Abstract
The aim of this study was to evaluate the efficacy of sanitizing fertile eggs with clove essential oil as an alternative to paraformaldehyde; effects on the reduction in eggshell microbial count, incubation yield, and neonatal chick quality were measured. A total of 1,460 brown fertile eggs with a mean weight of 58.64 ± 0.49 g (from 37-wk-old CPK [Pesadão Vermelho] breeder hens) were collected under aseptic conditions and randomly distributed into 4 treatments (nonsanitized and sanitized with grain alcohol, clove essential oil, and paraformaldehyde) before incubation. The count of total aerobic mesophilic bacteria was significantly lower after spraying with clove essential oil (2.30 ± 0.24 log10 CFU/mL) than on nonsanitized eggs (3.49 ± 0.34 log10 CFU/mL) or on eggs sprayed with grain alcohol (3.09 ± 0.14 log10 CFU/mL) but did not differ significantly from the count in the paraformaldehyde group (2.23 ± 0.29 log10 CFU/mL). The hatchability of fertile eggs differed significantly between the studied treatments. The mean values for the eggs treated with clove essential oil (84.69 ± 1.65%) and paraformaldehyde (81.87 ± 3.92%) were statistically similar but were higher than the negative control (74.03 ± 3.58%) and grain alcohol (73.59 ± 2.87%) values. In the Pasgar© score assessment, it was determined that the clove essential oil (9.21 ± 0.89%) had a superior effect on the physical quality of the chicks compared with the effects of the other treatments. Clove essential oil is effective and safe for eggs intended for incubation. Its use as an alternative to paraformaldehyde in the sanitation of fertile eggs is strongly recommended.
Key words: bacterial enumeration, clove essential oil, fertile eggs, hatching results, sanitizers
Introduction
There is a constant challenge to improve the productivity of the poultry production chain, whether in the prehatch, hatch, or posthatch stage. In this sense, maximizing the efficiency of incubation processes and maximizing the quality of day-old chicks are among the main objectives of broiler farming. For these goals to be achieved, we must identify the critical steps that may result in production losses.
One of the main strategic points at which the poultry industry can optimize the efficiency of production is the sanitation of fertile eggs. Reducing the microbial load of eggshells can minimize the occurrence and prevalence of pathogenic microorganisms, which are severely harmful to embryonic development and maximize hatchability and chick quality (Shahein and Sedeek, 2014). The sanitizing compound commonly used in farms and hatcheries is paraformaldehyde, which is effective for maintaining low contamination levels of eggshells (Williams, 1970; Whistler and Sheldon, 1989). However, paraformaldehyde is highly toxic to the health of the professionals who handle it and to chick embryos and harmful to the environment (Casteel et al., 1987; Roca et al., 2008; Cadirci, 2009; Unsaldi and Ciftci, 2010; Rhomberg, 2015). Zeweil et al. (2015), for example, observed that paraformaldehyde can cause malformations in developing chick embryos.
Clove essential oil (Syzygium aromaticum) may be an alternative for sanitizing fertile eggs (Oliveira and Santos, 2018; Oliveira et al., 2020). Usually, odoriferous and liquid essential oils are mixtures of lipophilic substances derived from secondary metabolites of plants. Chemically, most essential oils are composed of terpenoids, phenylpropanoids, or linear alkanes and alkenes (Dhifi et al., 2016). Clove essential oil consists of a mixture of aliphatic and cyclic volatile terpenes and phenylpropanoids (Oliveira et al., 2016), with eugenol being the major component, and has high antimicrobial activity (Dhara and Tripathi, 2013).
Further studies demonstrating the effectiveness of clove essential oil in the sanitation of fertile eggs are necessary, considering its chemical composition and antimicrobial activity, as well as the first promising results regarding the artificial incubation process of eggs sanitized with this oil (Oliveira et al., 2020). In addition, the development of new products to replace those considered harmful is fundamental for the advancement of the poultry sector. This study aimed to evaluate the efficacy of sanitizing fertile eggs with clove essential oil as an alternative to paraformaldehyde, measuring the reduction in eggshell microbial count, incubation yield, and neonatal chick quality.
Materials and methods
Ethics Approval
The present study was approved by the Ethics Committee on Animal Use of the University of Brasília under opinion No. 33/2019.
Experimental Procedure
A total of 1,460 brown fertile eggs with a mean weight of 58.64 ± 0.49 g (from 37-wk-old CPK [Pesadão Vermelho] breeder hens) were collected under aseptic conditions and randomly distributed into 4 treatments before incubation, as described in Table 1.
Table 1.
Description of treatments, chemical concentrations, and methods of application to eggs.
| Treatment | Concentration | Application | Number of eggs | |
|---|---|---|---|---|
| T1 | Nonsanitized1 | - | - | 365 |
| T2 | Grain alcohol | 93.5% | Spraying | 365 |
| T3 | Clove essential oil | 0.39% | Spraying | 365 |
| T4 | Paraformaldehyde | 6 g/m³ | Fumigation | 365 |
Negative control.
Internal egg quality was measured using the Haugh unit and yolk index of 80 eggs (20 per treatment). There was no significant difference among treatments, preventing egg quality from interfering with the incubation results. The mean Haugh unit of the eggs was 85.65 ± 7.31 (P = 0.2830; coefficient of variation [CV] = 8.30%), and they were classified as “AA”, that is, of excellent quality (USDA, 2000). The calculated yolk index was 0.39 ± 0.03 (P = 0.6647; CV = 7.33%). These analyses were also essential because the internal structure of the egg has the potential to meet the nutrient and energy demands of embryos until hatching.
Acquisition and Preparation of Clove Essential Oil-Based Sanitizing Agent
Dried clove flower buds were obtained from a commercial market in Brasília, Federal District, Brazil. The essential oil was extracted in a laboratory of natural product chemistry (Federal Institute of Brasília, Gama, Federal District, Brazil) by a method adapted from Ascenção and Filho (2013) involving hydrodistillation with the Clevenger extraction system (Vidrolabo, Poá, São Paulo, Brazil). The chemical analysis of the clove essential oil by means of gas chromatography coupled to mass spectrometry allowed the identification of 3 components, with eugenol (89.97%) being the main component (Table 2). The structures were defined on the basis of retention times, calculation of the Kovats index, the Wiley7, FFNSCl.3, and NIST08 databases and comparison with data from Adams (2017).
Table 2.
Chemical compounds identified in the clove essential oil.
| Peak | CRT (min) | Area (%) | CKI | TKI1 | Compound |
|---|---|---|---|---|---|
| 1 | 22.730 | 89.97 | 1,363 | 1,359 | Eugenol |
| 2 | 25.248 | 2.22 | 1,422 | 1,419 | β-Caryophyllene |
| 3 | 29.602 | 7.81 | 1,530 | 1,522 | Acetyleugenol |
Abbreviations: CTR, compound retention time; CKI, calculated Kovats index; TKI, tabulated Kovats index.
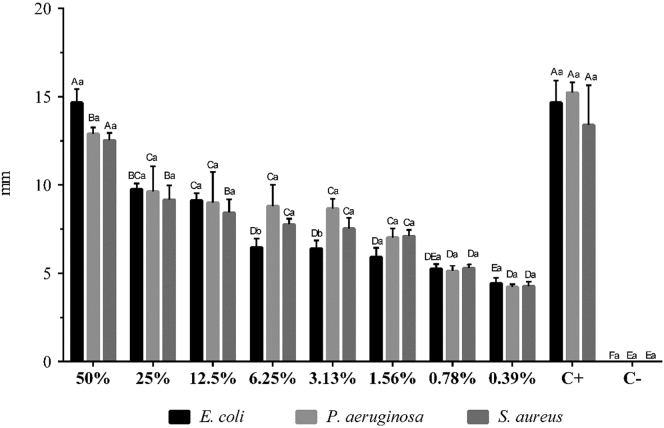
To prepare the sanitizer, the clove essential oil was diluted in 93.5% grain alcohol (Cromoline Química Fina, Diadema, São Paulo, Brazil) to a concentration of 0.39%. This concentration was chosen because it was the lowest concentration of the oil tested in vitro by the disc diffusion method recommended by Bauer et al. (1966) that showed an inhibitory effect against standard strains of Escherichia coli (ATCC 25,922), Pseudomonas aeruginosa (ATCC 27,853), and Staphylococcus aureus (ATCC 25,923) (Figure 1).
Figure 1.
Determination of the antimicrobial activity of clove essential oil against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus by the disk diffusion method.1A-F; a,b Means with different uppercase (bacteria) or lowercase (oil concentration) letters differ significantly (P < 0.05). Abbreviations: C+, positive control (30 μg of chloramphenicol); C−, negative control (5% DMSO); DMSO, dimethyl sulfoxide. 1Results are expressed as the mean diameter of the inhibition halos in millimeters (mm) for each concentration of clove oil (%) tested.
For this test, bacterial strains (ATCC, Manassas, VA) were activated in brain heart infusion broth (Neogen, Lansing, MI) and incubated for approximately 24 h at 36°C. Subsequently, they were standardized in sterile saline (NaCl 0.85%) until a turbidity compatible with grade 0.5 of the McFarland scale (1.5 × 108 CFU/mL) was obtained. The oil was weighed to determine the volume that comprised 100 mg. This amount was used in testing as the full-strength (100%) concentration and was then serially diluted in dimethyl sulfoxide (DMSO 5%; Sigma-Aldrich, Saint Louis, MO). The sterile filter paper discs (4 mm) were impregnated with 10 μL of clove essential oil in concentrations ranging from 50 to 0.39% (p/v) and then deposited with sterile forceps on the surface of the Petri dishes containing the culture medium (Mueller-Hinton agar, HiMedia, Mumbai, Maharashtra, India), which was previously inoculated with 100 μL of bacteria. A pure DMSO control was included with each test to ensure that microbial growth was not inhibited by DMSO itself. Chloramphenicol (30 μg/disc; Sigma-Aldrich) was used as a positive control. Plates were then inverted and incubated for approximately 24 h at 36°C, and the diameter of the inhibition zones was measured in millimeters using a digital caliper with 0.001-mm precision (Mitutoyo, Suzano, São Paulo, Brazil). Each test was performed with 3 replicates.
The grain alcohol used in this study served as the carrier vehicle of the clove essential oil. Therefore, its isolated effect on the sanitation of fertile eggs was also tested.
Egg Sanitation
Sanitation procedures were performed in a room at a commercial hatchery (Planaltina, Federal District, Brazil) 20 min after egg collection. The eggs from treatment T1 (negative control) were kept in the same room as the other treatments and did not receive any sanitation procedure. In the T2 (grain alcohol) and T3 (clove essential oil) treatments, eggs were homogeneously sprayed over their entire surface with manual sprayers. After spraying, the eggs were placed in sterile trays for drying at room temperature for 30 to 50 min. At the same time, the eggs from treatment T4 (paraformaldehyde) were sent for fumigation. In this process, a concentration of 6 g/m³ paraformaldehyde was used. Burning of the product, fumigation, and gas exhaustion proceeded for 20 min in a completely closed chamber, according to the guidelines of the commercial hatchery. The relative humidity and temperature in the chamber were 70% and 25°C, respectively.
Eggshell Microbial Count
A method adapted from Fasenko et al. (2009) was used to count microbes on the eggshell. One hour after sanitation, 20 eggs from each treatment were placed into sterile plastic bags (pooled sample of 4 eggs per bag) labeled according to treatment and transported under cooling to the laboratory of microbiology (Federal Institute of Brasília, Planaltina), where the analyses of eggshell microbial count were performed. Each bag containing a pooled sample of 4 eggs was reopened, and 220 mL of 0.1% peptone saline solution (Kasvi, São José dos Pinhais, Paraná, Brazil) was added. The eggs were manually massaged for 5 min to extract the microbial load. Then, a 1.0-mL aliquot was removed from each bag, and serial decimal dilutions in 0.1% peptone saline solution were performed for each sample. A 1.0-mL aliquot of each dilution was plated on standard plate count agar (Neogen), violet red bile glucose agar (Kasvi), and potato dextrose agar (HiMedia) to count of total aerobic mesophilic bacteria, Enterobacteriaceae, and molds and yeasts, respectively. The plates containing plate count agar and violet red bile glucose agar were incubated at 37°C for 48 h, and the plates with potato dextrose agar were incubated at 29°C for 6 D. After the incubation period, the colonies formed were counted, and the results are expressed in log10 CFU/mL of pooled sample of 4 eggs.
Incubation and Hatching
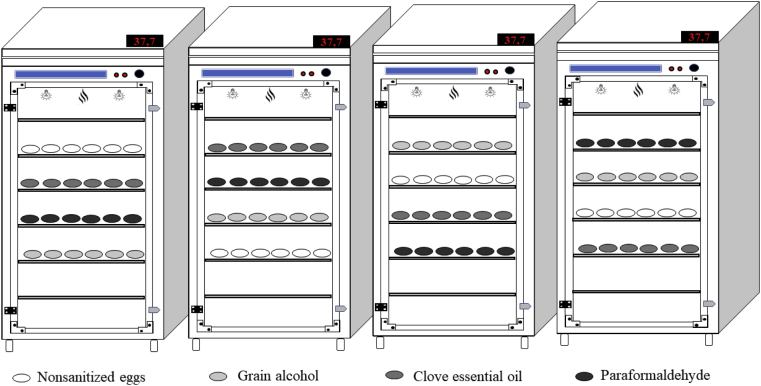
After the sanitation process, the eggs were transported to a laboratory of poultry science (Federal Institute of Brasília, Planaltina). The eggs were stored for 3 D at a temperature of 16°C to 18°C and a 55 to 60% relative humidity. The eggs were then separated by treatment into incubation trays with a capacity of 80 eggs each. For each treatment, 4 incubation trays were used, totaling 320 eggs. The incubation trays containing the eggs were individually weighed and randomly distributed into 4 single-stage setters (Luna 480, Chocmaster, Curitiba, Paraná, Brazil), as shown in Figure 2. The setters were in an air-conditioned room at 22°C to 24°C and 50 to 55% humidity. These meteorological variables were monitored by 2 thermohygrometers (Testo 608-H1, Campinas, São Paulo, Brazil) to ensure the proper functioning of the setters.
Figure 2.
Distribution of treatments in setters.
The setters were operated at a mean temperature of 37.7°C (99.86°F), a mean relative humidity of 60%, and with automatic turning every hour at a 45° angle for the first 18 D of incubation. On the eighth day (192 h of incubation), all eggs were candled to remove infertile eggs and eggs with early embryonic mortality. Starting on day 19 (456 h of incubation), the incubation trays were weighed again, and the incubators were operated at a mean temperature of 36.6°C (97.88°F) and a 65% relative humidity. After 21 D (504 h of incubation), the unhatched eggs were counted, opened, and evaluated to determine the number of infertile eggs, the period of embryonic mortality (early [0–7 D], mid [8–18 D], and late [19–21 D plus pipped]), and the number of contaminated eggs.
After the end of the incubation process, the percentages of egg weight loss (%), fertility (%), hatchability of set eggs (%), hatchability of fertile eggs (%), early dead (%), mid dead (%), late dead (%), contaminated eggs (%), and chick yield (%) were calculated per Aviagen (2011) and Baylan et al. (2018) using equations 1 to 9, respectively.
-
1)
Egg weight loss (%) = [(initial egg weight − egg weight measured on the transfer day)/initial egg weight] × 100.
-
2)
Fertility (%) = (number of fertilized eggs/number of eggs set) × 100.
-
3)
Hatchability of set eggs (%) = (number of hatched chicks/total number of set eggs) × 100.
-
4)
Hatchability of fertile eggs (%) = (number of hatched chicks/number of fertile eggs) × 100.
-
5)
Early dead (%) = (number of dead embryos on days 0–7 of incubation/number of fertile eggs) × 100.
-
6)
Mid dead (%) = (number of dead embryos on days 8–18 of incubation/number of fertile eggs) × 100.
-
7)
Late dead (%) = (number of dead embryos on days 19–21 of incubation/number of fertile eggs) × 100.
-
8)
Contaminated eggs (%) = (number of contaminated eggs/number of fertile eggs) × 100.
-
9)
Chick yield (%) = (chick weight on the day of hatch/initial egg weight) × 100.
Evaluation of Eggshell Thickness
A method adapted from Barbosa et al. (2012) was used to evaluate eggshell thickness. After the chicks hatched, 50 eggshells from each treatment were separated and dried at room temperature for 3 D. Then, the thickness of each eggshell was measured without removing its internal membranes, and means were obtained from 3 different points at the equatorial plane of the eggshell using a digital caliper with 0.001-mm precision (Mitutoyo).
Evaluation of Chick Quality
After removal of the chicks from the incubators, their quality was visually assessed using the Pasgar© score method adapted from Boerjan (2006). Typically, each bird started the evaluation with 10 points and lost 1 point for each trait (Table 3) considered to be poor by the examiner. This subjective assessment was performed by a single person to avoid interexaminer variation.
Table 3.
Assessment of chick quality according to the Pasgar© score.
| Observed parameter | Assessment |
|---|---|
| Navel area | Healing state |
| Legs | Presence of injury |
| Eyes | Brightness and wideness of the gape of the eyelid |
| Beak | Presence of injury |
| Venter | Degree of absorption of the yolk sac |
| Reflex | Ability to react to stimuli |
Source: Adapted from Boerjan (2006).
Experimental Design and Statistical Analysis
The experiment followed a randomized block design with 4 treatments. The analysis of incubation yield was based on 4 replicates per treatment, in which each tray of 80 eggs constituted a replicate. To analyze eggshell thickness and chick yield and quality, each egg and chick were considered a replicate. A completely randomized experimental design was used for eggshell bacterial count, with 5 replicates each, in which each pooled sample of 4 eggs was considered a replicate. Data were subjected to analysis of variance in SAS Studio University Edition software (SAS Inst. Inc., Cary, NC). Means were tested for significant differences by Tukey's test when the assumptions of normality and homoscedasticity were met. When the test for normality distribution or homogeneity of variances failed, the Kruskal-Wallis test was used. Statistical significance for all tests was considered at P < 0.05.
Results and discussion
Eggshell Microbial Count
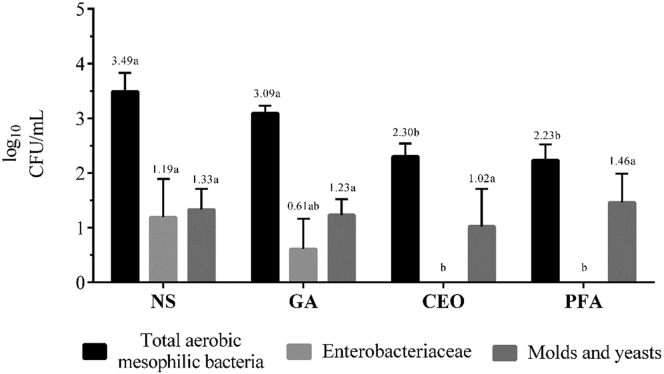
The count of total aerobic mesophilic bacteria was significantly lower (P < 0.0001; CV = 9.54%; Figure 3) after spraying with clove essential oil (2.30 ± 0.24 log10 CFU/mL) than on nonsanitized eggs (3.49 ± 0.34 log10 CFU/mL) or on eggs sprayed with grain alcohol (3.09 ± 0.14 log10 CFU/mL) but did not differ significantly from the count in the paraformaldehyde group (2.23 ± 0.29 log10 CFU/mL).
Figure 3.
Counts of total aerobic mesophilic bacteria (P < 0.0001; CV = 9.54%), Enterobacteriaceae (P = 0.0066; CV = 98.24%), and molds and yeasts (P = 0.6544; CV = 37.59%) on eggshell surfaces according to different treatments.1a,bMeans with different letters differ significantly (P < 0.05). Abbreviations: CEO, clove essential oil; CV, coefficient of variation; GA, grain alcohol; NS, nonsanitized; PFA, paraformaldehyde. 1Results are expressed in log10 CFU/mL pooled over 4 eggs.
Essential oils are effective in reducing the microbial contamination of eggshells intended for incubation (Copur et al., 2010; Ulucay and Yildirim, 2010), which is reinforced by the present results. In this study, the potent antimicrobial activity of clove essential oil was mainly because of its high phenolic compound content (Chaieb et al., 2007). Phenolic compounds react with the phospholipids of the bacterial cell membrane, making them more permeable. This change in membrane permeability leads to the loss of ions, a reduction in membrane potential, depletion of the function of proton pumps, and a reduction in adenosine triphosphate, causing cell death (Burt, 2004).
The count of Enterobacteriaceae was significantly affected (P = 0.0066; CV = 98.24%; Figure 3) by the treatments and ranged from 0.00 in the clove essential oil and paraformaldehyde groups to 1.19 ± 0.70 log10 CFU/mL for the negative control. These results show that the presence of Salmonella enterica serovar Enteritidis, and pathogenic E. coli was not observed in the eggshells after sanitation with clove essential oil or paraformaldehyde. Prabuseenivasan et al. (2006) reported that clove essential oil exhibited bioactivity mainly against gram-negative bacteria, which was confirmed by the results of this study. In general, the low count of Enterobacteriaceae indicated good hygienic conditions of the farm that provided the eggs (Musgrove et al., 2014).
The total count of molds and yeasts ranged from 1.02 ± 0.69 (clove essential oil) to 1.46 ± 0.53 log10 CFU/mL (paraformaldehyde). No significant differences were observed (P = 0.6544; CV = 37.59%; Figure 3) between any of the treatments. The low fungal load on the surface of the eggshells may be explained by the fact that the eggs were not exposed to a very humid environment on the farm (Board and Tranter, 1995).
Hatching Results
The percentage of egg weight loss during incubation did not differ between treatments in this study (P = 0.1495; CV = 3.00%, Table 4). This parameter is more strongly influenced by physical factors essential for incubation, such as temperature and relative humidity (Barott, 1937; Tullet and Burton, 1982; Meijerhof and van Beek, 1993). In this experiment, as the eggs were subjected to the same incubation conditions, no difference between the treatments was expected. In addition, evaluating egg weight loss during incubation allowed us to indirectly estimate the level of sanitizers damage to the cuticle, and, consequently, embryonic development (Brake and Sheldon, 1990; Peebles et al., 1998). Our findings suggest that the cuticle was not damaged by any of the sanitation processes.
Table 4.
Egg weight before setting and during transfer and the percentage of egg weight loss in eggs treated with different treatments.1
| Treatment | Egg weight before setting (g) | Egg weight during transfer (g) | Egg weight loss (%) |
|---|---|---|---|
| Nonsanitized | 58.55 ± 0.86 | 51.55 ± 0.90 | 11.95 ± 0.36 |
| Grain alcohol | 58.82 ± 0.30 | 52.13 ± 0.49 | 11.38 ± 0.45 |
| Clove essential oil | 58.66 ± 0.36 | 51.78 ± 0.32 | 11.72 ± 0.46 |
| Paraformaldehyde | 58.53 ± 0.45 | 52.09 ± 0.35 | 11.01 ± 0.29 |
| P-value | 0.7310 | 0.2920 | 0.1495 |
| Coefficient of variation (%) | 0.85 | 0.99 | 3.00 |
No significant differences existed between means (P > 0.05).
Results are expressed as the means ± SD.
There was no significant difference (P = 0.0546, CV = 1.76%) in the percentage of fertility (Table 5). The mean fertility was 82.62 ± 2.02%. This result was observed because the eggs were obtained from breeder hens of the same age that received the same management at the poultry house.
Table 5.
Fertility, hatchability of set eggs, hatchability of fertile eggs, early, mid, and late embryonic mortality, and contaminated eggs according to different treatments.1
| Treatment | Fert (%) | Hatch (%) | Hatch fert (%) | Early dead (%) | Mid dead (%) | Late dead (%) | Cont. (%) |
|---|---|---|---|---|---|---|---|
| Nonsanitized | 83.27 ± 2.31 | 61.63 ± 3.32b | 74.03 ± 3.58b | 7.09 ± 1.54 | 2.35 ± 1.99 | 13.41 ± 2.24a | 3.13 ± 1.25a |
| Grain alcohol | 81.96 ± 2.02 | 60.32 ± 2.89b | 73.59 ± 2.87b | 7.60 ± 1.46 | 2.40 ± 0.93 | 13.60 ± 1.00a | 2.81 ± 0.84a,b |
| Clove essential oil | 83.60 ± 1.77 | 70.81 ± 2.40a | 84.69 ± 1.65a | 4.32 ± 2.68 | 1.56 ± 1.24 | 8.67 ± 3.86b | 0.38 ± 0.76c |
| Paraformaldehyde | 81.63 ± 1.96 | 66.87 ± 4.48a,b | 81.87 ± 3.92a | 6.46 ± 1.46 | 1.48 ± 1.38 | 9.63 ± 2.92a,b | 0.81 ± 0.94b,c |
| P-value | 0.0546 | 0.0027 | 0.0043 | 0.0915 | 0.5443 | 0.0197 | 0.0330 |
| Coefficient of variation (%) | 1.76 | 4.07 | 3.86 | 25.68 | 69.77 | 18.69 | 57.54 |
Means in the same column with different superscript letters differ significantly (P < 0.05).
Abbreviations: Cont., contaminated; Fert, fertility; Hatch, hatchability of set eggs; Hatch fert, hatchability of fertile eggs.
Results are expressed as the means ± SD.
A significant difference was observed for the hatchability of set eggs (P = 0.0027, CV = 4.07%; Table 5). The eggs treated with clove essential oil hatched at a mean rate of 70.81 ± 2.40%, which was similar to the mean of 66.87 ± 4.48% observed in the group fumigated with paraformaldehyde and significantly higher than that in the negative control (61.63 ± 3.32%) and grain alcohol (60.32 ± 2.89%) groups. This result can be attributed to the effects of the treatments, as there was no significant difference in the fertility rate.
The hatchability of fertile eggs (P = 0.0043; CV = 3.86%) differed significantly between the studied treatments (Table 5). The mean values for the eggs treated with clove essential oil (84.69 ± 1.65%) and paraformaldehyde (81.87 ± 3.92%) were statistically similar but were higher than those for the negative control (74.03 ± 3.58%) and the eggs treated with grain alcohol (73.59 ± 2.87%).
Oliveira et al. (2020) compared the hatchability of fertile eggs between clove essential oil treatment at a concentration of 0.6 mg/mL (92.37 ± 3.25%) and paraformaldehyde treatment (94.44 ± 4.54%) and did not observe significant differences. Copur et al. (2010) evaluated the effect of oregano essential oil at 2 concentrations (0.55 and 0.75 mL/cm3) and 2 exposure times (3 and 6 h) on the sanitation of eggs intended for incubation and observed that the hatchability of these eggs (90.00%) did not differ significantly from the hatchability of formaldehyde-treated eggs (89.91%). Thus, sanitation with clove essential oil increased hatchability compared with that of nonsanitized eggs, possibly because of control of the bacterial load on the eggshell surface.
No significant differences in mortality were found between the treatments during the early (P = 0.0915; CV = 25.68%; Table 5) or middle (P = 0.5443; CV = 69.77%) incubation stage. However, there was a significant reduction (P = 0.0197; CV = 18.69%) in embryonic mortality during the late incubation stage in eggs sprayed with clove essential oil (8.67 ± 3.86%) compared with eggs sprayed with grain alcohol (13.60 ± 1.00) and eggs in the negative control group (13.41 ± 2.24%). In this context, Copur et al. (2011) and Baylan et al. (2018) reported that a decrease in early and late mortality may be the result of reduced eggshell contamination. Therefore, the lower mortality percentage observed in the late incubation stage of eggs treated with clove essential oil may be associated with decreased microbial populations on the eggshell because of the action of the chemical constituents present in the oil.
The rate of egg contamination during incubation was affected by the treatments (P = 0.0330; CV = 57.54%; Table 5). The largest percentage of contaminated eggs was observed in the negative control group (3.13 ± 1.25%), followed by the grain alcohol (2.81 ± 0.84%), paraformaldehyde (0.81 ± 0.94%), and clove essential oil (0.38 ± 0.76%) groups. These results reinforce that the clove essential oil-based sanitizing agent presents a broad spectrum of antimicrobial activity and may have been able to maintain low levels of microorganisms on the eggs during incubation, because according to Magwood (1964), there is a significant increase in the bacterial count on the eggshell during this period.
Chick initial weight (P = 0.0723; CV = 0.91%) and yield (P = 0.2122; CV = 1.05%) did not differ between treatments (Table 6). However, there was a significant difference (P < 0.0001; CV = 2.48%) in the physical quality score of the chicks. Chick weight is strongly associated with the weight of the egg from which the chick hatches (Morris et al., 1968). As no differences were observed in initial egg weight or weight loss, differences in the initial weight of the chicks were not expected.
Table 6.
Chick weight, percentage of chick yield, and chick physical quality score assessment according to different treatments.1
| Treatment | Chick weight (g) | Chick yield (%) | Pasgar Score© |
|---|---|---|---|
| Nonsanitized | 40.00 ± 0.68 | 68.34 ± 0.32 | 9.09 ± 0.81b,c |
| Grain alcohol | 40.51 ± 0.20 | 68.87 ± 0.54 | 9.06 ± 0.96c |
| Clove essential oil | 39.93 ± 0.40 | 68.08 ± 1.08 | 9.21 ± 0.89a |
| Paraformaldehyde | 40.32 ± 0.48 | 68.89 ± 1.08 | 9.13 ± 0.90b |
| P-value | 0.0723 | 0.2122 | <0.0001 |
| Coefficient of variation (%) | 0.91 | 1.05 | 2.48 |
Means in the same column with different superscript letters differ significantly (P < 0.05).
Results are expressed as the means ± SD.
Chick yield allows us to infer whether the incubation time and parameters were correct, with ideal chick yield values ranging between 67 and 68% (Aviagen, 2011). In the present experiment, all treatments presented yields classified as “slightly high” (68.55 ± 0.76%) but close to acceptable. The data observed in this study differ from those reported by Oliveira et al. (2020), who observed that eggs sanitized with grain alcohol (67.50 ± 1.92%), clove essential oil (67.90 ± 1.87%), or paraformaldehyde (67.80 ± 1.85%) showed chick yields classified as ideal. However, because in both experiments the eggs were subjected to ideal temperature and humidity conditions, a possible explanation for these contradictory results may be the egg weight loss observed in the present study, which, despite being within the ideal range reported in the literature (Molenaar et al., 2010), resulted in heavy chicks.
In the Pasgar© score assessment, it was determined that the clove essential oil (9.21 ± 0.89%) had a superior effect on the physical quality of the chicks compared with that of the other treatments. It is known that if eggs intended for incubation are not sanitized with effective products before being placed in the incubators, chick quality may decrease because of the high bacterial contamination that may occur, which may cause infection of the yolk sac (Harry, 1957; Cortés et al., 2004). Therefore, the microbial contamination level may be an indicator of chick quality. Thus, more viable chicks were obtained in the clove essential oil treatment because this sanitizer reduces microbial populations and does not adversely affect embryos or chicks.
Eggshell thickness is one of the factors that affects gas exchange and moisture loss during embryonic development (Ar et al., 1974; Veldsman et al., 2020). Therefore, any undesirable changes in this parameter may be harmful to embryos. In the present study, the similarity (P = 0.8502; CV = 7.79%; Table 7) between the means of eggshell thickness (0.365 ± 0.026 mm) showed that the tested treatments did not negatively affect this variable, even though the application of sanitizing agents to eggs can affect eggshell structure (Kim and Slavik, 1996). Oliveira et al. (2020) also did not observe significant differences in the thickness of eggshells treated with grain alcohol, clove essential oil, ethanolic extract of propolis, or paraformaldehyde (mean of 0.37 ± 0.029 mm).
Table 7.
Eggshell thickness according to different treatments.1
| Treatment | Eggshell thickness (mm) |
|---|---|
| Nonsanitized | 0.364 ± 0.024 |
| Grain alcohol | 0.365 ± 0.019 |
| Clove essential oil | 0.363 ± 0.031 |
| Paraformaldehyde | 0.368 ± 0.028 |
| P-value | 0.8502 |
| Coefficient of variation (%) | 7.79 |
No significant differences existed between means (P > 0.05).
Results are expressed as the means ± SD.
Conclusions
Clove essential oil is effective and safe for eggs intended for incubation. Its use as an alternative to paraformaldehyde in the sanitation of fertile eggs is strongly recommended because it reduces the eggshell microbial load, resulting in good incubation parameters and better neonatal chick quality. Furthermore, our data indirectly suggest that the application of clove essential oil does not negatively affect the structural integrity of the cuticle on the eggshell surface or the development of the embryo.
Acknowledgments
The authors wish to thank the University of Brasília and Federal Institute of Brasília for assistance. They also acknowledge Avifran - French Farming for making this study possible. Finally, the authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the granted scholarship.
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Adams R.P. 4.1 ed. Allured Pub. Corp.; Carol Stream, IL: 2017. Identification of Essential Oil Components by Gas Chromotograhpy/mass Spectrometry; p. 809. [Google Scholar]
- Ar A., Paganelli C.V., Reeves R.B., Greene D.G., Rahn H. The avian egg: Water vapor conductance, shell thickness and functional pore area. Condor. 1974;76:153–158. [Google Scholar]
- Ascenção V.L., Filho V.E.M. Extração, caracterização química e atividade antifúngica de óleo essencial Syzygium aromaticum (cravo da índia) [Extraction, chemical characterization and antifungal activity of Syzygium aromaticum (clove) essential oil] Cad. Pesqui. 2013;20:137–144. [Google Scholar]
- Aviagen How to measure chick yield. 2011. http://en.aviagen.com/assets/Tech_Center/BB_Resources_Tools/Hatchery_How_Tos/02HowTo2MeasureChickYield.pdf
- Barbosa V.M., Baião N.C., Mendes P.M.M., Rocha J.S.R., Pompeu M.A., Lara L.J.C., Martins N.R.S., Nelson D.L., Miranda D.J.A., Cunha C.E., Cardoso D.M., Cardeal P.C. Avaliação da qualidade da casca dos ovos provenientes de matrizes pesadas com diferentes idades [Evaluation of eggshell quality from broiler breeder hens with different ages] Arq. Bras. Med. Vet. Zootec. 2012;64:1036–1044. [Google Scholar]
- Barott H.G. Effects of temperature, humidity and other fac-tors on hatch of eggs and on energy metabolism of chick embryos. USDA Tech. Bull. 1937;553 [Google Scholar]
- Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Microbiol. 1966;40:2413–2415. [PubMed] [Google Scholar]
- Baylan M., Akpınar G.C., Canogullari S.D., Ayasan T. The effects of using garlic extract for quail hatching egg disinfection on hatching results and performance. Braz. J. Poult. Sci. 2018;20:343–350. [Google Scholar]
- Board R.G., Tranter H.S. The microbiology of eggs. In: Staldeman W.J., Cotterill O.J., editors. Egg Science and Technology. 4th ed. Binghamton; NY: 1995. pp. 81–104. [Google Scholar]
- Boerjan M. Chick vitality and uniformity. Int. Hatchery Pract. 2006;20:7–8. [Google Scholar]
- Brake J., Sheldon B.W. Effect of a quaternary ammonium sanitizer for hatching eggs on their contamination, permeability, water loss and hatchability. Poult. Sci. 1990;69:517–525. doi: 10.3382/ps.0690517. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Cadirci S. Disinfection of hatching eggs by formaldehyde fumigation—a review. Arch. Geflügelk. 2009;73:116–123. [Google Scholar]
- Casteel J.H., Vernon R.J., Bailey E.M.J. Formaldehyde: toxicology and hazards. Vet. Hum. Toxicol. 1987;20:31–33. [PubMed] [Google Scholar]
- Chaieb K., Hajlaoui H., zmantar T., Kahla-Nakbi A.B., Rouabhia M., Mahdouani K., Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother. Res. 2007;21:501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- Copur G., Arslan M., Baylan M., Canogullari S. Use ofallicin as an alternative hatching egg disinfectant versus formal-dehyde fumigation in broiler hatching eggs. Biotechnol. Equip. 2011;25:2494–2498. [Google Scholar]
- Copur G., Arslan M., Duru M., Baylan M., Canogullari S., Aksan E. Use of oregano (Origanum onites L.) essential oil as hatching egg disinfectant. Afr. J. Biotechnol. 2010;9:2531–2538. [Google Scholar]
- Cortés C.R., Tellez I.G., López C.C., Villaseca F.J.M., Eslava C.C. Bacterial isolation rate from fertile eggs, hatching eggs andneonatal broilers with yolk sac infection. Rev. Latinoam. Microbiol. 2004;46:12–16. [PubMed] [Google Scholar]
- Dhara L., Tripathi A. Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing Enterobacteriaceae by in vitro and molecular docking analysis. Eur. J. Integr. Med. 2013;5:527–536. [Google Scholar]
- Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Med. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasenko G.M., O’Dea Christopher E.E., McMullen L.M. Spraying hatching eggs with electrolyzed oxidizing water reduces eggshell microbial load without compromising broiler production parameters. Poult. Sci. 2009;88:1121–1127. doi: 10.3382/ps.2008-00359. [DOI] [PubMed] [Google Scholar]
- Harry E.G. The effect on embryonic and chick mortality of yolk contaminated with bacteria from the hen. Vet. Rec. 1957;69:1433–1439. [Google Scholar]
- Kim J.W., Slavik M.F. Use of blue lake as an indicator of bacterial penetration into eggs. J. Rapid Methods Automat. Microbiol. 1996;4:183–190. [Google Scholar]
- Magwood S.E. Studies in hatchery sanitation I. Fluctuations in microbial counts of air in poultry hatcheries. Poult. Sci. 1964;43:441–449. [Google Scholar]
- Meijerhof R., van Beek G. Mathematical modelling oftemperature and moisture loss of hatching eggs. J. Theor. Biol. 1993;165:27–41. [Google Scholar]
- Molenaar R., Reijrink I.A.M., Meijerhof R., Van den Brand H. Meeting embryonic requirements of broilers throughout incubation: a review. Rev. Bras. Cienc. Avic. 2010;12:137–148. [Google Scholar]
- Morris R.H., Hessels D.F., Bishop R.J. The relation-ship between hatching egg weight and subsequent performance of broiler chickens. Br. Poult. Sci. 1968;9:305–315. [Google Scholar]
- Morris M.T., Stephens C.B., Bourassa D.V., Cox N.A., Mauldin J.M., Berrang M.E., Buhr R.J. Enterobacteriaceae and Salmonella recovered from nonsanitized and sanitized broilerhatching eggs. J. Appl. Poult. Res. 2014;23:516–522. [Google Scholar]
- Oliveira G.S., Santos V.M. Sanitizantes alternativos ao uso do paraformaldeído para ovos incubáveis: revisão de literatura [Alternative sanitizers to the use of paraformaldehyde for hatching eggs: literature review] Nutritime Rev. Eletrônica. 2018;15:8254–8271. [Google Scholar]
- Oliveira M.S., Costa W.A., Pereira D.S., Botelho J.R.S., Menezes T.O.A., Andrade E.H.A., Silva S.H.M., Filho A.P.S.S., Carvalho R.N. Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J. Supercrit. Fluids. 2016;118:185–193. [Google Scholar]
- Oliveira G.S., Santos V.M., Nascimento S.T., Rodrigues J.C. Alternative sanitizers to paraformaldehyde for incubation of fertile eggs. Poult. Sci. 2020;99:2001–2006. doi: 10.1016/j.psj.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles E.D., Pansky T., Doyle S.M., Boyle C.R., Smith T.W., Latour M.A., Gerard P.D. Effects of dietary fat and eggshell cuticle removal on egg water loss and embryogrowth in broiler hatching eggs. Poult. Sci. 1998;77:1522–1530. doi: 10.1093/ps/77.10.1522. [DOI] [PubMed] [Google Scholar]
- Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhomberg L.R. Contrasting directions and directives on hazard identification for formaldehyde carcinogenicity. Regul. Toxicol. Pharm. 2015;73:829–833. doi: 10.1016/j.yrtph.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Roca A., Rodriguez-Herva J.J., Duque E., Ramos J.L. Physiological responses of Pseudomonas putida to formaldehyde during detoxification. Mol. Biotechnol. 2008;1:159–169. doi: 10.1111/j.1751-7915.2007.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahein E., Sedeek E.K. Role of spraying hatching eggs withnatural disinfectants on hatching characteristics and eggshell bacterial counts. Egypt. Poult. Sci. J. 2014;34:211–228. [Google Scholar]
- Tullet S.G., Burton F.G. Factors affecting the weight and water status of the chick at hatch. Br. Poult. Sci. 1982;23:361–369. [Google Scholar]
- Ulucay I.O., Yildirim I. Hatching traits of quail (Coturnix coturnix japonica) eggs disinfected with carvacrol, cinnamaldehyde or thymol. J. Appl. Anim. Res. 2010;38:139–142. [Google Scholar]
- Unsaldi E., Ciftci M.K. Formaldehyde and it using areas, risk group, harmful effects and protective precautions against it. Van Vet. J. 2010;21:71–75. [Google Scholar]
- USDA. Egg gradding manual. 2000. https://www.ams.usda.gov/sites/default/files/media/Egg%20Grading%20Manual.pdf Accessed Aug. 2020.
- Veldsman L.M., Kylin H., Bronkhorst P., Engelbrecht I., Bouwman H. A method to determine the combined effects of climate change (temperature and humidity) and eggshell thickness on water loss from bird eggs. Environ. Geochem. Health. 2020;42:781–793. doi: 10.1007/s10653-019-00274-x. [DOI] [PubMed] [Google Scholar]
- Whistler P.E., Sheldon B.W. Biocidal activity of ozone versus formaldehyde against poultry pathogens inoculated in a prototype setter. Poult. Sci. 1989;68:1068–1073. doi: 10.3382/ps.0681068. [DOI] [PubMed] [Google Scholar]
- Williams J.E. Effect of high-level formaldehyde fumigation on bacterial populations on the surface of chicken hatching eggs. Avian Dis. 1970;14:386–392. [PubMed] [Google Scholar]
- Zeweil H.S., Rizk R.E., Bekhet G.M., Ahmed M.R. Comparing the effectiveness of egg disinfectants against bacteria and mitotic indices of developing chick embryos. J. Basic Appl. Zool. 2015;70:1–15. [Google Scholar]