Abstract
Post-translational modifications (PTMs) of β-amyloid (Aβ) peptides are considered as triggering factors in sporadic Alzheimer’s diseases. However, studies to show the influences of pre-existed PTM-Aβ fibrils on wild-type Aβ peptides, which directly mimic the triggering scenarios, are rare. Here we show that three types of pathologically relevant PTM-Aβ variants with modifications in a similar segment (from D7 to V12) of the primary sequence lead to distinct impacts on the fibrillization of wild-type Aβ peptides. In general, the triggering effects are observed through cross-seeding between the PTM-Aβ seeds and wild-type peptides, which consequently induce modulations in the resultant wild-type fibril structures and elevations in the fibrillar cytotoxicity levels. Modifications with the similar chemical nature, such as the S8-phosphorylation and Y10-nitration, which both introduce additional side-chain negative charges, show comparable structural-modulation and cytotoxicity-elevation effects. The results imply the biological influences of PTM-Aβ variants on the formation of amyloid deposits through cross-seeded fibrillization.
Keywords: β-amyloid fibrils, post-translational modifications, structural polymorphisms, cytotoxicity, cross-seeded fibrillization
Graphical Abstract

Exocellular amyloid depositions with mainly fibrillar aggregates characterize the Alzheimer’s diseases (AD).1 The wild-type β-amyloid (wt-Aβ) peptides, along with multiple types of post-translational modified Aβ (PTM-Aβ) variants, are the main compositions in human AD plaques.2–5 Previous works showed that PTM-Aβ variants led to accelerated fibrillization with resultant aggregates that were more stable and/or more neurotoxic.6–15 Amyloid deposits with PTM-Aβ variants were identified in pathological plaques and vascular amyloids, and in many cases, co-localized with wt-Aβ alloforms.6,9,16–19 However, a fundamental question remains: how the presence of PTM-Aβ variants might influence the amyloidosis of wt-Aβ? Certain variant subtypes were shown to form amyloid aggregates at early stages of AD,8,9,20–22 and thus were proposed as triggering species that lead to accelerated amyloid deposition of wt-Aβs, especially in the sporadic AD.5 However, only few experimental evidences exist to explain the underlying molecular basis for such triggering effects. For instances, we and other groups showed that the cross-seeding between the S8-phosphorylated 40-residue Aβ (pS8-Aβ40) and the wt-Aβ40 was more efficient comparing to the self-seeding of wt-Aβ40.8,14 Another work on the E3-pyroglutamate Aβ40 also observed accelerated fibrillization when the variant seeds was added to wt-Aβ40 peptides, confirming the cross-seeding effect.23 However, more questions remain to be answered. For instances, whether the cross-seeding processes with different PTM-Aβ subtypes all have similar triggering effects to the fibrillization of wt-Aβ? What would be the biologically relevant consequences of the cross-seeding processes and how would they be related to the chemical nature of modifications?
Understanding of the influences of PTMs on the molecular structures of Aβ aggregates will help to answer these questions. This work focuses on three types of N-terminal PTM-Aβ variants: the pS8-Aβ40, the Y10-nitrated Aβ40 (nY10-Aβ40) and the Asp7-isomerized Aβ40 (isoD7-Aβ40). Pathologically, all these variants have been identified in human AD plaques and correlated to early-stage amyloid depositions.8,9,21 As shown in Figure 1, chemically, all three variants contain side chain modifications that are localized within a segment from D7 to V12. A previous report showed that this segment could manipulate the transition from oligomer to protofibril and the architectures of Aβ fibrils.24 Both pS8- and nY10-Aβ40 variants possess additional negative charges and decreased overall hydrophobicity (native-PAGE, Figure 1), while the isomerization at D7 does not have such effects. Most reported Aβ40 fibrillar molecular structures, including both Aβ40 and Aβ42 fibrils, showed that the first 10 N-terminal residues were disordered 25–27 In the two structural models with the entire Aβ40 sequence, the side chains of N-terminal residues interact with the loop region from E22 to G29.15,28 Interestingly, the side chains of S8 and Y10 show the same orientation, which are opposite to the side chain of D7, in both structures (Figure 1). A systematic characterization of these three variants tend to answer the questions about the similarities and specificities in the cross-seeding effects in individual PTM-Aβ40 subtypes with chemical natures of modifications. This work also focuses on the 40-residue alloform because only the Aβ40 fibrillar structural polymorphisms have been shown to correlate with clinical histories of AD patients.12,29,30
Figure 1.
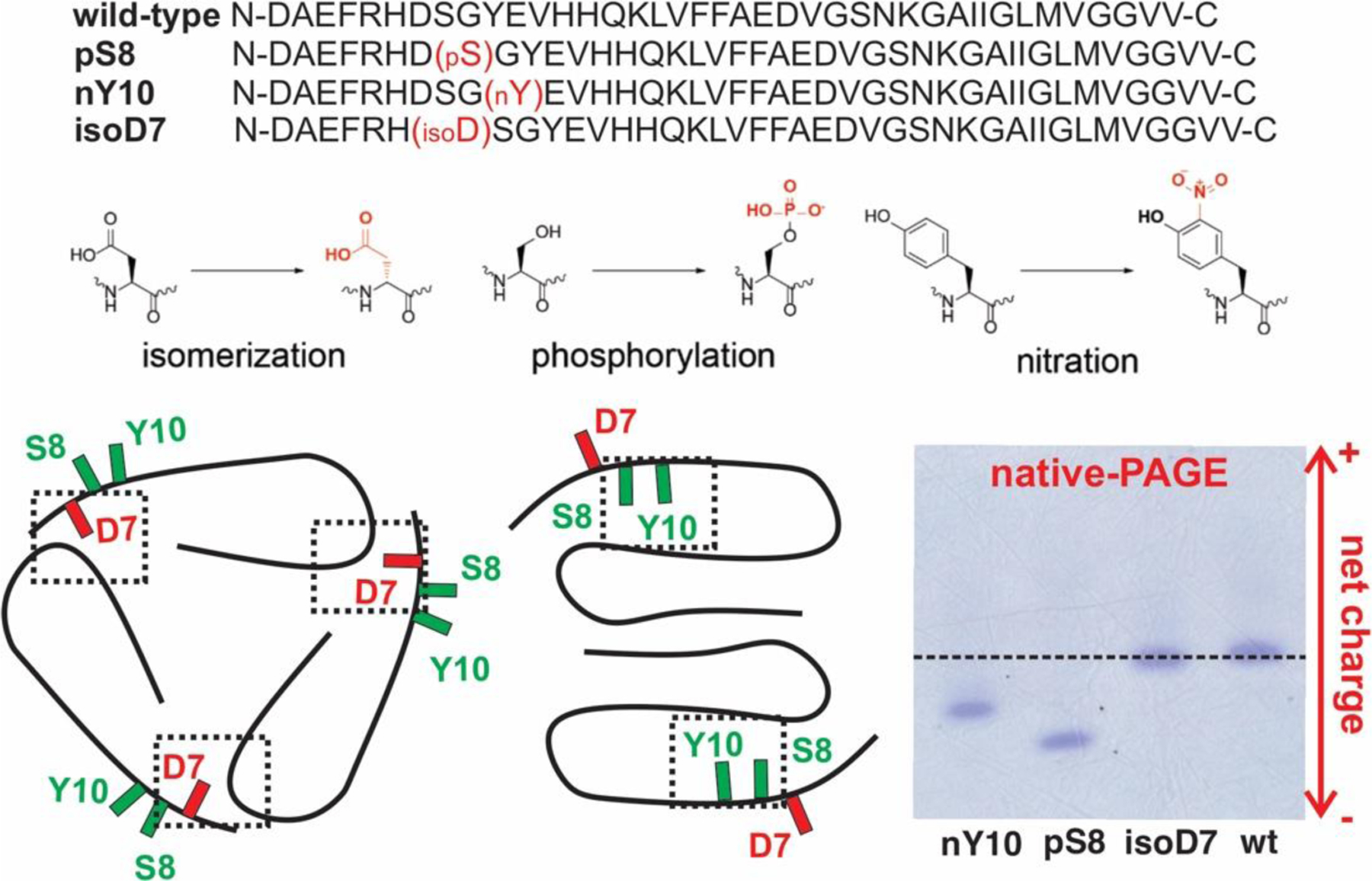
(Top) Sequences and the chemical modifications of the wt-, isoD7-, pS8-, and nY10-Aβ40 peptides. (Bottom Left) Sketches of reported Aβ40 fibril backbone structures with N-termini. The side chain orientations of D7 and S8/Y10 are highlighted in red and green respectively. The dashed rectangles show the inter- or intra-molecular packing that stabilize the fibril structures. (Bottom Right) Native-PAGE for the wt- and PTM-Aβ40 variants peptides.
The fibrilization kinetics, monitored by the thioflavin-T (ThT) fluorescence assay, for both nucleation-driven and seeded processes with the three PTM-Aβ40 variants, were plotted in Figure 2 (Supporting Information (SI) Figure S1 for wt-Aβ40). The nucleation-driven fibrillization was initiated from monomeric PTM and wt-Aβ40 peptides, which resulted in “parent fibrils”. The seeded fibrillization added the parent fibrillar seeds to fresh monomeric wt-Aβ40 peptides. Quantitative analyses were done by fitting individual curves to the sigmoidal function for nucleation-driven processes to determine the lag periods (tlag, defined as t1/2 – 2/k1) and the apparent ThT buildup rates (k1), shown in Figure 2D, and to fit the buildup rates of seeded fibrillization curves to the seeds’ concentrations linearly to obtain the elongation rate constants (kon), shown in Figure 2H. For the nucleation-driven processes, only nY10-Aβ40 showed significantly shortened tlag and faster k1 values comparing to wt-Aβ40. For the seeded fibrillization, all three variants possessed increased kon values comparing to the wt-Aβ40 peptides (kon,wt = 0.0451 hour−1μM−1), however, with different extents. Both pS8- and nY10-Aβ40 seeds induced ~ 10-fold increments (kon,pS8 = 0.3805 hour−1μM−1, kon,nY10 = 0.3905 hour−1μM−1) while isoD7-Aβ40 led to only moderate increments (kon,pS8 = 0.0804 hour−1μM−1). These results indicated that pS8- and nY10-Aβ40 could serve as more efficient seeds to trigger the fibrillization of wt-Aβ40 peptides through cross seeding processes. Thus, even the presence of small populations of these two variants would lead to rapid amyloid depositions of wt-Aβ40.
Figure 2.

(A–C) The ThT fluorescence kinetics curves for the nucleation-driven fibrillization of isoD7-Aβ40 (panel A), pS8-Aβ40 (panel B) and nY10-Aβ40 (panel C). Five repetitions were done for each sample. (D) Plots of the lag periods (left y-axis) and the ThT buildup rates (right y-axis) for the nucleation-driven fibrillization of different Aβ40 subtypes. Error bars represent the standard deviations from five independent measurements. (E–G) The ThT fluorescence kinetics curves for seeded fibrillization with seeds from isoD7-Aβ40 (panel E), pS8-Aβ40 (panel F) and nY10-Aβ40 (panel G). For all measurements, the concentration of monomeric wild-type Aβ40 was kept at 50 μM. Seeds with 1 mol%, 2.5 mol%, 5 mol% and 10 mol% were color-coded in purple, blue, red and black respectively. The brown curves contained no seeds as controls. Four repetitions were done for each sample condition. (H) plots of the linear relationship between the ThT buildup rates and the concentrations of seeds. The errors bar for individual data point represented the standard deviations from the four measurements shown in (E)–(G). Color-coding: black, self-seeding with wt; red, isoD7 cross-seeded; blue, pS8-cross-seeded; purple, nY10 cross-seeded.
Next, we investigated whether and to what extent the cross-seeding would modulate the molecular structures of resultant fibrils. Molecular level structural propagation was demonstrated by solid-state nuclear magnetic resonance (ssNMR) spectroscopy for self-seeding fibrillization in a previous study,31 but not for cross-seeding process. We showed that pS8-Aβ40 and wt-Aβ40 fibrils had different structures in terms of their extensions of parallel-β-sheets to the N-termini.15 Here we mapped the residue-specific inter-strand distances in all four parent fibrils using 13C-PITHIRDs-CT spectroscopy.32 Fast decays of 13C signals that reach minima at ~ 30 ms dipolar evolution times indicated parallel-β-sheet structures. Figure 3 showed that the residues in typical strand segments (e.g. L17-A21 and A30-V36, Figure 3A) of Aβ40 fibrils were in parallel β-sheets (Figure 3B, G33; Figure 3C, A21) and residues at the very N-termini were in non-parallel structures (Figure 3D, F4), as expected. But the inter-strand distances at residues G9 and V12, which were in the segments with modifications, showed diversities. V12 was in parallel β-sheets in pS8- and nY10-Aβ40 fibrils, but in non-parallel structures in isoD7- and wt-Aβ40 fibrils (Figure 3E). G9 was non-parallel in all fibrils, but the averaged inter-strand distances in pS8- and nY10-Aβ40 fibrils were shorter (e.g. faster decay curves) than in isoD7- and wt-Aβ40 fibrils (Figure 3F). Overall, the PITHIRDs results indicated the pS8-/nY10-Aβ40 parent fibrils adopted more extended fibril cores towards the N-termini comparing to the isoD7/wt-Aβ40 fibrils.
Figure 3.

(A) The cartoon model shows the typical core segments with parallel β-sheets (red and blue), the segment with species-specific structure (green) and the disordered segment (cyan). All labeled residues are given as purple circles. (B–F) 13C-PITHIRDs-CT dephasing curves for selectively isotope labeled residues (G33, panel B; A21, panel C; A2, panel D; V12, panel E; G9, panel F) in parent fibrils: wt-Aβ40 (black), isoD7-Aβ40 (red), pS8-Aβ40 (blue), and nY10-Aβ40 (purple). Faster decay (with maximum decay at ~ 30 ms) indicates parallel β-sheet with ~ 5Å inter-strand distance. (G and H) The 13C-PITHIRDs-CT dephasing curves for residue V12 (panel G) and G9 (panel H) in fibrils seeded from wt-Aβ40 (black), isoD7-Aβ40 (red), pS8-Aβ40 (blue) and nY10-Aβ40 (purple). In panel (B)-(H), the error bars represent the spectral noises instead of statistical uncertainties.
The structural similarities and diversities in seeded wt-Aβ40 fibrils were then explored by applying 13C-PITHIRDs-CT and two-dimensional (2D) 13C-13C spin diffusion spectroscopy. The PITHIRDs on V12 and G9 demonstrated the ability of cross-seeding to modulate the structures of wt-Aβ40 fibrils, especially to alter the extensions of fibril cores. At V12 (Figure 3G), the pS8-/nY10-seeded fibrils showed parallel and the isoD7-/wt-seeded ones showed non-parallel, agreeing well with their corresponding parent seeds shown in Figure 3E. At G9 (Figure 3H), the isoD7-/wt-seeded fibrils were non-parallel, the same as their parent seeds (Figure 3F). The pS8-/nY10-seeded fibrils adopted parallel β-sheets, while their seeds were in non-parallel. We previously reported that the residue G9 in pS8-Aβ40 fibrils was at the edge of parallel β-sheets.15 Thus, there might be a mixture of parallel and non-parallel inter-strand distances at this site and the parallel structure was selected through seeded fibril growth. Such “structural selection effect” was demonstrated for the self-seeded fibrillization of wt-Aβ40.33,34
The 2D spin diffusion spectroscopy further showed structural differences and similarities between fibrils seeded from wild-type or variants’ Aβ40 parent fibrils. Spectra with short-mixing time (i.e. 20 ms, SI Figure S2–3) indicated that there was not significant 13C chemical shift deviations for selected residues located in the typical β-strands (L17-A21 and A30-V36) and loop regions (E22- G29) of Aβ40 fibrils, including F19, F20, A21, G25, S26, A30, I32 and L34 (see Table S1 for 13C chemical shifts). Figure 4 plotted the 13C chemical shift deviations between pS8-/nY10-/isoD7-Aβ40-seeded fibrils and the wt-Aβ40-seeded fibril to confirm the similarity in secondary structures. No chemical shift differences were observed between isoD7- and wt-seeded fibrils. Only moderate deviations were seen between pS8-/nY10- and wt-seeded fibrils, which were on residues in the typical N-terminal β-strand and loop regions, but not the C-terminal β-strand segment. Additionally, the bulk morphologies of different parent fibrils and their seeded fibrils were also highly similar, confirmed by the negatively stained transmission electron microscopy (TEM, SI Figure S4).
Figure 4.

Plots of the 13C chemical shift differences between the nY10-seeded (top panel)/pS8-seeded (middle panel)/isoD7-seeded (bottom panel) Aβ40 and the self-seeded Aβ40 fibrils. For each residue, the chemical shift differences at C’, Cα, Cβ are considered and plotted from left to right. The error bars represent the line widths of peaks instead of statistical uncertainties. Rectangles and the arrows on top of the plots highlight the residues with significant chemical shift deviations.
Spectra with long-mixing time (i.e. 500 ms) confirmed similarities between different seeded fibrils in terms of the sidechain packing in the core segments, but some differences in the N-terminal regions. As shown in Figure 5A, inter-residues cross peaks were observed between the aromatic 13Cs of F19 and A21-Cβ/I32-Cγ1/Cδ for in all seeded fibrils, indicating their structural similarities at the molecular level. Interestingly, distinct spectral features were observed for residues closer to the N-termini, e.g. E3, G9 and V12. Spectral intensities (shown in Figure 5B) corresponding to the inter-residue interactions between G9-C’ and V12-Cα/Cγ, and between E3-Cδ and S26-Cα were seen in the pS8-/nY10-seeded fibrils, but not in the isoD7-/wt-seeded fibrils. In addition, the intra-residue cross peaks for the sidechains of E3 were more defined in the pS8-/nY10-seeded fibrils. These evidences supported that the fibrils seeded from pS8- and nY10-Aβ40 possessed a more ordered N-termini, consistent with their more extended N-terminal β-sheets determined by PITHIRDs-CT spectroscopy.
Figure 5.

The 2D 13C-13C spin-diffusion spectra with 500 ms mixing time. (A) Samples labeled at residues F19, A21, I32 and L34 for wt-, isoD7-, pS8- and nY10-Aβ40-seeded fibrils from top to bottom. The red and blue lines highlight the 1D slides (on the right side) with F19/I32 inter-residue cross peaks (green arrows in both 1D and 2D spectra). The orange and purple rectangles show the F19/A21 and I32/L34 cross peaks respectively. (B) Samples labeled at residues E3, G9, V12, F20, A21, G25 and S26 for wt-, isoD7-, pS8- and nY10-Aβ40-seeded fibrils from top to bottom. The red lines and 1D slides show the intra-residue C’/Cα cross peaks for G9. The green lines and 1D slides show the inter-residue G9/V12 cross peaks in the pS8-/nY10-Aβ40-seeded fibrils (black arrows in the bottom two 1D slides), but not in wt-/isoD7-Aβ40-seeded fibrils. The blue lines and 1D slides highlight the absence and presence of E3/S26 inter-residue cross peaks in pS8-/nY10-Aβ40-seeded (bottom two slides) and wt-/isoD7-Aβ40-seeded (top two slides) fibrils respectively. The purple rectangles show the intra-residue cross peaks from the side chains of E3.
Overall, the ssNMR results indicated that the three PTM variants affected mainly the molecular structures of the N-terminal segments of Aβ40 fibrils. Different extensions of fibril cores in these parent fibrils propagated to the seeded wt-Aβ40 fibrils, which altered the molecular structures of the seeded wild-type fibrils. Biologically, the combination of ThT kinetics and ssNMR data implied that the triggering effect of pS8-/nY10-Aβ40 variants would not only accelerate the fibrillar aggregation of wt-Aβ40 peptides, but also modulated the molecular polymorphisms of the resultant amyloid deposits.
The N-terminal modifications of Aβ were known to alter the toxicity levels of resulted variants’ fibrils. However, whether the cross-seeding processes would lead to different cytotoxicity levels for wt-Aβ fibrils remain unknown. Here we utilize the MTT cell proliferation assay and the model neuroblastoma Neuro2a (N2a) cells to explore the cytotoxicity associated with seeded fibrillization processes. There have been reports on the effects of Aβ peptides on the MTT reduction and the potential introduction of artifacts in this assay. Therefore, we kept a constant seed concentration and seed-to-monomer ratios and only focus on the relative differences between individual seeding systems and time points.35 The experimental groups contained a mixture of seeds and monomers with 1:20 (Figure 6) and 1:10 (SI Figure S5) molar ratios, which were added externally to cell cultures. The controls contained only wt-Aβ40 monomers or only different types of Aβ40 fibrillar seeds. The time-dependent cell viabilities were recorded.
Figure 6.

(A) Cell viabilities at 48-hour incubation for monomeric wt-Aβ40 peptides (brown), wt-Aβ40 seeds w. and w/o monomers (black), isoD7-Aβ40 seeds w. and w/o monomers (red), pS8-Aβ40 seeds w. and w/o monomers (blue), nY10-Aβ40 seeds w. and w/o monomers (purple). Error bars represent the standard deviations from five independent measurements. The p values were shown to highlight the pairs with statistically significant differences: **, p < 0.01; ***, p < 0.001. (B) The time-dependent cell viabilities for seeds (colored dashed lines) and seeds + monomers (colored solid lines) for wt-Aβ40 (top left), isoD7-Aβ40 (top right), pS8-Aβ40 (bottom left), nY10-Aβ40 (bottom right). The same colors are used as in panel (A) for different Aβ40 subtypes. The brown lines are added for monomeric peptides as negative controls.
At long incubation time (e.g. 48 hours, Figure 6A), all seeds showed comparable cytotoxicity levels while monomers were non-toxic. The mixtures of seeds and monomers exhibited further elevated cytotoxicity levels, which could be attributed to the aggregates induced by seeding processes. The elevations of cytotoxicity levels were distinct for different seeding systems, following the trend that nY10- > pS8- > isoD7 ≈ wt-Aβ40. The time-dependence of cytotoxicity levels were shown in Figure 5B for the monomers, the seeds and the mixtures. The monomers were non-toxic over the entire incubation period. The presence of seeds, although toxic to cells, did not possess significant time-dependent changes. As a comparison, the cytotoxicity levels for the mixtures increased as a function of incubation time, confirming that the dynamic seeded fibrillization process introduced elevated toxicities. Distinct time-dependences were noticed between isoD7-/wt-seeded and pS8-/nY10-seeded systems. No obvious elevation of cytotoxicity levels was observed in the former two systems within 24 hours and ~ 15% elevations were seen between 24 and 48 hours. In the latter two systems, however, ~10% elevations were detected within 24 hours, which was further increased to ~40% in 48 hours. Overall, these results indicated that the cross-seeding processes with nY10-/pS8-Aβ40 fibrillar seeds led to more rapidly elevated cytotoxicity levels than the ones with isoD7/wt-Aβ40 seeds, which had the same species-dependent trend as their seeding efficiencies to the wt-Aβ40 peptides determined by ThT fluorescence assays. Pathologically, it has been shown that mature fibrils are less toxic comparing to either the non-fibrillar intermediate states or the fibrillization process1 However, molecular dynamic simulations have shown that fibrils may serve as surfaces with catalytic functions to accelerate the formation of more toxic non-fibrillar aggregates.36–38 Our results here suggest that the cytotoxicity of fibrils could be dynamically modulated through cross-seeded fibrillization processes with PTM-Aβ variants. Even if the modifications occur after the formation of fibrils (i.e. on mature fibrils rather than monomeric peptides), the re-dissolving of plaques will still release the modified variants, which may alter in the cytotoxicity of plaques over time.
It is worth noting that among the three variants, pS8- and nY10-Aβ40 showed similar properties in terms of the cross-seeding abilities, the modulation effects to fibrillar structures and cytotoxicity levels. Although possessing a modification within a similar segment in primary sequence, the isoD7-Aβ40 exhibited significantly lower cross-seeding ability. In the meanwhile, the cytotoxicity elevation effect and the molecular structure of resultant fibril for cross-seeding with isoD7-Aβ40 was similar to the self-seeding of wt-Aβ40, but different from the pS8-/nY10-seeded fibrillization. These results suggested that the chemical nature of modifications could play important roles in the biological impacts of the PTM-Aβ variants. We previously determined the molecular structure of pS8-Aβ40 fibrils,15 which might provide insights on the similarities and differences between these modifications: The N-terminal segments, including the residues D7-Y10, are typically disordered or form inter-strand interactions in reported wt-Aβ40 fibril structures. However, the phosphorylation at S8 introduces intra-strand interactions that stabilize a more ordered N-terminus (SI Figure S6). Particularly, both S8 and Y10 side chains are oriented towards the interior of intra-molecular packing with close contacts to other polar residues (e.g. S8–S26 and Y10-E22 contacts). The site-specific phosphorylation and nitration, which introduce additional charges, are likely to strengthen such interactions and facilitate the stabilization of fibril cores. On the contrary, the side chain of D7 has an opposite orientation and is exposed to solvent. Therefore, the steric modification shows minimum effects on the overall structure and pathologically relevant properties of fibrils. Overall, our data suggested the biological relevance of N-terminal segments of Aβ sequences, which complemented the findings of the biological and/or pathological roles of β-sheets and loop segments in the inhibitor design and early-stage Aβ nucleation process.39,40
In summary, we showed in this work that certain PTM-Aβ variants, such as the pS8- and nY10-Aβ40, could potentially trigger the amyloid deposition of wt-Aβ40 through cross-seeding processes. Fibrils formed by these variants may serve as more efficient seeds comparing to the wt-Aβ40 fibril itself. Such cross-seeding process led to pathologically relevant consequences such as modulations of the molecular structures of resultant fibrils and elevations of the fibrillar cytotoxicity levels. Our results provide experimental evidences that demonstrate that the influence of PTM-Aβ variants on the amyloidosis of wt-Aβ and the biological impact of cross-seeding processes. Furthermore, the variants with similar chemical modifications in their primary sequence showed comparable cross-seeding efficiencies, as well as structural modulation and toxicity elevation effects. Differences in the molecular structures of the fibrils formed by these variants, especially their N-terminal interactions and the extension of fibril cores, might contribute to the biological properties because more extended fibril cores could serve as better structural templates through seeding, and more efficient seeding could cause accelerated amyloid depositions. Future studies of the molecular structural similarities and differences between these variants’ fibrils will lead to more information on the underlying molecular basis.
Methods
ThT Fluorescence kinetics Assays.
The growth of both parent and seeded fibrils were monitored by a microplate reader with 96-well plates at 37°C. Fluorescence emission intensities were recorded at 500 ± 13 nm with excitation wavelength 450 ± 25 nm. Five and four independent repetitions were performed for the parent and seeded fibrillization respectively.
Cell Viability Assay.
The viability of N2a cells cultured with different measured groups (e.g monomers, seeds or mixtures) were assessed using MTT cell proliferation assay. our experimental groups contained externally added wt-Aβ40 peptides and PTM variants fibrillar seeds to cell cultures. The controls included externally added monomeric wt-Aβ40 or different fibrillar seeds with the same concentrations as in the experimental groups. Cell viabilities were assessed with 6, 12, 24 and 48-hour incubation for all experimental groups and controls. The reported relative cell viabilities were normalized to corresponding blanks (with 20 mM phosphate buffer) that were incubated for the same time periods.
Solid-State NMR (ssNMR) Spectroscopy.
All ssNMR spectra were recorded on a 600 MHz Bruker Avance III spectrometer equipped with a 2.5 mm TriGamma Magic Angle Spinning (MAS) probe. All measurements were done with temperature controlling at 280 ± 5K using N2 air. Sample temperatures were monitored by measuring the H2O proton chemical shift before and after experiments. Spectra were processed using Topspin (1D spectra) and NMRPipe (2D spectra).
Supplementary Material
Acknowledgements
This work is supported by SUNY Research Foundation and the National Institutes of Health (R01-GM125853 to W.Q. and R15-GM111681 to L.V.) The NMR spectrometer was supported by the National Science Foundation Major Research Instrumentation Grant (NSF-0922815). We thank the help from the Microscopy Facility at University of Colorado at Denver for the TEM imaging.
Footnotes
Supporting Information
More details for the experimental procedures, as well as the complementary ssNMR, TEM and cytotoxicity results are provided in the Supporting Information.
References
- 1.Ricciarelli R & Fedele E The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol 15, 926–935 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgopoulou N, McLaughlin M, McFarlane I & Breen KC The role of post-translational modification in beta-amyloid precursor protein processing. Biochem. Soc. Symp 23–36 (2001) 10.1042/bss0670023. [DOI] [PubMed] [Google Scholar]
- 3.Atwood CS, Martins RN, Smith MA & Perry G Senile plaque composition and posttranslational modification of amyloid-beta peptide and associated proteins. Peptides 23, 1343–1350 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Kummer MP & Heneka MT Truncated and modified amyloid-beta species. Alzheimers. Res. Ther 6, 28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barykin EP, Mitkevich VA, Kozin SA & Makarov AA Amyloid β Modification: A Key to the Sporadic Alzheimer’s Disease? Front. Genet 8, 58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, & Kawashima S Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron 14, 457–466 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Schilling S, Lauber T, Schaupp M, Manhart S, Scheel E, Bohm G, & Demuth H On the seeding and oligomerization of pGlu-amyloid peptides (in vitro). Biochemistry 45, 12393–12399 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Kumar S & Walter J Phosphorylation of amyloid beta (Aβ) peptides - a trigger for formation of toxic aggregates in Alzheimer’s disease. Aging (Albany. NY) 3, 803–812 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D, Walter J, Pape H, Konig S, Roeber S, Jessen F, Klockgether T, Korte M, & Heneka M Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron 71, 833–844 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Rezaei-Ghaleh N, Kumar S, Walter J & Zweckstetter M Phosphorylation Interferes with Maturation of Amyloid-β Fibrillar Structure in the N Terminus. J. Biol. Chem 291, 16059–16067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wulff M, Baumann M, Thummler A, Yadav JK, Heinrich L, Knupfer U, Schlenzig D, Schierhorn A, Rahfeld J, Horn U, Balbach J, Demuth H & Fandrich M Enhanced Fibril Fragmentation of N-Terminally Truncated and Pyroglutamyl-Modified Aβ Peptides. Angew. Chem. Int. Ed. Engl 55, 5081–5084 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Qiang W, Yau W-M, Lu J-X, Collinge J & Tycko R Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 541, 217–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vugmeyster L, Au DF, Ostrovsky D, Kierl B, Fu R, Hu ZW & Qiang W Effect of Post-Translational Modifications and Mutations on Amyloid-β Fibrils Dynamics at N Terminus. Biophys. J 117, 1524–1535 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Z-W Ma MR, Chen YX, Zhao YF, Qiang W & Li YM Phosphorylation at ser8 as an intrinsic regulatory switch to regulate the morphologies and structures of alzheimer’s 40-residue β-Amyloid (Aβ40) fibrils. J. Biol. Chem 292, 2611–2623 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z-W Vugmeyster L, Au DF, Ostrovsky D, Sun Y & Qiang W Molecular structure of an N-terminal phosphorylated β-amyloid fibril. Proc. Natl. Acad. Sci. U. S. A 166, 11253–11258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca MI, Head E, Velazquez P, Cotman CW & Tenner AJ The presence of isoaspartic acid in beta-amyloid plaques indicates plaque age. Exp. Neurol 157, 277–288 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen J Mahler J, Beschorner N, Kaeser SA, Hasler LM, Baumann F, Nystrom S, Portelius E, Blennow K, Lashley T, Fox NC, Sepulveda-Falla D, Glazel M, Oblak AL, Ghetti B, Nilsson KPR, Hammarstrom P, Staufenbiel M, Walker LC & Jucker M Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A 114, 13018–13023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Garmendia R Hernandez-Zimbron LF, Morales MA, Luna-Munoz J, Mena R, Nava-Catorce M, Acero G, Vasilevko V, Viramontes-Pintos A, Cribbs DH & Gevorkian G Identification of N-terminally truncated pyroglutamate amyloid-β in cholesterol-enriched diet-fed rabbit and AD brain. J. Alzheimers. Dis 39, 441–455 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Chan P-C, Wei C-Y, Hung G-U & Chiu P-Y Reduced vascular risk factors in Parkinson’s disease dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Brain Behav 8, e00916 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S, Wirths O, Stuber K, Wunderlich P, Koch P, Theil S, Rezaei-Ghaleh N, Zweckstetter M, Bayer TA, Brustle O, Thal DR & Walter J Phosphorylation of the amyloid β-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity. Acta Neuropathol 131, 525–537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barykin EP, Petrushanko IY, Burnysheva KM, Makarov AA & Mitkevich VA Isomerization of Asp7 increases the toxic effects of amyloid β and its phosphorylated form in SH-SY5Y neuroblastoma cells. Mol. Biol. (Mosk) 50, 863–869 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Barykin EP, Garifulina AI, Kruykova EV, Spirova EN, Anashkina AA, Adzhubei AA, Shelukhina IV, Kasheverov IE, Mitkevich VA, Kozin SA, Hollmann M, Tsetlin VI & Makarov AA Isomerization of Asp7 in Beta-Amyloid Enhances Inhibition of the α7 Nicotinic Receptor and Promotes Neurotoxicity. Cells 8, 771 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jawhar S, Wirths O & Bayer TA Pyroglutamate amyloid-β (Aβ): a hatchet man in Alzheimer disease. J. Biol. Chem 286, 38825–38832 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haupt C, Leppert J, Ronicke R, Meinhardt J, Yadav JK, Ramachandran R, Ohlenschlager O, Reymann KG, Gorlach M & Fandrich M Structural basis of β-amyloid-dependent synaptic dysfunctions. Angew. Chem. Int. Ed. Engl 51, 1576–1579 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Tycko R Amyloid polymorphism: structural basis and neurobiological relevance. Neuron 86, 632–645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wälti MA, Ravotti F, Arai H, Glabe CG, Wall JS, Bockmann A, Guntert P, Meier BH & Riek R Atomic-resolution structure of a disease-relevant Aβ(1–42) amyloid fibril. Proc. Natl. Acad. Sci. U. S. A 113, E4976–84 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colvin MT, Silvers R, Frohm B, Su Y, Linse S & Griffin RG High resolution structural characterization of Aβ42 amyloid fibrils by magic angle spinning NMR. J. Am. Chem. Soc 137, 7509–7518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J-X, Qiang W, Yau WM, Schwieters CD, Meredith SC & Tycko R Molecular structure of β-amyloid fibrils in alzheimer’s disease brain tissue. Cell 154, 1257–1268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh U, Yau W-M & Tycko R Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer’s disease brain tissue. Chem. Commun. (Camb) 54, 5070–5073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollmer M, Close W, Funk L, Rasmussen J, Bsoul A, Schierhown A, Schmidt M, Sigurdson CJ, Jucker M & Fandrich M Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun 10, 4760 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP & Tycko R Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science 307, 262–265 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Tycko R Symmetry-based constant-time homonuclear dipolar recoupling in solid state NMR. J. Chem. Phys 126, 64506 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Qiang W, Yau W-M & Tycko R Structural evolution of Iowa mutant β-amyloid fibrils from polymorphic to homogeneous states under repeated seeded growth. J. Am. Chem. Soc 133, 4018–4029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiang W, Yau W-M, Luo Y, Mattson MP & Tycko R Antiparallel β-sheet architecture in Iowa-mutant β-amyloid fibrils. Proc. Natl. Acad. Sci. U. S. A 109, 4443–4448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rönicke R, Klemm A, Meinhardt J, Schroder UH, Fandrich M & Reymann KG Abeta mediated diminution of MTT reduction - an artefact of single cell culture? PLoS One 3, e3236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straub JE & Thirumalai D Toward a molecular theory of early and late events in monomer to amyloid fibril formation. Annu. Rev. Phys. Chem 62, 437–463 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurry T & Stultz CM Mechanism of amyloid-β fibril elongation. Biochemistry 53, 6981–6991 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Barz B & Strodel B Understanding Amyloid-β Oligomerization at the Molecular Level: The Role of the Fibril Surface. Chemistry 22, 8768–8772 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Brender JR, Ghose A, Kotler SA, Krishnamoorthy J, Bera S, Morris V, Sil TB, Garai K, Reif B, Bhunia A & Ramamoorthy A Probing transient non-native states in amyloid beta fiber elongation by NMR. Chem. Commun. (Camb) 55, 4483–4486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griner SL, Seidler P, Bowler J, Murray KA, Yang TP, Sahay S, Sawaya MR, Cascio D, Rodriguez JA, Philipp S, Sosna J, Glabe CG, Gonen T & Eisenberg DS Structure-based inhibitors of amyloid beta core suggest a common interface with tau. Elife 8, e46924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


