Abstract
Nutraceuticals are not only nutritionally beneficial for animals but also their use as feed supplements may reduce environmental contamination. The effect of fermented defatted “alperujo,” an olive oil by-product, supplementation on the intestinal health of broiler chickens was assessed by analyzing the intestinal mucosal morphology of the duodenum and the cecum. The microbiota of the cecum was also characterized by analyzing the V3-V4 region of the 16S rRNA gene on days 7, 14, 21, 28, 35, and 42. Supplemented broilers from 14 to 35 D of age showed an increase in villus height in the duodenum. This increase likely improved digestibility and absorption capacity during growth, leading to the observed increase in BW at day 35 of life. A progressive increase in crypt depth in both the duodenum and the cecum was also observed. This modification likely enhanced epithelial renewal, thus safeguarding the turnover capacity of the intestinal mucosa. Our molecular analysis of cecal microbiota suggests that this dietary supplement may favor the growth of certain bacteria and may control the spread of pathogenic bacteria by means of competitive exclusion.
Key words: intestinal health, olive oil by-product, fermented defatted “alperujo”, histology, microbiota
Introduction
A healthy gut is critical for the general health and welfare of poultry (Ducatelle et al., 2018). Intestinal health is a permanent balance that is influenced by the interactions between the gastrointestinal mucosa and environmental factors such as diet and infectious agents, which in turn, affect the microbiota of the gut (Yegani et al., 2008). Consequently, the mucosa and microbiota are the main components involved in determining intestinal health, as well as modulating the immune response (Yegani and Korver, 2008; Lee et al., 2017; Ducatelle et al., 2018).
The intestinal mucosa is a dynamic organ that undergoes continuous renewal. Damage to the epithelial surface leads to morphologic changes in the structure of villi and crypts (Ducatelle et al., 2018), thus interfering with the proper functioning of the intestinal mucosa. Epithelial injuries result in deficiencies in nutrient and water absorption and cause an unspecific immune response. A diet-based approach that establishes an adequate immune response during early production phases may prevent infectious diseases, a common cause of production losses in the poultry industry (Yegani and Korver, 2008; Rubio, 2019). On the other hand, the intestinal microbiota, and its interactions with the mucosa and other environmental factors, plays an important role in the intestinal development and physiology of poultry. Its role in digestion, absorption, and metabolism could directly influence immunity and performance (Yadav and Jha, 2019).
In general, diet composition is the main factor that influences the early development and health of the gastrointestinal tract (Rubio, 2019). Differential intake of components of poultry feed produces changes in both mucosal morphology and microbiota (Ducatelle et al., 2018) and can even induce the selection and growth of certain bacterial strains (Kelly et al., 2017; Lee et al., 2017; Yadav and Jha, 2019). Numerous dietary supplements, particularly those considered probiotic, prebiotic, or nutraceutical, have been added to broiler chicken feed.
Nutraceuticals are substances derived from natural sources that have benefits other than for nutrition (Nasri et al., 2014). In recent years, a main focus of animal research, particularly of poultry science, has been on the ability of natural compounds to promote a healthy gut by modifying mucosal morphology and microbiota, thereby improving the overall health status of chickens. Supplementation with natural compounds has improved the productive performance of some farms (Rubio, 2019). Some natural compounds, which are primarily obtained from the food industry, may not only be nutritionally valuable for animals but also their use as feed supplements may help reduce environmental contamination (Viveros et al., 2011; Berbel and Posadillo, 2018; Pappas et al., 2019). In particular, olive oil by-products, given their high polyphenolic content, have been associated with antioxidant, anti-inflammatory, antithrombotic, antimicrobial, and anti-coccidian effects in poultry (Alu'datt et al., 2010; Gerasopoulos et al., 2015; Nasopoulou et al., 2018).
The two main centrifugation systems used to obtain olive oil generate different types of wastes (Alburquerque et al., 2004). The three-phase extraction system generates olive mill wastewater and olive cake or pulp, which, as dietary supplements, have been shown to improve the organic antioxidant capacity, health status, and productive performance of broilers (Gerasopoulos et al., 2015; Sabino et al., 2018; Papadomichelakis et al., 2019). However, since the early 1990s, two-phase centrifugation has been the most implemented system, at least in the Spanish olive oil industry (Alburquerque et al., 2004). The two-phase system generates a waste product referred to as two-phase mill waste, “alperujo” or olive pomace. Of all the olive oil by-products, this one has been one of the most common to be tested as a supplement in animal feed (Berbel and Posadillo, 2018). Olive pomace extract has been reported, in particular, to have anti-inflammatory properties that enhance gut function in broilers (Herrero-Encinas et al., 2020).
Despite its beneficial effects and in contrast to other food industry wastes, further processing of “alperujo” is required before it can be used in animal feed (Berbel and Posadillo, 2018). “Alperujo” that had been previously fermented and defatted was recently shown to enhance intestinal health in laying hens by modifying the intestinal mucosa and microbiota, in addition to improving shell hardness (Rebollada-Merino et al., 2019). After degreasing, the free phenols present in “alperujo” appear to have the same level of antioxidant activities as those in full-fat olive cake (Alu'datt et al., 2010). This observation suggests that modified olive oil by-products, which can be used directly as dietary supplements, possess the same properties as unmodified ones. However, the effect of fermented defatted “alperujo” on broiler intestinal health has not been studied to date.
To evaluate the effect of feed supplementation on intestinal health, a multidisciplinary approach is required. In this context, histomorphologic and microbiota analyses have been shown to be reliable biomarkers in chickens (Viveros et al., 2011; Ducatelle et al., 2018). The aim of this experimental study was to analyze the potential effect of the modified olive oil by-product fermented defatted “alperujo” on the intestinal health of broilers by characterizing changes in the mucosal morphology of the duodenum and the cecum and in the microbiota of the cecum throughout the production cycle.
Materials and methods
Ethical Approval
Experimental procedures were approved by the Complutense University of Madrid Animal Care and Ethics Committee in compliance with the Community of Madrid (PROEX 152/19). Research met the guidelines approved by the Institutional Animal Care and Use Committee.
Birds, Rearing Conditions, and Diet
Sixty 1-day-old male Ross 308 broiler chickens were obtained from a commercial hatchery and raised in the laboratory facilities of the VISAVET Health Surveillance Center for 42 D under the same environmental and light conditions previously used for the species (Herrero-Encinas et al., 2020). Chickens were physically separated on arrival into 2 groups: a control group fed with conventional feed and a treated group whose feed was supplemented with 2% fermented defatted “alperujo” that was provided by Porres y Barios, S.A. (Córdoba, Spain) and the composition and processing to obtain fermented defatted “alperujo” was previously described by Rebollada-Merino et al. (2019). Animals had ad libitum access to feed and water for the duration of the experiment. They were monitored physically twice daily and at all times by video cameras.
Postmortem Study and Sample Collection
On posthatching days 7, 14, 21, 28, 35, and 42, five randomly selected animals from each group were sedated intramuscularly with diazepam and euthanized with an overdose of sodium pentobarbital by intravenous injection. Before the necropsy, each animal was weighed. A completed and systematic postmortem was performed, and duodenum and cecum samples were collected and fixed in a 10% formaldehyde-buffered solution (Panreac Química SLU, Barcelona, Spain). In addition, the cecal content was collected from each animal and preserved at −80°C for metagenomics studies.
Histomorphometric Study
After routine histopathologic processing and hematoxylin–eosin staining (Rebollada-Merino et al., 2019), sections of the duodenum and the cecum were examined under an optical microscope coupled with a digital camera, and a blind histomorphometric analysis was performed at 40 × magnification. Using an image analyzer (Leica Application Suite; Leica, Wetzlar, Germany), 20 intact and well-oriented villi and 20 crypts in the duodenum, as well as 20 crypts in the cecum, were evaluated per animal.
Statistical Analysis
Differences between the two groups by age were assessed using the Mann-Whitney test implemented in IBM SPSS Statistics v25 (IBM, Armonk, NY). Statistical significance was considered at P < 0.05.
Total DNA Extraction and 16S rRNA Library Preparation and Sequencing
Total DNA was extracted from 220 mg of the cecal content using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany), as per the manufacturer's instructions. DNA concentration was determined using a Qubit fluorometer (Invitrogen, Carlsbad, CA). Microbial diversity was studied by analyzing sequences of the V3-V4 region of the 16S rRNA gene. The primers and PCR conditions used for this analysis were as previously reported (Klindworth et al., 2013). Sample multiplexing, library purification, and sequencing were carried out as described in the “16S Metagenomic Sequencing Library Preparation” guide by Illumina (San Diego, CA). Libraries were sequenced on an Illumina MiSeq platform that provided 300-bp paired-end reads.
Bioinformatics and Data Analysis
Raw demultiplexed sequence data were processed using QiimeReporter (https://github.com/dabadgarcia/qiimereporter). This straightforward pipeline for the analysis of amplicon sequences integrates basic Qiime2 commands (Bolyen et al., 2019) with R programming language. In brief, the DADA2 package (Callahan et al., 2016) was used to filter reads, merge paired ends, remove chimeras, and assign amplicon sequence variants. Then, a pretrained Naïve Bayes classifier (Wang et al., 2007) was used to obtain the taxonomic assignment of the amplicon sequence variants, using the SILVA database v132 (Quast et al., 2013) as a reference, which resulted in a table containing the microbial composition for each of the samples. Raw reads are available in the BioProject database with ID PRJNA643396 (http://www.ncbi.nlm.nih.gov/bioproject/643396).
Results
Body Weight
On posthatching days 7, 14, 21, and 28, no BW differences were observed between the control and supplemented groups (Figure 1). However, at 35 D, the mean BW of the supplemented group was significantly higher than that of the control (P = 0.009). At 42 D, the mean weight of the supplemented group was still higher, although the difference was not statistically significant (P = 0.117).
Figure 1.
Effect of supplementation with 2% fermented defatted “alperujo” on broiler BW (in g) by age. At day 35, the mean BW was significantly higher in the treated group than that in the control group (P = 0.009).
Histomorphometric Study
The histomorphometric analysis of the duodenum revealed a significant increase (P < 0.05) in villus height on days 14, 21, 28, and 35 in the fermented defatted “alperujo”–supplemented group (Table 1). Duodenal crypts were significantly deeper in the control group on days 14 and 42 (P < 0.05); however, the mean values, although not significant (P > 0.05), were higher in the supplemented group for the other days. In the cecum, crypts were significantly deeper in the supplemented group for all samplings (P < 0.05), except on day 14 (Table 1, Figure 2).
Table 1.
Results of the histomorphometric analysis of the duodenum (villus height and crypt depth) and cecum of control (n = 100) and supplemented (n = 100) broilers. Number of samples (N), mean values in micrometers (Mean), SD, and P-value are shown for each age group.
| Control |
Supplemented |
P-Value1 | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Duodenal villus height | |||||
| 7 D | 702.31 | 134.75 | 670.70 | 236.95 | 0.653 |
| 14 D | 1,068.80 | 256.09 | 1,168.35 | 177.21 | 0.002 |
| 21 D | 857.38 | 302.76 | 1,303.24 | 441.13 | <0.001 |
| 28 D | 1,003.97 | 303.58 | 1,422.50 | 264.57 | <0.001 |
| 35 D | 990.49 | 343.05 | 1,199.55 | 286.47 | <0.001 |
| 42 D | 1,196.92 | 303.79 | 1,103.85 | 339.67 | 0.267 |
| Duodenal crypt depth | |||||
| 7 D | 99.13 | 24.54 | 105.41 | 46.84 | 0.869 |
| 14 D | 137.32 | 49.37 | 123.99 | 48.23 | 0.034 |
| 21 D | 170.49 | 138.68 | 177.10 | 123.20 | 0.197 |
| 28 D | 131.40 | 47.50 | 138.08 | 59.72 | 0.753 |
| 35 D | 115.35 | 38.71 | 122.11 | 88.47 | 0.882 |
| 42 D | 143.47 | 108.03 | 130.43 | 132.68 | 0.012 |
| Cecal crypt depth | |||||
| 7 D | 139.44 | 78.56 | 207.81 | 86.84 | <0.001 |
| 14 D | 217.02 | 52.60 | 207.60 | 59.38 | 0.178 |
| 21 D | 295.57 | 135.04 | 449.94 | 175.81 | <0.001 |
| 28 D | 391.66 | 204.00 | 492.70 | 245.29 | 0.007 |
| 35 D | 294.92 | 101.62 | 370.86 | 122.90 | <0.001 |
| 42 D | 276.64 | 106.88 | 617.00 | 236.68 | <0.001 |
The Mann-Whitney test was used to assess significant differences between control and supplemented animals.
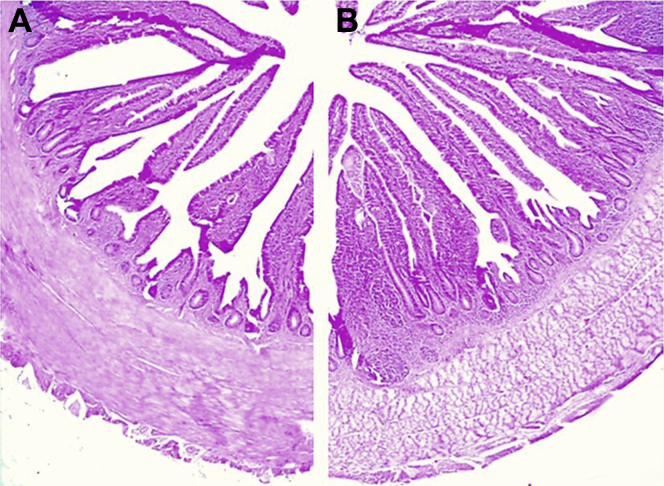
Figure 2.
Cecum of 28-day-old broilers. Hematoxylin–eosin stained section of a control (A) and a supplemented (B) cecum. Crypts were significantly deeper in the supplemented group than those in the control group. 40 × , scale bar: 500 μm.
Cecal Microbiota
Nonsignificant variability in bacterial taxonomic richness at the genus level was observed in the treated animals (Figure 3). The most abundant bacterial genera identified at day 7 were Clostridiales (relative abundance: 30.15%), Klebsiella (29.27%), and Escherichia-Shigella (22.99%) in the control group and Escherichia-Shigella (32.06%), Ruminococcus (25.63%), and Lachnospiraceae (16.22%) in the treated group. At day 14, Escherichia-Shigella (45.30 and 39.68% in control and treated, respectively) and Lachnospiraceae (20.21 and 9.71%) were most prevalent in both groups, though in the treated group, Oscillibacter (34.62%) was also highly abundant. At day 21, Escherichia-Shigella (48.78%) and Lactobacillus (11.45%) were the most abundant genera in the control group. By contrast, in the treated group, Escherichia-Shigella (22.11%), Oscillibacter (20.47%), and Lachnospiraceae (16.65%) were most abundant at this stage. At day 28, Lachnospiraceae (19.41 and 21.49% in control and treated, respectively) was predominant in both groups, followed by Escherichia-Shigella in the control (15.86%) and Ruminococcus in the treated group (14.66%). At day 35, Bacteroides (24.16%) and Lachnospiraceae (23.51%) were most abundant in the control and treated groups, respectively, followed by Escherichia-Shigella in both groups (13.21% in the control and 15.77% in the treated). Finally, at day 42, Bacteroides was the most abundant genus in both groups (35.87% in control and 53.52% in the treated), followed by Lachnospiraceae in the control group (15.64%) and Escherichia-Shigella in the treated group (8.13%).
Figure 3.
Bar chart showing the 11 most abundant bacterial groups found in the cecal content of control and treated broiler chickens. Each bar represents the relative abundance of bacteria by animal, diet treatment, and age. Relative abundance is shown on the vertical axis and age (7, 14, 21, 35, or 42) and diet (C, control; T, treatment) on the horizontal axis.
Discussion
Diet supplementation with nutraceuticals, natural compounds with positive health effects, is an active field of research in veterinary medicine, particularly in poultry science. Dietary changes have been shown to contribute to structural and bacterial modifications in the intestine that improve intestinal health and, consequently, poultry production performance (Viveros et al., 2011; Rebollada-Merino et al., 2019; Yadav and Jha, 2019). After fermented defatted “alperujo” supplementation, we observed a significant increase in BW at day 35 of life, which may be confirmed in further experiments with a higher sample size. Tufarelli et al. (2016) previously reported a BW increase after olive oil supplementation in broilers and suggested that lipid content directly influences weight gain and feed efficiency. Increased BW has also been attributed to polyphenols present in olive oil and olive oil by-products (Tufarelli et al., 2015). However, the results of our histomorphometric study of the duodenum and cecum suggest improved nutrient absorption in the intestine may be associated with morphologic changes in the mucosa in addition to the nutrient composition of the supplement. Histologic measurements are considered the gold standard for evaluating intestinal health (Samuel et al., 2017; Ducatelle et al., 2018) given that they are useful indicators of intestinal mucosal modifications that occur after dietary supplementation during the production cycle (Viveros et al., 2011).
We observed a significant increase in duodenal villus height in supplemented animals from the second to fifth week of life. This increase may result in a greater absorption capacity, which may partially explain the greater weight increase observed in this group, especially in the final week of production (Viveros et al., 2011; Samuel et al., 2017; Reis et al., 2018). A similar increase in villus height has been reported in broilers supplemented with the plant-derived phenol gallic acid (Samuel et al., 2017) and the essential oil carvacrol (Kelly et al., 2017). In addition, supplementation with Pulicaria gnaphalodes powder containing a high phenol content has been associated with a reduction of pathogenic bacteria in the small intestine, which may be due to increased mucus secretion by goblet cells (Shirani et al., 2019). Increases in villus height are thought to be caused by the action of more transport enzymes in the enterocytes (Viveros et al., 2011; Ma et al., 2018). However, a recent study has suggested that phenolic compounds inhibit digestive enzymes and transporters in the enterocytes, delaying the digestion of some macronutrients, such as proteins and carbohydrates, and even hindering fat absorption (Domínguez-Ávila et al., 2017). Our results indicate that villus growth in the duodenum of supplemented broilers is dynamic but not linear: a progressive increase in intestinal villus height was observed until it reached a maximum during the fourth week of life, after which it markedly decreased. Such dynamics have not been previously reported in broilers, mainly because other morphologic studies mainly focus on the last phases of the production cycle.
In chicken intestines, epithelial cells in the villi are continuously renewed by crypt stem cells (Ducatelle et al., 2018). In broilers, the presence of deep crypts generally implies a better response to potentially harmful events in the villi (Pourabedin and Zhao, 2015; Ducatelle et al., 2018; Sabino et al., 2018). In our study, crypts, particularly in the cecum, were deeper in the treated animals. By contrast, supplementation with grape products or olive oil wastewater (a three-phase mill waste), both of which contain high levels of polyphenols, decreases crypt depth in broilers (Viveros et al., 2011; Sabino et al., 2018). Compared with our study, in the olive oil wastewater study by Sabino et al. (2018), animals were supplemented with a lower by-product concentration for less time, and fewer samplings were taken for histologic analysis. Therefore, methodological differences may, in part, account for the contrasting observations between studies. Regardless, in our study, greater progressive growth in crypt depth was observed until the fifth week of life, suggesting that fermented defatted “alperujo” improves epithelial renewal, thus safeguarding the turnover capacity of crypts, which is critical in the response to potential mucosal injuries, secondary bacterial infections, or parasitic diseases.
Metagenomic studies of changes in cecal microbiota can provide insight into potential intestinal health benefits and host–microbiota interactions, despite only providing limited information on the effect of bioactive compounds on microbiota (Herrero-Encinas et al., 2020; Yadav and Jha, 2019; Rychlik, 2020). As physiological changes in intestinal microbiota have been associated with factors such as age and feed composition (Videnska et al., 2014; Lee et al., 2017; Ijaz et al., 2018; Richards et al., 2019; Rychlik, 2020), we evaluated the microbial composition of the cecum, a main site of bacterial metabolic activity (Cressman et al., 2010; Richards et al., 2019; Yadav and Jha, 2019; Rychlik, 2020), throughout the productive life of broilers.
In our microbiota analysis, Enterobacteriaceae was the most prominent family, consistent with the findings of a previous study analyzing cecal microbiota in broilers after Lactobacillus acidophilus supplementation (De Cesare et al., 2017). Specifically, during the first day of life, Escherichia-Shigella and Klebsiella were the most represented genera; however, their relative abundances decreased progressively thereafter. These results are in line with those reported by Ijaz et al. (2018) and Rychlik (2020). Some authors have hypothesized that a high level of dietary fiber accounts for the reduction of these bacteria in the gut (Walugembe et al., 2015). The fermented defatted “alperujo” supplement also has a high fiber content (Rebollada-Merino et al., 2019), supporting the idea that fiber hinders the growth of Enterobacteriaceae. In addition, some Enterobacteriaceae genera may be pathogenic (Ma et al., 2018), thus fermented defatted “alperujo” supplementation in broilers may act to reduce the abundance of these bacteria in the gut, resulting in less epithelial damage and inflammation. The reduced abundance of these bacteria in the cecum potentially explains the observed differences in crypt depth between 21 and 28 D of life.
In contrast, Oscillibacter abundance increased in the supplemented group from 14 to 35 D of life. Oscillibacter is a butyrate-producing bacterium that is hypothesized to increase short-chain fatty acid availability in the intestinal lumen (Rychlik, 2020).
At 42 D, the microbiota of supplemented broilers was, in general, similar to that of the control. One difference was the higher relative abundance of Bacteroides, at the expense of Lachnospiraceae and Ruminococcus, in the supplemented group. Bacteroides, which has been reported to be predominant in broilers around this age (Lee et al., 2017), increases propionic acid generation in the cecum, which may correspond to a reduction of fat synthesis in the liver (Qi et al., 2019) that, in turn, prevents hepatic steatosis.
We also noted some contradictory results in our analysis of microbiota composition with respect to the abundance of Ruminococcaceae and Lactobacillus and overall bacterial diversity. Ruminococcaceae has been associated with an increase in BW after Bacillus subtilis supplementation in broilers (Ma et al., 2018). In our study, Ruminococcaceae was underrepresented in the supplemented animals, yet we found significant differences in the BW of 35-day-old broilers between the two groups. This result suggests that low levels of Ruminococcaceae do not necessarily decrease BW and that the BW increase observed in fermented defatted “alperujo”–supplemented animals is likely influenced by some other microbial interaction. Lactobacillus appears to reduce the antigen load from resident bacteria, thus having an anti-inflammatory effect in the gut (De Cesare et al., 2019). Here, we observed a lower relative abundance of Lactobacillus in the supplemented group at all time points, except 35 D of life, which is also when crypt depth diminished in these animals. Finally, in contrast with other broiler studies, we found one of the highest levels of bacterial diversity at 42 D of life (Herrero-Encinas et al., 2020; Kumar et al., 2019).
To summarize, fermented defatted “alperujo” supplementation in broilers improves villus height in the duodenum at early life stages. This likely increases digestibility and absorption capacity during growth, leading to an increase in BW at day 35 of life. Moreover, we suggest that the greater progressive increase of crypt depth observed in both the duodenum and the cecum after supplementation leads to enhanced epithelial renewal, thus safeguarding the turnover capacity of the intestinal mucosa. Finally, the microbiota composition of supplemented broilers is dynamic: Enterobacteriaceae dominates during the first ws of life but then is replaced by Oscillibacter and Lachnospiraceae followed by Bacteroides at the end of the production cycle. These microbiota changes suggest that the fermented defatted “alperujo” dietary supplement may favor the growth of certain bacteria and may control the spread of pathogenic bacteria by means of competitive exclusion.
Acknowledgments
This study was conducted as part of the project “Promoting One Health in Europe through joint actions on foodborne zoonoses, antimicrobial resistance and emerging microbiological hazards”. This project received funding from the European Union Horizon 2020 research and innovation programme under Grant Agreement No 773830.
The authors acknowledge Luis Arrabal (Porres y Barrios, S.A.) for providing the fermented defatted “alperujo” and VISAVET Health Surveillance Centre personnel involved in the project. Specifically, we thank the Pathology and Forensic Veterinary Unit: M. C. Jiménez, S. Cruz, G. Torre, F. Mayoral, N. Porras, L. Barreno; the Foodborne Zoonoses and Antimicrobial Resistance Unit: M. García, E. Rivero, N. Maasoumi; and the Quality and Biosafety Unit: M. Mazariegos, L. Delgado. D. Duque, P. Alcubilla. We also thank English editing services provided by M. Modrell.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Alburquerque J.A., Gonzálvez J., García D., Cegarra J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004;91:195–200. doi: 10.1016/s0960-8524(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Alu’datt M.H., Alli I., Ereifej K., Alhamad M., Rahman Al-Tawaha A., Rababah T. Optimization, characterization and quantification of phenolic compounds in olive cake. Food Chem. 2010;123:117–122. [Google Scholar]
- Berbel J., Posadillo A. Review and analysis of alternatives for the valorization of agro-industrial olive oil by-products. Sustainability. 2018;10:237. [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodríguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., González A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., II, Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hassan S., van der Hooft J.J.J., Vargas F., Vázquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman M.D., Yu Z., Nelson M.C., Moeller S.J., Lilburn M.S., Zerby H.N. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 2010;76:6572–6582. doi: 10.1128/AEM.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesare A., Faria do Valle I., Sala S., Sirri F., Astolfi A., Castellani G., Manfreda G. Effect of a low protein diet on chicken ceca microbiome and productive performances. Poult. Sci. 2019;98:3963–3976. doi: 10.3382/ps/pez132. [DOI] [PubMed] [Google Scholar]
- De Cesare A., Sirri F., Manfreda G., Moniaci P., Giardini A., Zampiga M., Meluzzi A. Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens. PLoS One. 2017;12:e0176309. doi: 10.1371/journal.pone.0176309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Ávila J.A., Wall-Medrano A., Velderrain-Rodríguez G.R., Chen C.O., Salazar-López N.J., Robles-Sánchez M., González-Aguilar G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017;8:15–38. doi: 10.1039/c6fo01475e. [DOI] [PubMed] [Google Scholar]
- Ducatelle R., Goossens E., De Meyer F., Eeckhaut V., Antonissen G., Haesebrouck F., Van Immerseel F. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet. Res. 2018;49:43. doi: 10.1186/s13567-018-0538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasopoulos K., Stagos D., Kokkas S., Petrotos K., Kantas D., Goulas P., Kouretas D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015;82:42–49. doi: 10.1016/j.fct.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Herrero-Encinas J., Blanch M., Pastor J.J., Mereu A., Ipharraguerre I.R., Menoyo D. Effects of a bioactive olive pomace extract from Olea europaea on growth performance, gut function, and intestinal microbiota in broiler chickens. Poult. Sci. 2020;99:2–10. doi: 10.3382/ps/pez467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz U.Z., Sivaloganathan L., McKenna A., Richmond A., Kelly C., Linton M., Stratakos A.C., Lavery U., Elmi A., Wren B.W., Dorrell N., Corcionivoschi N., Gundogdu O. Comprehensive longitudinal microbiome analysis of the chicken 2 cecum reveals a shift from competitive to environmental drivers and 3 a window of opportunity for Campylobacter. Front. Microbiol. 2018;9:2452. doi: 10.3389/fmicb.2018.02452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Gundogdu O., Pircalabioru G., Cean A., Scates P., Linton M., Pinkerton L., Magowan E., Stef L., Simiz E., Pet I., Stewart S., Stabler R., Wren B., Dorrell N., Corcionivoschi N. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Path. Dis. 2017;14:341–349. doi: 10.1089/fpd.2016.2265. [DOI] [PubMed] [Google Scholar]
- Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Adhikari P., Oakley B., Kim W.K. Changes in cecum microbial community in response to total sulfur amino acid (TSAA: DL-methionine) in antibiotic-free and supplemented poultry birds. Poult. Sci. 2019;98:5809–5819. doi: 10.3382/ps/pez380. [DOI] [PubMed] [Google Scholar]
- Lee K.C., Kil D.Y., Sul W.J. Cecal microbiome divergence of broiler chickens by sex and body weight. J. Microbiol. 2017;55:939–945. doi: 10.1007/s12275-017-7202-0. [DOI] [PubMed] [Google Scholar]
- Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., Wu S., Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasopoulou C., Lytoudi K., Zabetakis I. Evaluation of olive pomace in the production of novel broilers with enhanced in vitro antithrombotic properties. Eur. J. Lipid Sci. Technol. 2018;120:1700290. [Google Scholar]
- Nasri H., Baradaran A., Shirzad H., Rafieian-Kopaei M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014;5:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- Papadomichelakis G., Pappas A.C., Tsiplakou E., Symeon G.K., Sotirakoglou K., Mpekelis V., Fegeros K., Zervas G. Effects of dietary dried olive pulp inclusion on growth performance and meat quality of broiler chickens. Livestock Sci. 2019;221:115–122. [Google Scholar]
- Pappas A.C., Tsiplakou E., Papadomichelakis G., Mitsiopoulou C., Sotirakoglou K., Mpekelis V., Haroutounian S.A., Fegeros K., Zervas G. Effects of olive pulp addition to broiler diets on performance, selected biochemical parameters and antioxidant enzymes. J. Hellenic Vet. Med. Soc. 2019;70:1687–1696. [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015;362:122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Shi S., Tu J., Li S. Comparative metagenomic sequencing analysis of cecum microbiotal diversity and function in broilers and layers. 3 Biotech. 2019;9:316. doi: 10.1007/s13205-019-1834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollada-Merino A., Bárcena C., Ugarte-Ruiz M., Porras N., Mayoral-Alegre F.J., Tomé-Sánchez I., Domínguez L., Rodríguez-Bertos A. Effects on intestinal mucosal morphology, productive parameters and microbiota composition after supplementation with fermented defatted alperujo (FDA) in laying hens. Antibiotics (Basel) 2019;8:e215. doi: 10.3390/antibiotics8040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J.H., Gebert R.R., Barreta M., Baldissera M.D., Dos Santos I.D., Wagner R., Campigotto G., Jaguezeski A.M., Gris A., de Lima J.L.F., Mendes R.E., Fracasso M., Boiago M.M., Stefani L.M., Dos Santos D.S., Robazza W.S., Da Silva A.S. Effects of phytogenic feed additive based on thymol, carvacrol and cinnamic aldehyde on body weight, blood parameters and environmental bacteria in broilers chickens. Microb. Pathog. 2018;125:168–176. doi: 10.1016/j.micpath.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Richards P., Fothergill J., Bernardeau M., Wigley P. Development of the caecal microbiota in three broiler breeds. Front. Vet. Sci. 2019;6:201. doi: 10.3389/fvets.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019;98:695–706. doi: 10.3382/ps/pey416. [DOI] [PubMed] [Google Scholar]
- Rychlik I. Composition and function of chicken gut microbiota. Animals. 2020;10:103. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino M., Cappelli K., Capomaccio S., Pascucci L., Biasato I., Verini-Supplizi A., Valiani A., Trabalza-Marinucci M. Dietary supplementation with olive mill wastewaters induces modifications on chicken jejunum epithelial cell transcriptome and modulate jejunum morphology. BMC Genomics. 2018;19:576. doi: 10.1186/s12864-018-4962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel K.G., Wang J., Yue H.Y., Wu S.G., Zhang H.J., Duan Z.Y., Qi G.H. Effects of dietary gallic acid supplementation on performance, antioxidant status, and jejunum intestinal morphology in broiler chicks. Poult. Sci. 2017;96:2768–2775. doi: 10.3382/ps/pex091. [DOI] [PubMed] [Google Scholar]
- Shirani V., Jazi V., Toghyani M., Ashayerizadeh A., Sharifi F., Barekatain R. Pulicaria gnaphalodes powder in broiler diets: consequences for performance, gut health, antioxidant enzyme activity, and fatty acid profile. Poult. Sci. 2019;98:2577–2587. doi: 10.3382/ps/pez010. [DOI] [PubMed] [Google Scholar]
- Tufarelli V., Bozzo G., Perillo A., Laucadio V. Effects of feeding different lipid sources on hepatic histopathology features and growth traits of broiler chickens. Acta Histochem. 2015;117:780–783. doi: 10.1016/j.acthis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Tufarelli V., Laudadio V., Casalino E. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens. Environ. Sci. Pollut. Res. Int. 2016;23:6197–6204. doi: 10.1007/s11356-015-5852-1. [DOI] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukak M., Faldynova M., Gerzova L., Cejkove D., Sisak F., Rychlik I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One. 2012;9:e115142. doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Walugembe M., Hsieh J.C.F., Koszewski N.J., Lamont S.J., Persia M.E., Rothschild M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult. Sci. 2015;94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]