Abstract
Heat stress (HS) causes significant economic losses in the poultry industry every year. However, the mechanisms for the adverse effects of HS on avian follicular development are largely unknown. The aim of this study was to test whether HS induces apoptosis of follicular cells and impairs egg production by activating the FasL/Fas and tumor necrosis factor (TNF)-α systems. To this end, Hy-Line Brown laying hens, at 32 wk of age, were either exposed to HS of 35°C to 37°C or maintained at 24°C to 26°C (control) for 5 D. At the end of the HS period, follicle numbers, apoptosis, FasL/Fas and TNF-α activation, oxidative stress, and hormone secretion were examined in ovarian follicles. Egg production was observed daily during both the stressed (day S1–S5) and the poststress recovery (day R1–R15) periods. The results demonstrated that HS on hens significantly 1) decreased laying rates from day S3 to R6; 2) reduced numbers of large yellow and hierarchical follicles; 3) triggered apoptosis while increasing the expression of FasL, Fas, TNF-α, and TNF-receptor 1 in small and large yellow follicles; and 4) increased levels of oxidative stress, corticotrophin-releasing hormone, and corticosterone while decreasing the estradiol/progesterone ratio in follicular fluid in small and large yellow follicles. Taken together, the results suggested that hen HS impaired egg production by reducing the number of follicles through inducing apoptosis and that it triggered apoptosis in follicular cells by activating the FasL/Fas and TNF-α systems.
Key words: heat stress, follicular cell apoptosis, FasL/Fas signaling, TNF-α signaling, laying hen
Introduction
In both the tropical areas and the temperate countries, heat stress causes significant economic losses in the poultry industry (Al-Saffar and Rose, 2002; Tan et al., 2010; Mignon-Grasteau et al., 2015). In the United States, for example, it has been estimated that heat stress may decrease egg production by 0.5 to 7.2%, leading to a significant yearly economic loss (Saint-Pierre et al., 2003). However, while it has been documented that high temperature influences the process of egg formation at both ovarian and reproductive tract levels including ovulation and oviposition (Rozenboim et al., 2007; Oguntunji and Alabi, 2010), the mechanisms by which heat stress negatively affects avian ovarian follicular development are largely unknown.
It is known that both the FasL/Fas signaling (Dhein and Walczak, 1995; Ju et al., 1995) and the tumor necrosis factor (TNF)-α signaling (Idriss and Naismith, 2000; Victor and Gottlieb, 2002) can trigger apoptosis in various tissues. It has been reported recently that restraint stress of female mice induced elevation of corticotrophin-releasing hormone (CRH) and corticosterone, which triggered apoptosis of ovarian cells and impairs oocyte competence through activating the Fas/FasL signaling (Yuan et al., 2016; Li et al., 2018). Heat stress of male mice significantly activated the Fas/FasL system in Sertoli cells (Guo et al., 2015). Immobilization stress of rats increased TNF-α expression in brain cortex (Madrigal et al., 2002). Furthermore, restraint stress of male mice triggered apoptosis with enhanced TNF-α and TNFR1 expression in sperm and spermatogenic cells (Zhang et al., 2020).
Because stress-enhanced expression of CRH (Cramer et al., 2015) and corticosterone (He et al., 2019) and activation of the FasL/Fas (Rauf et al., 2012; Guo et al., 2016a) and TNF-α signaling (Wride and Sanders, 1993; Onagbesan et al., 2000; Teng et al., 2019) during apoptosis have been observed in chicken, we thus hypothesized that heat stress might impair follicular development and consequently decrease egg production of laying hens through inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-α systems. The aim of this study was to test this hypothesis. Laying hens were either exposed to heat stress of 35°C to 37°C or maintained at normal temperature of 24°C to 26°C (for control) for 5 D before examination for follicle numbers, apoptosis, FasL/Fas and TNF-α activation, oxidative stress, and hormone secretion in ovarian follicles. Egg production was observed daily during both the stressed and poststress recovery periods.
Materials and methods
Hens and Temperature Treatment
The experimental procedures were carried out strictly in accordance with the guidelines approved by the Shandong Agricultural University Animal Care and Use Committee (Approval Number: SDAUA-2019-004). Thirty-wk-old Hy-Line Brown laying hens were housed in individual cages under ambient temperature, 55% to 65% of RH, and a light cycle of 16-hour light and 8-hour dark. The birds were fed ad libitum with a commercial layer feed that contained 59% corn and 24% soybean meal, among other ration ingredients, with a protein content of approximately 17.5%. At 32 wk of age, 180 Hy-Line Brown layers were randomly divided into stressed and control groups each containing 90 hens. The control and stressed groups were kept in different rooms of the same building. The temperature and humidity in the rooms were automatically controlled using air conditioners and humidifiers. While the temperature in the heat stress rooms was set to 35°C to 37°C, that in the control rooms was maintained at 24°C to 26°C. Humidity in both the stress and control rooms was kept at 55 to 65%. At the end of the 5-day heat stress period, blood and ovarian follicles were recovered to measure follicle numbers, apoptosis, FasL/Fas and TNF-α activation, oxidative stress, and hormone secretion. Egg production was observed daily during both the 5-day stress period (day S1–S5) and the 15-day poststress recovery period (day R1–R15).
Blood Collection
At 9 o’ clock in the morning, 3 mL of blood was aseptically drawn from the brachial vein into 5-mL vacuum tubes. The tubes were then kept standing at 4°C overnight for static settlement. On the next day, the samples were centrifuged (4°C, 900 × g) for 20 min, and the supernatant (serum) was recovered and stored at −20°C before use.
Counting, Recovery, and Processing of Ovarian Follicles
After hens were euthanized by jugular vein bleeding, the ovaries were quickly removed. Numbers of large white (LW, 2–5 mm in diameter), small yellow (SY, 6–8 mm), large yellow (LY, 9–12 mm), and hierarchical (>12 mm) follicles were counted and recorded. The follicles excised were either foxed in 4% formaldehyde for paraffin sectioning for apoptosis assessment by Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL), or used for reovery of follicular fluid and granulosa cells. To collect follicular fluid, the connective tissues on each follicle were removed, and the follicle was punctured at an avascular area with a scalpel. The follicular fluid thus released were collected and stored at −80°C until use. Granulosa cells were isolated from follicles following exactly the procedures reported by Gilbert et al (1977), and the granulosa cells obtained were stored at −80°C until use.
ELISA of CRH, Corticosterone, FasL, and TNF-α
Chicken CRH ELISA kit (Wuhan Colorful Gene Biotechnology Co., Ltd., JYM0155Ch), Corticosterone ELISA kit (Arbor Assays Co., Ltd., K014-H1), Chicken Tumor Necrosis Factor α (TNF-α) ELISA kit (Wuhan Colorful Gene Biotechnology Co., Ltd., JYM0033Ch), and Chicken Factor Related Apoptosis Ligand (FASL) ELISA kit (Shanghai Jianglai Biotechnology Co., Ltd., JL26226) were used to measure CRH, corticosterone, TNF-α and FasL, respectively, in serum and/or follicular fluid. The CRH detection range was 2 to 180 pg/mL (R > 0.92); the minimum level of corticosterone detection was 16.9 pg/mL; TNF-α detection range was 1.2 to 100 pg/mL (R > 0.92); and FasL detection range was 6.25 to 200 pg/mL. To start the measurement, 50 μL of standards or samples were added to wells of a microtiter plate. Then, after experimental procedures were performed in accordance with instructions of the respective kits, the plates were incubated at 37°C for 10 min (corticosterone was incubated at room temperature for 30 min). Finally, the optical density were read at 450 nm wavelength using a plate reader within 15 min after the reaction was terminated by adding 50 μL of the stop solution.
TUNEL Assays for Granulosa Cell Apoptosis
Follicles fixed in 4% formaldehyde solution were embedded individually in paraffin and sectioned into 5-μm-thick sections. Then, the sections were stained with hematoxylin and eosin. For TUNEL assay, the sections were stained using an in situ cell death detection kit (Roche, 11684817910). Briefly, the sections were 1) deparaffinized in xylene and rehydrated with reduced alcohol series; 2) incubated with proteinase K for 18 min at 37°C; 3) washed 3 times in PBS and blocked with 3% H2O2; 4) washed 3 times in PBS and incubated with terminal deoxynucleotidyl transferase enzyme at 4°C overnight; 5) washed 3 times in PBS and incubated in Streptavidin-HRP solution for 10 min at 37°C; 6) stained with 3,3′-diamino-benzidine components; and 7) observed under a microscope. Cells with nuclei stained brown were judged as apoptotic, whereas cells with nuclei stained blue were considered healthy. One section was randomly selected from each follicle, and 3 fields were observed in each section to calculate percentages of apoptotic cells from healthy cells using the Image-Pro plus 6.0 software.
Western Blotting
After being washed twice in cool PBS, 20 mg of granulosa cells from 4 SY follicles or 2-6 LY follicles were placed in a homogenizer containing 150 to 200 μL radioimmune precipitation assay buffer (R0010, Solarbio) and 10 μL phenylmethanesulfonyl fluoride and were homogenized for 30 min on ice. The homogenates were then centrifuged (14,000 × g) at 4°C for 5 min before the supernatant was collected. The total protein concentration of the supernatant was determined using a BCA Protein Assay Kit (P0009; Beyotime) and was adjusted to 2 μg/μL for further treatment. Then, 20 μL of sample was placed in a 0.5-mL microfuge tube and frozen at −80°C until use. For protein extraction, 5 μL of 5 × sodium dodecyl sulfate polyacrylamide gel electrophoresis loading buffer were added to each tube, and the tubes were boiled for 15 min. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was run on polyacrylamide gel to separate total proteins, and the proteins obtained were transferred electrophoretically onto polyvinylidene fluoride membranes. Then, the membranes were washed in TBST (150 mmol NaCl, 2 mmol KCl, 25 mmol Tris, and 0.05% Tween 20; pH 7.4), blocked with TBST containing 5% nonfat milk at 37°C for 2 h, and incubated at 4°C overnight with rabbit anti-GAPDH polyclonal antibodies (1:1,500; Abcam, ab181602), rabbit anti-active caspase-3 polyclonal antibodies (1:500; 9,664, CST), rabbit anti-FasL (1:1,000; Abcam, ab134401), mouse anti-Fas polyclonal antibodies (1:1,000; Abcam, ab82419), mouse anti-TNF-α polyclonal antibodies (1:,2000; Proteintech, 60291), or rabbit anti-TNF-R1 (1:1,000, Immunoway,YT4687). After being washed in TBST, the membranes were incubated for 2 h at 37°C with goat anti-mouse IgG HRP conjugated (1: 3,000; 7076, CST) or goat anti-rabbit IgG HRP conjugated (1: 3000; 7074, CST) secondary antibodies. Then, the membranes were washed in TBST and detected by the ImageQuant LAS 500 digital imaging system (GE Healthcare, Japan) using enhanced chemiluminescence detection regents ECL (Thermo). The relative quantities of proteins were determined with an Image-Pro Plus software by analyzing the sum density of each protein band image.
Quantitative Real-Time PCR
For RNA isolation, 50 to 100 mg granulosa cells were placed in a homogenizer containing 1 mL TRIzol reagent and homogenized for 10 min on ice. The homogenates were then centrifuged (13,800 × g) at 4°C for 10 min. The RNA isolated was resuspended in diethyl pyrocarbonate–treated MilliQ water and spectroscopically quantified at 260 nm. Purity and integrity of the RNA were assessed by determination of the A260/A280 ratio (1.8–2.0) and electrophoresis in 1% agarose, respectively.
Reverse transcription was performed in a total volume of 20 μL using a Transcriptor Reverse Transcriptase (TaKaRa; RR047 A). Briefly, 1 μg of each RNA sample, 2 μL 5×gDNA Eraser buffer, 1 μL gDNA Eraser, and 7 μL RNase-Free dH2O were mixed. The mixture was incubated at 42°C for 2 min to remove genomic DNA. Then, 1 μL PrimeScript RT Enzyme Mix I, 1 μL RT Primer Mix, 4 μL 5×PrimeScript Buffer 2, and 4 μL RNase-Free dH2O were added to the mixture and incubated at 37°C for 15 min and 85°C for 5 s to obtain cDNA, which was stored at −20°C until use.
A real-time PCR instrument (Roche, LightCycler96) was used to quantify Bax and Bcl-2 mRNAs. The gene-specific primers used are shown in Table 1. Amplification reactions were performed in a 20-μL reaction volume containing 2 μL of cDNA, 10 μL of 2 × TB Green Premix Ex Taq (TaKaRa, R820 A), 6.4 μL of RNase-free water, and 0.8 μL each of forward and reverse gene-specific primers (10 μmol). Cycle amplification conditions consisted of an initial denaturation step at 95°C for 30 s followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. The PCR products were analyzed by sequencing, dissociation curve analysis, and gel electrophoresis to determine specificity of the reaction. Gene expression was normalized to the β-actin internal control. All values were then expressed relative to the calibrator samples using the 2−(ΔΔCT) method.
Table 1.
Primer sequences from Gallus gallus (chicken) used for real-time PCR analysis.
| Gene name | Gene ID | Primer sequence | Size (bp) |
|---|---|---|---|
| Bax | XM_025145467.1 | F: TTCGGCTGTTTCTCAC | 234 |
| R: ATCCTTATCTCCGCTCT | |||
| Bcl-2 | NM_205339.2 | F: TACCAGAGGGACTTCGC | 213 |
| R: GTCATCCAGGTGGCAAT | |||
| β-actin | NM_205518.1 | F: TGTCCCTGTATGCCTCTGGT | 355 |
| R: GGGCACCTGAACCTCTCATT |
Spectrophotometry for Malondialdehyde and Superoxide Dismutase
A malondialdehyde (MDA) Detection Kit (A003-1; Nanjing Jiancheng Bioengineering Institute) was used to determine the MDA level in follicular fluid. Briefly, MDA in follicular fluid were allowed to react with thiobarbituric acid for 40 min at 95°C in acidic conditions. The MDA–thiobarbituric acid conjugate formed was then measured at 532 nm using a plate reader (EPOCH; Bio-Tek). All the data were normalized to nmol/mL sample. A superoxide dismutase (SOD) Detection Kit (A001-1; Nanjing Jiancheng Bioengineering Institute) was used to measure the SOD activity in follicular fluid. The samples were allowed to react with hydroxylamine hydrochloride, xanthine, and xanthine oxidase for 40 min at 25°C. The SOD activity was then measured at 550 nm using a plate reader (EPOCH; Bio-Tek). All the data were normalized to U/mL sample.
Chemiluminescence for Progesterone and 17-β Estradiol
Progesterone (P4) and 17-β estradiol (E2) were measured using the ARCHITECT Progesterone kit (Cat. No. 7K77) and ARCHITECT estradiol kit (Cat. No. 7K72), respectively, and the kits were purchased from Abbott Ireland Diagnostics Division. The sample was mixed with the sample diluent, the project diluent, and the P4 (or E2) antibody-coated paramagnetic microparticles. The acridinium ester–conjugated P4 (or E2) conjugate was added to the reaction mixture. After further incubation and washing, the reaction mixture was mixed with pre-excitation and excitation fluids. The resulting chemiluminescence reaction was measured using the architect I system and expressed as relative luminescence units. For P4 measurement, 100 μL of the sample was used; the analytical sensitivity was ≤0.1 ng/mL, and the detection range was 0.1 to 40 ng/mL. For E2 determination, 200 μL sample was used; the analytical sensitivity was ≤10 pg/mL, and the detection range was 10 to 5,000 pg/mL. Concentrations of P4 and E2 were calculated using the 4PLC software.
Data Analysis
Each treatment contained at least 3 replicates. Independent sample t test was used because of each measure had only 2 groups. All the data were analyzed using the SPSS (Statistics Package for Social Sciences) software (SPSS 13.0, SPSS Inc. Chicago, IL) and were expressed as mean ± SEM. Difference was considered significant only when the P value was less than 0.05.
Results
Effects of Heat Exposure on Egg Production and Follicle Development of Laying Hens
Laying rates were significantly (P < 0.05) lower in stressed than in control hens from day S3 to day R6, and the difference became insignificant (P > 0.05) from day R7 onward (Figure 1A). According to Gilbert et al (1983), at peak laying periods, growth of hen follicles took 3 D from 3 to 5 mm in diameter, 2 D from 5 to 8 mm, and 6 D from 8 mm to ovulation. In hen ovaries, the diameters of LW, SY, LY, and hierarchical follicles are 2 to 5 mm, 6 to 8 mm, 9 to 12 mm, and >13 mm, respectively (Li et al., 2017). Thus, the SY, LY and hierarchical follicles that had been affected during the heat stress period would ovulate during the decreased egg production period (Figure 1C), suggesting that our heat stress protocol decreased egg production via damaging mainly the SY, LY and hierarchical follicles and/or ovulation.
Figure 1.
Effects of heat stress of laying hens on laying rates and follicle development. Graph (A) shows percentages of laying/total hens on each heat stressed (S) or poststress recovering (R) day in control (Ctrl) or stressed (Strs) hens. In this experiment, 120 layers were randomly divided into stressed and control groups each containing 60 hens. Egg production was observed daily during both the stress period (day S1 to S5) and the poststress recovery period (day R1 to R15). On each day, each treatment was repeated 20 times with each replicate containing 3 hens. Graph (B) compares numbers of large white (LW), small yellow (SY), large yellow (LY), and hierarchical (Hie) follicles between Ctrl and Strs hens on day 5 of heat stress. Each treatment was repeated 10 times with each replicate containing one ovary. ∗ indicates significant difference (P < 0.05) from control values within d or follicle types. Panel (C) shows the estimated ovulation times for LW, SY, LY, and hierarchical follicles from the onset of heat stress exposure. From the onset of heat stress, it took 11 D for the LW follicles of 3 to 5 mm in diameter, 8 D for the SY follicles of 5 to 8 mm in diameter, 6 D– for the LY follicles of 8 to 12 mm in diameter, and about 3 to 5 D for the F3 to F5 hierarchical follicles of >12 mm in diameter to grow and mature before ovulation (Δ).
We therefore compared numbers of LW, SY, LY, and hierarchical follicles between control and stressed hens immediately at the end of the heat stress. The results showed that numbers of LY and hierarchical follicles were significantly (P < 0.05) lower in stressed than in control hens, although numbers of LW and SY follicles did not differ significantly (P > 0.05) between the 2 groups (Figure 1B). We proposed that the heat stress might have reduced the number of LY and hierarchical follicles directly by triggering their apoptosis and/or indirectly by causing apoptosis in LW and SY follicles, which halted their growth into LY and hierarchical follicles. We therefore examined apoptosis and related mechanisms in SY and LY follicles in the following experiments, as both of the 2 follicle types were involved in reduced egg production, and apoptosis of the SY follicles might contribute to reduced numbers of the LY and hierarchical follicles after heat stress.
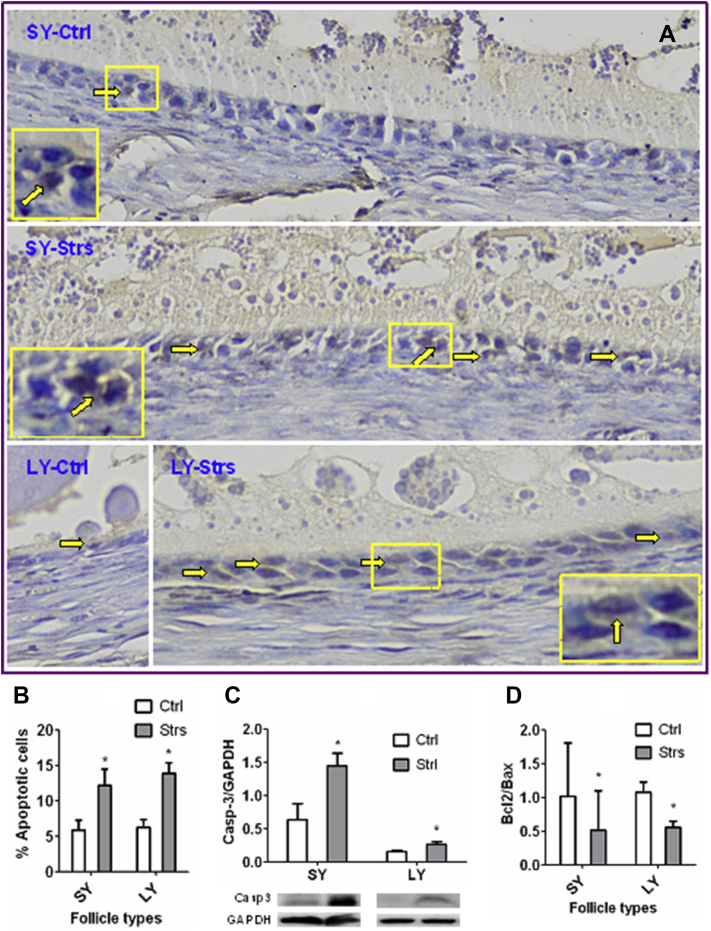
Heat Stress of Laying Hens Triggered Apoptosis of Mural Granulosa Cells
Apoptosis of mural granulosa cells (MGC) in SY and LY follicles was analyzed at the end of the heat stress. Our TUNEL staining of follicle sections showed that while the TUNEL-negative nuclei of healthy cells appeared blue, the TUNEL-positive nuclei of apoptotic MGC were stained brown after TUNEL staining (Figure 2A). In both SY and LY follicles, percentages of TUNEL-positive MGC were significantly (P < 0.05) higher in stressed than in control hens (Figure 2B). In both follicle types, while the level of active caspase-3 was significantly higher (Figure 2C), the ratio of Bcl2/Bax was significantly lower (Figure 2D) in stressed than in control hens. Together, the results confirmed that exposure of laying hens to heat stress induced significant apoptosis in MGCs.
Figure 2.
Effects of heat stress of laying hens on apoptosis of follicles. Panel (A) shows images of follicular wall sections after TUNEL staining of SY or LY follicles from control (Ctrl) or stressed (Strs) hens. In the images, while the TUNEL-positive nuclei of mural granulosa cells (MGC) were stained brown (arrows), the TUNEL-negative nuclei appear blue. Boxed regions in the image are shown as 2-fold enlarged insets to highlight the TUNEL-positive brown nuclei. Graphs (B), (C), and (D) compare percentages of apoptotic cells (TUNEL-positive), levels of active caspase-3 (Caspase-3/GAPDH ratio, Western blotting), and Bcl2/Bax ratio (RT-PCR results), respectively, in MGC of SY or LY follicles between Ctrl and Strs hens on day 5 of heat stress. In graph (B), each treatment was repeated 6 times with each replicate containing 1 hen contributing 2 SY or 1 or 2 LY follicles. In graph (C), each treatment was repeated 5 times with each replicate containing 2 hens each contributing 2 SY follicles or 6 times with each replicate containing 2 hens each contributing 1–3 LY follicles. In graph (D), each treatment was repeated 8 times with each replicate containing 1 SY or LY follicle from 1 hen. ∗ indicates significant difference (P < 0.05) from control values within follicle types. Abbreviations: LY, large yellow; SY, small yellow; TUNEL, Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling.
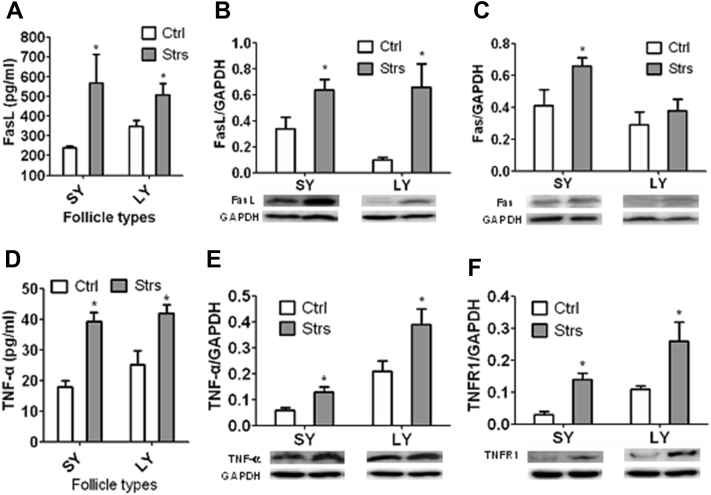
Heat Stress of Laying Hens Increased Follicular Expression of FasL/Fas and TNF-α/TNFR1
Expression of FasL, Fas, TNF-α, and TNFR1 in MGC was measured by Western blotting, and FasL and TNF-α concentrations in follicular fluid were measured by ELISA, in SY and LY follicles at the end of the heat stress. Heat stress significantly (P < 0.05) increased contents of FasL and TNF-α in follicular fluid and their protein expression in MGC in both SY and LY follicles (Figure 3). Heat stress also increased protein expression of Fas and TNFR1 in MGC in both SY and LY follicles although the difference in Fas expression in LY MGC did not reach a significant level statistically (P = 0.36) between control and stressed hens. Taken together, the results have unequivocally confirmed that heat stress of laying hens activated both the Fas and TNF-α systems in ovarian follicles.
Figure 3.
Effects of heat stress of laying hens on FasL/Fas and TNF-α/TNFR1 expression in ovarian follicles. Graphs (A) and (D) show ELISA results comparing FasL and TNF-α levels, respectively, in follicular fluid from SY or LY follicles between Ctrl and Strs hens on day 5 of heat stress. Each treatment was repeated 6 times and each replicate contained 1 hen contributing pooled follicular fluid from 3 SY or 1–3 LY follicles. Graphs (B)/(C) and (E)/(F) show Western blotting results comparing levels of FasL/Fas (Fasl or Fas/GAPDH ratio) and TNF-α/TNFR1 (TNF-α or TNFR1/GAPDH ratio), respectively, in MGC from SY or LY follicles between Ctrl and Strs hens. Each treatment was repeated 7 times with each replicate including MGC from 2 hens each contributing 2 SY follicles, or repeated 8 times with each replicates including 2 hens each contributing 1–3 LY follicles. ∗ indicates significant difference (P < 0.05) from control values within follicle types. Abbreviations: Ctrl, control hens; LY, large yellow; Strs, stressed hens; SY, small yellow; TNF, tumor necrosis factor.
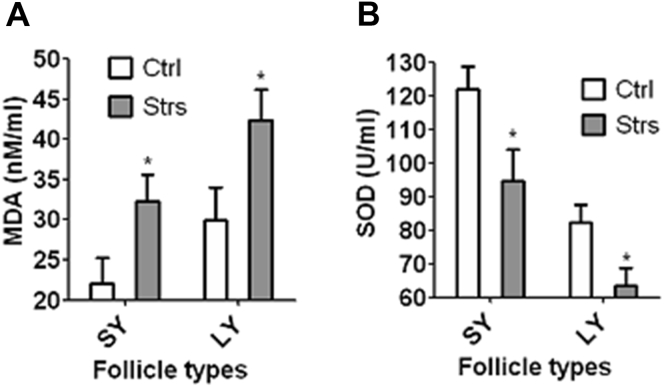
Heat Exposure of Laying Hens Increased Oxidative Stress in SY and LY Follicles
Levels of MDA and SOD in follicular fluid of SY and LY follicles were analyzed by spectrophotometry. In both SY and LY follicles, while the level of MDA was higher, the level of SOD was lower significantly (P < 0.05) in stressed than in control hens (Figure 4), suggesting that heat stress of laying hens caused significant oxidative stress in SY and LY follicles through impairing their antioxidant potential.
Figure 4.
Effects of heat stress of laying hens on redox status of SY and LY follicles. Graphs (A) and (B) show malondialdehyde (MDA) and superoxide dismutase (SOD) levels, respectively, in follicular fluid from SY and LY follicles of control (Ctrl) or stressed (Strs) hens on day 5 of heat stress. In graph (A), each treatment was repeated 8 times with each replicate containing 1 hen contributing pooled follicular fluid from 3 SY follicles or 1–3 LY follicles. In graph (B), each treatment was repeated 5 times with each replicate containing 1 hen contributing pooled follicular fluid from 3 SY or 1-3 LY follicles. ∗ indicates significant difference (P < 0.05) from control values within follicle types. Abbreviations: LY, large yellow; SY, small yellow.
Effects of Heat Stress on Hormone Levels in Follicular Fluid and Serum of Laying Hens
In follicular fluid of both SY and LY follicles, the concentration of E2 was significantly lower in stressed than in control hens (Figure 5A). Although the P4 concentration in LY follicles was significantly higher in stressed than in control hens, that in SY follicles was very low and did not differ between stressed and control hens (Figure 5B). As a result, the E2/P4 ratio in LY follicles was significantly (P < 0.05) lower in stressed than in control hens (Figure 5C). The E2/P4 ratio in SY follicles was also lower in stressed than in control hens, and the difference was close to a significant level (P = 0.096). Thus, while the stress-induced decrease in the E2/P4 ratio resulted mainly from a significant increase in P4 production in LY follicles, it was attributed mainly to a decreased production of E2 in the SY follicles. Both E2 and P4 levels in serum were significantly lower in stressed than in control hens, although the E2/P4 ratio did not differ between stressed and control hens. Both CRH (Figure 5D) and corticosterone (Figure 5E) levels in serum and SY and LY follicular fluid were significantly higher in stressed than in control hens, suggesting that heat stress of hens damaged ovarian cells by increasing secretion of the stress hormones.
Figure 5.
Effects of heat stress of laying hens on hormone levels in serum and follicular fluid. Hormone concentrations were measured by ELISA. Graphs (A), (B), and (C) compare E2 and P4 concentrations and the E2/P4 ratio, respectively, in serum or follicular fluid from SY or LY follicles between Ctrl and Strs hens on day 5 of heat stress. Each treatment was repeated 5 times with each replicate containing 1 serum sample from 1 hen, or repeated 9 times with each replicate containing follicular fluid from 1 follicle from 1 hen. Graphs (D) and (E) compare levels of CRH and corticosterone, respectively, in serum or follicular fluid from SY or LY follicles between Ctrl and Strs hens. Each treatment was repeated 6 times with each replicate containing 1 hen providing 1 serum sample or follicular fluid from 3 SY or 1–3 LY follicles. ∗ indicates significant difference (P < 0.05) from control values within serum or follicle types. Abbreviations: CRH, corticotrophin-releasing hormone; Ctrl, control hens; E2, estradiol; LY, large yellow; P4, progesterone; Strs, stressed hens; SY, small yellow.
Discussion
The present results demonstrated that heat stress of laying hens activated the hypothalamus–pituitary–adrenal axis with an elevation of CRH and corticosterone in both blood and follicular fluid. Downing and Bryden (2008) observed that heat exposure of laying hens increased albumen corticosterone significantly on day 4 and 9 after initial exposure and that corticosterone concentration in albumen was closely correlated with that in serum. Alhenaky et al (2017) reported that both chronic and acute heat stress of broiler chickens increased serum concentrations of corticosterone. Furthermore, He et al (2019) observed that serum corticosterone in broilers increased significantly after 7 D of heat stress. Whether in chickens or in mammals, we could find few articles reporting the effects of heat stress on CRH secretion. Nakamura et al (2000) reported that heat treatment increased blood CRH in pregnant rats. Cramer et al (2015) observed that CRH mRNA expression in the hypothalamus was increased after heat challenge of chicks trained to be vulnerable to heat.
The present results showed that heat stress of laying hens with elevation in CRH and corticosterone induced apoptosis with increased oxidative stress in follicular cells. It was reported that heat stress could cause apoptosis in spleen cells (Xu et al., 2017) and aortic endothelial cells (Cui et al., 2019) of chicken. Increasing evidence suggests that oxidative stress can trigger apoptosis through both the mitochondria-dependent and mitochondria-independent pathways (Sinha et al., 2013). Heat stress increased the production of ROS and MDA in rats (Yu et al., 2013) and decreased SOD and catalase in ducks (Zeng et al., 2014). Although we could not find any report in chicken, we did find many articles reporting that CRH and corticosterone induced apoptosis, sometimes with increased oxidative stress, in various mammalian tissues. For example, the glucocorticoid-induced apoptosis of rat Leydig cells was associated with increased generation of ROS (Gao et al., 2003). Preimplantation restraint stress of mice, which significantly elevated both CRH and glucocorticoids, induced apoptosis in oviducts and embryos with increased oxidative stress (Zheng et al., 2016). Furthermore, injection of females with cortisol (Yuan et al., 2016) or culture of cells with CRH (Li et al., 2018; Zhao et al., 2020) or corticosterone (Yuan et al., 2020) triggered apoptosis in MGC, cumulus cells, and/or oocytes in mice.
This study indicated that heat stress of laying hens with elevation of CRH and corticosterone activated the Fas and the TNF-α signaling in follicular cells. Guo et al (2016b) reported that nickel chloride significantly increased hepatic apoptosis with increased expression of Fas, FasL, and TNF-α in broiler chickens. Peng et al (2016) observed that aflatoxin B1 triggered thymocyte apoptosis in chickens with upregulated FasL and Fas expression. Furthermore, heat stress significantly increased TNF-α expression in serum in laying hens (Deng et al., 2012) and broiler chickens (Alhenaky et al., 2017). In mice, restraint stress or injection of females with cortisol (Yuan et al., 2016; 2020) or culture of cells with CRH (Li et al., 2018; Zhao et al., 2020) or corticosterone (Yuan et al., 2020) triggered apoptosis in MGC and/or oocytes through activating the Fas and TNF-α systems.
The current results showed that the SY follicles of laying hens produced a small amount of P4 but normal amount of E2, whereas the LY follicles produced a large quantity of both hormones. Heat stress of laying hens decreased both E2 and P4 levels in serum, and it decreased the E2/P4 ratio in follicular fluid. It has been reported that small follicles of hens produce small amount of P4 but normal amount of E2. For example, Robinson and Etches (1986) reported that 1- to 10-mm follicles did not produce any P4 although they were a major E2 producer. Etches and Duke (1984) and Tilly et al (1991) observed that P4 production by granulosa cells increased with follicular growth and maturation. Previous studies indicated that heat stress of hen decreased P4 and E2 in the blood. For example, Rozenboim et al (2007) observed a significant decrease in plasma P4 and a mild decline in plasma E2 after laying hens were exposed to heat stress. Novero et al (1991) reported that levels of plasma P4 and basal P4 production by granulosa cells were lower in heat stressed than in unstressed hens. Furthermore, in mammals, the E2/P4 ratio in follicular fluid has been found to be an important estimator for the degree of follicular health. For example, the level of E2 and the ratio of E2/P4 were significantly higher in healthy follicles than in atretic follicles (Yu et al., 2004; Wei and Shi, 2013; Hernández-Coronado et al., 2015).
In summary, this study demonstrated that heat stress of laying hens activated the hypothalamus–pituitary–adrenal axis with an elevation of CRH and corticosterone in both blood and follicular fluid. The heat stress and its associated elevation in CRH and corticosterone might have increased oxidative stress, reduced the E2/P4 ratio, and induced apoptosis in follicular cells via activating the Fas and the TNF-α signaling, leading to a reduction in follicle numbers and then in egg production. The data are important not only theoretically for our understanding of the mechanisms by which heat stress negatively affects avian follicular development but also practically for developing measures to reduce the heat stress–caused economic losses in the poultry industry.
Acknowledgments
Funding was provided by The Jinan Layer Experiment Station of China Agriculture Research System (CARA-40-S12), China, National Key Research and Development Program of China (2017YFC1702700), China, Natural Science Foundation of Shandong Province (ZR2019MC022), China, and China National Natural Science Foundation (Nos. 31772599 and 31702114), China.
Conflict of Interest Statement: The authors declare no competing of interest.
References
- Alhenaky A., Abdelqader A., Abuajamieh M., Al-Fataftah A.R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017;70(Pt B):9–14. doi: 10.1016/j.jtherbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Al-Saffar A.A., Rose S.P. Ambient temperature and the egg laying characteristics of the laying fowl. World’s Poult. Sci. J. 2002;58:317–331. [Google Scholar]
- Cramer T., Kisliouk T., Yeshurun S., Meiri N. The balance between stress resilience and vulnerability is regulated by corticotropin-releasing hormone during the critical postnatal period for sensory development. Dev. Neurobiol. 2015;75:842–853. doi: 10.1002/dneu.22252. [DOI] [PubMed] [Google Scholar]
- Cui Y., Wu G., Wang Z., Huang F., Ning Z., Chu L., Yang S., Lv Q., Hu J. Effects of Taurine on broiler aortic endothelial apoptosis induced by heat stress. Adv. Exp. Med. Biol. 2019;1155:391–406. doi: 10.1007/978-981-13-8023-5_37. [DOI] [PubMed] [Google Scholar]
- Deng W., Dong X.F., Tong J.M., Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012;91:575–582. doi: 10.3382/ps.2010-01293. [DOI] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K.M., Krammer P.H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Downing J.A., Bryden W.L. Determination of corticosterone concentrations in egg albumen: a non-invasive indicator of stress in laying hens. Physiol. Behav. 2008;95:381–387. doi: 10.1016/j.physbeh.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Etches R.J., Duke C.E. Progesterone, androstenedione and oestradiol content of theca and granulosa tissues of the four largest ovarian follicles during the ovulatory cycle of the hen (Gallus domesticus) J. Endocrinol. 1984;103:71–76. doi: 10.1677/joe.0.1030071. [DOI] [PubMed] [Google Scholar]
- Gao H.B., Tong M.H., Hu Y.Q., You H.Y., Guo Q.S., Ge R.S. Hardy MP. Mechanisms of glucocorticoid- induced Leydig cell apoptosis. Mol. Cell. Endocrinol. 2003;199:153–163. doi: 10.1016/s0303-7207(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Gilbert A.B., Evans A.J., Perry M.M., Davidson M.H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus) J. Reprod. Fertil. 1977;50:179–181. doi: 10.1530/jrf.0.0500179. [DOI] [PubMed] [Google Scholar]
- Gilbert A.B., Perry M.M., Waddington D., Hardie M.A. Role of atresia in establishing the follicular hierarchy in the ovary of the domestic hen (Gallus domesticus) J. Reprod. Fertil. 1983;69:221–227. doi: 10.1530/jrf.0.0690221. [DOI] [PubMed] [Google Scholar]
- Guo H., Cui H., Fang J., Zuo Z., Deng J., Wang X., Zhao L., Chen K., Deng J. Nickel chloride (NiCl2) in hepatic toxicity: apoptosis, G2/M cell cycle arrest and inflammatory response. Aging (Albany NY) 2016;8:3009–3027. doi: 10.18632/aging.101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Cui H., Fang J., Zuo Z., Deng J., Wang X., Zhao L., Wu B., Chen K., Deng J. Nickel chloride-induced apoptosis via mitochondria- and Fas-mediated caspase-dependent pathways in broiler chickens. Oncotarget. 2016;7:79747–79760. doi: 10.18632/oncotarget.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Chi S., Cong X., Li H., Jiang Z., Cao R., Tian W. Baicalin protects Sertoli cells from heat stress-induced apoptosis via activation of the Fas/FasL pathway and Hsp72 expression. Reprod. Toxicol. 2015;57:196–203. doi: 10.1016/j.reprotox.2015.06.049. [DOI] [PubMed] [Google Scholar]
- He X., Lu Z., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress alters hypothalamus integrity, the serum indexes and attenuates expressions of hypothalamic appetite genes in broilers. J. Therm. Biol. 2019;81:110–117. doi: 10.1016/j.jtherbio.2019.02.025. [DOI] [PubMed] [Google Scholar]
- Hernández-Coronado C.G., Guzmán A., Espinosa-Cervantes R., Romano M.C., Verde-Calvo J.R., Rosales-Torres A.M. Sphingosine-1-phosphate and ceramide are associated with health and atresia of bovine ovarian antral follicles. Animal. 2015;9:308–312. doi: 10.1017/S1751731114002341. [DOI] [PubMed] [Google Scholar]
- Idriss H.T., Naismith J.H. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ju S.T., Panka D.J., Cui H., Ettinger R., el-Khatib M., Sherr D.H., Stanger B.Z., Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373(6513):444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- Li C.Y., Li Z.B., Kong Q.Q., Han X., Xiao B., Li X., Chang Z.L., Tan J.H. Restraint-induced corticotrophin-releasing hormone elevation triggers apoptosis of ovarian cells and impairs oocyte competence via activation of the Fas/FasL system. Biol. Reprod. 2018;99:828–837. doi: 10.1093/biolre/ioy091. [DOI] [PubMed] [Google Scholar]
- Li P., Yu X., Xie J., Yao X., Liu W., Yao J., Zhu Z., Lyu L. Expression of cocaine- and amphetamine-regulated transcript (CART) in hen ovary. Biol. Res. 2017;50:18. doi: 10.1186/s40659-017-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal J.L., Hurtado O., Moro M.A., Lizasoain I., Lorenzo P., Castrillo A., Boscá L., Leza J.C. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Mignon-Grasteau S., Moreri U., Narcy A., Rousseau X., Rodenburg T.B., Tixier-Boichard M., Zerjal T. Robustness to chronic heat stress in laying hens: a meta-analysis. Poult. Sci. 2015;94:586–600. doi: 10.3382/ps/pev028. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Nagase H., Ogino K., Hatta K., Matsuzaki I. Heat produces uteroplacental circulatory disturbance in pregnant rats through action of corticotropin releasing hormone (CRH) Placenta. 2000;21:510–515. doi: 10.1053/plac.2000.0536. [DOI] [PubMed] [Google Scholar]
- Novero R.P., Beck M.M., Gleaves E.W., Johnson A.L., Deshazer J.A. Plasma progesterone, luteinizing hormone concentrations, and granulosa cell responsiveness in heat-stressed hens. Poult. Sci. 1991;70:2335–2339. doi: 10.3382/ps.0702335. [DOI] [PubMed] [Google Scholar]
- Oguntunji O.M., Alabi A.O. Influence of high environmental temperature on egg production and shell quality, a review. World’s Poult. Sci. J. 2010;66:739–774. [Google Scholar]
- Onagbesan O.M., Mast J., Goddeeris B., Decuypere E. Effect of TNF-alpha on LH and IGF-I modulated chicken granulosa cell proliferation and progesterone production during follicular development. J. Reprod. Fertil. 2000;120:433–442. [PubMed] [Google Scholar]
- Peng X., Yu Z., Liang N., Chi X., Li X., Jiang M., Fang J., Cui H., Lai W., Zhou Y., Zhou S. The mitochondrial and death receptor pathways involved in the thymocytes apoptosis induced by aflatoxin B1. Oncotarget. 2016;7:12222–12234. doi: 10.18632/oncotarget.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf A., Khatri M., Murgia M.V., Saif Y.M. Fas/FasL and perforin-granzyme pathways mediated T cell cytotoxic responses in infectious bursal disease virus infected chickens. Results Immunol. 2012;2:112–119. doi: 10.1016/j.rinim.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson F.E., Etches R.J. Ovarian steroidogenesis during follicular maturation in the domestic fowl (Gallus domesticus) Biol. Reprod. 1986;35:1096–1105. doi: 10.1095/biolreprod35.5.1096. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J.A., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Saint Pierre N.R., Cobanov B., Schnitkey G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003;86:E52–E77. [Google Scholar]
- Sinha K., Das J., Pal P.B., Sil P.C. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- Tan G.Y., Yang L., Fu Y.Q., Feng J.H., Zhang M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010;89:115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Teng X., Zhang W., Song Y., Wang H., Ge M., Zhang R. Protective effects of Ganoderma lucidum triterpenoids on oxidative stress and apoptosis in the spleen of chickens induced by cadmium. Environ. Sci. Pollut. Res. Int. 2019;26:23967–23980. doi: 10.1007/s11356-019-05638-5. [DOI] [PubMed] [Google Scholar]
- Tilly J.L., Kowalski K.I., Johnson A.L. Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol. Reprod. 1991;44:305–314. doi: 10.1095/biolreprod44.2.305. [DOI] [PubMed] [Google Scholar]
- Victor F.C., Gottlieb A.B. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J. Drugs Dermatol. 2002;1:264–275. [PubMed] [Google Scholar]
- Wei Q., Shi F. Cleavage of poly (ADP-ribose) polymerase-1 is involved in the process of porcine ovarian follicular atresia. Anim. Reprod. Sci. 2013;138:282–291. doi: 10.1016/j.anireprosci.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Wride M.A., Sanders E.J. Expression of tumor necrosis factor-alpha (TNF alpha)-cross-reactive proteins during early chick embryo development. Dev. Dyn. 1993;198:225–239. doi: 10.1002/aja.1001980308. [DOI] [PubMed] [Google Scholar]
- Xu D., Li B., Cao N., Li W., Tian Y., Huang Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget. 2017;8:70394–70405. doi: 10.18632/oncotarget.19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Liu F., Yin P., Zhao H., Luan W., Hou X., Zhong Y., Jia D., Zan J., Ma W., Shu B., Xu J. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Stress. 2013;16:99–113. doi: 10.3109/10253890.2012.680526. [DOI] [PubMed] [Google Scholar]
- Yu Y.S., Sui H.S., Han Z.B., Li W., Luo M.J., Tan J.H. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res. 2004;14:341–346. doi: 10.1038/sj.cr.7290234. [DOI] [PubMed] [Google Scholar]
- Yuan H.J., Han X., He N., Wang G.L., Gong S., Lin J., Gao M., Tan J.H. Glucocorticoids impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the Fas system. Sci. Rep. 2016;6:24036. doi: 10.1038/srep24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.J., Li Z.B., Zhao X.Y., Sun G.Y., Wang G.L., Zhao Y.Q., Zhang M., Tan J.H. Glucocorticoids impair oocyte competence and trigger apoptosis of ovarian cells via activating the TNF-a system. Reproduction. 2020;160:129–140. doi: 10.1530/REP-20-0025. [DOI] [PubMed] [Google Scholar]
- Zeng T., Li J.J., Wang D.Q., Li G.Q., Wang G.L., Lu L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress Chaperones. 2014;19:895–901. doi: 10.1007/s12192-014-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kong D.L., Xiao B., Yuan H.J., Kong Q.Q., Han X., Luo M.J., Tan J.H. Restraint stress of male mice triggers apoptosis in spermatozoa and spermatogenic cells via activating the TNF-α system. Zygote. 2020;28:160–169. doi: 10.1017/S0967199419000844. [DOI] [PubMed] [Google Scholar]
- Zhao X.Y., Li Z.B., Yuan H.J., Han X., Wu J.S., Feng X.Y., Zhang M., Tan J.H. Restraint stress and elevation of corticotrophin-releasing hormone in female mice impair oocyte competence through activation of the tumor necrosis factor a (TNF-α) system. Reprod. Fertil. Dev. 2020;32:862–872. doi: 10.1071/RD20002. [DOI] [PubMed] [Google Scholar]
- Zheng L.L., Tan X.W., Cui X.Z., Yuan H.J., Li H., Jiao G.Z., Ji C.L., Tan J.H. Preimplantation maternal stress impairs embryo development by inducing oviductal apoptosis with activation of the Fas system. Mol. Hum. Reprod. 2016;22:778–790. doi: 10.1093/molehr/gaw052. [DOI] [PubMed] [Google Scholar]