Abstract
L-theanine (γ-Glutamylethylamide) is a nonprotein water soluble amino acid (AA) mostly found in leaves of Camellia sinensis (green tea). This is a key component of green tea and is considered as the most abundant form of total AAs in green tea (i.e., about 50%). L-theanine is an exclusive taste ingredient of tea producing an attractive flavor and aroma in tea. It has biological effects such as antioxidant, growth promoter, immune booster, anti-stresser, hepatoprotective, antitumor, antiaging, antimicrobial, anti-inflammatory, and antianxiety activities that are worth noticing. It could reduce the oxidative impairment by reducing the synthesis of reactive oxygen species, oxidative parameters, and lipid damage as well as increasing the activity of antioxidant enzymes. The oral ingestion of L-theanine enhanced γδ T-cell proliferation. Therefore, it is being considered an essential compound of green tea that has the ability to improve immune function. The L-theanine can be used as a potential treatment for hepatic injury and immune-related liver diseases via the downregulation of the inflammatory response through the initiation of nitric oxide synthesis and glutathione production which are likely to be critical for the control of hepatic diseases as well as for the improvement of immune function. In addition, it could be used as a best natural feed additive with a potent antistressor by decreasing the levels of corticosterone, dopamine, and noradrenaline. After systematically reviewing the literature, it is noticed that most studies were carried out on mice, pig, human, and butterfly; while dietary supplementation studies of L-theanine in animal and poultry especially among broilers are very limited because of less awareness of this AA. So, the aim of this review is to encourage the veterinarian and poultry researchers to conduct more research at the molecular level about this AA to expose its more beneficial effects and its mechanism of absorption for potential use of this unique green tea AA in poultry nutrition.
Key words: L-theanine, tea plant, amino acid, biological effect, poultry
Introduction
Tea is derived from the leaves of Camellia sinensis (green tea) and is one of the utmost traditionally consumed beverages throughout the globe. Historically, tea has been used as a medicinal herb dating back to 4,700 yr in China and is regarded as a healthy practice, especially for improving liver function. Although caffeine and catechins are the primary biological components considered to contribute to the beneficial effects of tea, the health benefits of theanine have also become prominent in recent years. L-theanine is a nonproteinic amino acid (AA) that is found in the tea plant. The International Union of Pure and Applied Chemistry proposed the name of theanine as 2-amino-4-(ethylcarbamoyl) butyric acid (Vuong et al., 2011). Similar to other natural AAs, L-theanine is a chiral type and is mainly observed in the L-enantiomer form (Wan et al., 2008). L-theanine is a unique taste element of tea with an attractive aroma and a caramel flavor that aid to attenuate the bitterness of caffeine (Eschenauer and Sweet, 2006). Toxicological and technical assessment tests proposed that L-theanine is a nontoxic and safe phytogenic food additive. L-theanine is well known with several different names including γ–glutamylethylamide and γ-ethylamino-l-glutamic acid, and it also has a commercial name called Suntheanine (Facts and Comparisons, 2007). L-theanine (γ-glutamylethylamide) is a non–protein-derived AA that is bountiful in leaves of green tea (Deng et al., 2010). It (γ-glutamylethylamide) has been evaluated as a natural food additive for food fortification in relation to human nutrition and health benefits. It has many biological and pharmacological activities such as cerebral ischemia/reperfusion injury protection, antitumor, stress modulator, antiaging, and antianxiety properties (Higashiyama et al., 2011; Saito et al., 2011; Gong et al., 2012; Culetu et al., 2016). In addition, Cooper (2012) pointed out that dietary inclusion of L. theanine is an easy way to alleviate ROS (reactive oxygen species)-induced damage.
In the tea, L-theanine is biosynthesized from ethylamine and glutamic acid via the enzyme theanine synthetase (Deng et al., 2008). It represents nearly 50% of the total AAs in leaves of tea. It covers approximately 1 to 2% of the total dry weight (DM) of green tea leaves with one cup of green tea containing about 8 to 30 mg of theanine (de Mejia et al., 2009). L-theanine is readily bioavailable on consumption and is quickly absorbed in the intestinal tract followed by its metabolism in the liver (Owen et al., 2008; Van der Pijl et al., 2010). Chronic and acute toxicity tests carried out on the safety of L-theanine have not so far confirmed its toxicity (Turkozu and Sanlier, 2017).
L-theanine is available as a dietary supplement and has been approved as “generally recognized as safe” by the U.S. Food and Drug Administration Authority (Wang et al., 2017). Several researches have stated that L-theanine decreases the oxidative impairment by reducing the production of ROS, oxidative parameters, and lipid damage as well as enhancing the activity of antioxidative enzymes (Ben et al., 2015). Furthermore, L-theanine improved hepatocyte antioxidant capacity by inhibiting the malondialdehyde (MDA) formation and increasing the antioxidant enzymes activities such as catalase (CAT) and superoxide dismutase (SOD) and reduced GSH during liver damage in in vivo rat model and in vitro studies (Li et al., 2012; Thangarajan et al., 2014). Its intraduodenal and intraruminal administration in cattle has been proved effective against liver diseases (Winkler et al., 2015). Several previous animal studies, epidemiological studies, and human interventions support the conclusion that tea and tea extracts have a promising protective effect on liver tissue (Mehana et al., 2012). For example, in the study of Ruhl and Everhart (2005), they reported that the ingestion of tea (>2 cups per day) is linked to a lower occurrence of chronic liver disease in the United States. Although some studies on tea have focused on catechins, L-theanine may be the compound that mediates the hepatoprotective capabilities of tea. Several other published reports indicated the hepatoprotective activities of L-theanine on toxicity-induced hepatic injury and its pharmacokinetic and pharmacodynamic properties. Yang et al. (2013) reported that L-theanine when fed to fruit fly resulted in a significant effect against stress via dry and wet starvation in males. Li et al. (2016) reported that supplementation of L-theanine at the rate of 400 mg/kg BW/d for 14 d in rat positively improved immune function by increasing the spleen function and decreasing the corticosterone (CORT) level in the serum of rat.
In this review, theanine will be referred to as specifically L-theanine. The review consisted of available scientific evidences about L-theanine pertaining to its chemical composition, antistressor and immune functions, and effects on performance in different animal models such as mice, pigs, and so on. To the best of the authors' knowledge, most of the previous studies were conducted in human, mice, and pigs, and there is a serious gap of information in literature on the use of L-theanine in farm animal and birds especially in broiler production. So, this is the first review article on L-theanine which would provide exclusively a new insight regarding this AA that could be used as a best natural feed additive with antistressor and hepatoprotective properties in poultry farming. This article compiled its natural sources, mechanism of actions, chemical composition, and beneficial applications in animal and poultry health. Moreover, this review will be useful for poultry nutritionists, veterinarians, and researchers, to expose its more beneficial effects, so it could be used as dietary supplement in poultry feed.
Sources and structure of L-theanine
Naturally, this AA (theanine) is derived from a nonedible mushroom, Xerocomus badius and Camellia genus including C. sinensis var. assamica, C. sinensis var. sinensis, Camellia sasanqua, and Camellia japonica (Deng et al., 2008). It shares in tea aroma in a great concentration and particularly it is linked to the umami taste of the tea (Narukawa et al., 2014). L-theanine consists of 50% of the free AAs in tea. The level of L-theanine in the tea represents 1 to 3% of the dry tea, and that level varies depending on many factors such as the geographic region in which the tea is grown, conditions of cultivation, variety of tea, time and harvest type, and so on (Vuong et al., 2011). The type of tea is essential as well in terms of theanine amount, for example, comparing with C. sinensis var. assamica, C. sinensis var. sinensis have higher contents of L-theanine as confirmed by Chu (1997). In addition, the harvested tea in the early period of the summer has greater L-theanine contents in contrast to tea cultivated in the late summer season (Vuong et al., 2011).
For synthetic L-theanine (Suntheanine), it is typically produced as racemic combination of L- and D-forms from food-borne ethylamine and L-glutamine by glutaminase enzyme as described by Juneja et al. (1999). The structural formula of L-theanine is shown in Figure 1.
Figure 1.
Chemical structure of L-theanine.
The chemical structure of L-theanine is N-ethyl-L-glutamine; (2S)-2-ammonio-5-(ethylamino)-5-oxopentanoate, which was gained from the related proteinogenic L-AA glutamic acid (Scheid et al., 2012). The structural formula of L-theanine is shown in Figure 1.
Pharmacokinetics of L-theanine
Animal studies have indicated that L-theanine absorption by the small intestine is facilitated by sodium-coupled active transporters (Kitaoka et al., 1996) and has been found that L-theanine was absorbed rapidly in the gastrointestinal tract (Van der Pijl et al., 2010). L-theanine, also called as Suntheanine, is known for its effect on alpha waves, which are produced in the brain of an individual during relaxed state. It was observed that 50 or 200 mg of L-theanine improved the alpha waves in occipital and parietal regions of the brain within 40 min after administration (Kakuda, 2002). The time to peak absorption after oral administration is 0.5 to 2 h in rats (Unno et al., 1999; Kakuda, 2002). The metabolism of L-theanine is even less clear. In 1966, ethylamine was detected in the urine of rats that were administered theanine, but the metabolic fate of theanine could not be determined (Tsuge et al., 2003). It is theorized that L-theanine undergoes hydrolysis in the kidneys and it is converted to glutamic acid (l-glutamate) and ethylamine by phosphate-independent glutaminase (Unno et al., 1999). Because theanine is metabolized by the kidneys, a large percentage of its byproducts are immediately excreted by the kidneys and not present in large quantities in the plasma (Tsuge et al., 2003). Theanine diffuses through the blood-brain barrier via the leucine-preferring transport mechanism (Yokogoshi et al., 1998; Juneja et al., 1999; Yokogoshi and Terashima, 2000). After dietary intake, L-theanine is absorbed through the small intestine and then hydrolyzed into ethylamine and glutamic acid in the intestine and liver followed by urinary excretion. Therefore, it is considered to function as a donor that supplies glutamic acid to the body (Tsuge et al., 2003). Aforementioned studies show that L-theanine has a strong anxiety effect, so it may be used as an antistressor agent for poultry industry.
Beneficial effects of L-theanine
In previous studies, L-theanine has been reported as a functional ingredient with significant potential as food additive in designer foods (Vuong et al., 2011). Its health benefits have been associated with a number of pharmacological and biological properties such as antioxidant, growth promoter, immune booster, antistresser, hepatoprotective, antitumor, antiging, antimicrobial, anti-inflammatory, and antianxiety activities, observed in rats, nematodes, and in vitro studies (Saito et al., 2011; Gong et al., 2012; Culetu et al., 2016), and a summary of those available studies is presented in Table 1.
Table 1.
Summary of the different study findings showing health-enhancing potential of tea amino acid L-theanine.
| Agent | Species | Dosage | Study outcomes | Suggested/Observed effect | References |
|---|---|---|---|---|---|
| L-theanine | Mice | 50, 100, 200 mg/kg | The tea amino acid theanine reduced the levels of liver enzymes (AST and ALT) and MDA, however, increased the activities of CAT, SOD, and GR | Antioxidant, hepatoprotective | Li et al., 2012 |
| L-theanine | Mice | 10 mg/kg | It reduced the levels of AST, ALT, and the expression of Bax and cleaved caspase-3. | Hepatoprotective | Nagai et al., 2015 |
| L-theanine | Mice | 50, 100, and 200 mg/kg | Tea amino acid theanine reduced the levels of bilirubin, AST, ALT, TNF-α, and IL-1β and also increased the activities of antioxidant enzymes and GSH contents. | Hepatoprotective, antioxidant | Jiang et al., 2012 |
| L-theanine | Mice | 10 and 100 mg/kg | L-theanine increased the contents of hepatic and heart glutamate without affecting in tumors, and also GSH. | Antioxidant, anticancer | Sugiyama and Sadzuka, 2004 |
| L-theanine | Rats | 8 mg/kg | Unique tea amino acid theanine reduced the different levels of aminotransferase, IL-1β, γ-GT, TGF-β, IL-6, and CTGF and the degree of lipid peroxidation. | Anti-inflammatory, hepatoprotective | Pérez-Vargas et al., 2016 |
| L-theanine | Broiler | 100, 200, and 300 mg/kg | It decreased the relative concentrations of cytokines IFN-γ and IL-2 in serum, and mRNA expression of IL-6 and TNF-α in thymus as well as IL-2 and IFN-γ in spleen. L-theanine also improved the antioxidative status by improving the serum GSH-Px, SOD, and relative CAT levels. In addition, it improved performance in broilers. | Immunomodulatory, antioxidant | Saeed et al., 2018 |
| L-theanine | Broiler | 100, 200, and 300 mg/kg | It has positive effects on immunity and gut microbe by increasing the community of commensal bacteria in poultry. | Antibacterial and immune modulator | Saeed et al., 2019 |
| L-theanine | Broiler | 400 | It is a safe feed supplement up to 400 mg/kg and could boost the immune function of broilers. | Immunomodulatory | Wen et al., 2012 |
| L-theanine | rats | 4 g/kg (review) | It is found as promising liver protective agent via improving/restoring the antioxidant enzyme activities like SOD, CAT, and GSH, and inhibiting the production of MDA. | Antioxidant, hepatoprotective | Liang et al., 2015 |
| L-theanine | Piglets | 80 mg/kg | Tea amino acid (theanine) improved body weight gain, and anti-inflammatory cytokine production including TNF-α, IFN-γ, and IL-10. | Anti-inflammatory, growth promoter | Hwang et al., 2008 |
| L-theanine | Poultry | Review | L-theanine amino acid as a powerful natural antistressor can also enhance productivity and health status in poultry industry. | Growth promoter, antistressor | Saeed et al., 2017 |
| L-theanine | Human | 10 or 100 mg/kg | L-theanine impeded the adriamycin (ADR) efflux from Ehrlich ascites carcinoma cells and sustained the concentration of ADR in cancer cells, however in normal tissue like liver and heart ADR increase was not noted. | Antitumor | Sadzuka et al., 1996 |
| L-theanine | Mice | 2 and 4 mg/kg | L-theanine significantly inactivated the NF-κB and ERK/p38, and prevent the oxidative damage of neuronal cells. | Memory enhancing | Kim et al., 2009 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAT, catalase; CTGF, connective tissue growth factor; ERK, extracellular signal-regulated kinase; GR, glutathione reductase; GSH, glutathione; IL, interleukin; IFN, interferon; MDA, malondialdehyde; NF, nuclear factor; SOD, superoxide dismutase; TGF, transforming growth factor; TNF, tumor necrosis factor. TGF, transforming growth factor; ERK, extracellular signal-regulated kinase; CTGF,connective tissue growth factor; NF, nuclear factor;
Lipid-Lowering Properties
Several experimental trials had proposed that L-theanine may have lipid-lowering properties. Zhang et al. (2002) studied the effect of theanine on hepatic tumor-induced hyperlipidemia in rats and found a significant decrease in all lipid indices. In vitro scientific evidences suggest that L-theanine may inhibit low-density lipoprotein peroxidation, thus decreasing the likelihood of developing atherosclerosis (Yokozawa and Dong, 1997). As such, the role of this AA (L-theanine) in lipid lowering may be similar to its proposed effects on blood pressure in mice; thus, theanine is a minor component contributing to an overall effect (Zheng et al., 2004).
Antihypertension Effects
L-theanine AA of tea plant has proved to have antihypertensive effects in spontaneously hypertensive rats (Yokogoshi et al., 1995, 1998). Also in another study by Yokogoshi et al. (1995), they showed that high doses of L-theanine at 1,500 and 2,000 mg/kg resulted in significant decreases in blood pressure in spontaneously hypertensive rats, but glutamic acid and glutamine had no observable antihypertensive effects. In a clinical trial, 200 mg of L-theanine significantly reduced blood pressure increases in Japanese individuals during mental task (Yoto et al., 2012). A similar effect of L-theanine was observed in a double-blind randomized placebo-controlled study carried out in United Kingdom that showed L-theanine could antagonize the caffeine-induced blood pressure rise (Rogers et al., 2008). Although theanine may slightly reduce blood pressure, these studies suggested that other components in green tea might exert more of an antihypertensive effect.
Immunomodulatory Effects
It is noticed in many studies that L-theanine and tea have favorable effects on immune function in animal models, cell cultures, epidemiological studies, and human interventions as well (Chattopadhyay et al., 2012; da Silva Pinto, 2013; Williams et al., 2019). In humans, γδ lymphocytes are a subset of T cells and considered as the first line of defense against many pathogens. The ethylamine is formed by the acid hydrolysis of L-theanine in the gastrointestinal tract and then via enzymatic breakdown mediated by the amidases in the liver, which are accomplished of expanding γδ T cells (Asatoor, 1966). Moreover, a previous clinical study recognized that the oral consumption of theanine enhanced γδ T-cell proliferation (Rowe et al., 2007). Therefore, this green tea AA that is known as L-theanine is being considered an essential compound for teas that have a strong ability to improve immune function. The immunomodulative actions of L-theanine are therefore very important for combating various infections and allergic diseases and hypersensitivity reactions. The results of another study have shown that the daily intragastric ingestion of theanine at 400 mg/kg could improve immune response through an increase in the splenic organ index and a decrease in the contents of interleukin-4/6/10 and CORT and the ratio of interleukin-4/interferon-γ in the serum of rats (Li et al., 2016).
Increased Resistance Against Pathogenic Bacteria
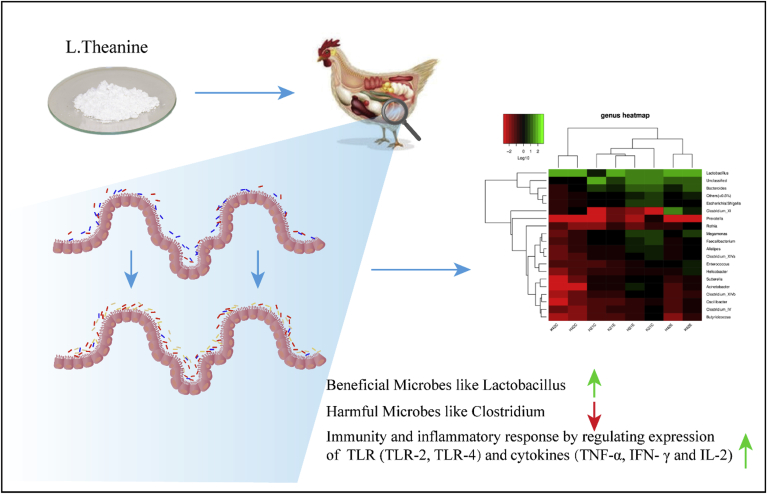
It was assumed that drinking L-theanine–containing green tea could prime the immune system to react to bacterial infections by boosting the γδ T cells' infection-fighting abilities. This hypothesis was studied in 11 non–tea-drinking volunteers who drank about 1.3 mmol of theanine daily for either 2 or 4 wk (Kamath et al., 2003). In our previous study, we found that a diet with L-theanine revealed a potent positive impact on intestinal microbial communities by supporting useful bacteria such as Lactobacillus while decreasing harmful bacteria such as Clostridium as shown in Figure 2 (Saeed et al., 2019).
Figure 2.
Beneficial effect of L-theanine on commensal bacteria. (Figure originally published in Saeed et al., 2019.)
Neuroprotective and Memory-Enhancement Properties
L-theanine offers a promising neuroprotective effect by binding to glutamate receptors and their receptor subtypes, that is, N-methyl-D-aspartate (NMDA), thereby preventing the excess calcium release into the extracellular space (Kakuda, 2002). Its ability to antagonize the NMDA receptor has gained peak gratitude because the introduction of memantine, an NMDA-receptor antagonist, indicated for the treatment of various diseases. L-theanine is also a more potent antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate and kainate receptors than NMDA receptors (Kakuda et al., 2002). The neuroprotective impact of L-theanine has been evaluated in many animal studies that primarily addressed its key pharmacologic effects (Kakuda et al., 2000). As described by Jamwal and Kumar (2017) who observed that when theanine was given to rats at 50 mg/kg/d, it exerts as neuroprotective effect as well as helps with preservation of striatal neurotransmitters homeostasis, antioxidant activity, anti-inflammatory potential, and free radical scavenging, which saves the neurons from death. L-theanine, at the rate of 2 to 4 mg/kg for 5 wk, notably attenuates the amyloid-induced memory impairment and neurons death. It was suggested that the beneficial effects of L-theanine on memory and learning capacities are due to the inactivation of extracellular signal–regulated kinase/p38 and nuclear factor kappa-light-chain-enhancer of activated B cells and decreased oxidative damage to neuronal cells (Deb et al., 2019).
Antistressor Effect and Relaxation Properties
Green tea contains a large number of AAs including L-theanine, which represents around 50% of the total AAs (Horanni and Engelhardt, 2013). This AA (L-theanine) can protect the body cells from free radical species and prevent anxiety by increasing the levels of dopamine (DA) and serotonin in the nerve cells as well as helping to induce relaxation (Khan, 2014). Under heat condition, the supplementation of 200 mg/kg L-theanine and 200 mg/kg tea polyphenols in laying hen diets is the most appropriate technique for antioxidant impact. L-theanine remarkably improved CAT activity and decreased MDA in different organs and tissues (Wang and Cai, 2013). The alpha brain waves are being considered to be a measure of relaxation (Juneja et al., 1999). Their activity is associated to improved performance under stress, increased creativity, improved concentration and learning, and reduced anxiety (PDR Health, 2004). The effect of L-theanine on relaxation in humans was studied in 8 female college students suffering from low- and high-grade anxiety. It was observed that 200-mg dose of L-theanine significantly improved the alpha waves generation in the brains' parietal and occipital regions (Kobayashi et al., 1998). In the study of Takeda et al. (2012), they also reported that drinking water containing 0.3% L-theanine after birth markedly decreased the serum CORT concentration. Lee et al. (2010) stated that oral administration of L-theanine declines the levels of CORT (DA and noradrenaline), but increases 5-HT content in the brain cortex, striatum of mice, and hippocampus. Hence, L-theanine plays an imperative role to help in the secretion of neurotransmitters, hormones, and immune cytokines. Moreover, Tian et al. (2013) reported that L-theanine exerts a wide range of effects including natural antioxidant and neuroprotective effects against cognitive impairments in mice.
The results of White et al. (2016) encourage the antistress impact of L-theanine. Where, individual stress response to a cognitive stressor was notably decreased after 1 h of administration of L-theanine at 200 mg, and cortisol (CORT) response was outstandingly decreased 3 h after administration. In line with this, Kimura et al. (2007) found that individual stress and anxiety response to a cognitive stressor significantly reduced after receiving L-theanine (200 mg) compared to placebo. Also, a decrease in response of individual stress to a cognitive stressor was observed after administration of 200 mg of L-theanine (Yoto et al., 2012).
Research has demonstrated that long-term administration of L-theanine (400 mg daily) had antistress effects by reducing the salivary α-amylase and subjective stress (Unno et al., 2013). In addition, Steptoe et al. (2007) observed that administration of black tea for 6 wk decreased CORT levels and platelet activation in response to behavioral and cognitive stressors compared to control. The results of White et al. (2016) were consistent with some reports (Kimura et al., 2007; Yoto et al., 2012) of stress-reducing impact in response to an acute stressor, but others have stated treatment-related alterations in resting mood (Lu et al., 2004; Haskell et al., 2008). The discrepancy may be due to the variations in measurement instruments used and laboratory stressors; however, growing evidence supports a stress-reducing impact of L-theanine.
The fluctuation of blood levels of α1-acid glycoprotein and CORT could indicate physiological stress of poultry and animals. Regulation of stress was strongly correlated to the pituitary gland (hypothalamus) (Koolhaas et al., 1999; Dickerson and Kemeny, 2004). CORT is a glucocorticoid hormone, which plays an important role in mitigating stress. If animals were subjected to any external stimulation, the serum level of CORT would increase and metabolism of nutrients would be enhanced to cover the necessity of normal physiological functions (Nakamura et al., 1998; Liu et al., 2015).
L-theanine in broiler diets mitigated the elevated serum level of α1-acid glycoprotein on 25 d of age, concentration of IL-6 on 24 and 26 d of age, and the decreased the content of mucosal secretory immunoglobulin A in jejunum on 28 d of age of the lipopolysaccharide-challenged broilers (Li et al., 2018). Some reports indicated that L-theanine AA could enter through the blood-brain barrier to contribute in reducing responses of physiological and psychological stresses (Cho et al., 2008; Haskell et al., 2008; Yoto et al., 2012).
Hepatoprotective and Antioxidant Effects
Hepatic injury is mostly caused by sustained exposure of the liver to harmful exogenous entities, including viruses, alcohol, toxicants, and also other biotransformed metabolites, which induced chronic inflammation of the liver, leading to diseases such as cirrhosis, fibrosis, and hepatocellular carcinoma.
To the best of our knowledge, tea is the only main dietary source of L-theanine. The most commonly consumed teas across the globe are nonfermented green tea and intensively fermented black tea (Bokuchava and Skobeleva, 1980). Nowadays, it has been proved that the green tea could provide protection against hepatic injury in toxic murine models including carbon tetrachloride, (Cui et al., 2014), dextran sodium sulfate (Inoue et al., 2013), microcystin (Xu et al., 2007), and alcohol (Park et al., 2012). In green tea, catechins are considered to be the most important functional components for liver protection. Compared with green tea, black tea exhibits a near complete destruction of catechins. However, black tea has also been shown to protect against carbon tetrachloride (Sun et al., 2013) and aflatoxin (Jha et al., 2013) and liver injury in mice. In general, theanine content seems to be minimally influenced by the fermentation process because both black and green teas contain similar theanine levels (Syu et al., 2008). Li et al. (2012) examined the effect of L-theanine on alcoholic liver injury both in vivo and in vitro. In ethanol-treated human hepatic cells, the L-theanine surprisingly protected hepatocytes against ethanol-induced cell cytotoxicity and apoptosis through the prevention of ethanol-triggered ROS and MDA. When mice were treated with ethanol, the level of hepatic enzymes such as alanine aminotransferase, aspartate aminotransferase, and MDA augmented as well as the activities of glutathione reductase, CAT, and SOD also diminished. However, when this AA (L-theanine) was administered to mice before ethanol exposure, all the baseline functions were restored. These results suggested that L-theanine may prevent ethanol-induced liver damage, possibly through enhanced hepatocyte antioxidative capacities (Li et al., 2012). Hence, it has been confirmed that the L-theanine is likely to be the compound responsible for hepatoprotection (Wang et al., 2017). The many possible beneficial effects of theanine consumption have been demonstrated by many animal studies and human trials. Regarding the possible mechanisms of actions of theanine on the downregulation of the inflammatory response, the induction of NO production and GSH synthesis are likely to be critical for the prevention of hepatic diseases as well as for the improvement of immune function as shown in the study by Wang et al., 2017.
Usage of tea AA (L-theanine) in poultry
Poultry industry is providing the good-quality protein to human beings all over the globe. In many parts of the world, particularly in developing countries, poultry nutritionist are using antibiotics as growth promoters in poultry diet to improve the productive performance of birds; however, they are facing severe criticism by the consumers to replace synthetic antibiotics with safe natural alternatives (Ahossi et al., 2016; Saeed et al., 2017, 2018; Rehman et al., 2019). In a study on broiler chickens, Biswas and Wakita (2001) used 4 levels of C. sinensis powder (0.5, 0.75, 1.0, and 1.5%) in broiler diets and found that body weight gain and feed intake tended to decrease at a higher dose, while feed conversion ratio improved. Similarly, Uuganbayar (2004) claimed that 1.0 to 1.5% of green tea supplementation in broiler feed reduced body weight gain. Yang et al. (2003) conducted a trial to examine the effect of graded levels of green tea by-product on growth performance of broiler chickens; the results showed insignificant improvement in feed efficiency. Authors added that the inclusion of C. sinensis by-product to broiler diets decreased blood low-density lipoprotein cholesterol comparing to the control group and increased HDL and docosahexaenoic acid levels in blood. Moreover, cholesterol content in meat tended to decrease because of dietary inclusion of green tea by-product. In a most recent study, Zhang et al. (2020) reported the improved body weight gain, relative weight of intestine, intestinal histomorphology, antioxidant status, and intestinal mRNA levels of AA and peptide transporters while decreased the MDA content, crypt depth, jejunal protein carbonyls, and serum d-lactic acid by dietary administration of L-theanine in broilers. Similarly, in another study, 600 mg/kg supplementation of L-theanine in broilers ameliorated the effects of transport stress on meat quality by improving muscle pH, color, antioxidative status, and glycogen content while it decreased the drip loss and lactate and MDA contents of meat (Zhang et al., 2019a). A duck study used 300 to 1,500 mg/kg of L-theanine and reported 600 and 900 mg/kg as best supplementation levels to improve growth performance, immunity, intestinal morphology, and antioxidant status (Zhang et al., 2019b).
Also, Cao et al. (2005) demonstrated that feed consumption, feed conversion ratio, and body weight gain from 28 to 42 d of age were not enhanced, while mortality was expressively declined by supplemental C. sinensis by-products in broilers. Sarker et al. (2010) reported a statistical increase in body weight gain (1210.61 g/bird) in broiler chickens within the finishing period at the 0.5% level compared to the 1.0% (1033.36 g/bird) level of C. sinensis powder. Using a liquid hydroalcoholic extract of fresh C. sinensis, Erener et al. (2011) supplemented broiler diets with 0.1 g/kg or 0.2 g/kg extract of C. sinensis and reported the improved values of live body weight, feed conversion, dressing percentage, and carcass weight. The aforementioned authors attributed the improved growth performance with added green tea extract to physiological mechanisms such as the regulation of the microflora in caeca. On the other hand, the immune parameters (antibody level, serum lysozyme activity, intestinal secretory immunoglobulin, and serum interferon-γ and interleukin-2) of broilers were improved by the diets supplemented with L-theanine at 100, 200, 400, and 800 mg/kg diet (Wen et al., 2012).
The live body weight, feed intake, and ileal digestibility of nutrients were not expressively affected by 10 g/kg supplementation of green tea in diet in comparison with control in broiler chickens. Plasma cholesterol and triglyceride were lowered with supplemental green tea compared to control birds (Afsharmanesh and Sadaghi, 2014). In the same context, El-Deek et al. (2012) concluded that dietary addition of green tea (1.5 and 3 g/kg diet) did not affect performance and meat quality of broiler. But, environmental emission and pollution via decreasing excreted nitrogen were overcome by the green tea treatments. Birds fed diets supplemented with graded concentrations of green tea extract (125, 250, 500, 1,000, and 2,000 mg/kg) had improved antioxidant and immunostimulant traits for broiler chickens (Farahat et al., 2016). Furthermore, the antibody titer against Newcastle disease virus vaccines was increased in chicks fed diets supplemented with green tea (Farahat et al., 2016). No adverse impacts on egg weight and egg production rate comparing to the control was observed when hens were fed diets containing 2.0% of green tea powder (Uuganbayar et al., 2005). Kojima and Yoshida (2008) did not find any important difference in egg production rate, egg mass, or egg weight between 1.0 and 0.0% green tea-supplemented laying hens. However, increasing the level of green tea up to 5.0 and 10.0% produced little effects in terms of egg production and egg contents. The same trend was observed by Biswas et al. (2000), who observed that long-term inclusion of layer diet with 0.60% Japanese green tea did not affect egg production or egg contents ratio. Ariana et al. (2011) postulated that enriching layer feeds with 1.50% powder of green tea and 0.50% extract of green tea did not significantly affect egg weight, feed intake, and egg production. On the other hand, Al-Harthi (2004) assured that dietary supplementation of green tea at 0.20% produced statistically improved egg weight and egg production comparing to the control group. In comparing the impacts of 2 levels (1 or 2%) of Japanese, Chinese, and Korean green tea on the performance of laying hens, it was found that egg yield of hens offered diets supplemented with 1 or 2% powders of green tea were statistically enhanced comparing to that of the control (Uuganbayar et al., 2006). Abdo et al. (2010) studied the influence of supplementing green tea extract (0.5–2.5 L/100 kg of diet) and leaves (1–5% of diet) in laying hen. The results showed significant improvements in feed conversion ratio, egg mass, and egg production due to supplementing the diet with 1% of green tea leaves compared to the control. With regard to egg quality criteria, Biswas and Wakita (2001) observed that using 0.3% of green tea powder as a dietary supplement improved the albumen percentage and Haugh unit score. The flow diagram indicating the possible beneficial effects of L-theanine in poultry in terms of growth promotion and stress reduction has been presented in Figure 3. By far, there is little work done on L-theanine into poultry, especially broiler chicken. There is a dire need to debate on the antistress effects of this AA to combat the stress problem of poultry industry.
Figure 3.
Flow diagram showing the proposed beneficial effects of L-theanine in poultry farming. (Figure originally published in Saeed et al., 2018.)
Toxicology effect of L-theanine
Regular or modest intake of green tea is safe (Boehm et al., 2009), but the ingestion of high doses (10–29 mg/kg/d) of green tea extract causes liver toxicity in humans (Lambert et al., 2007). The side effects of L-theanine in animal were not reported in any published study (Kakuda et al., 2000). It has been proposed that the side effects of all dietary additives, depend upon the sources, species, exposure duration and inclusion (Unl, 2012). As higher inclusion of L-theanine may lower blood pressure, appetite loss, difficulty concentrating, dizziness, diarrhea, headaches, nausea, and gastrointestinal discomfort (Haskell et al., 2008; Giesbrecht et al., 2010; Yoto et al., 2012). Chronic administration of L-theanine (250 mg/d for 8 wk) has also been found to be safe in patients with major depressive disorder, it even exhibited multiple beneficial effects against major depressive disorder symptoms including cognitive impairments, sleep disturbance, anxiety, and so on (Hidese et al., 2017). Similarly a 13-wk rodent study dealing with 1.5, 3, and 4 g/kg BW of L-theanine demonstrated no side effect on organ weight and gross pathology or histopathology (Borzelleca et al., 2006). In another study, maximum tolerable dose (0–5%) of L-theanine was added in the diet of rats that were analyzed for subacute (for 13 wk) and chronic (for 78 wk) toxicity. The results demonstrated zero side effects of L-theanine on feed intake, weight gain, or survival rate. Moreover, chronic administration (78 wk) significantly reduced the tumor number and tumor incidence without showing any toxicological effects (Fujii and Inai, 2008). However, there is no reported toxicological effect of L-theanine in in vivo and in vitro studies, and the Food and Drug Agency of the United States suggested that maximum consumption of L-theanine should not exceed 1,200 mg daily (Turkozu and Sanlier, 2017).
Conclusion
L-theanine is a non–protein-derived AA that is abundant in leaves of C. sinensis. It could improve immune function by improving the spleen function and decreasing the CORT level in the serum. It acts as an antioxidant, growth promoter, immune booster, antistresser, hepatoprotective, antitumor, antiaging, antimicrobial, anti-inflammatory, and antianxiety agents. It could reduce the oxidative impairment via decreasing the lipid peroxidation, ROS production, and oxidative deterioration of macromolecules and increasing the glutathione concentrations. Furthermore, L-theanine might improve hepatocyte antioxidant efficiency via restoring the activities of antioxidant enzymes such as SOD, GSH, and CAT and inhibiting the formation of MDA in liver injury disorder. It has an attractive taste that can affect meat flavor and colors positively. To the best of our knowledge, most of earlier studies have been carried out in humans, pigs, and mice, but there is still a serious gap of information in literature on the use of L-theanine in farm animals and poultry, especially in broilers. So, this is the first comprehensive article on L-theanine with a new insight about the potential of this AA in poultry nutrition. We are hopeful that this article will be valuable for poultry scientist and nutritionist for potential use of L-theanine as a natural feed additive with potent antistressor functionality via decreasing the levels of CORT, DA, and noradrenaline and hepatoprotective properties. Future studies should be carried out to find its effective level that could be used on commercial level in poultry industry.
Acknowledgments
The author is very grateful to Major National Scientific Research Projects (2015CB943102) and the National Nature Science Foundation of China (31572365) and Key Sci-tech innovation team of Shaanxi province (2017KCT-24). The authors are also very thankful to, Cholistan University of Veterinary and Animal Sciences, Bahawalpur 63100, Pakistan.
Conflict of Interest Statement: The authors declared that they don't have any conflict of interest.
Contributor Information
Muhammad Sajjad Khan, Email: drsajjad2@yahoo.com.
Sun Chao, Email: sunchao2775@163.com.
References
- Abdo Z.M., Hassan R., El-Salam A.A., Helmy S.A. Effect of adding green tea and its aqueous extract as natural antioxidants to laying hen diet on productive, reproductive performance and egg quality during storage and its content of cholesterol. Egypt. Poult. Sci. J. 2010;30:1121–1149. [Google Scholar]
- Afsharmanesh M., Sadaghi B. Effects of dietary alternatives (probiotic, green tea powder, and Kombucha tea) as antimicrobial growth promoters on growth, ileal nutrient digestibility, blood parameters, and immune response of broiler chickens. Comp. Clin. Pathol. 2014;23:717–724. [Google Scholar]
- Ahossi P., Dougnon J., Kiki P., Houessionon J. Effects of Tridax procumbens powder on zootechnical, biochemical parameters and carcass characteristics of Hubbard broiler chicken. J. Anim. Health Prod. 2016;4:15–21. [Google Scholar]
- Al-Harthi M. Responses of laying hens to different levels of amoxicillin, hot pepper or green tea and their effects on productive performance, egg quality and chemical composition of yolk and blood plasma constituents. Egypt. Poult. Sci. J. 2004;24:845–868. [Google Scholar]
- Ariana M., Samie A., Edriss M.A., Jahanian R. Effects of powder and extract form of green tea and marigold, and-tocopheryl acetate on performance, egg quality and egg yolk cholesterol levels of laying hens in late phase of production. J. Med. Plants Res. 2011;5:2710–2716. [Google Scholar]
- Asatoor A. Tea as a source of urinary ethylamine. Nature. 1966;210:1358. doi: 10.1038/2101358b0. [DOI] [PubMed] [Google Scholar]
- Ben P., Zhang Z., Xuan C., Sun S., Shen L., Gao Y., Cao X., Zhou Y., Lan L., Yin Z., Luo L. Protective effect of L-theanine on cadmium-induced apoptosis in PC12 cells by inhibiting the mitochondria-mediated pathway. Neurochem. Res. 2015;40:1661–1670. doi: 10.1007/s11064-015-1648-4. [DOI] [PubMed] [Google Scholar]
- Biswas M.A., Miyazaki Y., Nomura K., Wakita M. Influences of long-term feeding of Japanese green tea powder on laying performance and egg quality in hens. Asian Austral. J. Anim. Sci. 2000;13:980–985. [Google Scholar]
- Biswas A.H., Wakita M. Effect of dietary Japanese green tea powder supplementation on feed utilization and carcass profiles in broilers. J. Poult. Sci. 2001;38:50–57. [Google Scholar]
- Boehm K., Borrelli F., Ernst E., Habacher G., Hung S.K., Milazzo S., Horneber M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2009;3:CD005004. doi: 10.1002/14651858.CD005004.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokuchava M.A., Skobeleva N.I. The biochemistry and technology of tea manufacture. Crit. Rev. Food Sci. Nutr. 1980;12:303–370. doi: 10.1080/10408398009527280. [DOI] [PubMed] [Google Scholar]
- Borzelleca J., Peters D., Hall W. A 13-week dietary toxicity and toxicokinetic study with L-theanine in rats. Food Chem. Toxicol. 2006;44:1158–1166. doi: 10.1016/j.fct.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Cao B., Karasawa Y., Guo Y. Effects of green tea polyphenols and fructo-oligosaccharides in semi-purified diets on broilers’ performance and caecal microflora and their metabolites. Asian Austral. J. Anim. Sci. 2005;18:85–89. [Google Scholar]
- Chattopadhyay C., Chakrabarti N., Chatterjee M., Mukherjee S., Sarkar K., Chaudhuri A.R. Black tea (Camellia sinensis) decoction shows immunomodulatory properties on an experimental animal model and in human peripheral mononuclear cells. Pharmacognosy. Res. 2012;4:15. doi: 10.4103/0974-8490.91029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.S., Kim S., Lee S.Y., Park J.A., Kim S.J., Chun H.S. Protective effect of the green tea component, L-theanine on environmental toxins-induced neuronal cell death. Neurotoxicol. 2008;29:656–662. doi: 10.1016/j.neuro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Chu D. Chemistry and Applications of Green Tea. CRC Press; Boca Raton, FL: 1997. Green tea—its cultivation, processing of the tea leaves for drinking materials, and kinds of green tea; pp. 1–11. [Google Scholar]
- Cooper R. Green tea and theanine: health benefits. Int. J. Food Sci. Nutr. 2012;63:90–97. doi: 10.3109/09637486.2011.629180. [DOI] [PubMed] [Google Scholar]
- Cui Y., Yang X., Lu X., Chen J., Zhao Y. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem. Biol. Interact. 2014;220:75–83. doi: 10.1016/j.cbi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Culetu A., Fernandez-Gomez B., Ullate M., del Castillo M.D., Andlauer W. Effect of theanine and polyphenols enriched fractions from decaffeinated tea dust on the formation of Maillard reaction products and sensory attributes of breads. Food Chem. 2016;197:14–23. doi: 10.1016/j.foodchem.2015.10.097. [DOI] [PubMed] [Google Scholar]
- da Silva Pinto M. Tea: a new perspective on health benefits. Food Res. Int. 2013;53:558–567. [Google Scholar]
- de Mejia E.G., Ramirez-Mares M.V., Puangpraphant S. Bioactive components of tea: cancer, inflammation and behavior. Brain Behav. Immun. 2009;23:721–731. doi: 10.1016/j.bbi.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Deb S., Dutta A., Phukan B.C., Manivasagam T., Justin-Thenmozhi A., Bhattacharya P., Paul R., Borah A. Neuroprotective attributes of L-theanine, a bioactive amino acid of tea, and its potential role in Parkinson's disease therapeutics. Neurochem. Int. 2019;129:104478. doi: 10.1016/j.neuint.2019.104478. [DOI] [PubMed] [Google Scholar]
- Deng W.-W., Ogita S., Ashihara H. Biosynthesis of theanine (γ-ethylamino-l-glutamic acid) in seedlings of Camellia sinensis. Phytochem. Lett. 2008;1:115–119. [Google Scholar]
- Deng W.W., Ogita S., Ashihara H. Distribution and biosynthesis of theanine in Theaceae plants. Plant Physiol. Biochem. 2010;48:70–72. doi: 10.1016/j.plaphy.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- El-Deek A., Al-Harthi M., Osman M., Al-Jassas F., Nassar R. Effect of different levels of green tea (Camellia sinensis) as a substitute for oxytetracycline as a growth promoter in broilers diets containing two crude protein levels. Arch. Geflugelk. 2012;76:88–98. [Google Scholar]
- Erener G., Ocak N., Altop A., Cankaya S., Aksoy H.M., Ozturk E. Growth performance, meat quality and caecal coliform bacteria count of broiler chicks fed diet with green tea extract. Asian Austral. J. Anim. Sci. 2011;24:1128–1135. [Google Scholar]
- Eschenauer G., Sweet B.V. Pharmacology and therapeutic uses of theanine. Am. J. Health Syst. Pharm. 2006;63:28–30. doi: 10.2146/ajhp050148. [DOI] [PubMed] [Google Scholar]
- Facts and Comparisons. L-theanine. eFacts monograph. 2007. www.efactsweb.com
- Farahat M., Abdallah F., Abdel-Hamid T., Hernandez-Santana A. Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response. Br. Poult. Sci. 2016;57:714–722. doi: 10.1080/00071668.2016.1196339. [DOI] [PubMed] [Google Scholar]
- Fujii S., Inai K. Tumorigenicity study of L-theanine administrated orally to mice. Food Chem. 2008;110:643–646. [Google Scholar]
- Giesbrecht T., Rycroft J.A., Rowson M.J., De Bruin E.A. The combination of L-theanine and caffeine improves cognitive performance and increases subjective alertness. Nutr. Neurosci. 2010;13:283–290. doi: 10.1179/147683010X12611460764840. [DOI] [PubMed] [Google Scholar]
- Gong Y., Luo Y., Huang J.-A., Zhang J., Peng Y., Liu Z., Baolu Z. Theanine improves stress resistance in Caenorhabditis elegans. J. Funct. Foods. 2012;4:988–993. [Google Scholar]
- Haskell C.F., Kennedy D.O., Milne A.L., Wesnes K.A., Scholey A.B. The effects of L-theanine, caffeine and their combination on cognition and mood. Biol. Psychol. 2008;77:113–122. doi: 10.1016/j.biopsycho.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Hidese S., Ota M., Wakabayashi C., Noda T., Ozawa H., Okubo T., Kunugi H. Effects of chronic L-theanine administration in patients with major depressive disorder: an open-label study. Acta Neuropsychiatr. 2017;29:72–79. doi: 10.1017/neu.2016.33. [DOI] [PubMed] [Google Scholar]
- Higashiyama A., Htay H.H., Ozeki M., Juneja L.R., Kapoor M.P. Effects of L-theanine on attention and reaction time response. J. Funct. Foods. 2011;3:171–178. [Google Scholar]
- Horanni R., Engelhardt U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Comp. Anal. 2013;31:94–100. [Google Scholar]
- Hwang Y., Park B., Lim J., Kim M., Song I., Park S., Jung H., Hong J., Yun H. Effects of β-Glucan from paenibacillus polymyxa and L-theanine on growth performance and immunomodulation in weanling piglets. Asian Austral. J. Anim. Sci. 2008;21:1753–1759. [Google Scholar]
- Inoue H., Maeda-Yamamoto M., Nesumi A., Tanaka T., Murakami A. Low and medium but not high doses of green tea polyphenols ameliorated dextran sodium sulfate-induced hepatotoxicity and nephrotoxicity. Biosci. Biotechnol. Biochem. 2013;77:1223–1228. doi: 10.1271/bbb.121003. [DOI] [PubMed] [Google Scholar]
- Jamwal S., Kumar P. L-theanine, a component of green tea prevents 3-nitropropionic acid (3-NP)-induced striatal toxicity by modulating nitric oxide pathway. Mol. Neurobiol. 2017;54:2327–2337. doi: 10.1007/s12035-016-9822-5. [DOI] [PubMed] [Google Scholar]
- Jha A., Krithika R., Manjeet D., Verma R.J. Protective effect of black tea infusion on aflatoxin-induced hepatotoxicity in mice. J. Clin. Exp. Hepatol. 2013;3:29–36. doi: 10.1016/j.jceh.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Gao M., Sun S., Bi A., Xin Y., Han X., Wang L., Yin Z., Luo L. Protective effect of L-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem. Biophys. Res. Commun. 2012;422:344–350. doi: 10.1016/j.bbrc.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Juneja L.R., Chu D.-C., Okubo T., Nagato Y., Yokogoshi H. L-theanine—a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci. Technol. 1999;10:199–204. [Google Scholar]
- Kakuda T. Neuroprotective effects of the green tea components theanine and catechins. Biol. Pharm. Bull. 2002;25:1513–1518. doi: 10.1248/bpb.25.1513. [DOI] [PubMed] [Google Scholar]
- Kakuda T., Nozawa A., Sugimoto A., Niino H. Inhibition by theanine of binding of [3H]AMPA, [3H]kainate, and [3H]MDL 105,519 to glutamate receptors. Biosci. Biotechnol. Biochem. 2002;66:2683–2686. doi: 10.1271/bbb.66.2683. [DOI] [PubMed] [Google Scholar]
- Kakuda T., Yanase H., Utsunomiya K., Nozawa A., Unno T., Kataoka K. Protective effect of gamma-glutamylethylamide (theanine) on ischemic delayed neuronal death in gerbils. Neurosci. Lett. 2000;289:189–192. doi: 10.1016/s0304-3940(00)01286-6. [DOI] [PubMed] [Google Scholar]
- Kamath A.B., Wang L., Das H., Li L., Reinhold V.N., Bukowski J.F. Antigens in tea-beverage prime human Vγ2Vδ2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6009–6014. doi: 10.1073/pnas.1035603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.H. The use of green tea (Camellia sinensis) as a phytogenic substance in poultry diets. Onderstepoort. J. Vet. Res. 2014;81:01–08. doi: 10.4102/ojvr.v81i1.706. [DOI] [PubMed] [Google Scholar]
- Kim T.I., Lee Y.K., Park S.G., Choi I.S., Ban J.O., Park H.K., Nam S.-Y., Yun Y.W., Han S.B., Oh K.W. L-Theanine, an amino acid in green tea, attenuates β-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-κB pathways. Free Radic. Biol. Med. 2009;47:1601–1610. doi: 10.1016/j.freeradbiomed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Kimura K., Ozeki M., Juneja L.R., Ohira H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007;74:39–45. doi: 10.1016/j.biopsycho.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Hayashi H., Yokogoshi H., Suzuki Y. Transmural potential changes associated with the in vitro absorption of theanine in the Guinea pig intestine. Biosci. Biotechnol. Biochem. 1996;60:1768–1771. doi: 10.1271/bbb.60.1768. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Nagato Y., Aoi N., Juneja L., Kim M., Yamamoto T., Sugimoto S. Effects of L-theanine on the release of alpha-brain waves in human volunteers. J. Agric. Chem. Soc. Jpn. (Japan) 1998;72:153–157. [Google Scholar]
- Kojima S., Yoshida Y. Effects of green tea powder feed supplement on performance of hens in the late stage of laying. Int. J. Poult. Sci. 2008;7:491–496. [Google Scholar]
- Koolhaas J.M., Korte S.M., De Boer S.F., Van Der Vegt B.J., Van Reenen C.G., Hopster H., De Jong I.C., Ruis M.A., Blokhuis H.J. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Lambert J.D., Sang S., Yang C.S. Possible controversy over dietary polyphenols: benefits vs risks. Chem. Res. Toxicol. 2007;20:583–585. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- Lee W.K., Kim T.I., Park S.-k., Park H.K., Hong J.T. Anxiolytic effect of a combination of green tea extract and L-theanine. Lab. Anim. Res. 2010;26:63–68. [Google Scholar]
- Li R., Song Z., Zhao J., Huo D., Fan Z., Hou D.X., He X. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers. Anim. Nutr. 2018;4:265–272. doi: 10.1016/j.aninu.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Tong H., Yan Q., Tang S., Han X., Xiao W., Tan Z. L-theanine improves immunity by altering TH2/TH1 cytokine balance, brain neurotransmitters, and expression of phospholipase C in rat hearts. Med. Sci. Monit. 2016;22:662–669. doi: 10.12659/MSM.897077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Ye Y., Kang J., Yao X., Zhang Y., Jiang W., Gao M., Dai Y., Xin Y., Wang Q. L-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem. Toxicol. 2012;50:363–372. doi: 10.1016/j.fct.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Liang Y.-R., Liu C., Xiang L.-P., Zheng X.-Q. Health benefits of theanine in green tea: a review. Trop. J. Pharm. Res. 2015;14:1943–1949. [Google Scholar]
- Liu L., Shen J., Zhao C., Wang X., Yao J., Gong Y., Yang X. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int. J. Biol. Macromol. 2015;72:624–632. doi: 10.1016/j.ijbiomac.2014.08.057. [DOI] [PubMed] [Google Scholar]
- Lu K., Gray M.A., Oliver C., Liley D.T., Harrison B.J., Bartholomeusz C.F., Phan K.L., Nathan P.J. The acute effects of L-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum. Psychopharmacol. 2004;19:457–465. doi: 10.1002/hup.611. [DOI] [PubMed] [Google Scholar]
- Mehana E.E., Meki A.R.M., Fazili K.M. Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp. Toxicol. Pathol. 2012;64:291–295. doi: 10.1016/j.etp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Nagai K., Oda A., Konishi H. Theanine prevents doxorubicin-induced acute hepatotoxicity by reducing intrinsic apoptotic response. Food Chem. Toxicol. 2015;78:147–152. doi: 10.1016/j.fct.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mitarai Y., Yoshioka M., Koizumi N., Shibahara T., Nakajima Y. Serum levels of interleukin-6, alpha1-acid glycoprotein, and corticosterone in two-week-old chickens inoculated with Escherichia coli lipopolysaccharide. Poult. Sci. 1998;77:908–911. doi: 10.1093/ps/77.6.908. [DOI] [PubMed] [Google Scholar]
- Narukawa M., Toda Y., Nakagita T., Hayashi Y., Misaka T. L-Theanine elicits umami taste via the T1R1 + T1R3 umami taste receptor. Amino Acids. 2014;46:1583–1587. doi: 10.1007/s00726-014-1713-3. [DOI] [PubMed] [Google Scholar]
- Owen G.N., Parnell H., De Bruin E.A., Rycroft J.A. The combined effects of L-theanine and caffeine on cognitive performance and mood. Nutr. Neurosci. 2008;11:193–198. doi: 10.1179/147683008X301513. [DOI] [PubMed] [Google Scholar]
- Park J.H., Kim Y., Kim S.H. Green tea extract (Camellia sinensis) fermented by Lactobacillus fermentum attenuates alcohol-induced liver damage. Biosci. Biotechnol. Biochem. 2012;76:2294–2300. doi: 10.1271/bbb.120598. [DOI] [PubMed] [Google Scholar]
- PDR Health. ltheanine. 2004. www.pdrhealtcom
- Pérez-Vargas J., Zarco N., Vergara P., Shibayama M., Segovia J., Tsutsumi V., Muriel P. L-theanine prevents carbon tetrachloride-induced liver fibrosis via inhibition of nuclear factor κB and down-regulation of transforming growth factor β and connective tissue growth factor. Hum. Exp. Toxicol. 2016;35:135–146. doi: 10.1177/0960327115578864. [DOI] [PubMed] [Google Scholar]
- Rehman A., Khan A., Khan S., Maris H., Khan N. Effect of quinolones on blood glucose level and blood profile of laying hens. J. Anim. Health Prod. 2019;7:51–57. [Google Scholar]
- Rogers P.J., Smith J.E., Heatherley S.V., Pleydell-Pearce C.W. Time for tea: mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacol. (Berl) 2008;195:569–577. doi: 10.1007/s00213-007-0938-1. [DOI] [PubMed] [Google Scholar]
- Rowe C.A., Nantz M.P., Bukowski J.F., Percival S.S. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances γδ T cell function: a randomized, double-blind, placebo-controlled study. J. Am. Coll. Nutr. 2007;26:445–452. doi: 10.1080/07315724.2007.10719634. [DOI] [PubMed] [Google Scholar]
- Ruhl C.E., Everhart J.E. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Sadzuka Y., Sugiyama T., Miyagishima A., Nozawa Y., Hirota S. The effects of theanine, as a novel biochemical modulator, on the antitumor activity of adriamycin. Cancer Lett. 1996;105:203–209. doi: 10.1016/0304-3835(96)04282-6. [DOI] [PubMed] [Google Scholar]
- Saeed M., El-Hack M.E.A., Alagawany M., Naveed M., Arain M.A., Arif M., Soomro R.N., Kakar M., Manzoor R., Tiwari R. Phytochemistry, modes of action and beneficial health applications of green tea (Camellia sinensis) in humans and animals. Int. J. Pharmacol. 2017;13:698–708. [Google Scholar]
- Saeed M., Yatao X., Hassan F.U., Arain M.A., Abd El-Hack M.E., Noreldin A.E., Sun C. Influence of graded levels of L-theanine dietary supplementation on growth performance, carcass traits, meat quality, organs histomorphometry, blood chemistry and immune response of broiler chickens. Int. J. Mol. Sci. 2018;19:462. doi: 10.3390/ijms19020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Yatao X., Tiantian Z., Qian R., Chao S. 16S ribosomal RNA sequencing reveals a modulation of intestinal microbiome and immune response by dietary L-theanine supplementation in broiler chickens. Poult. Sci. 2019;98:842–854. doi: 10.3382/ps/pey394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Ikeda M., Kametani H. Theanine in the tea roots attenuates memory deficits in the aged rats: 125. Free Radic. Bio. Med. 2011;51:S58. [Google Scholar]
- Sarker M., Kim G., Yang C. Effect of green tea and biotite on performance, meat quality and organ development in Ross broiler. Egypt. Poult. Sci. J. 2010;30:77–88. [Google Scholar]
- Scheid L., Ellinger S., Alteheld B., Herholz H., Ellinger J., Henn T., Helfrich H.-P., Stehle P. Kinetics of L-theanine uptake and metabolism in healthy participants are comparable after ingestion of L-theanine via capsules and green tea–4. J. Nutr. 2012;142:2091–2096. doi: 10.3945/jn.112.166371. [DOI] [PubMed] [Google Scholar]
- Wen H., Wei S., Zhang S., Hou D., Xiao W., He X. Effects of L-theanine on performance and immune function of yellow-feathered broilers. Chin. J. Anim. Nutr. 2012;24:1946–1954. [Google Scholar]
- Steptoe A., Gibson E.L., Vuononvirta R., Williams E.D., Hamer M., Rycroft J.A., Erusalimsky J.D., Wardle J. The effects of tea on psychophysiological stress responsivity and post-stress recovery: a randomised double-blind trial. Psychopharmacol. (Berl) 2007;190:81–89. doi: 10.1007/s00213-006-0573-2. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Sadzuka Y. Theanine, a specific glutamate derivative in green tea, reduces the adverse reactions of doxorubicin by changing the glutathione level. Cancer Lett. 2004;212:177–184. doi: 10.1016/j.canlet.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Sun Y., Yang X., Lu X., Wang D., Zhao Y. Protective effects of Keemun black tea polysaccharides on acute carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food Chem. Toxicol. 2013;58:184–192. doi: 10.1016/j.fct.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Syu K.Y., Lin C.L., Huang H.C., Lin J.K. Determination of theanine, GABA, and other amino acids in green, oolong, black, and Pu-erh teas with dabsylation and high-performance liquid chromatography. J. Agric. Food Chem. 2008;56:7637–7643. doi: 10.1021/jf801795m. [DOI] [PubMed] [Google Scholar]
- Takeda A., Tamano H., Suzuki M., Sakamoto K., Oku N., Yokogoshi H. Unique induction of CA1 LTP components after intake of theanine, an amino acid in tea leaves and its effect on stress response. Cell Mol. Neurobiol. 2012;32:41–48. doi: 10.1007/s10571-011-9732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangarajan S., Deivasigamani A., Natarajan S.S., Krishnan P., Mohanan S.K. Neuroprotective activity of L-theanine on 3-nitropropionic acid-induced neurotoxicity in rat striatum. Int. J. Neurosci. 2014;124:673–684. doi: 10.3109/00207454.2013.872642. [DOI] [PubMed] [Google Scholar]
- Tian X., Sun L., Gou L., Ling X., Feng Y., Wang L., Yin X., Liu Y. Protective effect of l-theanine on chronic restraint stress-induced cognitive impairments in mice. Brain Res. 2013;1503:24–32. doi: 10.1016/j.brainres.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Tsuge H., Sano S., Hayakawa T., Kakuda T., Unno T. Theanine, γ-glutamylethylamide, is metabolized by renal phosphate-independent glutaminase. Biochim. Biophys. Acta. 2003;1620:47–53. doi: 10.1016/s0304-4165(02)00504-4. [DOI] [PubMed] [Google Scholar]
- Turkozu D., Sanlier N. L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit. Rev. Food Sci. Nutr. 2017;57:1681–1687. doi: 10.1080/10408398.2015.1016141. [DOI] [PubMed] [Google Scholar]
- Unl Toxicology and exposure guidelines. UNL Environ. Health Saf. 2012;40:472–4925. [Google Scholar]
- Unno T., Suzuki Y., Kakuda T., Hayakawa T., Tsuge H. Metabolism of theanine, gamma-glutamylethylamide, in rats. J. Agric. Food Chem. 1999;47:1593–1596. doi: 10.1021/jf981113t. [DOI] [PubMed] [Google Scholar]
- Unno K., Tanida N., Ishii N., Yamamoto H., Iguchi K., Hoshino M., Takeda A., Ozawa H., Ohkubo T., Juneja L.R. Anti-stress effect of theanine on students during pharmacy practice: positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem. Behav. 2013;111:128–135. doi: 10.1016/j.pbb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Uuganbayar D. Sunchon National University; Sunchon: 2004. A Study on the Utilization of Green Tea for Laying Hens and Broiler Chicks. A Dissertation for the Degree of Doctor of Philosophy. [Google Scholar]
- Uuganbayar D., Bae I., Choi K., Shin I., Firman J., Yang C. Effects of green tea powder on laying performance and egg quality in laying hens. Asian Austral. J. Anim. Sci. 2005;18:1769. [Google Scholar]
- Uuganbayar D., Shin I., Yang C. Comparative performance of hens fed diets containing Korean, Japanese and Chinese green tea. Asian Austral. J. Anim. Sci. 2006;19:1190. [Google Scholar]
- Van der Pijl P., Chen L., Mulder T. Human disposition of L-theanine in tea or aqueous solution. J. Funct. Foods. 2010;2:239–244. [Google Scholar]
- Vuong Q.V., Bowyer M.C., Roach P.D. L-Theanine: properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011;91:1931–1939. doi: 10.1002/jsfa.4373. [DOI] [PubMed] [Google Scholar]
- Wan X., Zhengzhu Z., Daxiang L. Tea and Tea Products: Chemistry and Health-Promoting Properties. CRC Press; Boca Raton, FL: 2008. 16 Chemistry and Biological Properties of Theanine; pp. 255–274. [Google Scholar]
- Wang L., Cai H. Effects of tea polyphenol and ltheanine on growth performance and antioxidant performance of egg-laying chickens under heat stress. China Anim. Husband. Vet. Med. 2013;40:76–80. [Google Scholar]
- Wang D., Gao Q., Wang T., Qian F., Wang Y. Theanine: the unique amino acid in the tea plant as an oral hepatoprotective agent. Asia Pac. J. Clin. Nutr. 2017;26:384. doi: 10.6133/apjcn.032017.11. [DOI] [PubMed] [Google Scholar]
- White D.J., de Klerk S., Woods W., Gondalia S., Noonan C., Scholey A.B. Anti-stress, behavioural and magnetoencephalography effects of an L-theanine-based nutrient drink: a randomised, double-blind, placebo-controlled, crossover trial. Nutrients. 2016;8:53. doi: 10.3390/nu8010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Sergi D., McKune A.J., Georgousopoulou E.N., Mellor D.D., Naumovski N. The beneficial health effects of green tea amino acid l-theanine in animal models: promises and prospects for human trials. Phytother. Res. 2019;33:571–583. doi: 10.1002/ptr.6277. [DOI] [PubMed] [Google Scholar]
- Winkler A., Gessner D.K., Koch C., Romberg F.J., Dusel G., Herzog E., Most E., Eder K. Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Arch. Anim. Nutr. 2015;69:425–441. doi: 10.1080/1745039X.2015.1093873. [DOI] [PubMed] [Google Scholar]
- Xu C., Shu W.Q., Qiu Z.Q., Chen J.A., Zhao Q., Cao J. Protective effects of green tea polyphenols against subacute hepatotoxicity induced by microcystin-LR in mice. Environ. Toxicol. Pharmacol. 2007;24:140–148. doi: 10.1016/j.etap.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Yang H., Li W., Yu H., Yu an R., Yang Y., Pung K., Li P., Xue L. Physiological effects of L-theanine on Drosophila melanogaster. Molecules. 2013;18:13175–13187. doi: 10.3390/molecules181113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Yang I., Oh D., Bae I., Cho S., Kong I., Uuganbayar D., Nou I., Choi K. Effect of green tea by-product on performance and body composition in broiler chicks. Asian Austral. J. Anim. Sci. 2003;16:867–872. [Google Scholar]
- Yokogoshi H., Kato Y., Sagesaka Y.M., Takihara-Matsuura T., Kakuda T., Takeuchi N. Reduction effect of theanine on blood pressure and brain 5-hydroxyindoles in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 1995;59:615–618. doi: 10.1271/bbb.59.615. [DOI] [PubMed] [Google Scholar]
- Yokogoshi H., Kobayashi M., Mochizuki M., Terashima T. Effect of theanine, r-glutamylethylamide, on brain monoamines and striatal dopamine release in conscious rats. Neurochem. Res. 1998;23:667–673. doi: 10.1023/a:1022490806093. [DOI] [PubMed] [Google Scholar]
- Yokogoshi H., Terashima T. Effect of theanine, r-glutamylethylamide, on brain monoamines, striatal dopamine release and some kinds of behavior in rats. Nutrition. 2000;9:776–777. doi: 10.1016/s0899-9007(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Yokozawa T., Dong E. Influence of green tea and its three major components upon low-density lipoprotein oxidation. Exp. Toxicol. Pathol. 1997;49:329–335. doi: 10.1016/S0940-2993(97)80096-6. [DOI] [PubMed] [Google Scholar]
- Yoto A., Motoki M., Murao S., Yokogoshi H. Effects of L-theanine or caffeine intake on changes in blood pressure under physical and psychological stresses. J. Physiol. Anthropol. 2012;31:28. doi: 10.1186/1880-6805-31-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Chen K.K., Zhao X.H., Wang C., Geng Z.Y. Effect of L-theanine on the growth performance, immune function, and jejunum morphology and antioxidant status of ducks. Animal. 2019;13:1145–1153. doi: 10.1017/S1751731118002884. [DOI] [PubMed] [Google Scholar]
- Zhang C., Geng Z.Y., Chen K.K., Zhao X.H., Wang C. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status. Poult. Sci. 2019;98:4648–4655. doi: 10.3382/ps/pez164. [DOI] [PubMed] [Google Scholar]
- Zhang G., Miura Y., Yagasaki K. Effects of dietary powdered green tea and theanine on tumor growth and endogenous hyperlipidemia in hepatoma-bearing rats. Biosci. Biotechnol. Biochem. 2002;66:711–716. doi: 10.1271/bbb.66.711. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang C., Chen K.K., Zhao X.H., Geng Z.Y. Effect of l-theanine on growth performance, intestinal development and health, and peptide and amino acid transporters expression of broilers. J. Sci. Food Agric. 2020;100:1718–1725. doi: 10.1002/jsfa.10192. [DOI] [PubMed] [Google Scholar]
- Zheng G., Sayama K., Okubo T., Juneja L.R., Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo. 2004;18:55–62. [PubMed] [Google Scholar]