatch window was calculated by subtracting the hatching time of the last chick from that of the first chick.
Hatching Performance
The effects of light treatments and strains of birds on hatching performance characteristics are shown in Table 2. Light had no significant effect on hatchability of fertile egg, early death, late death, activity, unhealed navel, dirty feather, or total chick quality score (P > 0.05). Columbia Rock and BR had lower hatchability of fertile egg than the strains of WL and RIR (P < 0.05). Rhode Island Red had the highest early death, followed by CR and BR, and WL was the lowest (P < 0.05). Barred Rock and CR had the highest late death, followed by WL, and RIR was the lowest. Hatched chicks of CR showed lesser activity, more unhealed navel, and lower chick quality score (P < 0.05) than other 3 stains. However, there was no significant interaction effect between light treatments and strains of birds on those hatching performance characteristics (P > 0.05).
Table 2.
Hatch performance characteristics in relation to light treatments and strains of birds.
| Factor | Level | Hatchability of fertile egg (%) | Early death (%) | Late death (%) | Chick weight/egg weight (%) | Activity (%) | Unhealed navel (%) | Dirty feather (%) | Chick quality score |
|---|---|---|---|---|---|---|---|---|---|
| Light treatment (L) | Dark | 79.99 | 5.28 | 14.73 | 62.78 | 86.07 | 12.92 | 1.00 | 97.11 |
| Light | 81.48 | 4.44 | 14.09 | 62.77 | 87.49 | 15.93 | 2.53 | 96.89 | |
| SEM | 2.05 | 0.95 | 2.62 | 0.65 | 1.92 | 3.33 | 0.49 | 0.48 | |
| Strain (S) | WL | 85.12a | 2.64c | 12.24b | 69.03b | 90.14a | 7.52b | 2.45 | 97.52a |
| RIR | 86.68a | 8.37a | 4.94c | 69.03b | 89.82a | 8.53b | 0.76 | 97.93a | |
| CR | 76.56b | 5.25b | 18.20a | 71.86a | 80.14b | 28.19a | 2.28 | 95.58b | |
| BR | 74.58b | 3.18b,c | 22.25a | 72.25a | 87.02a | 13.45b | 1.57 | 96.97a,b | |
| SEM | 0.90 | 0.70 | 1.38 | 0.50 | 1.85 | 1.99 | 0.78 | 0.53 | |
| P-value | L | 0.15 | 0.29 | 0.69 | 0.78 | 0.38 | 0.07 | 0.05 | 0.14 |
| S | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.35 | <0.01 | |
| L × S | 0.84 | 0.75 | 0.77 | 0.64 | 0.10 | 0.08 | 0.51 | 0.43 |
Data are the mean of 2 replicates.
Within columns, values with no common letters (a, b, c) are significantly different (P < 0.05).
Abbreviations: BR, Barred Rock; CR, Columbia Rock; Early death, the death from E0 to E10; Late death, the death from E11 to E21; RIR, Rhode Islands Red; WL, White Leghorn.
Posthatch Growth
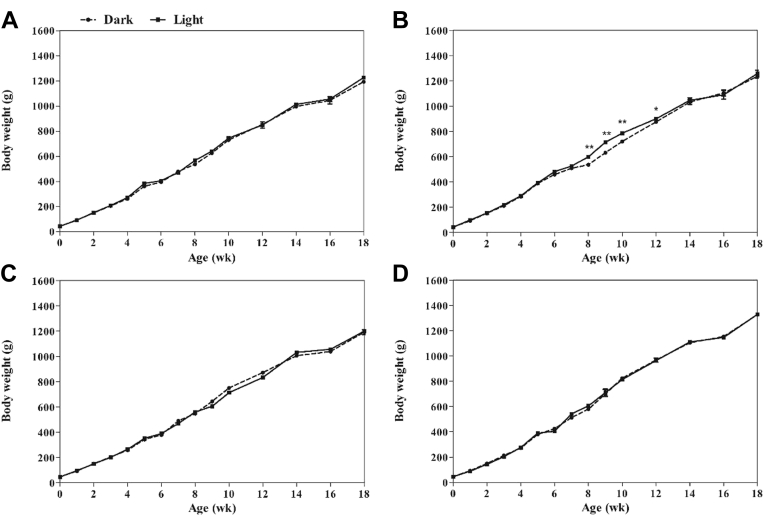
Figure 2 showed the posthatch BW of the 4 strains of pullets from different light treatment groups during the first 18 wk after hatching. For the 3 strains of WL, CR and BR, light stimulation had no significant effect on BW from hatch to 18 wk of age (P > 0.05). For RIR, the BW in the light group was heavier than those from the dark group at 8 to 12 wk of age (P < 0.05), and this difference disappeared from week 14.
Figure 2.
BW of pullets in dark and light groups from week 0 to week 18 of White Leghorn (A), Rhode Island Red (B), Columbia Rock (C) and Barred Rock (D). Data are presented as mean ± SEM (2 replicates of each group at each week of age). Asterisk indicates significant difference between groups at a given specific week (∗P < 0.05, ∗∗P < 0.01). Abbreviations: dark group, 0L:24D; light group, 12L:12D.
Discussion
Light is an important environmental factor in poultry production. It shows physiological and biological significance via the regulation of circadian rhythms and providing time for rest and regeneration (Zawilska et al., 2006, 2007; Malleau et al., 2007). Light has been widely used in different stages of commercial poultry production except for incubation period. Fertilized eggs are incubated in complete darkness commercially owing to concerns about potential adverse effects of light stimulation on performance and economics. The present study evaluated the lighted-incubation effects and explored whether adverse effects would occur on hatching or posthatch growth performance by using 4 typical strains of layer breeder eggs.
Several studies had shown that light stimulation during embryogenesis accelerated chick embryo development and shortened hatching time in layers (Siegel et al., 1969; Adam and Dimond, 1971), broilers (Walter and Voitle, 1973; Shafey and Al-Mohsen, 2002), and quails (Walter and Voitle, 1973). Because fluorescent and incandescent light used in these studies can release heat and alter the incubator environment, the effects were supposed to be caused by additional heat from the light source. In the present study, the low-power LED strip lights (0.29 W/m2) were used to verify the potential effects of lighting on hatching time. To evaluate the impact of monochromatic green light on hatching time, 3 indicators including hatch time, time reaching 90% hatch, and average hatch time, which represent the hatching characteristics, were compared between groups. Overall, monochromatic green light stimulation shortened the hatching time, which was consistent in the 4 layer strains. These observations were similar with those of the study by Tong et al. (2018) who reported a 3.4-h shortened time in broilers using the monochromatic green LED light. This further confirmed the subsistent effect of light on hatching time. Shafey et al. (2005) proposed that the amount and spectrum could be affected by the different eggshell colors, which may cause different lighting effects on hatching process. White eggs of WL and brown eggs of RIR, CR, and BR were used in the study, and the results here showed that the effects on hatching time were consistent among strains with different eggshell color. This suggests that monochromatic green light shortens the hatching time regardless of differently pigmented eggs on layers.
The hatching time distribution affected most on chick qualities and physiological traits of a batch of hatched chicks (Careghi et al., 2005; Wang et al., 2014). The hatch window ranges from 24 to 48 h, and early hatched chicks may suffer from feed and water deprivation for 72 h after hatch by considering the spread of hatch window, chick handling, and transport time (Noy and Sklan, 1997; Dibner et al., 1998; Careghi et al., 2005). It has been reported that early feeding improves the initiation growth in neonatal chicks after hatch, and this effect may last until marketing (Noy and Sklan, 1997; Bigot et al., 2003). Layer chicks with 48-h feed and water deprivation had a lower BW and decreased concentration of serum glucose, total protein, and triglycerides up to 56 D (Gaglo-Disse et al., 2010). The scattered hatching period may impair day-old chick quality and posthatch performance from the welfare aspect (Hulet et al., 2007). Hatch window and peak hatching period, representing the intervals and concentration of hatching time of a batch hatched chicks, respectively, were not affected by monochromatic green light stimulation during embryogenesis as observed in the present study. However, peak hatching period was tending to be narrowed by the lighted incubation (P < 0.1). The results were similar to the findings by Tong et al. (2018). This suggests that light stimulation shortens the hatching time without scattering the hatching period.
Hatchability is highly related to economic benefits of hatcheries. Cooper (1972) and Gold and Kalb (1976) reported that lighted incubation using incandescent light bulbs of 2,500 lx decreased hatchability. This may be due to the secondary heating. Potential adverse effect of light stimulation on hatchability had aroused our concerns. There are some studies reported that green LED light did not affect hatchability and mortality in broilers (Özkan et al., 2012a; Zhang et al., 2012; Archer, 2017) or turkeys (Rozenboim et al., 2003). Consistent with these reports, this study showed that monochromatic green light stimulation did not affect hatchability, early mortality, or late mortality of 4 layer strains. However, there were differences in hatchability among different strains here that CR and BR showed lower hatchability than WL and RIR. It has been reported that large eggs produce more heat than small eggs (Rahn et al., 1974; Vleck and Vleck, 1980; Hoyt, 1987; Vleck and Vleck, 1987; Meijerhof and Beek, 1993) and face more difficulties to remove the surplus heat during incubation (French, 1997), resulting in higher embryo temperatures. It seems true that the difference in hatchability and late mortality of different strains may be caused by the egg size, as the average egg weight of low hatchability strains (64.10 ± 3.0 g for CR and 63.00 ± 3.0 g for BR) were heavier than that of high hatchability strains (62.5 ± 3.0 g for WL and 61.5 ± 3.0 g for RIR). These could be related to the decreasing ratio between egg surface and egg content with the increasing of egg size (Vogel and Gollub, 1995; Lourens et al., 2006) and reducing air velocity over eggs in incubators (French, 1997), resulting in less water loss during the hatching process as confirmed by their higher ratio of chick weight/egg weight in the present study.
Several previous studies found lighted incubation improved chick quality in broilers (Archer et al., 2009; Özkan et al., 2012b; Huth and Archer, 2015), layers (Fairchild and Christensen, 2000; Shafey, 2004b), and wild birds (Cooper et al., 2011). Most of this improvement was due to increased navel maturation resulting in less unhealed navels and navel tags (Shafey et al., 2004a; Cooper et al., 2011). Navel maturation had been shown to be influenced by light stimulation during incubation, which could be related to the more of internalizing the yolk, resulting in accelerated navel healing than birds incubated in darkness (Shafey and Al-Mohsen, 2002). Chick quality, however, does not appear to be strongly affected by green light stimulation during incubation. On the one hand, chick quality was performed within 4 to 6 h after each hatch here, whereas on the other hand, other studies were performed after all hatch of a batch. Thus, the differences in the assessment time may be the reason of different effects on chick quality from the previous studies. However, the chick quality score of CR strain was lower than that of other 3 strains. The activity and unhealed navel were affected among the parameters of chick quality assessment. It maybe also related to the difficulties to remove the surplus heat in larger eggs.
Green light stimulation during embryogenesis had no significant effect on posthatch growth of pullets among 3 strains (WL, CR, and BR) during the entire period of 0 to 18 wk after hatching. This observation in broilers and layers agreed with that of the studies by Archer et al. (2009), Huth and Archer (2015), and Archer (2017). These literatures proposed that lighted-incubation effects on posthatch growth were very limited and did not result in larger or more efficient birds. BW of RIR in the light group was increased at 8 to 12 wk, and the difference disappeared at week 14. This observation in broilers agrees with that of the study by Shafey and Al-Mohsen (2002), Zhang et al. (2012), and Rozenboim et al. (2004). The results suggest that different strains may have differential responses to light stimulation during embryogenesis on posthatch growth.
In summary, the present study confirmed that stimulation with monochromatic green light during embryogenesis shortened the hatching time on 4 layer strains. Adverse effects were not found on hatching and posthatch pubertal growth performance. Further research is needed to determine the molecular mechanism of lighted-incubation effects on hatching time and to explore the synergistic effects of different prehatch and posthatch photoperiods on posthatch growth.
Acknowledgments
Financial support of this study was provided by The National Key Research and Development Program of China (grant number 2016YFD0500502), Beijing Municipal Science and Technology Project (grant number D171100007817005), China Agriculture Research Systems (grant number CARS-40), and Agricultural Science and Technology Innovation Program (grant number ASTIP-IAS04).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Contributor Information
Xiaolin Liu, Email: liuxiaolin@nwsuaf.edu.cn.
Jilan Chen, Email: chen.jilan@163.com.
References
- Adam J.H., Dimond S.J. Influence of light on the time of hatching in the domestic chick. Anim. Behav. 1971;19:226–229. [Google Scholar]
- Archer G. Exposing broiler eggs to green, red and white light during incubation. Animal. 2017;11:1203–1209. doi: 10.1017/S1751731117000143. [DOI] [PubMed] [Google Scholar]
- Archer G.S., Shivaprasad H.L., Mench J.A. Effect of providing light during incubation on the health, productivity, and behavior of broiler chickens. Poult. Sci. 2009;88:29–37. doi: 10.3382/ps.2008-00221. [DOI] [PubMed] [Google Scholar]
- Bigot K., Mignon-Grasteau S., Picard M., Tesseraud S. Effects of delayed feed intake on body, intestine, and muscle development in neonate broilers. Poult. Sci. 2003;82:781–788. doi: 10.1093/ps/82.5.781. [DOI] [PubMed] [Google Scholar]
- Bohren B., Siegel P. Light effects during incubation on lines of White Leghorns selected for fast and slow hatching. Poult. Sci. 1975;54:1372–1374. doi: 10.3382/ps.0541372. [DOI] [PubMed] [Google Scholar]
- Buys N., Buyse J., Hassanzadeh-Ladmakhi M., Decuypere E. Intermittent lighting reduces the incidence of ascites in broilers: an interaction with protein content of feed on performance and the endocrine system. Poult. Sci. 1998;77:54–61. doi: 10.1093/ps/77.1.54. [DOI] [PubMed] [Google Scholar]
- Careghi C., Tona K., Onagbesan O., Buyse J., Decuypere E., Bruggeman V. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 2005;84:1314–1320. doi: 10.1093/ps/84.8.1314. [DOI] [PubMed] [Google Scholar]
- Cooper J. Effect of light during incubation on hatchability of turkey eggs. Poult. Sci. 1972;51:1105–1108. doi: 10.3382/ps.0511105. [DOI] [PubMed] [Google Scholar]
- Cooper C.B., Voss M.A., Ardia D.R., Austin S.H., Robinson W.D. Light increases the rate of embryonic development: implications for latitudinal trends in incubation period. Funct. Ecol. 2011;25:769–776. [Google Scholar]
- Dibner J.J., Knight C.D., Kitchell M.L. Early feeding and development of the immune system in neonatal poultry. J. Appl. Poult. Res. 1998;7:425–436. [Google Scholar]
- Fairchild B.D., Christensen V.L. Photostimulation of turkey eggs accelerates hatching times without affecting hatchability, liver or heart growth, or glycogen content. Poult. Sci. 2000;79:1627–1631. doi: 10.1093/ps/79.11.1627. [DOI] [PubMed] [Google Scholar]
- Farghly M., Mahrose K. Effects of light during storage and incubation periods on pre and post hatch performance of Japanese quail. Egypt Poult. Sci. 2012;32:947–958. [Google Scholar]
- French N. Modeling incubation temperature: the effects of incubator design, embryonic development, and egg size. Poult. Sci. 1997;76:124–133. doi: 10.1093/ps/76.1.124. [DOI] [PubMed] [Google Scholar]
- Gaglo-Disse A., Tona K., Aliou S., Debonne M., Aklikokou K., Gbeassor M., Decuypere E. Effect of delayed feed access on production and blood parameters of layer-type chicks. Acta Vet. Hung. 2010;58:211–219. doi: 10.1556/AVet.58.2010.2.7. [DOI] [PubMed] [Google Scholar]
- Garwood V.A., Thornton E.J., Lowe P.C. The effect of continuous illumination of incubating chicken eggs on embryonic development. Poult. Sci. 1973;52:337. doi: 10.3382/ps.0520337. [DOI] [PubMed] [Google Scholar]
- Ghatpande A., Ghatpande S., Khan M.J.C. Effect of different intensities of fluorescent light on the early development of chick embryos in ovo. Cell. Mol. Biol. Res. 1995;41:613–621. [PubMed] [Google Scholar]
- Gold P.S., Kalb J. Secondary heating of chicken eggs exposed to light during incubation. Poult. Sci. 1976;55:34–39. [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1062–R1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Hoyt D.F. A new model of avian embryonic metabolism. J. Exp. Zool. Suppl. 1987;1:127–138. [PubMed] [Google Scholar]
- Hulet R., Gladys G., Hill D., Meijerhof R., El-Shiekh T. Influence of egg shell embryonic incubation temperature and broiler breeder flock age on posthatch growth performance and carcass characteristics. Poult. Sci. 2007;86:408–412. doi: 10.1093/ps/86.2.408. [DOI] [PubMed] [Google Scholar]
- Huth J.C., Archer G.S. Effects of LED lighting during incubation on layer and broiler hatchability, chick quality, stress susceptibility and post-hatch growth. Poult. Sci. 2015;94:3052–3058. doi: 10.3382/ps/pev298. [DOI] [PubMed] [Google Scholar]
- Lourens A., Molenaar R., van den Brand H., Heetkamp M.J., Meijerhof R., Kemp B. Effect of egg size on heat production and the transition of energy from egg to hatchling. Poult. Sci. 2006;85:770–776. doi: 10.1093/ps/85.4.770. [DOI] [PubMed] [Google Scholar]
- Malleau A.E., Duncan I.J.H., Widowski T.M., Atkinson J.L. The importance of rest in young domestic fowl. Appl. Anim. Behav. Sci. 2007;106:52–69. [Google Scholar]
- Meijerhof R., Beek G.V. Mathematical modelling of temperature and moisture loss of hatching eggs. J. Theor. Biol. 1993;165:27–41. [Google Scholar]
- Noy Y., Sklan D. Posthatch development in poultry. J. Appl. Poult. Res. 1997;6:344–354. [Google Scholar]
- Özkan S., Yalçın S., Babacanoğlu E., Kozanoğlu H., Karadaş F., Uysal S. Photoperiodic lighting (16 hours of light: 8 hours of dark) programs during incubation: 1. Effects on growth and circadian physiological traits of embryos and early stress response of broiler chickens. Poult. Sci. 2012;91:2912–2921. doi: 10.3382/ps.2012-02426. [DOI] [PubMed] [Google Scholar]
- Özkan S., Yalçın S., Babacanoğlu E., Kozanoğlu H., Karadaş F., Uysal S. Photoperiodic lighting (16 hours of light:8 hours of dark) programs during incubation: 2. Effects on early posthatching growth, blood physiology, and production performance in broiler chickens in relation to posthatching lighting programs. Poult. Sci. 2012;91:2922–2930. doi: 10.3382/ps.2012-02427. [DOI] [PubMed] [Google Scholar]
- Rahn H., Paganelli C.V., Ar A. The avian egg: air-cell gas tension, metabolism and incubation time. Respir. Physiol. 1974;22:297–309. doi: 10.1016/0034-5687(74)90079-6. [DOI] [PubMed] [Google Scholar]
- Rogers L.J., Andrew R.J., Burne T.H.J. Light exposure of the embryo and development of behavioural lateralisation in chicks, I: olfactory responses. Behav. Brain Res. 1998;97:195–200. doi: 10.1016/s0166-4328(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Halawani M.E., Kashash Y., Piestun Y., Halevy O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen. Comp. Endocrinol. 2013;190:214–219. doi: 10.1016/j.ygcen.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Huisinga R., Halevy O., Halawani M.E. Effect of embryonic photostimulation on the posthatch growth of turkey poults. Poult. Sci. 2003;82:1181–1187. doi: 10.1093/ps/82.7.1181. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Piestun Y., Mobarkey N., Barak M., Hoyzman A., Halevy O. Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poult. Sci. 2004;83:1413. doi: 10.1093/ps/83.8.1413. [DOI] [PubMed] [Google Scholar]
- Sabuncuoglu K.M., Korkmaz F., Gurcan E.K., Narinc D., Samli H.E. Effects of monochromatic light stimuli during embryogenesis on some performance traits, behavior, and fear responses in Japanese quails. Poult. Sci. 2018;97:2385–2390. doi: 10.3382/ps/pey105. [DOI] [PubMed] [Google Scholar]
- Shafey T., Al-Mohsen T. Embryonic growth, hatching time and hatchability performance of meat breeder eggs incubated under continuous green light. Asian Austral. J. Anim. 2002;15:1702–1707. [Google Scholar]
- Shafey T., Al-Mohsen T., Al-Sobayel A. The effect of lighting during incubation of turkey and chicken eggs on embryonic growth, hatchability traits and post-hatch performance of chickens. J. King Saud Univ. Sci. 2004;16:61–82. [Google Scholar]
- Shafey T.M. Effect of lighted incubtion on embryonic growth and hatchability performance of two strains of layer breeder eggs. Br. Poult. Sci. 2004;45:223–229. doi: 10.1080/00071660410001715821. [DOI] [PubMed] [Google Scholar]
- Shafey T., Al-Batshan H., Ghannam M., Al-Ayed M. Effect of intensity of eggshell pigment and illuminated incubation on hatchability of brown eggs. Br. Poult. Sci. 2005;46:190–198. doi: 10.1080/00071660500065789. [DOI] [PubMed] [Google Scholar]
- Shi L., Sun Y., Xu H., Liu Y., Li Y., Huang Z., Ni A., Chen C., Wang P., Ye J., Ma H., Li D., Chen J. Effect of age at photostimulation on reproductive performance of Beijing-you chicken breeders. Poult. Sci. 2019;98:4522–4529. doi: 10.3382/ps/pez267. [DOI] [PubMed] [Google Scholar]
- Siegel P.B., Isakson S.T., Coleman F.N., Huffman B.J. Photoacceleration of development in chick embryo. Comp. Biochem. Physiol. 1969;28:753–758. [Google Scholar]
- Sun Y., Li Y., Li D., Chen C., Bai H., Xue F., Chen J. Responses of broilers to the near-continuous lighting, constant 16-h lighting, and constant 16-h lighting with a 2-h night interruption. Livest. Sci. 2017;206:135–140. [Google Scholar]
- Sun Y., Tang S., Chen Y., Li D., Bi Y., Hua D., Chen C., Luo Q., Yang L., Chen J. Effects of light regimen and nutrient density on growth performance, carcass traits, meat quality, and health of slow-growing broiler chickens. Livest. Sci. 2017;198:201–208. [Google Scholar]
- Tona K., Bamelis F., Ketelaere B.D., Bruggeman V., Moraes V., Buyse J., Onagbesan O., Decuypere E. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Tong Q., McGonnell I., Demmers T., Roulston N., Bergoug H., Romanini C., Verhelst R., Guinebretière M., Eterradossi N., Berckmans D. Effect of a photoperiodic green light programme during incubation on embryo development and hatch process. Animal. 2018;12:765–773. doi: 10.1017/S1751731117002117. [DOI] [PubMed] [Google Scholar]
- Vleck C.M., Vleck V.D.F. Patterns of metabolism and growth in avian embryos. Am. Zool. 1980;20:405–416. [Google Scholar]
- Vleck C.M., Vleck D. Metabolism and energetics of avian embryos. J. Exp. Zool. 1987;1:111–125. [PubMed] [Google Scholar]
- Vogel S., Gollub J. Life in moving fluids. Phys. Today. 1995;48:84. [Google Scholar]
- Walter J., Voitle R. Effects of photoperiod during incubation on embryonic and post-embryonic development of broilers. Poult. Sci. 1972;51:1122–1126. doi: 10.3382/ps.0511122. [DOI] [PubMed] [Google Scholar]
- Walter J.H., Voitle R.A. Effects of photoperiod during incubation on embryonic and post-embryonic development of quail and chickens. Br. Poult. Sci. 1973;14:533–540. doi: 10.1080/00071667308416062. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li Y., Willems E., Willemsen H., Franssens L., Koppenol A., Guo X., Tona K., Decuypere E., Buyse J. Spread of hatch and delayed feed access affect post hatch performance of female broiler chicks up to day 5. Animal. 2014;8:610–617. doi: 10.1017/S175173111400007X. [DOI] [PubMed] [Google Scholar]
- Yeager R.L., Franzosa J.A., Millsap D.S., Angell-Yeager J.L., Heise S.S., Wakhungu P., Lim J., Whelan H.T., Eells J.T., Henshel D.S. Effects of 670-nm phototherapy on development. Photomed. Laser Surg. 2005;23:268–272. doi: 10.1089/pho.2005.23.268. [DOI] [PubMed] [Google Scholar]
- Zawilska J.B., Lorenc A., Berezińska M.G., Vivien-Roels B., Pévet P., Skene D.J. Diurnal and circadian rhythms in melatonin synthesis in the turkey pineal gland and retina. Gen. Comp. Endocrinol. 2006;145:162–168. doi: 10.1016/j.ygcen.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Zawilska J.B., Lorenc A., Bereziñska M., Vivien-Roels B., Pévet P., Skene D.J. Photoperiod-dependent changes in melatonin synthesis in the turkey pineal gland and retina. Poult. Sci. 2007;86:1397–1405. doi: 10.1093/ps/86.7.1397. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H., Qiao X., Yue H., Wu S., Yao J., Qi G. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Yu Y., Jin S., Pan J. Effects of mixing eggs of different initial incubation time on the hatching pattern, chick embryonic development and post-hatch performance. PeerJ. 2018;6:e4634. doi: 10.7717/peerj.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]