Abstract
Sodium bisulfate (SB) was evaluated on its ability to improve broiler growth and intestinal structure with(out) a coccidia challenge. One thousand two hundred Cobb500 day-old males were randomly assigned within 4 experimental groups with a 2 × 2 factorial design, with (out) SB in the diet and with(out) a day 0 coccidia challenge using a 10× dose of a commercial vaccine. At day 7, oocysts per gram of feces were determined. At day 0, 14, 28, and 41, BW and feed consumption were measured. At day 21, 20 birds per treatment were subjectively scored for coccidia lesions, and jejunal histologic samples were collected for villi measurements. Twenty additional birds were given fluorescein isothiocyanate-dextran to determine gut permeability. At day 41, 10 birds per treatment had histologic samples collected. Statistical analysis was conducted in JMP Pro 14 using GLM procedure to compare disease state and diet. Means were separated using Dunnett's test (P ≤ 0.05) with the nonchallenged standard diet treatment that is considered the control. All parameters measured indicated an effect due to the coccidia inoculation. Therefore, effects of diet on (non)challenged treatments were determined using a Student t test (P ≤ 0.05). Limited differences due to diet were seen for the nonchallenged production data. Sodium bisulfate had a thinner villi base width (P = 0.04) on day 21 and greater villi height (P = 0.03), smaller base width (P = 0.04), thicker muscularis (P = 0.03), and lower crypt: height ratio (P = 0.01) on day 41. Challenged SB had similar gut permeability to the nonchallenged control (P = 0.94) on day 21. There was no difference in flock uniformity, feed intake, oocysts per gram of feces, or lesion scores between challenged treatments. Challenged SB had greater BW on day 14 (P < 0.0001), 28 (P < 0.0001), and 41 (P = 0.02). Feed conversion ratio from day 0 to 14 was also lower (P = 0.0002). Challenged SB had smaller crypts (P = 0.02) and therefore a smaller crypt: height ratio (P = 0.03) on day 21. Challenged control had a larger apical width (P = 0.03) and thicker muscularis (P = 0.04) on day 41. Overall, the addition of SB during coccidial enteropathy aided in BW, feed conversion ratio, and villi health with no observed effects on parasite cycling.
Key words: broiler, coccidiosis, sodium bisulfate
Introduction
Sodium bisulfate is an acidic compound that has been conventionally applied to poultry litter and used as a feed additive (Pope and Cherry, 2000; Line, 2002; Ruiz-Feria et al., 2011; Kassem et al., 2012; Hunolt et al., 2015). This compound has shown positive impacts on poultry health and rearing, with binding to ammonia to improve air and litter quality, while having various effects on bacteria in the broiler and the broiler's environment (Pope and Cherry, 2000; Line, 2002; Kassem et al., 2012; Williams et al., 2012; Hunolt et al., 2015). Some studies have indicated a positive correlation with broiler growth if given sodium bisulfate in the diet with or without a disease challenge (Line, 2002; Ruiz-Feria et al., 2011; Kassem et al., 2012).
Sodium bisulfate dissociates into its chemical matrix of sodium, hydrogen, and sulfate. Sodium and hydrogen are important ions that are regularly transported between the lumen and gut epithelia for acid–base and electrolyte homeostasis. Within the ileum, absorption of sugars and fluids is predominately driven by sodium uptake (Gennari and Weise, 2008). Sulfate is an anion well documented in mammalian plasma that acts as a key component for the maintenance of tissues (Markovich, 2001). It is also essential for sulfonated carbohydrates in mucin, the protective barrier between the lumen and the epithelium of the gastrointestinal tract (Dawson et al., 2009). Most sulfates come from exogenous sources, either as an inorganic source in the diet or from metabolized sulfur-containing amino acids (Whittamore and Hatch, 2017). Anion substitution of sulfate for chloride has been considered in poultry, mainly during a period of stress that alters gut homeostasis leading to increased electrolyte losses (Hooge et al., 1999; Ahmad et al., 2006; Zdunczyk et al., 2012). During diarrheal states, fluid losses of electrolytes are common owing to improper absorption and sloughing of villi (Gennari and Weise, 2008). Decreased sulfomucins have been identified in humans suffering from gastrointestinal distress (Dawson et al., 2009). Therefore, increased availability of these compounds during enteropathy could aid the host.
Coccidiosis in poultry is a parasitic disease caused by multiple species in the genus Eimeria that compromises the intestinal barrier functionality leading to diarrhea (McDougald, 1998; Yun et al., 2000; Williams, 2005; Assis et al., 2010) Coccidia are ingested by the host, invade the mucosa and cause various degrees of damage to the epithelial cells of the intestine, initiating inflammation and macroscopic lesions (Yun et al., 2000; Assis et al., 2010). Other clinical signs of coccidiosis include dehydration, nutrient malabsorption, and therefore reduced weight gain because of this parasite's disruptive properties (McDougald 1998). Intestinal maintenance is of high importance for nutrient absorption and therefore is high energy cost for the bird. Aiding the gut lining and villi during a coccidial infection is essential to maintain performance (Assis et al., 2010). It is hypothesized that the addition of sodium bisulfate will aid coccidia-challenged broilers in growth and intestinal integrity. Therefore, the objective of this study was to determine how the addition of sodium bisulfate in the diet changes broiler production and gut morphology and permeability with or without a multiple coccidia species challenge.
Materials and methods
Animal Care and Housing
A total of 1,200 day-of-hatch broilers from the North Carolina State University Chicken Education Unit (Raleigh, NC) were randomly divided into 4 experimental groups (10 pens/treatment) with a 2 × 2 factorial design, which included combinations of diet and disease (300 birds/treatment). The treatments included challenged birds on the sodium bisulfate diet (CSB), challenged birds on the standard diet (standard), nonchallenged birds on the sodium bisulfate diet (SB), and nonchallenged birds on the standard diet (control). The experiment was conducted in 4 rooms, 2 rooms were designated for the coccidia vaccination challenge, whereas 2 rooms were given no vaccination. Each room had diets randomly distributed between 10 pens, with 5 pens on the standard diet and 5 pens on the sodium bisulfate diet. Birds were placed into individual floor rearing pens dressed with new pine shavings. Broilers were reared during the spring in North Carolina, with optimal temperatures, humidity, and lighting programs set and maintained in each room based on the Cobb500 management standards. Animal welfare, temperature, humidity, and feed and water were checked twice daily over the duration of this study. All experimental procedures for broiler chickens were performed following the guidelines of the North Carolina State University Animal Care and Use Committee.
Diet
The starter diets were fed as crumbles, and the grower and finisher diets were fed as pellets. Standard-diet birds (challenged or not) were fed a corn- and soy-based feed. Sodium bisulfate–treated birds (challenged or not) were fed corn- and soy-based feed with 0.5% feed-grade sodium bisulfate (Jones-Hamilton Co., Walbridge, OH) that replaced sodium chloride (Table 1). Feed and water were available ad libitum with diets meeting or exceeding the NRC (1994) requirements. No coccidiostats, antibiotics, or enzymes were used.
Table 1.
Feed ingredients and their inclusion levels for the 2 diets used in this study.
| Ingredient | Starter |
Grower |
Finisher |
|||
|---|---|---|---|---|---|---|
| Standard/Control | (C)SB | Standard/Control | (C)SB | Standard/Control | (C)SB | |
| Corn (yellow, grain) | 53.8 | 53.6 | 60.4 | 60.1 | 63.6 | 63.4 |
| Poultry fat | 4.12 | 4.12 | 3.92 | 3.92 | 4.03 | 4.03 |
| Soybean meal | 37 | 37 | 31 | 31 | 28 | 28 |
| Calcium carbonate | 1.5 | 1.5 | 1.2 | 1.2 | 1.1 | 1.1 |
| Monodicalcium phosphate | 1.8 | 1.8 | 1.7 | 1.7 | 1.6 | 1.6 |
| Salt (plain) | 0.4 | 0.15 | 0.4 | 0.15 | 0.4 | 0.15 |
| Lysine | 0.21 | 0.21 | 0.28 | 0.28 | 0.23 | 0.23 |
| Methionine | 0.38 | 0.38 | 0.36 | 0.36 | 0.35 | 0.35 |
| Selenium premix | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Trace mineral premix | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Threonine | 0.15 | 0.15 | 0.15 | 0.15 | 0.1 | 0.1 |
| Vitamin premix | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium bisulfate | 0 | 0.5 | 0 | 0.5 | 0 | 0.5 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
Abbreviation: (C)SB= (challenged) sodium bisulfate treatments.
Performance
Broilers were individually weighed at hatch, randomly assigned a treatment, and then placed in the corresponding pen. Individual BW were also collected on day 14, 28, and 41 to analyze for treatment growth and flock uniformity. Feed added and leftover during each diet phase change was recorded to determine feed intake and feed conversion ratio (FCR). Mortality was recorded twice daily with necropsies performed to determine the cause of death.
Coccidia Inoculation
At placement, 20 pens (10 pens per room, 2 rooms per inoculation) were orally inoculated with 10× of a commercial coccidia vaccine (Merck & Co., Inc., Kenilworth, NJ). Each dose contained 4 Eimeria species: Eimeria mivati, Eimeria tenella, Eimeria acervulina, and 2 strains of Eimeria maxima. For birds not challenged with coccidia, 20 pens (10 pens per room, 2 rooms per inoculation) were sham inoculated with water.
Oocysts Shedding
Fecal material was collected from each treatment on day 7 to determine fecal shedding of oocysts. Fresh feces from the same treatment group were collected from multiple pens to obtain a pooled sample of approximately 500 g. Fecal material in each bag was massaged to evenly distribute the collection. Four 1-gram replicates per treatment of feces were added to a 50-mL conical flask and saturated in 2 mL of 2% potassium dichromate following the general guidelines of Haug et al. (2006). To count, samples were filled with 58% sugar solution until the sample solution volume reached a total of 30 mL. Each sample was vortexed to ensure even oocysts distribution followed by microscopic counting using a McMaster egg chamber. Each sample had 2 replicates counted to determine the average number of oocysts per gram of feces (OPG) per treatment.
Gross Lesion Scoring
On day 21, 20 birds per treatment (representing the mean treatment population BW) were euthanized by cervical dislocation, and their intestinal tract was isolated. Because the treatments included a mixed infection with Eimeria species having overlapping colonization sites, 1 researcher examined the upper intestines, midintestine, and ceca for macroscopic lesions associated with the coccidia species in the vaccination. Birds were subjectively scored based on severity of lesions through the intestinal tract using the Poultry Coccidiosis Diagnostic and Testing Procedures as a guide (Conway and McKenzie, 2007). Briefly, a 0 to 4 scale was used to assess macroscopic damage at the duodenal loop/upper intestines to approximately 1 inch above Meckel's diverticulum, the midintestines approximately 1 inch above and 1 inch below Meckel's diverticulum, and each cecum. A score of “0” represented no visual lesions, “1” was minimal lesions, “2” was moderate lesions, “3” was severe lesions, and “4” was extremely severe. The researcher was blind to which birds correlated with which treatment.
Histopathology
Twenty birds per treatment on day 21 and 10 birds per treatment on day 41 were euthanized, and a tissue sample was isolated by cutting at Meckel's diverticulum and approximately 2.5 cm toward the duodenal loop. Intestinal tissue was flushed with PBS (Fisher Scientific, Waltham, MA) and then placed into approximately 10 mL of 10% buffered formalin and stored at room temperature for 3 wk. Samples were then transferred to 70% ethanol for 3 D and then sent to the College of Veterinary Medicine, North Carolina State University for processing and hematoxylin–eosin staining. Samples on slides were microscopically measured using an AmScope, version 3.7 (Irvine, California). Three villi per bird were examined for oocysts presence, and the following villi measurements were collected: villus height, villus apical width, villus base width, crypt depth, and muscularis thickness.
Intestinal Permeability
Intestinal macromolecular permeability testing was carried out using a similar method to Baxter et al. (2017) with minor modifications. Permeability was determined by giving an oral gavage of 4 mg of fluorescein isothiocyanate-dextran (FITC-D) (4 kDa; Sigma-Aldrich Co., St. Louis, MO) dissolved in distilled water per kilogram of broiler BW. Twenty birds per treatment were randomly selected on day 21, weighed, and then dosed with the appropriate volume of FITC-D solution. Marked birds were placed back into their pens for 1.5–2 h with free access to feed and water. After, 2 mL of blood per bird was collected from the ulnar vein using a heparinized syringe and stored in a capped, heparinized tube. Blood samples were stored in a cooler to decrease light exposure during sampling. Samples were centrifuged (5,000 g for 5 min), and the plasma was isolated. Fluorescence in plasma samples was read using a FilterMax F5 microplate reader (Molecular Devices, San Jose, California).
Statistical Analysis
Data were analyzed as a 2 × 2 factorial in JMP Pro 14 using GLM (SAS Institute Inc., Cary, NC). For the gut permeability data, all 4 treatments had their means separated with Dunnett's test (P ≤ 0.05) with the nonchallenged birds fed the standard diet considered the control. Because a treatment effect was found with coccidiosis, analysis of diets with treatments having the same disease state was compared using a Student t test (P ≤ 0.05). These data included growth performance, OPG counts, lesion scoring, histology, and gut permeability. Due to the necessity of separating coccidia-challenged and nonchallenged treatments, each dietary treatment was also compared by room to determine if there was a room effect.
Results
Growth and Feed Intake
Growth and feed intake were negatively impacted by the coccidiosis challenge (Table 2, Table 3, Table 4). BW were significantly heavier for CSB on day 14 (P < 0.0001), 28 (P < 0.0001), and 41 (P = 0.002). A room effect was observed on day 28 for the BW of both the challenged treatments. Feed conversion ratio was significantly lower for CSB on day 0 to 14 (P = 0.002). There was no difference in flock uniformity for challenged treatments. Nonchallenged birds had no difference in individual BW, FCR, or flock uniformity after placement. The SB treatment was significantly lighter than the control at placement (P = 0.05), but growth and feed intake was not altered because of this difference based on subsequent measurements. The SB treatment had a room effect on day 28 for BW.
Table 2.
The mean individual BW (kilograms) and SEM of each treatment group before diet-phase changes.
| Treatment | Day 0 | P value | Day 14 | P value | Day 28∗ | P value | Day 41 | P value |
|---|---|---|---|---|---|---|---|---|
| CSB | 0.049 | 0.77 | 0.58 | <0.0001 | 1.81 | <0.0001 | 3.29 | 0.02 |
| Standard | 0.049 | 0.54 | 1.74 | 3.23 | ||||
| SB | 0.048 | 0.05 | 0.62 | 0.96 | 1.87 | 0.46 | 3.36 | 0.17 |
| Control | 0.049 | 0.62 | 1.87 | 3.39 | ||||
| SEM | 0.0002 | 0.003 | 0.001 | 0.016 |
Abbreviations: CSB, challenged sodium bisulfate treatment group; SB, nonchallenged sodium bisulfate treatment group.
Differences were considered significant at P ≤ 0.05.
A room effect was observed.
Table 3.
The average FCR with the SEM of each treatment group before diet-phase changes.
| Treatment | Day 0–14 | P value | Day 14–28 | P value | Day 28–41 | P value | Day 0–41 | P value |
|---|---|---|---|---|---|---|---|---|
| CSB | 1.22 | 0.002 | 1.72 | 0.59 | 1.8 | 0.34 | 1.63 | 0.06 |
| Standard | 1.25 | 1.75 | 1.85 | 1.68 | ||||
| SB | 1.18 | 0.47 | 1.53 | 0.62 | 1.77 | 0.94 | 1.63 | 0.51 |
| Control | 1.19 | 1.57 | 1.76 | 1.64 | ||||
| SEM | 0.01 | 0.02 | 0.04 | 0.02 |
Abbreviations: CSB, challenged sodium bisulfate treatment group; FCR, feed conversion ratio; SB, nonchallenged sodium bisulfate treatment group.
Differences were considered significant at P ≤ 0.05.
Table 4.
The CV calculated to determine treatment flock uniformity at 3 time periods.
| Treatment | Day 14 | P value | Day 28 | P value | Day 41 | P value |
|---|---|---|---|---|---|---|
| CSB | 11 | 0.75 | 8 | 0.26 | 7 | 0.64 |
| Standard | 12 | 9 | 7 | |||
| SB | 8 | 0.33 | 7 | 0.38 | 6 | 0.08 |
| Control | 8 | 8 | 7 | |||
| SEM | 0.7 | 0.4 | 0.5 |
Abbreviations: CSB, challenged sodium bisulfate treatment group; SB, nonchallenged sodium bisulfate treatment group.
Differences were considered significant at P ≤ 0.05.
Oocysts Shedding and Lesion Scores
None of the nonchallenged treatments had oocysts found in their feces or had lesion scores associated with the coccidia species (Table 5). Therefore, data presented are based on the challenged treatments. There was no significant difference in the average OPG between diets (P = 0.72) nor was there a difference in subjective lesion scoring for the duodenal loop (P = 0.07), midintestine (P = 0.25), or ceca (P = 1). No room effect was observed for the oocyst shedding or lesion scores. No correlations were seen between the subjective lesion scores and the BW, gut morphology, or permeability.
Table 5.
Oocysts per gram (OPG) counts from day 7 and lesion scoring of the intestine on day 21 for challenged treatments.
| Day 7 |
Day 21 |
|||
|---|---|---|---|---|
| OPG | Upper intestine | Midintestine | Ceca | |
| CSB | 46,000 | 1.3 | 0.2 | 0.75 |
| Standard | 50,000 | 0 | 0.05 | 0.75 |
| P-value | 0.72 | 0.07 | 0.25 | 1 |
Abbreviations: CSB, challenged sodium bisulfate treatment group; OPG, oocysts per gram of feces.
Differences were considered significant at P ≤ 0.05.
Gut Morphology and Permeability
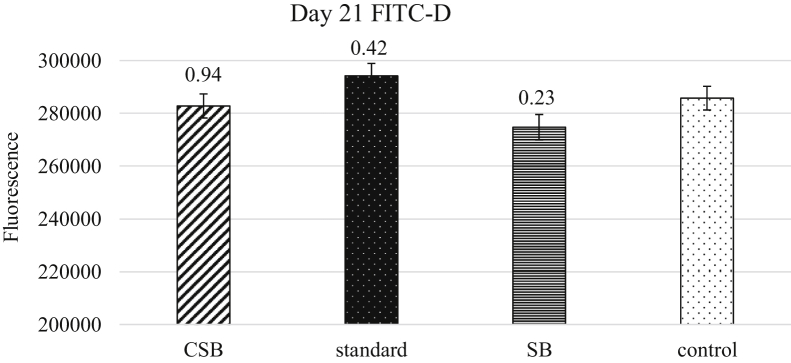
Histology measurements were compared between treatments with the same challenge but on varying diets (Table 6). For the challenged treatments, the crypt depth of CSB was significantly smaller (P = 0.02) leading to a smaller crypt: height ratio (P = 0.03) on day 21. On day 41, CSB had smaller apical width (P = 0.03) and a thinner muscularis (P = 0.04) compared with the standard. For the nonchallenged treatments, the control treatment had significantly larger base width (P = 0.04) than SB on day 21. On day 41, control treatment had shorter villus height (P = 0.03), thicker base width (P = 0.04), thinner muscularis (P = 0.03), and a greater crypt:height ratio (P = 0.01). Fluorescence levels of blood samples collected on day 21 were compared between the 4 treatments to determine gut integrity where CSB had the closest florescence value to the control (P = 0.94) with no statistical differences found between the treatment groups (Figure 1). No room effect was observed for gut morphology or permeability.
Table 6.
Average villi measurements (μm) of the 4 treatments from day 21 and 41.
| Day 21 |
Day 41 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSB | Standard | P value | SB | Control | P value | CSB | Standard | P value | SB | Control | P value | |
| Villus height | 839 | 820 | 0.45 | 868 | 891 | 0.46 | 1,026 | 1,060 | 0.52 | 1,123 | 997 | 0.03 |
| Apical width | 152 | 151 | 0.92 | 154 | 151 | 0.73 | 106 | 126 | 0.03 | 110 | 127 | 0.13 |
| Base width | 141 | 141 | 0.98 | 122 | 128 | 0.04 | 107 | 116 | 0.4 | 113 | 137 | 0.04 |
| Crypt | 165 | 196 | 0.02 | 114 | 116 | 0.73 | 138 | 157 | 0.24 | 130 | 155 | 0.11 |
| Muscularis | 149 | 151 | 0.8 | 188 | 179 | 0.29 | 169 | 200 | 0.04 | 238 | 231 | 0.03 |
| Crypt: height ratio | 0.2 | 0.24 | 0.03 | 0.14 | 0.14 | 0.48 | 0.14 | 0.16 | 0.53 | 0.13 | 0.16 | 0.01 |
Abbreviations: CSB, challenged sodium bisulfate treatment group; SB, sodium bisulfate treatment group.
Differences were considered significant at P ≤ 0.05.
Figure 1.
Fluorescence of plasma measured on day 21 (mean ± SEM) with treatment groups compared using the Duncan's test (P ≤ 0.05) with the nonvaccinated control group designated as the control treatment. P-values are indicated above each treatment bar. Abbreviations: CSB, challenged sodium bisulfate treatment group; FITC-D, fluorescein isothiocyanate-dextran; SB, nonchallenged sodium bisulfate treatment group.
Discussion
Performance
Sodium bisulfate has shown to improve feed conversion in broilers (Ruiz-Feria et al., 2011). Other studies have shown that the addition of sodium bisulfate in litter increases BW, while decreasing the number of broilers with signs of ascites or respiratory distress (Terzich et al., 1998; Line, 2002). These studies were focused on applying sodium bisulfate to the litter, whereas the present study was focused on applying sodium bisulfate into the feed. Limited differences in performance were seen with nonchallenged broilers on the varying diets in this study. Hooge et al., 1999 showed that the addition of a sodium sulfate, not bisulfate, source in the broiler diet had similar effects on broiler growth and lesion scores compared with other salts if birds were given an acute coccidial challenge. The present study indicates a benefit in growth if sodium bisulfate was supplemented in the feed during a prolonged challenge. Coccidiosis is known for reducing early live performance by increasing FCR while decreasing BW (Christaki et al., 2004). A lower feed conversion was observed during the starter period for SB-challenged birds (P = 0.002), the time period at which many broiler producers are concerned about performance when vaccinating meat-producing broilers. A significant increase in BW for CSB was observed when compared with the standard treatment on day 14, 28, and 41 (Table 2). A room effect was observed for the challenged treatments on day 28. The light timer broke at the beginning of week 4, resulting in no dark cycle for approximately 2 days in one of the challenged rooms. There was no change in the monitored environment to justify the room effect for the SB treatment in the nonchallenged rooms. It was understood that environmental effects could alter performance results of the study when using multiple rearing facilities. However, owing to the inability to control cross contamination of oocysts in a pen trial, separation of broilers inoculated with coccidia from the nonchallenged broilers was necessary to ensure that there was no contamination. Even with the room effect, the combination of improved feed efficiency and increased BW with CSB suggests some alleviation of the negative clinical signs of coccidiosis during early rearing. Growth performance may be altered by factors other than the coccidia challenge, which highlights the necessity to incorporate multiple parameters to measure intervention strategies against coccidiosis within a study (Chasser et al., 2020).
Disease Signs
Macroscopic and microscopic pathology were used to determine if the addition of SB altered parasite cycling in broilers. Oocyst counts in the feces on day 7 and subjective lesion scoring on day 21 indicated no difference in disease state due to the diet (Table 5). For this study, a combination vaccine containing E. acervulina, E. maxima (2 strains), E. mivati, and E. tenella was used to mimic field conditions, where most cases of coccidiosis have more than one infective species (Reisinger et al., 2011). Microscopic observations were not used to differentiate species of oocysts in the feces or intestinal tract. The prepatent period of Eimeria species used in this study vary from 4 to 6 days after inoculation, and oocysts can be shed into the litter several days afterward (McDougald, 2013). Day 7 was selected to measure OPG so that a representative population of the Eimeria species could be in the fecal samples. Fecal shedding of the oocysts is an indicator of how parasite load is affected by treatments (Chasser, 2020). Based on no significant differences found in OPG between diets, it can be inferred that sodium bisulfate did not alter the shedding of these parasites. Damage of the digestive tract and not damage due to a specific species was analyzed owing to the inoculation containing Eimeria species that can colonize in similar areas of the digestive tract. Day 21 was selected for measuring macroscopic lesions owing to this time corresponding with the greatest intestinal damage due to coccidiosis if Eimeria species are routinely cycling (McDougald, 2013). The recorded intensity of visual lesion damage was low, but this could be due to the subjective nature of the scoring system and the single-day observation. Lesion scoring has been suggested to understate the Eimeria species impact on gut health indicating that observing only lesion scores may not be indicative of disease (Chasser et al., 2020). Based on no significant differences detected on the oocysts shedding and the lesion scoring, it can be inferred that SB had limited effects on the life cycle of the Eimeria species.
Oocysts alter villi functionality by invading the protective mucosal layer and enterocytes. This leads to innate immune responses and intestinal morphologic changes, causing villi sloughing to remove damaged cells, a decrease in nutrient absorption, and an inflammatory response (Yun et al., 2000). In this study, oocysts were identified in both inoculated treatments invading villi on day 21 at the third (and greatest) peak of the cocci cycling. As expected, villi were shortened owing to the challenge independent of the diet (Table 6). Shortening of the villi and increased crypt depth to compensate for epithelial cell loss has been recorded during a coccidia challenge (Fernando and McCraw, 1973; Al-Sheikhly and Al-Saieg, 1980). This correlates with decreased BW, potentially due to the increased cell turnover from the crypts, leading to immature and nonspecialized enterocytes and therefore nutrient malabsorption (Fernando and McCraw, 1973). The SB-challenged birds had greater BW with a smaller crypt (P = 0.02) and smaller crypt: height ratio (P = 0.03) on day 21, supporting the idea that this feed additive favorably altered the intestinal morphology if coccidia were present. On day 41, all measurements were increased for the challenged birds consuming the control diet. This could indicate compensatory hypertrophy, which has been seen in other coccidiosis models (Fernando and McCraw, 1973). A thinner muscularis was observed on day 41 for SB-challenged birds (P = 0.04), whereas the nonvaccinated birds consuming SB had a thicker muscularis (P = 0.03). Broilers consuming antibiotic growth promotors tend to have a thinner intestinal wall, potentially owing to fewer accumulation of inflammatory cells (Kuttappan et al., 2015). The changes in muscularis thickness in this study could be due to inflammation. However, further research is warranted before conclusions can be made. Intestinal morphology at 21 and 41 D indicated a favorable response with SB if birds are suffering from coccidiosis.
Intestinal Permeability
During infection, intestinal permeability is altered where plasma proteins leak from the lumen into the bloodstream of challenged broilers because of damage of the mucosa. The intestinal lumen also becomes acidified during a coccidia infection, which decreases gut passage time while altering absorption (Williams, 2005; Chapman, 2014). Fluorescein isothiocyanate-dextran is a fluorescent marker that is passively absorbed through tight junctions of the intestinal epithelia and can be detected in blood plasma or serum using a fluorescent reader. Metabolic and immune parameters of the host affect the permeability of FITC-D, and it is common to restrict feed in determining intestinal integrity with this marker (Kuttappan et al., 2015; Baxter et al., 2017; Woting and Blaut, 2018). This was not performed in our model because of the concern with an interaction in feed restriction and disease state as well as feed-to-gain ratio in the broilers. A disease state will cause a change in gut permeability (Kuttappan et al., 2015). The challenged SB birds had permeability similar to the nonvaccinated control (P = 0.94) (Figure 1). At this point in the coccidia cycling, the greatest intestinal damage is expected. Based on our findings for intestinal permeability using FITC-D, sodium bisulfate consumed during a disease state had intestinal integrity similar to that of a broiler without a disease challenge.
Conclusion
Nutritional status of the bird influences its response to stressed states, such as coccidiosis (Yun et al., 2000). Sodium bisulfate may indirectly improve growth performance of coccidia-challenged broilers by supporting intestinal structure and function. No significant differences in parasite shedding or lesion scores between treatments indicate that sodium bisulfate had no direct effect on Eimeria oocysts or their replication. This study indicates that the addition of sodium bisulfate is efficacious for broiler performance during a coccidia challenge if birds are reared in ideal environmental conditions.
Acknowledgments
The authors thank Jones Hamilton Co for financial support for this project.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Ahmad T., Mushtaq T., Mahr Un N., Sarwar M., Hooge D.M., Mirza M.A. Effect of different non-chloride sodium sources on the performance of heat-stressed broiler chickens. Br. Poult. Sci. 2006;47:249–256. doi: 10.1080/00071660600753342. [DOI] [PubMed] [Google Scholar]
- Al-Sheikhly F., Al-Saieg A. Role of Coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 1980;24:324–333. [PubMed] [Google Scholar]
- Assis R.C., Luns F.D., Beletti M.E., Assis R.L., Nasser N.M., Faria E.S., Cury M.C. Histomorphometry and macroscopic intestinal lesions in broilers infected with Eimeria acervulina. Vet. Parasitol. 2010;168:185–189. doi: 10.1016/j.vetpar.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Baxter M.F.A., Merino-Guzman R., Latorre J.D., Mahaffey B.D., Yang Y.C., Teague K.D., Graham L.E., Wolfenden A.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Optimizing fluorescein isothiocyanate dextran measurement as a Biomarker in a 24-h feed restriction model to Induce gut permeability in broiler chickens. Front Vet. Sci. 2017;4:56. doi: 10.3389/fvets.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.D. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Chasser K.M., Duff A.F., Wilson K.M., Briggs W.N., Latorre J.D., Barta J.R., Bielke L.R. Research Note: Evaluating fecal shedding of oocysts in relation to body weight gain and lesion scores during Eimeria infection. Poult. Sci. 2020;99:886–892. doi: 10.1016/j.psj.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christaki E., Florou-Paneri P., Giannenas I., Papazahariadou M., Botsoglou N., Spais A. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim. Res. 2004;53:137–144. [Google Scholar]
- Conway D.P., McKenzie M.E. 2007. Poultry Coccidiosis Diagnostic Testing Procedures, Blackwell Publishing. Oxford, United Kingdom. [Google Scholar]
- Dawson P.A., Huxley S., Gardiner B., Tran T., McAuley J.L., Grimmond S., McGuckin M.A., Markovich D. Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut. 2009;58:910–919. doi: 10.1136/gut.2007.147595. [DOI] [PubMed] [Google Scholar]
- Fernando M.A., McCraw B.M. Mucosal morphology and cellular renewal in the intestine of chickens following a single infection of Eimeria acervulina. J. Parasitol. 1973;59:493–501. [PubMed] [Google Scholar]
- Gennari F.J., Weise W.J. Acid-base disturbances in gastrointestinal disease. Clin. J. Am. Soc. Nephrol. 2008;3:1861–1868. doi: 10.2215/CJN.02450508. [DOI] [PubMed] [Google Scholar]
- Haug A., Williams R.B., Larsen S. Counting coccidial oocysts in chicken faeces: a comparative study of a standard McMaster technique and a new rapid method. Vet. Parasitol. 2006;136:233–242. doi: 10.1016/j.vetpar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Hooge D.M., Cummings K.R., McNaughton J.L. Evaluation of sodium bicarbonate, chloride, or sulfate with a coccidiostat in corn-soy or corn-soy-meat diets for broiler chickens. Poult. Sci. 1999;78:1300–1306. doi: 10.1093/ps/78.9.1300. [DOI] [PubMed] [Google Scholar]
- Hunolt A.E., Maguire R.O., Ogejo J.A., Badgley B.D., Frame W.H., Reiter M.S. Multiple Applications of sodium bisulfate to broiler litter affect ammonia Release and litter properties. J. Environ. Qual. 2015;44:1903–1910. doi: 10.2134/jeq2015.05.0214. [DOI] [PubMed] [Google Scholar]
- Kassem, Sanad Y.M., Stonerock R., Rajashekara G. An evaluation of the effect of sodium bisulfate as a feed additive on Salmonella enterica serotype Enteritidis in experimentally infected broilers. Poult. Sci. 2012;91:1032–1037. doi: 10.3382/ps.2011-01935. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Berghman L.R., Vicuna E.A., Latorre J.D., Menconi A., Wolchok J.D., Wolfenden A.D., Faulkner O.B., Tellez G.I., Hargis B.M., Bielke L.R. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 2015;94:1220–1226. doi: 10.3382/ps/pev114. [DOI] [PubMed] [Google Scholar]
- Line J.E. Campylobacter and Salmonella populations associated with chickens raised on acidified litter. Poult. Sci. 2002;81:1473–1477. doi: 10.1093/ps/81.10.1473. [DOI] [PubMed] [Google Scholar]
- Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol. Rev. 2001;81:1499–1533. doi: 10.1152/physrev.2001.81.4.1499. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. Intestinal protozoa important to poultry. Poult. Sci. 1998;77:1156–1158. doi: 10.1093/ps/77.8.1156. [DOI] [PubMed] [Google Scholar]
- McDougald L.R. In: Protozoal Infections in Diseases of Poultry. Swayne D.E., editor. John Wiley & Sons; Ames, Iowa: 2013. [Google Scholar]
- National Research Council. The National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Pope M.J., Cherry T.E. An evaluation of the presence of pathogens on broilers raised on poultry litter treatment-treated litter. Poult. Sci. 2000;79:1351–1355. doi: 10.1093/ps/79.9.1351. [DOI] [PubMed] [Google Scholar]
- Reisinger N., Steiner T., Nitsch S., Schatzmayr G., Applegate T.J. Effects of a blend of essential oils on broiler performance and intestinal morphology during coccidial vaccine exposure. J. Appl. Poult. Res. 2011;20:272–283. [Google Scholar]
- Ruiz-Feria C.A., Larrison E., Davis M., Farnell M., Carey J., Grimes J.L., Pitts J. Supplementation of feed Grade sodium bisulfate in broiler diets improves feed efficiency. Int. J. Poult. Sci. 2011;10:670–676. [Google Scholar]
- Terzich M., Quarles C., Goodwin M.A., Brown J. Effect of Poultry Litter Treatment(R) (PLT(R)) on the development of respiratory tract lesions in broilers. Avian Pathol. 1998;27:566–569. doi: 10.1080/03079459808419385. [DOI] [PubMed] [Google Scholar]
- Whittamore J.M., Hatch M. Loss of the anion exchanger DRA (Slc26a3), or PAT1 (Slc26a6), alters sulfate transport by the distal ileum and overall sulfate homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;313:G166–G179. doi: 10.1152/ajpgi.00079.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Williams Z.T., Blake J.P., Macklin K.S. The effect of sodium bisulfate on Salmonella viability in broiler litter. Poult. Sci. 2012;91:2083–2088. doi: 10.3382/ps.2011-01976. [DOI] [PubMed] [Google Scholar]
- Woting A., Blaut M. Small intestinal permeability and gut-Transit time determined with low and high Molecular weight fluorescein isothiocyanate-Dextrans in C3H Mice. Nutrients. 2018;10:685. doi: 10.3390/nu10060685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C.H., Lillehoj H.S., Lillehoj E.P. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000;24:303–324. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]
- Zdunczyk Z., Jankowski J., Juskiewicz J., Kwiecinski P. The response of the gastrointestinal tract of broiler chickens to different dietary levels and sources of sodium. Veterinarija Ir Zootechnika. 2012;60:92–98. [Google Scholar]