Abstract
The treatment of patients with early breast cancer has always been characterised by escalation by new therapies and de-escalation through identification of better treatment regimens or introduction of better tools to estimate prognosis. Efforts in some of these areas in the last few years have led to solid data. The results of the large studies of de-escalation through use of multi-gene tests are available, as are the results of some studies that investigated the new anti-HER2 substances T-DM1 and pertuzumab in the early treatment situation. Several large-scale studies examining the role of CDK4/6 inhibitors will soon be concluded so innovations can be anticipated in this area also. This review article will summarise and classify the results of the latest publications.
Key words: early breast cancer, prevention, treatment, prognosis, immunotherapy, digital medicine
Introduction
The treatment of patients with early breast cancer has changed in recent years, especially of patients with HER2-positive tumours through the introduction of T-DM1 and pertuzumab. CDK4/6 inhibitors for HER2-negative, hormone receptor-positive tumours could also be added soon, though the patient population is unclear since one of the adjuvant studies announced a negative study outcome (PALLAS) according to the press release and another study announced a positive result, likewise by press release (MonarchE). Another study in this indication is still recruiting (NataLEE). Until further major changes are possibly implemented clinically in the (neo-)adjuvant situation, there have in the meantime been other interesting insights into the mode of action of existing therapies for many clinical scenarios. This review article will summarise the recent publications from international conferences.
Prevention
Use of knowledge of risk factors for prevention
Epidemiological studies and the recording of genetic and other risk factors are becoming increasingly detailed and comprehensive so that a relatively good estimate of the magnitude of the individual breast cancer risk can be made. One in eight women will develop breast cancer up to the age of 85 years. Even though mortality is decreasing because of improved early diagnosis and treatment, the incidence of breast cancer has not fallen but has even increased in Western industrialised countries. With all the scientific efforts of recent decades, the question arises of how the knowledge can be used actually to reduce the incidence of breast cancer (primary prevention).
Among the genetic risk factors, a distinction is currently made between high-penetrance, moderate-penetrance and low-penetrance genetic changes. Most high-penetrance and moderate-penetrance genes are already being investigated today in panel gene tests as part of predictive genetic diagnostics. In addition to BRCA1 and BRCA2, which are still the most important for planning individual prevention, other genes such as PALB2, CHEK2, ATM and others have been genotyped 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 . Since these gene changes are present very rarely in the general population, however, it is difficult to envisage that broad genotyping of these genes can contribute to a reduction in disease rates.
In addition to the high- and moderate-penetrance genes, further risk variants have been identified in over 150 genomic regions 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 . Although these low-penetrance risk variants occur relatively frequently in the population, they have only a slight effect individually on the individual breast cancer risk. All genetic risk variants together explain roughly 40% of the increased familial breast cancer risk.
Among the non-genetic risk factors, mammographic density has the greatest effect on breast cancer risk. High mammographic density (> 50%) is present in ca. 20% of women. These have an approximately three-fold increased incidence of breast cancer 27 , 28 . Similarly to some genetic changes, this risk is not the same for all molecular subtypes 29 , 30 . Mammographic density is also correlated with several genetic and non-genetic risk factors 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 . The few relevant protective factors include an early first childbirth, prolonged breast-feeding and possibly sports 37 .
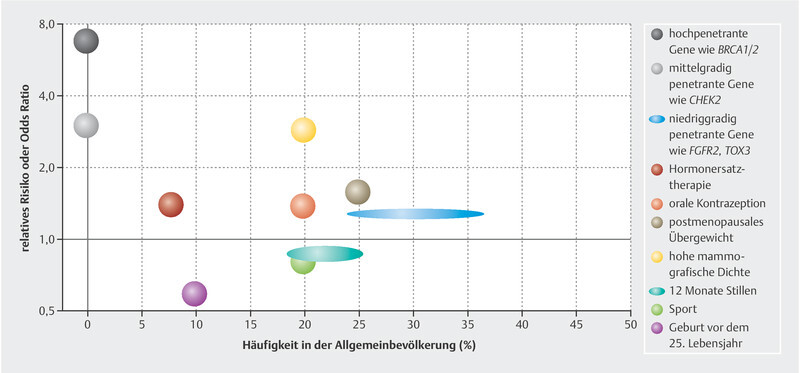
As mentioned above for genetic risk factors, the risk factors that have a large effect on disease risk occur rather rarely and the risk factors that have a small effect occur frequently in the population. This means that a marked increase in risk applies for only a few individuals in a population ( Fig. 1 ). There are several models that attempt to integrate the genetic and non-genetic risk factors in risk models that can better quantify the individual risk. However, these have not yet been integrated in studies or treatment concepts 34 , 36 , 38 , 39 , 40 .
Fig. 1.

Risk relationship and frequency of different factors that increase or decrease the risk of breast cancer.
Neoadjuvant Therapy
Monitoring of neoadjuvant therapy in patients with HER2-positive breast cancer
Patients with HER2-positive breast cancer are among the patients in whom pathological complete remission (pCR) correlates very strongly with a good prognosis after neoadjuvant therapy 41 , 42 , 43 . Against this background there is great interest in identifying patients early during neoadjuvant therapy in order to continue de-escalated therapy until surgery if appropriate. The recently reported PHERGAIN study was conducted in this context 44 . The study design is complex and shown in Fig. 2 . The HER2-positive patients received either a standard treatment with 6 cycles of taxane, platinum, trastuzumab and pertuzumab (TCHP) or therapy adjusted to the response, which was designed to examine whether a group of patients can be spared the chemotherapy and treatment with trastuzumab and pertuzumab alone suffices. This response was measured by a PET scan before and after 2 cycles of treatment. All patients initially received treatment with 2 chemotherapy-free cycles with pertuzumab and trastuzumab and continued this treatment until surgery if a treatment response was shown in the PET scan after 2 cycles. If no response was seen, these patients received 6 cycles of TCHP therapy until the operation. The results with regard to pCR rates in these arms are shown in Fig. 3 . Patients who had received treatment with 6 cycles of TCHP regardless of the assessment after 2 weeks achieved pCR in 57.7% of cases 44 . This corresponds roughly to real-world results from Germany (52.8% 45 ). If treatment was chemotherapy-free and consisted of trastuzumab and pertuzumab after a response following 2 cycles, pCR was recorded in 37.9% of cases. Among the patients who had started with chemotherapy-free treatment and then switched to TCPH after 2 cycles, pCR was seen in 25.9% of cases 44 . The pCR rate in the group who received 6 cycles of TCHP regardless of response and did not show any response in the PET after 2 cycles was only 10%. However, there were only 10 patients in this group.
Fig. 2.

PHERGAIN study design. C: Carboplatin; D: Docetaxel; EVC: Early breast cancer; ETx: Endocrine therapy (Letrozole post-menopausal/Tamoxifen pre-menopausal) Adjuvant ETx up to 3 years from surgery; PET: 18 F-fluorodeoxyglucose positron emission tomography/computed tomography; H: Trastuzumab SC; HER2: Human Epidermal Growth Factor Receptor 2; iDFS: Invasive disease-free survival; MRI: Magnetic resonance Imaging; P: Pertuzumab IV; R: Randomization; TCHIP: Trastuzumab, Pertuzumab, Docetaxel, and Carboplatin. † All hormonal receptor-positive patients will receive ETx concomitantly with PH (except on chemotherapy) PET responders: RECIST responders after cycle 2 with SUV max reduction ≥ 40%. pCR: Pathological complete response (ypT0/isN0). Modified after 44 .
Fig. 3.

Results of the PHERGAIN study with regard to one of the primary study aims. * These patients received TCHP. a Logistic regression model adjusted by hormonal status, based on the Wald test. PET: 18 F-fluorodeoxyglucose positron emission tomography/computed tomography; pCR: pathological complete response. Modified after 44 .
The clinical relevance of this study is apparent when the adverse effect rates are considered. The rate of grade 3 – 4 adverse events was 58.8% in the group of patients who had received 6 cycles of TCHP regardless of the PET assessment, and the rate was 3.1% in patients in the chemotherapy-free group 44 .
Even if the pCR rate in the patients in the chemotherapy-free treatment arm was ca. 20% below that of the patients who had received TCHP regardless of PET, the approach of the PHERGAIN study shows the way forward for planning future treatment concepts. The long-term prognosis of the different treatment arms remains to be seen. These results will be reported in the future.
Neoadjuvant platinum therapy instead of anthracycline therapy in the pertuzumab era
The BCRIG006/TRIO study showed that anthracycline can be replaced by carboplatin in the treatment of early HER2-positive breast cancer to avoid cardiac toxicity without risking lower effectiveness of the therapy 46 , 47 . Anthracyclines are still used frequently in the treatment of early HER2-positive breast cancer, however. The TRAIN-2 study has again addressed this question in a time when dual blockade with trastuzumab and pertuzumab is frequently employed in neoadjuvant anti-HER2 therapy when excellent pCR rates can also be achieved in the real-world setting 45 , 48 . The TRAIN-2 study randomised HER2-positive patients to treatment with 9 cycles of paclitaxel/trastuzumab/carboplatin/pertuzumab (PTCPtz) vs. treatment with 3 cycles of FEC, followed by 6 cycles of PTCPtz. The pCR rates in both randomisation arms were similarly high at 68 vs. 67% 49 . The 3-year survival rates have now been reported, which included 438 patients. The event-free survival did not differ between the randomisation arms. The hazard ratio was 0.9 (95% CI: 0.50 – 1.63) 50 . The results in patients with positive lymph node status are also noteworthy. They were 92.7% in the anthracycline-containing arm and 93.7% in the anthracycline-free treatment arm. As regards toxicity, a LVEF decrease below 50% or a LVEF decrease of at least 10% was seen in 36% of the patients who received anthracyclines and in only 22% of the patients who had been treated without anthracycline. This difference was statistically significant (p = 0.0016) 50 . These data therefore confirm that the replacement of anthracyclines by carboplatin even with the addition of pertuzumab can be justified in the context of avoiding cardiac toxicity.
Neoadjuvant CDK4/6 inhibitor therapy
The use of endocrine-based therapy in the neoadjuvant situation represents an alternative to chemotherapy for a certain patient population and is currently being investigated intensively in studies 51 , 52 , 53 . In particular, a neoadjuvant study can investigate how certain resistance mechanisms are overcome by CDK4/6 inhibitors. Data regarding abemaciclib were already available from the Neo-Monarch study that showed that abemaciclib leads to marked cell cycle arrest in the neoadjuvant setting 54 . In this connection, the FELINE study, which investigated neoadjuvant use of ribociclib, has now been reported 55 . Patients with primary HER2-negative, HR-positive breast cancer were randomised to 3 treatment arms, each lasting for 6 months:
Letrozole monotherapy,
Letrozole + continuous ribociclib,
Letrozole + intermittently paused ribociclib.
The primary study aim was the frequency of a PEPI score of zero (0) after the neoadjuvant therapy 56 . Interestingly, the frequency of patients with a PEPI score of 0 did not differ between the letrozole monotherapy and the CDK4/6 therapy arms. The percentage was 25.8% in the letrozole monotherapy arm and 25.4% in the two ribociclib arms together (p = 0.96). It was shown, however, that complete cell cycle arrest was attained in 91.9% of patients treated with ribociclib after 14 days of treatment, while this was detectable in only 51.7% of the patients on letrozole monotherapy (p < 0.0001). This difference was smaller by the time of surgery (71.4% after 6 months of therapy containing ribociclib and 61.3% after 6 months of letrozole monotherapy, p = 0.4225). The FELINE study thus delivers interesting insights into how cell cycle arrest behaves when endocrine monotherapy is compared with CDK4/6 inhibitor therapy + ET.
Locoregional Therapies
Surgery of the primary tumour as part of primary treatment even if M1 at initial diagnosis?
Roughly 6 – 10% of patients newly diagnosed with breast cancer already have distant metastases at the time of diagnosis. For these patients the question arises as to whether surgery of the local disease should be performed as part of the initial treatment. A few retrospective studies had implied this, but the analyses were not balanced. Patients who had had surgery were generally younger, had smaller tumours, and had more often had hormone receptor-positive disease and less advanced malignant disease 57 . Two prospective randomised studies yielded conflicting results 58 , 59 . In this connection the new E2108 study has been reported 57 . This study randomised 256 patients who had shown no progression with primary systemic therapy. 131 of these patients continued to receive the systemic therapy and 125 patients had surgery after initial systemic therapy. The overall survival, which was the primary study aim, did not differ between the two randomisation arms. The hazard ratio was 1.09 (90% CI: 0.80 – 1.49). There was no difference in progression-free survival either. With regard to locoregional recurrence, this occurred in 10.2% of cases in the surgery arm and locoregional recurrence or progression occurred in 5 and 20.6% of cases in the randomisation arms without surgery. However, this did not affect quality of life. The authors of the study concluded that when the disease is well controlled by systemic therapy, surgery could take place only when the local disease progresses.
Adjuvant Therapy
T-DM1 to avoid adjuvant chemotherapy?
The antibody-drug conjugate (ADC) T-DM1 is in clinical use both in patients with advanced HER2-positive breast cancer and in the post-neoadjuvant situation 60 , 61 . This naturally raised the question of whether T-DM1, possibly in combination with pertuzumab, could replace a therapy that includes conventional chemotherapy. KRISTINE/TRIO-021 is a study in this connection that has already been conducted in the neoadjuvant setting. In this neoadjuvant study, treatment with TCHP was compared to treatment with T-DM1 + pertuzumab. Treatment with TCHP led to a significantly higher pCR (56%) compared with 44% in the T-DM1 + pertuzumab arm 62 . With regard to the prognosis, more events were apparent in the T-DM1 + pertuzumab arm than in the TCHP arm, most probably due to preoperative progression 63 .
In this connection, the KAITLIN study has now investigated the combination of T-DM1 + pertuzumab in the adjuvant situation also 64 . The KAITLIN study compared treatment with AC-THP with treatment with T-DM1 + pertuzumab in a mainly node-positive HER2-positive population of primary breast cancer patients after surgery. 1846 patients were included in the study. The invasive recurrence-free survival (primary study aim) did not differ between the two treatments. The hazard ratio was 0.97 (95% CI: 0.71 – 1.32). It must be commented that the invasive recurrence-free 3-year survival was very good for the high-risk patients included in the study, at 94.1% in the AC-THB arm and 92.8% in the AC-TDM1/P arm. Grade 3/4 adverse effects were similar in the two arms. This rate was 55.4% in the AC-THP arm and 51.8% of the patients in the AC-TDM1/P arm suffered a grade 3/4 adverse effect. The authors concluded that treatment with AC-THP continues to be the standard treatment for patients with HER2-positive breast cancer.
Consolidated data from the MINDACT study for decision-making regarding adjuvant therapy
Besides the TailorX study, which investigated a 21-gene score with regard to decision-making in HER2-negative, hormone receptor-positive patients 65 , 66 , the MINDACT study is the second large study that addresses the question of whether and which patients in this population can be spared chemotherapy. The MINDACT study bases its genomic analyses on a 70-gene risk score 67 . The study design is shown in Figs. 4 and 5 shows a comparison of the TailorX and MINDACT study populations. The primary aim of the MINDACT study was reached 67 , which was defined that patients with a high clinical recurrence risk and who had not received any chemotherapy based on the genomic assessment should have a better metastasis-free 5-year survival than 92%. The metastasis-free 5-year survival at the time was 94.7% (95% CI: 92.5 – 96.2%) 67 . Long-term follow-up observations after 8.7 years have now been presented. The primary analysis was confirmed with the more mature data. The metastasis-free 5-year survival was 95.1% (95% CI: 93.1 – 96.6%) 68 .
Fig. 4.

MINDACT study design. HER2: Human Epidermal Growth Factor Receptor 2; HR: hormone receptor; TNBC: triple negative breast cancer. Modified after 68 .
Fig. 5.

Comparison of the TailorX and Mammaprint study populations. HER2: Human Epidermal Growth Factor Receptor 2; HR: hormone receptor; TN: triple negative. Modified after 68 .
Consideration of the four groups who were observed in the MINDACT study confirmed the results of the primary analysis ( Fig. 6 ). Patients who have a good prognosis according to both assessment methods (clinical and genomic) have an excellent prognosis without chemotherapy. Patients who have a poor prognosis as assessed clinically and genomically had a poor prognosis even despite chemotherapy. Both groups with a discordant estimate of prognosis had a similar prognosis; chemotherapy can therefore be avoided for this group.
Fig. 6.

Survival rates in the 4 groups of the MINDACT study. c: clinical; g: genomic; H: high; L: low. Modified after 68 .
Biomarkers
T-DM1 to avoid adjuvant chemotherapy?
The introduction of T-DM1 after neoadjuvant anti-HER2 therapy without pCR has signified a marked improvement in treatment results for these patients 61 . The study that delivered these results (KATHERINE) integrated a very comprehensive translational research programme prospectively in the study procedure. This offers the possibility of investigating the resistance mechanisms of different anti-HER2 therapies in order to identify patients who may react particularly well to adjuvant therapy with T-DM1 after a suboptimal response to neoadjuvant therapy. An analysis has now been presented that examined tumour samples before and after the neoadjuvant therapy. This focused on the PI3K signalling pathway and the gene expression profiles of the immune signalling pathways 69 . Whether PIK3CA mutations have an influence on the prognosis of the patients in the study was to be investigated. In the overall population, no influence was seen on invasive recurrence-free survival. The hazard ratios were similar in all groups ( Fig. 7 ) and were 0.48 (95% CI: 0.35 – 0.65) in patients with non-mutated tumours and 0.54 (95% CI: 0.32 – 0.90) in mutated patients 69 . Post-neoadjuvant therapy is therefore independent of the PIK3CA mutation status.
Fig. 7.

Effect of T-DM1 therapy in the KATHERINE study according to PIK3CA mutation status. CI: confidence interval; HR: hazard ratio; IDFS: invasive disease-free survival; T-DM1: trastuzumab emtansine 69 .
For the immunological analyses, samples were examined at the time of surgery with regard to the expression of HER2, PD-L1, CD8 and T cell effector molecules. None of these gene expression signatures was able to identify a group of patients in which T-DM1 had different effectiveness than in other subgroups.
It was interesting that high HER2 expression after neoadjuvant anti-HER2 therapy provided evidence for resistance for adjuvant trastuzumab. In patients with high HER2 expression after neoadjuvant therapy the risk for a recurrence event was twice as high compared with patients with low HER2 expression (hazard ratio: 2.02; 95% CI: 1.32 – 3.11). This was not the case in patients who had received adjuvant T-DM1 (hazard ratio: 1.01; 95% CI: 0.56 – 1.83) 69 .
Outlook
Even though there are few data at present regarding an improvement of the treatment of patients with triple-negative cancer, an attempt is now being made to translate the results of studies in the advanced therapy situation to the treatment of early breast cancer. The ADC sacituzumab govitecan, which has shown clear efficacy in metastatic TNBC 70 , is currently being integrated in a larger study of HER2-negative patients in the post-neoadjuvant setting (SASCIA study 71 ). The results of the large adjuvant CDK4/6 inhibitor studies will also be interesting as there is a high probability that these will change routine clinical practice in the near future.
Acknowledgements
This work was developed in part as a result of support from Pfizer and Hexal, as well as the PRAEGNANT network, which is supported by Pfizer, Hexal, Celgene, Daiichi-Sankyo, Merrimack, Eisai, Astra Zeneca and Novartis. None of the companies played a part in drafting this manuscript. The authors alone are responsible for the content of the manuscript.
Danksagung
Diese Arbeit entstand teilweise in Folge von Förderungen der Firmen Pfizer und Hexal sowie des PRAEGNANT-Netzwerks, das von den Firmen Pfizer, Hexal, Celgene, Daiichi-Sankyo, Merrimack, Eisai, Astra Zeneca und Novartis unterstützt wird. Keine der Firmen hatte einen Anteil bei der Verfassung dieses Manuskriptes. Für den Inhalt des Manuskriptes sind alleine die Autoren verantwortlich.
Footnotes
Conflict of Interest/Interessenkonflikt A. D. H. received speaker and consultancy honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi-Sankyo, Hexal and Pfizer. F. O. received speaker and consultancy honoraria from Amgen, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Chugai, Celgene, Cellex, Eisai, Gilead, Hexal, Ipsen, Janssen-Cilag, Merck, MSD, Novartis, Novonordisc, Riemser, Roche, Servier, Shire, Tesaro, TEVA. H.-C. K. received honoraria from Carl Zeiss meditec, Teva, Theraclion, Novartis, Amgen, AstraZeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, Roche and Genomic Health. P. A. F. received honoraria from Novartis, Pfizer, Roche, Amgen, Celgene, Daiichi Sankyo, AstraZeneca, Merck-Sharp & Dohme, Eisai, Puma and Teva. His institution conducts research with funding from Novartis and Biontech. H. T. received honoraria from Novartis, Roche, Celgene, Teva, Pfizer and travel support from Roche, Celgene and Pfizer. J. E. received honoraria from AstraZeneca, Roche, Celgene, Novartis, Lilly, Pfizer, Pierre Fabre, Teva and travel support from Celgene, Pfizer, Teva and Pierre Fabre. M. P. L. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Genomic Health and Roche and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Pfizer, Genomic Health, AstraZeneca, medac and Eisai. V. M. received speaker honoraria from Amgen, Astra Zeneca, Celgene, Daiichi Sankyo, Eisai, Pfizer, Novartis, Roche, Teva, Janssen-Cilag and consultancy honoraria from Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, Novartis, MSD, Daiichi Sankyo and Eisai, Lilly, Tesaro and Nektar. E. B. received honoraria from Novartis, Hexal and onkowissen.de for consulting, clinical research management or medical education activities. A. S. received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Zuckschwerdt Verlag GmbH, Georg Thieme Verlag, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH and promedicis GmbH. W. J. received honoraria and research grants from Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, Sanofi, Daichi, Tesaro. F. S. participated on advisory boards for Novartis, Lilly, Amgen and Roche and received honoraria for lectures from Roche, AstraZeneca, MSD, Novartis and Pfizer. A. W. participated on advisory boards for Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro, Eisai and received honoraria for lectures from Novartis, Pfizer, Aurikamed, Roche, Celgene. D. L. received honoraria from Amgen, AstraZeneca, Celgene, Lilly, Loreal, MSD, Novartis, Pfizer, Tesaro, Teva T. N. F. has participated on advisory boards for Amgen, Daiichi Sankyo, Novartis, Pfizer, and Roche and has received honoraria for lectures from Amgen, Celgene, Daiichi Sankyo, Roche, Novartis and Pfizer. M. T. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer, Genomic Health and Roche and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Pfizer, Genomic Health, and AstraZeneca M. W. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer and Roche. J. H. reports receiving speakers bureau honoraria from Celgene, Novartis, and Roche, and is a consultant/advisory board member for Amgen, Celgene, Novartis and Roche./ A. D. H. hat von AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi-Sankyo, Hexal und Pfizer Honorare für Referenten- und Beratungstätigkeiten erhalten. F. O. hat von Amgen, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Chugai, Celgene, Cellex, Eisai, Gilead, Hexal, Ipsen, Janssen-Cilag, Merck, MSD, Novartis, Novonordisc, Riemser, Roche, Servier, Shire, Tesaro und Teva Honorare für Referenten- und Beratungstätigkeiten erhalten. H.-C. K. hat von Carl Zeiss meditec, Teva, Theraclion, Novartis, Amgen, AstraZeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, Roche und Genomic Health Honorare erhalten. P. A. F. hat von Novartis, Pfizer, Roche, Amgen, Celgene, Daiichi-Sankyo, AstraZeneca, Merck-Sharp & Dohme, Eisai, Puma und Teva Honorare erhalten. Seine Institution betreibt Forschung mit finanzieller Unterstützung durch Novartis und Biontech. H. T. hat von Novartis, Roche, Celgene, Teva und Pfizer Honorare und von Roche, Celgene und Pfizer Reisebeihilfen erhalten. J. E. hat von AstraZeneca, Roche, Celgene, Novartis, Lilly, Pfizer, Pierre Fabre und Teva Honorare und von Celgene, Pfizer, Teva und Pierre Fabre Reisebeihilfen erhalten. M. P. L. hat für AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Genomic Health und Roche in Beratungsgremien mitgewirkt und von MSD, Lilly, Roche, Novartis, Pfizer, Genomic Health, AstraZeneca, medac und Eisai Vortragshonorare erhalten. V. M. hat von Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Pfizer, Novartis, Roche, Teva und Janssen-Cilag Honorare für Referententätigkeiten und von Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, Novartis, MSD, Daiichi-Sankyo und Eisai, Lilly, Tesaro und Nektar Beratungshonorare erhalten. E. B. hat von Novartis, Hexal und onkowissen.de für Beratungstätigkeiten, Leitung klinischer Forschung oder medizinische Schulungsaktivitäten Honorare erhalten. A. S. hat von Roche, Celgene, AstraZeneca, Novartis, Pfizer, Zuckschwerdt Verlag GmbH, Georg Thieme Verlag, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH und promedicis GmbH Honorare erhalten. W. J. hat von Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, Sanofi, Daichi und Tesaro Honorare und Forschungszuschüsse erhalten. F. S. hat für Novartis, Lilly, Amgen und Roche in Beratungsgremien mitgewirkt und von Roche, AstraZeneca, MSD, Novartis und Pfizer Vortragshonorare erhalten. A. W. hat für Novartis, Lilly, Amgen, Pfizer, Roche, Tesaro und Eisai in Beratungsgremien mitgewirkt und von Novartis, Pfizer, Aurikamed, Roche und Celgene Vortragshonorare erhalten. D. L. hat von Amgen, AstraZeneca, Celgene, Lilly, Loreal, MSD, Novartis, Pfizer, Tesaro und Teva Honorare erhalten. T. N. F. hat für Amgen, Daichi Sankyo, Novartis, Pfizer und Roche in Beratungsgremien mitgewirkt und von Amgen, Celgene, Daichi Sankyo, Roche, Novartis und Pfizer Vortragshonorare erhalten. M. T. hat für AstraZeneca, Lilly, MSD, Novartis, Pfizer, Genomic Health und Roche in Beratungsgremien mitgewirkt und von MSD, Lilly, Roche, Novartis, Pfizer, Genomic Health und AstraZeneca Vortragshonorare erhalten. M. W. hat für AstraZeneca, Lilly, MSD, Novartis, Pfizer und Roche in Beratungsgremien mitgewirkt. J. H. berichtet, von Celgene, Novartis, und Roche Honorare für Referententätigkeiten erhalten zu haben und ist Berater/Beiratsmitglied von Amgen, Celgene, Novartis und Roche.
References/Literatur
- 1.Ditsch N, Untch M, Thill M. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2019. Breast Care (Basel) 2019;14:224–245. doi: 10.1159/000501000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thill M, Jackisch C, Janni W. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2019. Breast Care (Basel) 2019;14:247–255. doi: 10.1159/000500999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welslau M, Hartkopf A D, Muller V. Update Breast Cancer 2019 Part 5 – Diagnostic and Therapeutic Challenges of New, Personalised Therapies in Patients with Advanced Breast Cancer. Geburtshilfe Frauenheilkd. 2019;79:1090–1099. doi: 10.1055/a-1001-9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schutz F, Fasching P A, Welslau M. Update Breast Cancer 2019 Part 4 – Diagnostic and Therapeutic Challenges of New, Personalised Therapies for Patients with Early Breast Cancer. Geburtshilfe Frauenheilkd. 2019;79:1079–1089. doi: 10.1055/a-1001-9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolberg H C, Schneeweiss A, Fehm T N. Update Breast Cancer 2019 Part 3 – Current Developments in Early Breast Cancer: Review and Critical Assessment by an International Expert Panel. Geburtshilfe Frauenheilkd. 2019;79:470–482. doi: 10.1055/a-0887-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wunderle M, Olmes G, Nabieva N. Risk, Prediction and Prevention of Hereditary Breast Cancer – Large-Scale Genomic Studies in Times of Big and Smart Data. Geburtshilfe Frauenheilkd. 2018;78:481–492. doi: 10.1055/a-0603-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimelis H, LaDuca H, Hu C. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J Natl Cancer Inst. 2018 doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couch F J, Shimelis H, Hu C. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol. 2017;3:1190–1196. doi: 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fachal L, Aschard H, Beesley J. Fine-mapping of 150 breast cancer risk regions identifies 191 likely target genes. Nat Genet. 2020 doi: 10.1038/s41588-019-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L, Shi W, Long J. A transcriptome-wide association study of 229,000 women identifies new candidate susceptibility genes for breast cancer. Nat Genet. 2018 doi: 10.1038/s41588-018-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milne R L, Kuchenbaecker K B, Michailidou K. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat Genet. 2017;49:1767–1778. doi: 10.1038/ng.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michailidou K, Lindstrom S, Dennis J. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day F R, Thompson D J, Helgason H. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49:834–841. doi: 10.1038/ng.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michailidou K, Beesley J, Lindstrom S. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47:373–380. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day F R, Ruth K S, Thompson D J. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47:1294–1303. doi: 10.1038/ng.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pharoah P D, Tsai Y Y, Ramus S J.GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer Nat Genet 201345362–370.370e1–370e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michailidou K, Hall P, Gonzalez-Neira A.Large-scale genotyping identifies 41 new loci associated with breast cancer risk Nat Genet 201345353–361.361e1–361e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Closas M, Couch F J, Lindstrom S.Genome-wide association studies identify four ER negative-specific breast cancer risk loci Nat Genet 201345392–398.398e1–398e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bojesen S E, Pooley K A, Johnatty S E.Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer Nat Genet 201345371–384.384e1–384e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghoussaini M, Fletcher O, Michailidou K. Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet. 2012;44:312–318. doi: 10.1038/ng.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haiman C A, Chen G K, Vachon C M. A common variant at the TERT-CLPTM1 L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011;43:1210–1214. doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antoniou A C, Wang X, Fredericksen Z S. A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet. 2010;42:885–892. doi: 10.1038/ng.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghoussaini M, French J D, Michailidou K. Evidence that the 5p12 Variant rs10941679 Confers Susceptibility to Estrogen-Receptor-Positive Breast Cancer through FGF10 and MRPS30 Regulation. Am J Hum Genet. 2016;99:903–911. doi: 10.1016/j.ajhg.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couch F J, Kuchenbaecker K B, Michailidou K. Identification of four novel susceptibility loci for oestrogen receptor negative breast cancer. Nat Commun. 2016;7:11375. doi: 10.1038/ncomms11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purrington K S, Slager S, Eccles D. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2014;35:1012–1019. doi: 10.1093/carcin/bgt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens K N, Fredericksen Z, Vachon C M. 19p13.1 is a triple-negative-specific breast cancer susceptibility locus. Cancer Res. 2012;72:1795–1803. doi: 10.1158/0008-5472.CAN-11-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd N F, Guo H, Martin L J. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 28.Heusinger K, Loehberg C R, Haeberle L. Mammographic density as a risk factor for breast cancer in a German case-control study. Eur J Cancer Prev. 2011;20:1–8. doi: 10.1097/CEJ.0b013e328341e2ce. [DOI] [PubMed] [Google Scholar]
- 29.Heusinger K, Jud S M, Haberle L. Association of mammographic density with the proliferation marker Ki-67 in a cohort of patients with invasive breast cancer. Breast Cancer Res Treat. 2012;135:885–892. doi: 10.1007/s10549-012-2221-3. [DOI] [PubMed] [Google Scholar]
- 30.Heusinger K, Jud S M, Haberle L. Association of mammographic density with hormone receptors in invasive breast cancers: results from a case-only study. Int J Cancer. 2012;131:2643–2649. doi: 10.1002/ijc.27515. [DOI] [PubMed] [Google Scholar]
- 31.Bayer C M, Beckmann M W, Fasching P A. Updates on the role of receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand/osteoprotegerin pathway in breast cancer risk and treatment. Curr Opin Obstet Gynecol. 2017;29:4–11. doi: 10.1097/GCO.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 32.Hack C C, Stoll M J, Jud S M. Correlation of mammographic density and serum calcium levels in patients with primary breast cancer. Cancer Med. 2017;6:1473–1481. doi: 10.1002/cam4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph A, Fasching P A, Behrens S. A comprehensive evaluation of interaction between genetic variants and use of menopausal hormone therapy on mammographic density. Breast Cancer Res. 2015;17:110. doi: 10.1186/s13058-015-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vachon C M, Pankratz V S, Scott C G. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015;107:dju397. doi: 10.1093/jnci/dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vachon C M, Scott C G, Fasching P A. Common breast cancer susceptibility variants in LSP1 and RAD51L1 are associated with mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:1156–1166. doi: 10.1158/1055-9965.EPI-12-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vachon C M, Scott C G, Tamimi R M. Joint association of mammographic density adjusted for age and body mass index and polygenic risk score with breast cancer risk. Breast Cancer Res. 2019;21:68. doi: 10.1186/s13058-019-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Britt K L, Cuzick J, Phillips K A. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020 doi: 10.1038/s41568-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 38.Kapoor P M, Mavaddat N, Choudhury P P. Combined associations of a polygenic risk score and classical risk factors with breast cancer risk. J Natl Cancer Inst. 2020 doi: 10.1093/jnci/djaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavaddat N, Michailidou K, Dennis J. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am J Hum Genet. 2018 doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mavaddat N, Pharoah P DP, Michailidou K. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107:djv036. doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortazar P, Zhang L, Untch M. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 42.von Minckwitz G, Untch M, Blohmer J U. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 43.Fasching P A, Heusinger K, Haeberle L. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortes J, Gebhart G, Borrego M R. Chemotherapy (CT) de-escalation using an FDG-PET/CT (F-PET) and pathological response-adapted strategy in HER2[+] early breast cancer (EBC): PHERGain Trial. J Clin Oncol. 2020;38:503–503. [Google Scholar]
- 45.Fasching P A, Hartkopf A D, Gass P. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat. 2018 doi: 10.1007/s10549-018-5008-3. [DOI] [PubMed] [Google Scholar]
- 46.Slamon D, Eiermann W, Robert N. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slamon D J, Eiermann W, Robert N J. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC -> T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC -> TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+early breast cancer. Cancer Res. 2016 doi: 10.1158/1538-7445.SABCS1115-S1155-1104. [DOI] [Google Scholar]
- 48.Gianni L, Pienkowski T, Im Y H. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 49.van Ramshorst M S, van der Voort A, van Werkhoven E D. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1630–1640. doi: 10.1016/S1470-2045(18)30570-9. [DOI] [PubMed] [Google Scholar]
- 50.van der Voort A, van Ramshorst M S, van Werkhoven E. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2-blockade for HER2-positive breast cancer (TRAIN-2): A randomized phase III trial. J Clin Oncol. 2020;38:501–501. [Google Scholar]
- 51.Fasching P A, Jud S M, Hauschild M. FemZone trial: a randomized phase II trial comparing neoadjuvant letrozole and zoledronic acid with letrozole in primary breast cancer patients. BMC Cancer. 2014;14:66. doi: 10.1186/1471-2407-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fasching P A, Abad M F, Garcia-Saenz J A.Biological and clinical effects of abemaciclib in a phase 2 neoadjuvant study for postmenopausal patients with HR+/HER2-breast cancer Oncol Res Treat 201740225–226.28365697 [Google Scholar]
- 53.Mayer I A, Prat A, Egle D. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB) Clin Cancer Res. 2019;25:2975–2987. doi: 10.1158/1078-0432.CCR-18-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurvitz S A, Martin M, Press M F. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR(+)/HER2(−) Breast Cancer. Clin Cancer Res. 2020;26:566–580. doi: 10.1158/1078-0432.CCR-19-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan Q J, OʼDea A, Bardia A. Letrozole + ribociclib versus letrozole + placebo as neoadjuvant therapy for ER+ breast cancer (FELINE trial) J Clin Oncol. 2020;38:505–505. [Google Scholar]
- 56.Ellis M J, Tao Y, Luo J. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan S A, Zhao F, Solin L J. A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: A trial of the ECOG-ACRIN Research Group (E2108) J Clin Oncol. 2020;38:LBA2. [Google Scholar]
- 58.Soran A, Ozmen V, Ozbas S. Randomized Trial Comparing Resection of Primary Tumor with No Surgery in Stage IV Breast Cancer at Presentation: Protocol MF07-01. Ann Surg Oncol. 2018;25:3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
- 59.Badwe R, Hawaldar R, Nair N. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16:1380–1388. doi: 10.1016/S1470-2045(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 60.Verma S, Miles D, Gianni L. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Minckwitz G, Huang C S, Mano M S. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 62.Hurvitz S A, Martin M, Symmans W F. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–126. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 63.Hurvitz S A, Martin M, Jung K H. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. doi:10.1200/JCO.19.00882. J Clin Oncol. 2019;37:2206–2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harbeck N, Im S-A, Barrios C H. Primary analysis of KAITLIN: A phase III study of trastuzumab emtansine (T-DM1) + pertuzumab versus trastuzumab + pertuzumab + taxane, after anthracyclines as adjuvant therapy for high-risk HER2-positive early breast cancer (EBC) J Clin Oncol. 2020;38:500–500. [Google Scholar]
- 65.Sparano J A, Gray R J, Makower D F. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sparano J A, Gray R J, Makower D F. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cardoso F, vanʼt Veer L J, Bogaerts J. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 68.Cardoso F, vanʼt Veer L, Poncet C. MINDACT: Long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. J Clin Oncol. 2020;38:506–506. [Google Scholar]
- 69.Denkert C, Lambertini C, Fasching P A. Biomarker data from KATHERINE: A phase III study of adjuvant trastuzumab emtansine (T-DM1) versus trastuzumab (H) in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer. J Clin Oncol. 2020;38:502–502. [Google Scholar]
- 70.Bardia A, Mayer I A, Vahdat L T. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380:741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 71.Marme F.Phase-III-Studie zur postneoadjuvanten Behandlung mit dem Antikörper-Medikamenten-Konjugat Sacituzumab-Govitecan bei Frauen mit frühem, HER2-negativem Brustkrebs und hohem Rückfallrisiko nach einer Standardbehandlung im neoadjuvantenSetting – SASCIA 2020. Online (Stand: 16.07.2020):https://www.gbg.de/wAssets/docs/events-vortraege/2020_JT/23_SASCIA.pdf