Abstract
In recent years, the blood-based analysis of circulating tumour cells (CTCs) and nucleic acids (DNA/RNA), otherwise known as liquid biopsy, has become increasingly important in breast cancer. Numerous trials have already underscored the high prognostic significance of CTC detection in both early and metastatic stages. Moreover, the changes in CTC levels and circulating tumour DNA (ctDNA) during the course of the disease correlate with the response to treatment. Research currently focuses on liquid-biopsy based therapeutic interventions in metastatic breast cancer. In this context, alpelisib, a PI3K inhibitor, was the first agent to be approved by FDA and EMA.
Key words: liquid biopsy, breast cancer, circulating tumour DNA, circulating tumour cell, targeted therapy
Introduction
In the last century, scientific and clinical advances in the diagnosis and treatment of breast cancer have resulted in a significant paradigm shift. The so-called “Halsted Doctrine”, which viewed breast cancer as a local event with healing only possible by the most radical surgery, was replaced by the “Fischer Doctrine”, which regards even early stage breast cancer as a systemic disease 1 . Today we know that haematogenous dissemination already takes place in very early stages of breast cancer and that the circulating tumour cells (CTCs) will even be found in patients with pre-invasive breast lesions 2 . At the same time the hypothesis of metastatic inefficiency states that most of these cells are eliminated by the immune system or the mechanical shear forces in the blood 3 , 4 . Only a small CTC subpopulation can persist for long time in the blood or other “homing sites”, such as the bone marrow, and is considered a surrogate marker of minimal residual disease (MRD). These cells also exhibit long-term tumour cell dormancy and may be detected in the peripheral blood even many years after the initial diagnosis of the disease 5 .
It still remains unclear which characteristics contribute to certain CTCs being able to “wake up” and survive several steps of the metastatic cascade in order to later evolve into distant metastases. One theory currently under discussion states that these cells are particularly aggressive and undergo the so-called epithelial-mesenchymal transition (EMT). This process involves a number of changes, for example, the loss of polarity and intercellular adhesion, an increase in mobility and invasiveness, and finally the loss of the epithelial, and acquisition of the mesenchymal, phenotype 6 . Furthermore, EMT can generate cells with stem cell characteristics that have resistance mechanisms as well as a very high potential of self-renewal and may be regarded as actual precursors of distant metastasis 7 , 8 .
The Concept of Liquid Biopsy
In breast cancer patients peripheral blood samples may contain not only circulating tumour DNA (ctDNA) and/or RNA fragments (non-coding RNA, ncRNA) but also intact CTCs. The ctDNA/RNA fragments are released continuously into the bloodstream by the primary cancer and/or the metastatic lesion itself and by the decaying CTCs. Liquid biopsy is defined as the detection and analysis of these blood-borne biomarkers in cancer patients. Thus, in heterogeneous tumours such as breast cancer, oncogene mutations and amplifications may be detected more effectively than with tissue biopsies, which only represent a limited tumour area 9 , 10 . Further benefits compared to tissue biopsy include the possibility of serial analyses by simple blood sampling and the detectability of multiple or hard to reach metastases. In addition, due to its low invasiveness liquid biopsy acceptance is very high among patients. This paper presents the current data on clinical significance as well as the potential applications of liquid biopsy in primary and metastatic breast cancer.
Detection Techniques
Circulating tumour cells
In principle CTC detection involves two independent steps: Isolation of the cells from the whole blood and their actual detection. Isolation of these cells from the other constituents of the blood draws on various cell characteristics, mainly physical (size- and/or density-based) and biological (specific antigen expression). Examples include the so-called density gradient technique with Ficoll and antigen-based modalities with epithelial (e.g., EpCAM, cytokeratins) and/or tumour-specific markers such as CEA or HER2. In antibody-based isolation, the specific antibodies are mostly coupled to magnetic particles, with the enriched cells then being separated by a magnetic field for further analysis.
The subsequent CTC detection may then be based again on nucleic acid (RT-PCR, qRT-PCR, multiplex RT-PCR) or the antigen characteristics (immunocytochemistry, immunofluorescence, immunofluorescence flow cytometry) of the CTCs. While the sensitivity and specificity of nucleic-acid based detection is rather high, their drawback is CTC lysis during the analysis, making it impossible to assess cell morphology or perform further cell analysis.
The most common technique for CTC detection in clinical trials is the CellSearch system (Menarini Silicon Biosystems, Inc.), which has been approved by the U. S. Food and Drug Administration (FDA) for metastatic breast, colon and prostate cancer. With this standardised assay, EpCAM-positive CTCs are isolated immunomagnetically from 7.5 ml whole venous blood and subsequently stained by immunofluorescence with antibodies against cytokeratins (CK) 8, 18, 19, the specific leukocyte marker CD45, and the nuclear marker DAPI. The sample is then scanned by a semi-automatic fluorescence microscope and the potential CTCs are automatically imaged. Finally, the analysis is performed by an experienced examiner: CK 8/18/19 and DAPI-positive as well as CD45-negative cells with specific morphology are identified as CTCs ( Table 1 , Fig. 1 ). The CTCs detected by the CellSearch system can be harvested by micromanipulation and are available for further analysis (e.g., single-cell gene expression analysis). The downside of this technique is on the one hand the subjective assessment by the examiner, and on the other hand also the fact that it is an EpCAM-based technique and the cells cannot be detected after EMT due to the loss of epithelial characteristics 11 , 12 .
Table 1 Examples of typical cytomorphological criteria for the identification of isolated tumour cells (based on 13 ).
| Cell morphology/phenotype |
|---|
|
|
|
|
|
|
|
|
Fig. 1.
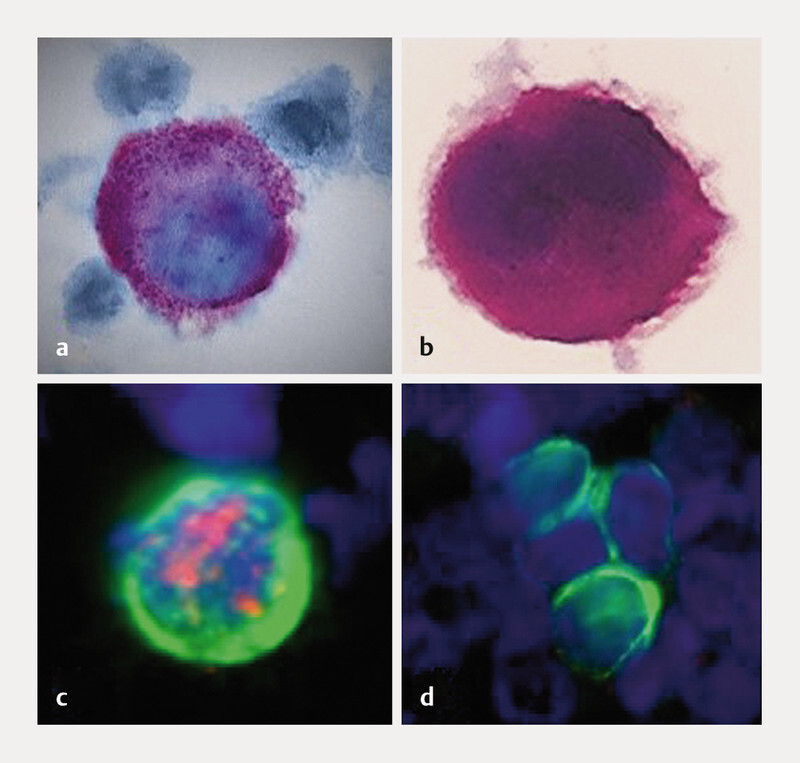
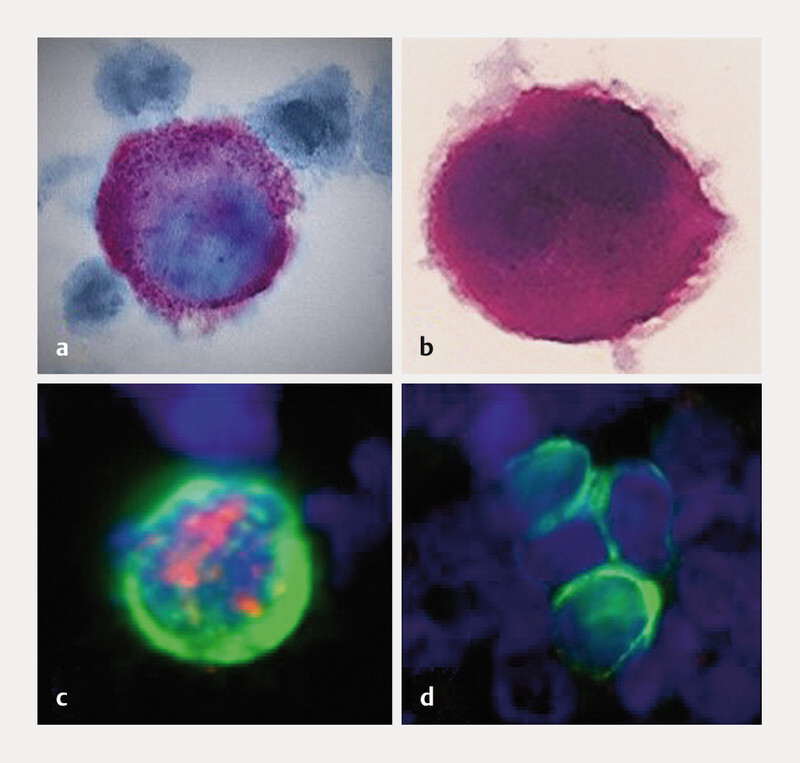
Detection of isolated tumour cells by various techniques: a Apoptotic tumour cell stained by immunocytochemistry (M30 antibody [AK]); b Vital tumour cell stained by immunocytochemistry (anti-cytokeratin [CK]-AK); c Immunofluorescence, double staining (anti-CK-AK: green, anti-oestrogen receptor-AK: red); d Immunofluorescence-stained tumour cell cluster (anti-CK-AK).
Another challenge in CTC diagnostic work-up is the low detection rate, primarily in non-metastatic patient populations. Particularly in view of the known heterogeneity of the tumour, it is advisable to analyse as many CTCs of a patient as possible. In this context, many enrichment/isolation techniques have been explored in recent years to increase the detection rate. Another possible approach is the analysis of larger blood volumes, for example, as part of diagnostic leukapheresis (DLA). On average, this extracorporeal filtration procedure will filter 2770 ml of blood. The CTCs are isolated by density gradient and an aliquot of the DLA product is then analysed with the CellSearch system. This significantly increases the CTC detection rate and number of CTCs identified 14 . However, analysis of large blood volumes requires considerable time on the part of the patient: the procedure takes about one hour. Lab panel changes after DLA have also been observed, e.g., slight decreases in leukocyte and haemoglobin counts, though these are not clinically significant. It remains to be seen to what extent the collection of several thousand CTCs will have an impact on clinical significance and whether DLA will improve CTC-based diagnostic work-up.
Circulating tumour DNA
The term circulating tumour DNA (ctDNA) refers to free DNA fragments, originating from the tumour (primary tumour, metastasis, isolated tumour cells), which can be detected in blood and other body fluids. This must be distinguished from the broader term cell-free DNA (cfDNA) or free circulating DNA (fcDNA) , which refers to all non-tumour specific cell-free DNA fragments. Decaying normal cells can also release cfDNA into the blood. While the older trials primarily focused on the detection of the total amount of freely circulating DNA in the blood, more recent studies have addressed the specific detection techniques for ctDNA.
Detection is usually carried out on blood plasma. Detection is determined by the percentage of ctDNA compared to cell-free DNA from normal cells. Since this percentage may be quite small, ctDNA detection requires highly sensitive techniques. These include digital-droplet PCR (ddPCR), beads amplification magnetics-PCR (BEAMing-PCR), and digital next-generation sequencing (dNGS) 15 , 16 , 17 . Here, ddPCR and BEAMing-PCR are the so-called targeted detection techniques (targeted approach), in which only a few gene loci can be examined simultaneously. In other words, the search is targeted at specific, already known tumour mutations, e.g., in genes PIK3CA, ESR1, AKT1, ERBB2 and PTEN. The term dNGS, on the other hand, summarises several non-targeted gene analysis techniques (untargeted approach), where large DNA molecules are sequenced and thus numerous unknown genetic alterations and mutations can be detected. These include, for example, array CGH (array-comparative genomic hybridization), whole-genome sequencing and exome sequencing. These techniques usually identify gene mutations that may contribute to the assessment of developing resistance 15 , 16 .
Clinical Applications of Liquid Biopsy in Early Breast Cancer
Improved prediction of prognosis
Individual tumour cells reaching the blood vessels and their subsequent dissemination are important steps in the metastatic cascade. According to studies, pre-invasive lesions of the breast, such as ductal carcinoma in situ (DCIS), can be accompanied by tumour cell dissemination 2 , 18 , so that haematogenous tumour cell dissemination is considered an early event in the course of malignant disease. The CellSearch system can detect circulating tumour cells in 20 – 30% of patients with non-metastatic breast cancer 19 ( Table 2 ). In a pooled analysis, Janni et al. were able to evaluate the data of 3173 patients with stage I – III breast cancer and demonstrated that the detection of at least one CTC in 7.5 ml blood predicts a significantly worse clinical outcome 19 . CTC-positive patients were twice as likely to die from breast cancer as women who did not have CTCs at the time of diagnosis (hazard ratio [HR]: 2.04; 95% CI: 1.52 – 2.75). CTC-positivity also correlated with a statistically significant shorter overall survival (HR 1.97; 95% CI: 1.51 – 2.59), disease-free survival (HR 1.82; 95% CI: 1.47 – 2.26) and distant disease-free survival (HR 1.89; 95% CI: 1.49 – 2.40).
Table 2 Prognostic significance of circulating tumour cells in breast cancer: the most important trials.
| Trial | Number of patients | Stage | Positivity rate n (%) |
Assay | Correlation with prognosis |
|---|---|---|---|---|---|
|
1
defined as ≥ 1 CTC per 7.5 ml
2 defined as ≥ 5 CTCs per 7.5 ml Acronyms: BCSS – breast cancer-specific survival; DDFS – distant disease-free survival; DFS – disease-free survival; LRRFS – loco-regional recurrence-free survival; OS – overall survival; pCR – pathological complete response | |||||
| Janni et al. 19 | 3173 | Stage I – III | 641 (20%) 1 | CellSearch | DFS, DDFS, BCSS, OS |
| Cristofanilli et al. 20 | 2436 | Stage IV | 1099 (45.1%) 2 | CellSearch | OS |
| Bidard et al. 21 | 1574 | Stage I – III before neoadjuvant chemotherapy | 398 (25.2%) 1 | CellSearch | OS, DDFS, LRRFS; pCR rate higher in case of CTC positivity (24.2% vs. 17.4%) |
While large meta-analyses ( Table 2 ) have confirmed the prognostic relevance of CTCs, the existing data on circulating DNA is much less extensive ( Table 3 ). A meta-analysis published in 2018 by Tan et al. included a total of 1127 patients from 10 trials, the majority with non-metastatic disease 22 . Unlike in the pooled analyses on CTC significance, the populations here were markedly smaller (336 patients maximum). In addition, the heterogeneity of the different assays used hampered direct comparison of the trials considerably. Some of the trials analysed the total amount of cfDNA, while the others studied the detection of pre-defined genomic alterations. Despite these shortcomings, the meta-analysis suggests a possible prognostic significance of cfDNA and mutation detection in non-metastatic breast cancer.
Table 3 Prognostic significance of circulating DNA in breast cancer patients (based on: 22 , only trials with at least 100 patients were included).
| Trial | Number of patients | Setting | Technique: | Prognostic significance: OS |
|---|---|---|---|---|
|
1
Serum
2 Plasma Acronyms: dPCR – digital PCR; HR – hazard ratio; OSMSP – one-step methylation-specific PCR; PCR-SSCP – PCR-single-strand conformation polymorphism; RFS – relapse-free survival | ||||
| Fujita et al. 23 | 336 | Stage I – II |
OSMSP
1
Met-DNA (±)/Total cfDNA (high/low) |
OS:
Yes (HR for Met-DNA 3.17; for total cfDNA 4.03) DFS/RFS: Met-DNA: No (HR 2.3) Total cfDNA: Yes (2.70) |
| Fernandaz-Garcia et al. 24 | 194 | Stage IV | TaqMan, RT-PCR Total cfDNA (high/low) |
OS:
yes (HR 2.296) |
| BRE12–158 25 | 151 | Stage I – III triple-negative, non-pCR |
FoundationOne Liquid
2
ctDNA (±) |
OS:
yes (HR 2.7) DDFS: yes (HR 3.1) |
| Garcia et al. 26 | 142 | Stage I – III |
PCR-SSCP
2
ctDNA (±) |
OS:
no (HR 1.60) DFS/RFS: yes (HR 2.70) |
| Fujita et al. 27 | 120 | Stage II – III post-therapy |
OSMSP
1
Met-DNA (±)/Total cfDNA (high/low) |
OS:
yes (HR for Met-DNA 4.91; for total cfDNA 4.11) DFS/RFS: yes (HR for Met-DNA 4.23; for total cfDNA 1.93) |
| Shaw et al. 28 | 112 | Stage IV |
ddPCR
2
Total cfDNA |
OS:
yes (HR 2.20) |
| Shaw et al. 29 | 110 | Stage I – III |
dPCR
1
mut. PIK3CA (±) |
OS:
no (HR 3.92) DFS/RFS: yes (HR 4.78) |
Liquid biopsy treatment monitoring
Non-invasive diagnostic blood tests allow serial studies which can be repeated as often as needed during treatment or after its completion. This provides unique insight into the current cancer situation. Since systemic treatment exerts a selection pressure on MRD, blood samples can help assess the persistent tumour cell population 30 . This way, it is possible to monitor the response to treatment and identify those patients with increased risk of relapse who might benefit from additional therapeutic approaches.
We now know that CTCs can persist beyond (neo)adjuvant therapy (so-called persistent CTCs, Table 4 ). The SUCCESS trial, initiated in Germany, was the largest analysis of CTC persistence in non-metastatic disease to date 31 . In 2026 patients, CTCs were examined with the CellSearch system before the patients started adjuvant chemotherapy. At least one CTC was detected in 21.5% of the women. After completion of chemotherapy, a blood sample was taken from 1493 patients. The positivity rate was 22.1%. The detection of CTCs prior to chemotherapy correlated with clinical outcome (DFS, DDFS, BCSS and OS), but only to a limited extent with CTC persistence. For example, 76 patients had a positive CTC status both before and after chemotherapy. In 936 women both blood samples were CTC-negative. In 491 patients the CTC status changed (+ → − in 238 cases and − → + in 253 cases). The detection of persistent CTCs predicted unfavourable clinical outcomes (DFS: hazard ratio 1.124, p = 0.02, OS: hazard ratio 1.162, p = 0.06). This suggests the development of effective resistance mechanisms by the tumour cells, allowing them to evade the effects of cytotoxic treatment 21 , 31 . In addition, neoadjuvant studies have shown that CTC response does not correlate with primary tumour response to treatment.
Table 4 Clinical significance of persistent CTCs in early breast cancer.
| Trial | Number of patients | Setting | Positivity rate (%) |
Correlation with survival |
|---|---|---|---|---|
|
1
Analysed with CellSearch
2 Analysed with AdnaTest Acronyms: DDFS – distant disease-free survival; DFS – disease-free survival; NACT – neoadjuvant chemotherapy, OS – overall survival | ||||
| Rack et al. 31 | 1493 | Stage I – III N+ or high-risk N0 Blood test after adjuvant chemotherapy |
22% 1 | yes: DFS, OS |
| Bidard et al. 21 | 1200 | Stage I – III Blood test after NACT |
15% 1 | yes: OS, DDFS |
| Riethdorf et al. 32 | 207 | high-risk Blood test after NACT |
11% 1 | not analysed |
| Kasimir-Bauer et al. 33 | 133 | Stage II – III Blood test before and after NACT |
8% 2 | no |
Numerous smaller studies have explored the question of how the detection of circulating DNA may complement treatment monitoring in early breast cancer ( Table 5 ). Li et al. studied plasma samples before, during and after neoadjuvant chemotherapy in 52 patients and demonstrated that ctDNA analysis after 2 cycles of treatment correlated better with pCR probability than radiological diagnostic work-up 34 . Similar results were reported by Magbanua et al., who performed ctDNA ultra-deep sequencing in 84 patients treated in the I-SPY 2 trial 35 . Within 3 weeks after treatment was started, the ctDNA positivity rate dropped sharply from 73 to 35%. All patients who achieved pCR were ctDNA negative after chemotherapy. In the non-pCR subset, the detection of persistent ctDNA after NACT predicted a significantly increased risk of metastasis (hazard ratio 10.4).
Table 5 Clinical significance of circulating DNA during and after neoadjuvant therapy in early breast cancer: Summary of the key trials.
| Trial | Number of patients | Technique | Positivity rate (%) | Results |
|---|---|---|---|---|
| Acronyms: AUC – area under the curve; DFS – disease-free survival; ddPCR – digital droplet PCR; dPCR – digital PCR; DDFS – distant disease-free survival; EFS – event-free survival; NACT – neoadjuvant chemotherapy; NGS – next generation sequencing; OS-MSP – one-step methylation-specific PCR; RCB – residual cancer burden | ||||
| Takahashi et al. 36 | 87 | OS-MSP | 23% before NACT | ctDNA persistence associated with RCB |
| Magbanua et al. 35 | 84 | Ultra-deep sequencing | 73% before NACT 9% after NACT |
Persistent ctDNA 3 weeks after start of NACT associated with pCR (pCR rate 17% vs. 48%, p = 0.012); ctDNA detection after NACT associated with DDFS |
| NeoALTTO trial 37 | 69 | Mutation analysis PIK3CA and TP53 (ddPCR) | 41% before NACT 20% 2 weeks after start 5% after NACT |
Persistent ctDNA 2 weeks after start of NACT associated with a lower probability of pCR, but not EFS |
| Garcia-Murillas et al. 38 | 55 | dPCR, high-depth DNA sequencing | 69% before NACT 19% 2 – 4 weeks postop. |
ctDNA persistence 2 – 4 weeks postop. associated with DFS (hazard ratio 25.1) |
| Li et al. 34 | 52 | NGS panel of 1021 genes | 48% before NACT (most mutations in genes TP53, PIK3CA, GAB2, and IRS2); ctDNA persistence in 70% of initially ctDNA-positive pts. | ctDNA after 2 cycles predicted the pathological response (AUC 0.81); higher rate of relapse in case of persistent ctDNA (50 vs. 33%) |
| Sharma et al. 39 | 30 | Methylation analysis (genes BRCA1, MGMT, GSTP1, stratifin, MDR1) | Methylation before NACT: 76% at least 1 gene; 53% (BRCA1), 37% (MGMT), 43% (GSTP1), 83% (stratifin), 60% (MDR1) (76%) | Tumour response associated with increased methylation before NACT and decreased methylation after NACT |
| Moss et al. 40 | 30 | Methylation analysis | 80% before NACT | marked decrease in cfDNA under NACT; cfDNA associated with pCR in last month of NACT (p = 0.006) |
Liquid biopsy potential in follow-up
After completion of the primary therapy, which usually comprises surgery and, depending on the subtype and the extent of surgical treatment, radiotherapy and chemotherapy, possibly in combination with targeted therapy, when trying to estimate the remaining risk of relapse, the attending physician can nowadays only refer back to the characteristics of the disease at the time of the initial diagnosis. There are no other tools available allowing individualised risk estimation. On the other hand, during follow-up patients have a strong desire to learn their chances of recovery. In this context some women demand analysis of the classical tumour markers (e.g., CEA, CA 15-3), a step which the current guidelines expressly discourage 41 , 42 . Several trials have explored how liquid biopsy can improve the prediction of prognosis in the follow-up of asymptomatic patients.
In 2018, the data from two large-scale adjuvant therapy trials were published, which studied the significance of CTC testing 5 years post-diagnosis in translational subprojects ( Table 6 ). The CellSearch system was used in both trials. Interestingly enough, the prognosis in women with persistent CTCs was significantly poorer than in the CTC-negative population, especially in the subgroup of HR-positive tumours. For example, the U. S. trial demonstrated that the annual risk of recurrence was 21.4% when at least one CTC was detected, compared to only 2% in CTC-negative women.
Table 6 Clinical significance of persistent CTC in follow-up.
| Trial | Number of patients | Date of CTC analysis | Positivity rate (%) | Median follow-up period | Correlation with prognosis |
|---|---|---|---|---|---|
| ECOG-ACRIN E5103 47 , 48 | 547 HER2-negative stage II – III |
4.5 – 7.5 years post-diagnosis | 4.8% | 2.6 years | Risk of relapse 12.7 × higher in patients with persistent CTCs; risk of relapse per patient/year in the HR-positive population: 21.4 vs. 2.0% |
| SUCCESS-A 49 | 206 Stage I – III (high-risk) |
median 62 months post-diagnosis | 7.8% | 1 year | In HR-positive population: risk of relapse higher in CTC-positive pts. (hazard ratio 5.95) |
According to smaller trials the circulating DNA may also contribute to risk stratification in the follow-up of asymptomatic patients 43 , 44 , 45 , 46 . In a group of 101 women Garcia-Murillas et al. were able to establish that serial ctDNA measurements can predict the risk of recurrence 44 . Blood tests were repeated every 3 months in the first year and then every 6 months for 5 years. ctDNA detection was based on the somatic mutations in the tumour tissue, which were analysed by dPCR in the blood. Patients in whom ctDNA was detected during the course of chemotherapy experienced a markedly shorter recurrence-free survival (hazard ratio 16.7, p < 0.001), with the first blood samples after completion of chemotherapy being initially ctDNA-negative in most patients. Clinical recurrence or distant metastasis was observed a median of 10.7 months after the first ctDNA-positive blood test. Interestingly enough, since ctDNA progression did not correlate with the presence of brain metastases, this location may remain “mute” in liquid biopsy-based detection.
The EBLIS trial also confirmed the potential of ctDNA-based monitoring in follow-up 45 . Here, in the first 4 years after completion of chemotherapy, blood tests were performed every 6 months. ctDNA detection relied on the detection of individual tumour-specific mutation signatures, which were established by analysing the primary tumours. Clinical assessment of the course of the first 49 patients revealed that ctDNA detection occurred a median 8.9 months before local or distant relapse.
It is still unclear how these findings can be implemented in routine clinical practice and which diagnostic or therapeutic consequences might derive from a positive ctDNA blood test result. In the British c-TRAK TN trial (NCT03145961), monitoring by ctDNA testing is performed every 3 months after completion of therapy in triple negative disease. Patients with persistent ctDNA are randomized to pembrolizumab or observation. In Germany, the SURVIVE trial initiated by the Department of Obstetrics & Gynaecology at Ulm University Medical Centre plans to study the significance of liquid biopsy in follow-up.
Liquid biopsy-based therapeutic interventions
The adoption of blood-based biomarkers in the practice of early breast cancer has been hampered to date by the uncertainty regarding the clinical consequences. One of the few trials exploring possible CTC-based therapeutic intervention in non-metastatic disease was the multicentre Treat CTC trial 50 . Here, patients with HER2-negative primary tumour and persistent CTCs after completion of (neo)adjuvant chemotherapy were randomised to 6 cycles of trastuzumab or observation. A total of 1317 patients were screened. CTCs were detected in 95 women, of which 63 were successfully randomised. Since the primary endpoint (successful CTC elimination by trastuzumab) was not met, the trial was terminated. Nor did HER2-targeted therapy improve clinical outcome: after a median follow-up of 13 months, invasive disease-free and overall survival were the same in both arms. The precise inclusion criteria must be taken into account when assessing this result. The HER2 status of the CTCs did not impact on possible enrolment in the trial. Thus, patients with HER2-negative CTCs who, as expected, did not benefit from trastuzumab were also randomised. When planning future trials, the phenotype and genotype characteristics of the detected cells should be included, if possible.
Clinical Applications of Liquid Biopsy in Metastatic Breast Cancer
Improved prediction of prognosis
In the metastatic setting, CTC detection can complement the prediction of prognosis as well. In 2019, the course in 2436 patients with metastatic disease was evaluated in a retrospective pooled analysis of 18 centres 20 ( Table 2 ). 54% of patients had already received systemic therapy for their metastatic disease. CTC detection in all trials analysed was performed on the CellSearch system. However, due to the higher concentration of CTCs in metastatic disease, a different cutoff proved to be effective: in most trials 5 or more CTCs per 7.5 ml of blood was considered elevated (or CTC-high). In the pooled analysis, patients with ≥ 5 CTCs per 7.5 ml blood were classified as CTC aggressive and those with < 5 CTCs as CTC indolent . The analysis confirmed that the presence of increased CTC levels in metastatic disease correlated significantly with shorter overall survival (median OS: 36.3 in CTC aggressive vs. 16.0 months in CTC indolent , p < 0.0001) and this association was observed in all subtypes of the tumour 20 . In multivariate analysis, the following factors were associated with shorter OS: prior treatment, poor differentiation, triple-negative phenotype, visceral metastasis, and presence of ≥ 5 CTCs, with the CTC count as the strongest predictor of OS (HR 2.71, 95% CI 2.35 – 3.12, p < 0.0001).
Few trials explored the prognostic value of circulating DNA in metastatic disease ( Table 3 ). Shaw et al. analysed blood samples from 112 patients with prior treatment 28 . Elevated cfDNA levels correlated with shorter overall survival. In addition, ctDNA and CTCs were detected. ctDNA was identified by detecting mutations in the genes PIK3CA, TP53, ESR1, and KRAS. Interestingly enough, elevated cfDNA levels were usually detected in patients with high ctDNA levels. In terms of the mutation profile, the ctDNA reflected the mutations that were detected in the CTCs.
Liquid biopsy treatment monitoring
According to several trials, clinical response in metastatic disease is reflected by changes in the CTC numbers 16 . A marked decrease in CTCs is usually noted even after the first cycle of palliative chemotherapy. Smerage et al. and Martin et al. were able to demonstrate that after the first cycle of therapy < 5 CTCs were detected in 47 – 57% of patients with initial counts of ≥ 5 CTCs 51 , 52 . Consistently high CTC numbers 3 – 4 weeks after the start of treatment on the other hand indicate an increased risk of progression. This allows faster assessment of the response than with conventional radiological diagnostic work-up. However, the clinical consequences to be derived from consistently high CTC counts remain unclear. This question was explored by the randomised phase-III trial of the US SWOG study group 51 . A total of 595 patients with metastatic breast cancer received their first-line chemotherapy in the trial. 43% of the 319 women with elevated CTC levels before treatment was initiated continued to have ≥ 5 CTCs after the first cycle. These patients were randomised either to a different treatment regimen or to continued treatment without change. The best survival was observed in patients with low initial CTC levels (35 months), followed by women whose high initial CTC levels dropped after the first cycle (23 months) and patients with persistent CTC (13 months). Interestingly enough, since the change in treatment did not yield the hoped-for improvement in prognosis, the CTC persistence predicts resistance to traditional cytotoxic agents. It is possible that patients with sustained high CTC levels could benefit from immunological, targeted or experimental approaches.
The detection of circulating DNA was integrated into the translational adjunct programmes of several trials. In the BEECH trial, Hrebien et al. addressed the question of how serial ctDNA measurements could complement monitoring under chemotherapy 53 . In this randomized phase-II trial, patients with ER-positive, HER2-negative tumours received first-line treatment with paclitaxel and the AKT inhibitor capivasertib versus placebo. First, mutations in the baseline samples were evaluated, with the latter then undergoing detection by mutation-specific ddPCR. As early as one week after the start of treatment, changes in ctDNA levels were observed that could predict progression-free survival. The best correlation with progression-free survival (PFS) was observed for blood samples taken on day 1 of the second treatment cycle (q4w) (11.1 vs. 6.4 months, hazard ratio 0.2). Both arms showed an equally strong drop in ctDNA levels, reflecting the lack of clinical benefit of capivasertib that has since been demonstrated.
The PALOMA-3 trial also confirmed the high significance of early ctDNA testing 54 . In this phase-III trial, a total of 521 patients progressing under endocrine therapy were randomised to treatment with fulvestrant and the CDK4/6 inhibitor palbociclib vs. placebo. In 455 patients enrolled in the trial, the blood sample drawn before starting treatment was analysed by multiplex dPCR for hotspot mutations in the PIK3CA gene. At least one mutation was detected in 100 women, in 73 patients a blood sample was also analysed on day 15 of treatment. In the palbociclib arm, the drop in ctDNA between baseline and day 15 was markedly more pronounced than in the placebo arm. Based on these results, it may be surmised that ctDNA dynamics may serve as a suitable surrogate parameter for the early benefit assessment of new agents.
At the ASCO Symposium 2020 the results of two CDK4/6 trials were presented, which had studied ctDNA measurements in their translational adjunct programmes 55 , 56 . The PADA-1 trial evaluated the clinical significance of the ESR1 mutation detected in cell-free DNA of 1017 patients under treatment with aromatase inhibitors and palbociclib 56 . All patients presented with ER-positive, HER2-negative metastatic disease and received first-line treatment in the trial. The serial blood samples were analysed by ddPCR. Before treatment was initiated, the ESR1 mutation was detected in 3.2% of patients, most of whom had already undergone adjuvant treatment with an aromatase inhibitor. In 78% of the patients the mutation was eliminated from the cfDNA within the first 5 months of treatment. Patients with a mutation in the ESR1 gene before starting treatment had a shorter PFS than women without a mutation in the cfDNA. It remains to be seen how these results might affect treatment decisions in ESR1 mut patients in everyday clinical practice. In addition, ctDNA analysis was presented at the ASCO Symposium 2020 in the context of the three trials on ribociclib, MONALEESA-2, -3 and -7 55 . The blood samples of 1503 patients in total were analysed by NGS for alterations in 557 genes before endocrine-based treatment was initiated. Alterations in the genes FRS2, PRKCA, MDM1, ERBB2, AKT1, and BRCA1/2 predicted a stronger PFS benefit for ribociclib (statistical trend), while patients with alterations in genes CHD4, BCL11B, ATM, and CDKN2A/2B/2C benefited little or not at all from ribociclib. Future trials must establish whether the genes studied can contribute to the early detection of resistance in the context of treatment monitoring.
Liquid biopsy-based therapeutic interventions
Today, treatment of the patient with metastatic disease is based on the predictive properties of the primary tumour or metastases. This assumes the need for invasive tissue sampling to obtain material for histological examination and may be associated with complications in case of difficult locations. On the other hand, the clonal heterogeneity of the malignant disease must be taken into account. Thus, individual metastases may differ from each other in terms of phenotype and genotype, but different populations can also be present within a metastatic mass. The analysed markers may also vary over time. A meta-analysis of 39 trials demonstrated that 22.5% of patients with initially ER-positive primary tumours developed ER-negative metastases 57 . Loss of HER2 status was observed in 21.3% of patients. In contrast, 9.5% of patients with HER2-negative primary tumours developed HER2-positive metastasis. For this reason, the Breast Committee of the Working Group for Gynaecological Oncology (AGO-Kommission Mamma) recommends a re-evaluation of the receptor status in metastatic disease.
Assuming that the blood-based biomarkers reflect the characteristics of the dominant tumour populations, the possible use of the liquid biopsy as foundation for treatment decisions is under intense discussion.
The STIC CTC study is one of the most important trials looking at CTC-based treatment choice 58 . In this phase-III trial, a total of 778 women with hormone-receptor-positive HER2-negative disease were randomised before starting their first-line therapy. In the CTC arm, the choice of treatment was based solely on the results of the blood test: Patients with < 5 CTCs received endocrine monotherapy, while women with ≥ 5 CTCs underwent chemotherapy. Blood was also taken in the control arm and examined with the CellSearch system, but the result remained blinded. In this arm, treatment was decided by the attending physician based on the usual clinical criteria. As the study was initiated in 2012, the study design did not include CDK4/6 inhibitors, which were only approved later. The STIC CTC trial met its primary endpoint: the CTC-based choice of treatment was not inferior to the physicianʼs decision, referred to as “treatment of physicianʼs choice”. The clinical outcome (PFS and OS) was the same in both arms. Interestingly enough, patients with discordant risk assessment (clinically low-risk but with high CTC counts or clinically high-risk with low CTC counts) benefited from chemotherapy in terms of overall survival. Since the STIC CTC trial did not include any endocrine-based combined treatment with CDK4/6 inhibitors, the first-line therapy selected most often at present in the hormone receptor-positive population, this trial does not currently provide any recommendation for everyday clinical practice.
Unlike the STIC CTC trial, which analysed the CTC counts as the basis for treatment decisions, the CirCe T-DM1 study explored the question of how the characteristics of CTCs can affect the choice of treatment 59 . It studied whether patients with histologically HER2-negative disease (determined for the primary tumour and/or metastases) and HER2-positive CTC status could benefit from HER2-targeted treatment. A total of 154 women with prior treatment for breast cancer were FisH-screened for HER2-amplified CTCs. In 14 cases at least one HER2-positive CTC was detected, 11 of which were target-treated with T-DM1 in the trial. The response rate was rather low at 9.1%. Only partial remission was achieved. The median PFS was 4.8 months and the OS 9.5 months. The lack of benefit of HER2-targeted therapy may possibly be explained by the heterogeneous nature of the CTCs. The majority of CTCs detected had a negative HER2 status and only 4.4% of CTCs exhibited HER2 amplification.
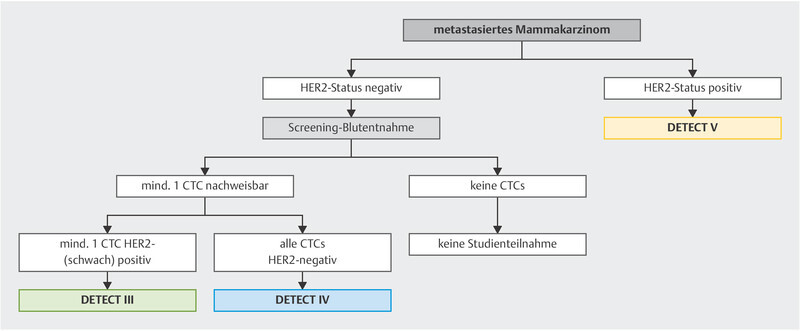
Other CTC-based treatment concepts are currently being studied in the DETECT trials, the worldʼs largest trial programme on CTC-based treatment interventions 50 ( Fig. 2 ).
Fig. 2.
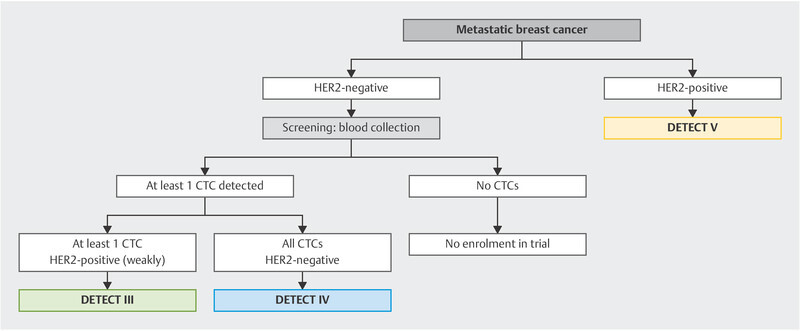
Trial algorithm in the DETECT programme.
Unlike in diagnostic CTC work-up, ctDNA can already be considered in the routine clinical practice when choosing a particular treatment. According to several trials, somatic genetic alterations in the PIK3CA, ESR1, HER2, and PTEN genes can be detected and specifically targeted as “targetable mutations” ( Table 7 ).
Table 7 Possible alterations detectable in the ctDNA and their potential significance.
| Gene/Marker | potential clinical significance |
|---|---|
| ESR1 | Imparting endocrine resistance and unfavourable prognosis, depending on the variant resistance to everolimus (ESR1 D538G and Y 537S ), exemestan ( D538G , ESR1 mut ), fulvestrant ( Y537S, Y537C ), relative fulvestrant sensitivity (ESR1 mut ) |
| PIK3CA | Prediction of response to therapy with PI3K inhibitor (alpelisib approved in Europe in 2020); potentially useful in treatment monitoring |
| AKT1 | Prediction of response to AKT inhibitors such as capivasertib (AKT1 E17K ) |
| HER2 |
Prediction of the response to neratinib in HER2
mut
Treatment monitoring under anti-HER2 therapy |
| TP53 | Imparting resistance to treatment |
| PTEN | Imparting resistance to treatment |
| BRCA | Prediction of the response to PARP inhibitors and platinum salts in somatic BRCA mutations in the ctDNA |
| Microsatellite instability (MSI), loss of heterozygosity (LOH) | Prediction of the response to targeted and immunological therapeutic approaches |
The first agent with liquid biopsy-based indication was approved as a result of the SOLAR 1 trial 30 . Here, the PI3K inhibitor alpelisib in combination with fulvestrant was studied in patients with hormone receptor-positive HER2-negative disease. Alpelisib inhibits the enzyme PI3 kinase coded by the PIK3CA gene. Mutations in the PIK3CA gene are seen in up to 40% of patients in advanced stages and result in increasingly aggressive disease by activating the PI3K/Akt/mTOR signaling cascade. In the SOLAR 1 trial, a total of 572 patients were randomised to treatment with fulvestrant and alpelisib versus fulvestrant and placebo. The patients suffered from metastatic disease and had already undergone prior endocrine treatment. In all women, the PIK3CA mutation status was determined in the tumour tissue and in some cases also in the ctDNA. Detection of a mutation was associated with a significant PFS benefit when alpelisib was added, regardless of whether the mutation was detected in the tissue or in the ctDNA. Alpelisib has already been approved by the U. S. FDA in 2019 and by the European Medicines Agency in 2020.
With modern sequencing techniques, it is possible to study several potentially treatment-relevant mutations simultaneously. The PlasmaMatch trial 60 followed this approach, in which blood samples from a total of 1044 women with metastatic breast cancer were screened. A number of genetic alterations in the ctDNA were detected. The TP53 gene was most often affected (44.1%), followed by PIK3CA (34.9%), ESR1 (oestrogen receptor 1) (33.1%), PTEN (6.9%), HER2 (6.4%), and AKT1 (5.0%). The study design provided for mutation-triggered targeted treatment. For example, patients with ESR1 gene mutations were treated with fulvestrant, while women with HER2 gene mutations received anti-HER2 treatment with neratinib. In case of mutations in the PTEN or AKT1 gene, treatment with capivasertib was initiated. After analysis of the response, treatment with neratinib and capivasertib proved particularly promising (response rates: neratinib 25%, capivasertib 22 – 33%).
Table 8 summarises the current knowledge on liquid biopsy in early and advanced breast cancer.
Table 8 Clinical significance of CTCs and circulating DNA in breast cancer.
| CTCs | Circulating DNA | ||
|---|---|---|---|
| M0 | Prognostic significance | Very high (confirmed in meta-analyses) | Probably high; limitation: small case numbers, rather brief follow-up, differences in methodology |
| Treatment monitoring | Persistence after (neo)adjuvant chemotherapy predicts worse outcome | Persistence after (neo)adjuvant chemotherapy predicts worse outcome; ctDNA dynamics correlate with pCR | |
| Follow-up complement | Potentially significant, especially in HR positive disease: CTC detection within 5 years post-diagnosis predicts an increased risk of relapse | Potentially significant: ctDNA positivity predicts an increased risk of relapse | |
| Liquid-biopsy based therapeutic interventions | No positive trials to date, potential unclear; TREAT CTC trial without benefit of trastuzumab in persistent CTC (HER2 status of CTCs not considered) | No positive trials to date, potential unclear | |
| M1 | prognostic significance | Very high (confirmed in meta-analyses) | Probably high; limitation: few trials, small case numbers, differences in methodology |
| Treatment monitoring | High potential: Persistence after 1st cycle chemotherapy correlates with response, clinical consequence unclear | High potential: Persistence after 1st cycle of treatment correlates with response, clinical consequence unclear | |
| Liquid biopsy-based therapeutic interventions | First positive trial (STIC CTC): CTC-based choice of first-line therapy is not inferior to oncologistʼs choice; clinical consequence unclear; further trials pending (e.g. DETECT study programme) | Main area of use: Detection of somatic mutations in the ctDNA as basis of indication for targeted therapy: Alpelisib approved in the USA and Europe for patients with a mutation in the PIK3CA gene | |
Summary
Liquid biopsy refers to the study of circulating tumor cells and nucleic acids (DNA/RNA) in the blood.
The prognostic significance of CTC detection in patients with breast cancer is very high in both early and metastatic stages.
The dynamics of CTCs and ctDNA correlate with response to palliative treatment.
Oncologic research currently focuses on liquid biopsy-based therapeutic interventions in metastatic breast cancer. The PI3K inhibitor alpelisib is the first agent approved in this context.
Footnotes
Conflict of Interest/Interessenkonflikt The authors declare that they have no conflict of interest./Die Autorinnen/Autoren geben an, dass kein Interessenkonflikt besteht.
References/Literatur
- 1.Fisher B, Ravdin R G, Ausman R K. Surgical adjuvant chemotherapy in cancer of the breast: results of a decade of cooperative investigation. Ann Surg. 1968;168:337–356. doi: 10.1097/00000658-196809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banys M, Hahn M, Gruber I. Detection and clinical relevance of hematogenous tumor cell dissemination in patients with ductal carcinoma in situ. Breast Cancer Res Treat. 2014;144:531–538. doi: 10.1007/s10549-014-2898-6. [DOI] [PubMed] [Google Scholar]
- 3.Luzzi K J, MacDonald I C, Schmidt E E. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehes G, Witt A, Kubista E. Circulating breast cancer cells are frequently apoptotic. Am J Pathol. 2001;159:17–20. doi: 10.1016/S0002-9440(10)61667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng S, Tripathy D, Frenkel E P. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 6.Krawczyk N, Meier-Stiegen F, Banys M. Expression of stem cell and epithelial-mesenchymal transition markers in circulating tumor cells of breast cancer patients. Biomed Res Int. 2014;2014:415721. doi: 10.1155/2014/415721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mani S A, Guo W, Liao M J. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell. 2012;22:699–701. doi: 10.1016/j.ccr.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzenbach H, Pantel K, Kemper B. Comparative evaluation of cell-free tumor DNA in blood and disseminated tumor cells in bone marrow of patients with primary breast cancer. Breast Cancer Res. 2009;11:R71. doi: 10.1186/bcr2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzenbach H, Hoon D S, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 11.Ignatiadis M, Riethdorf S, Bidard F C. International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res. 2014;16:R43. doi: 10.1186/bcr3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allard W J, Matera J, Miller M C. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 13.Fehm T, Braun S, Muller V. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer. 2006;107:885–892. doi: 10.1002/cncr.22076. [DOI] [PubMed] [Google Scholar]
- 14.Fehm T N, Meier-Stiegen F, Driemel C. Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytometry A. 2018;93:1213–1219. doi: 10.1002/cyto.a.23669. [DOI] [PubMed] [Google Scholar]
- 15.Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 16.München: LUKON Verlagsgesellschaft mbH; 2019. Colloquium Senologie 2019/2020. [Google Scholar]
- 17.Jung A, Kirchner T. Liquid Biopsy in Tumor Genetic Diagnosis. Dtsch Arztebl Int. 2018;115:169–174. doi: 10.3238/arztebl.2018.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husemann Y, Geigl J B, Schubert F. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Janni W J, Rack B, Terstappen L W. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res. 2016;22:2583–2593. doi: 10.1158/1078-0432.CCR-15-1603. [DOI] [PubMed] [Google Scholar]
- 20.Cristofanilli M, Pierga J Y, Reuben J. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit Rev Oncol Hematol. 2019;134:39–45. doi: 10.1016/j.critrevonc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Bidard F C, Michiels S, Riethdorf S. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J Natl Cancer Inst. 2018;110:560–567. doi: 10.1093/jnci/djy018. [DOI] [PubMed] [Google Scholar]
- 22.Tan G, Chu C, Gui X. The prognostic value of circulating cell-free DNA in breast cancer: A meta-analysis. Medicine (Baltimore) 2018;97:e0197. doi: 10.1097/MD.0000000000010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita N, Nakayama T, Yamamoto N. Methylated DNA and total DNA in serum detected by one-step methylation-specific PCR is predictive of poor prognosis for breast cancer patients. Oncology. 2012;83:273–282. doi: 10.1159/000342083. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Garcia D, Hills A, Page K. Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res. 2019;21:149. doi: 10.1186/s13058-019-1235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radovich M, Jiang G, Chitambar C. Detection of circulating tumor DNA (ctDNA) after neoadjuvant chemotherapy is significantly associated with disease recurrence in early-stage triple-negative breast cancer (TNBC): Preplanned correlative results from clinical trial BRE12-158. San Antonio Breast Cancer Symposium 2019; Abstr. GS5-02
- 26.Garcia J M, Garcia V, Silva J. Extracellular tumor DNA in plasma and overall survival in breast cancer patients. Genes Chromosomes Cancer. 2006;45:692–701. doi: 10.1002/gcc.20334. [DOI] [PubMed] [Google Scholar]
- 27.Fujita N, Kagara N, Yamamoto N. Methylated DNA and high total DNA levels in the serum of patients with breast cancer following neoadjuvant chemotherapy are predictive of a poor prognosis. Oncol Lett. 2014;8:397–403. doi: 10.3892/ol.2014.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw J A, Guttery D S, Hills A. Mutation Analysis of Cell-Free DNA and Single Circulating Tumor Cells in Metastatic Breast Cancer Patients with High Circulating Tumor Cell Counts. Clin Cancer Res. 2017;23:88–96. doi: 10.1158/1078-0432.CCR-16-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oshiro C, Kagara N, Naoi Y. PIK3CA mutations in serum DNA are predictive of recurrence in primary breast cancer patients. Breast Cancer Res Treat. 2015;150:299–307. doi: 10.1007/s10549-015-3322-6. [DOI] [PubMed] [Google Scholar]
- 30.Fehm T, Neubauer H, Banys-Paluchowski M. München: LUKON Verlag; 2019. Liquid Biopsy. [Google Scholar]
- 31.Rack B, Schindlbeck C, Juckstock J. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106:dju066. doi: 10.1093/jnci/dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riethdorf S, Muller V, Zhang L. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16:2634–2645. doi: 10.1158/1078-0432.CCR-09-2042. [DOI] [PubMed] [Google Scholar]
- 33.Kasimir-Bauer S, Bittner A K, Konig L. Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res. 2016;18:20. doi: 10.1186/s13058-016-0679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Lai H, Liu J. Circulating Tumor DNA Predicts the Response and Prognosis in Patients With Early Breast Cancer Receiving Neoadjuvant Chemotherapy. JCO Precision Oncology. 2020;4:244–257. doi: 10.1200/PO.19.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magbanua M JM, Brown-Swigart L, Wu H-T. Circulating tumor DNA in neoadjuvant treated breast cancer reflects response and survival. medRxiv. 2020 doi: 10.1101/2020.02.03.20019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi H, Kagara N, Tanei T. Correlation of Methylated Circulating Tumor DNA With Response to Neoadjuvant Chemotherapy in Breast Cancer Patients. Clin Breast Cancer. 2017;17:61–69000. doi: 10.1016/j.clbc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Rothe F, Silva M J, Venet D. Circulating Tumor DNA in HER2-Amplified Breast Cancer: A Translational Research Substudy of the NeoALTTO Phase III Trial. Clin Cancer Res. 2019;25:3581–3588. doi: 10.1158/1078-0432.CCR-18-2521. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Murillas I, Schiavon G, Weigelt B. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 39.Sharma G, Mirza S, Parshad R. DNA methylation of circulating DNA: a marker for monitoring efficacy of neoadjuvant chemotherapy in breast cancer patients. Tumour Biol. 2012;33:1837–1843. doi: 10.1007/s13277-012-0443-y. [DOI] [PubMed] [Google Scholar]
- 40.Moss J, Zick A, Grinshpun A. Circulating breast-derived DNA allows universal detection and monitoring of localized breast cancer. Ann Oncol. 2020;31:395–403. doi: 10.1016/j.annonc.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Diagnostik und Therapie früher und fortgeschrittener Mammakarzinome. Herausgegeben von der Kommission Mamma (vertreten durch: Wolfgang Janni) der Arbeitsgemeinschaft Gynäkologische Onkologie e. V. in der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe e. V. sowie in der Deutschen Krebsgesellschaft e.V. 2020
- 42.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.3, AWMF Registernummer: 032–045OL 2020. Online (last access: 18.09.2020):http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/
- 43.Fiegl H, Millinger S, Mueller-Holzner E. Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 2005;65:1141–1145. doi: 10.1158/0008-5472.CAN-04-2438. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Murillas I, Chopra N, Comino-Mendez I. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coombes R C, Page K, Salari R. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin Cancer Res. 2019;25:4255–4263. doi: 10.1158/1078-0432.CCR-18-3663. [DOI] [PubMed] [Google Scholar]
- 46.Olsson E, Winter C, George A. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015;7:1034–1047. doi: 10.15252/emmm.201404913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sparano J A, OʼNeill A, Alpaugh K. Circulating tumor cells (CTCs) five years after diagnosis are prognostic for late recurrence in operable stage II – III breast cancer. San Antonio Breast Cancer Symposium 2017; Abstr. GS6-03
- 48.Sparano J, OʼNeill A, Alpaugh K. Association of Circulating Tumor Cells With Late Recurrence of Estrogen Receptor-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2018.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janni W, Rack B, Fasching P.Persistence of circulating tumor cells in high risk early breast cancer patients five years after adjuvant chemotherapy and late recurrence: Results from the adjuvant SUCCESS A trial J Clin Oncol 201836 (15_suppl)515.doi:10.1200/JCO.2018.36.15_suppl.51529267131 [Google Scholar]
- 50.Banys-Paluchowski M, Fehm T, Janni W. Circulating and Disseminated Tumor Cells in Breast Carcinoma: Report from the Consensus Conference on Tumor Cell Dissemination during the 39th Annual Meeting of the German Society of Senology, Berlin, 27 June 2019. Geburtshilfe Frauenheilkd. 2019;79:1320–1327. doi: 10.1055/a-1031-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smerage J B, Barlow W E, Hortobagyi G N. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–3489. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin M, Custodio S, de Las Casas M L. Circulating tumor cells following first chemotherapy cycle: an early and strong predictor of outcome in patients with metastatic breast cancer. Oncologist. 2013;18:917–923. doi: 10.1634/theoncologist.2012-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hrebien S, Citi V, Garcia-Murillas I. Early ctDNA dynamics as a surrogate for progression-free survival in advanced breast cancer in the BEECH trial. Ann Oncol. 2019;30:945–952. doi: 10.1093/annonc/mdz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.OʼLeary B, Hrebien S, Morden J P. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun. 2018;9:896. doi: 10.1038/s41467-018-03215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andre F, Su F, Solovieff N.Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials J Clin Oncol 202038(Suppl.)Abstr.. 1009. [DOI] [PubMed] [Google Scholar]
- 56.Bidard F C, Callens C, Dalenc F. Prognostic impact of ESR1 mutations in ER+ HER2- MBC patients prior treated with first line AI and palbociclib: An exploratory analysis of the PADA-1 trial. doi:10.1200/JCO.2020.38.15_suppl.1010 J Clin Oncol. 2020;38 (no. 15_suppl):1010. [Google Scholar]
- 57.Schrijver W, Suijkerbuijk K PM, van Gils C H. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. J Natl Cancer Inst. 2018;110:568–580. doi: 10.1093/jnci/djx273. [DOI] [PubMed] [Google Scholar]
- 58.Bidard F-C, Jacot W, Dureau S. Abstract GS3-07: Clinical utility of circulating tumor cell count as a tool to chose between first line hormone therapy and chemotherapy for ER+ HER2- metastatic breast cancer: Results of the phase III STIC CTC trial. Cancer Res. 2019 doi: 10.1158/1538-7445.SABCS18-GS3-07. [DOI] [Google Scholar]
- 59.Jacot W, Cottu P, Berger F. Actionability of HER2-amplified circulating tumor cells in HER2-negative metastatic breast cancer: the CirCe T-DM1 trial. Breast Cancer Res. 2019;21:121. doi: 10.1186/s13058-019-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner N, Kingston B, Kilburn L. Results from the plasmaMATCH trial: A multiple parallel cohort, multi-centre clinical trial of circulating tumour DNA testing to direct targeted therapies in patients with advanced breast cancer (CRUK/15/010). San Antonio Breast Cancer Symposium 2019; Abstr. GS3-06