Abstract
In this study, we aimed to assess the feasibility of the lactic acid bacterium Lactobacillus kefiranofaciens DN1 (LKF_DN1) and the yeast Kluyveromyces marxianus KU140723-05 (KMA5), recently isolated from kefir, as probiotics. Specifically, we evaluated the effect of early administration of these 2 microbes on the inhibition of Salmonella Enteritidis (SE) colonization in neonatal chicks. We also examined the effects of exposure of chicks to probiotics before SE exposure on the reduction in the number of gut SE. A total of 108 1-day-old specific-pathogen-free male layer chicks were used for 3 independent experiments. The experimental chicks were randomly divided into 6 groups (negative control: basal diet [BD] without probiotics and SE; positive control: BD; probiotic group [PG] 1: BD + LKF_DN1; PG2: BD + KMA5; PG3: BD + LKF_DN1 + KMA5; and PG4: BD+ a commercial product IDF-7), all of which, except negative control, were coadministered with SE strain resistant to rifampicin (SERR). We found that the administration of LKF_DN1 and/or KMA5 reduced the number of viable cells of the SERR strain in chicks by up to 1.90 log10, relative to positive control chicks. Compared with late administration (day [D] 10 and D11), early administration (D1 and D2) of the probiotics was more effective in reducing SERR cell numbers in the gut. Furthermore, we detected no significant difference in the reduction of gut SERR cell numbers in chicks from the same groups exposed to the probiotics at D10 and D11 before and after administration with SERR. Collectively, our findings indicate that, as dietary additives, LKF_DN1 and KMA5 showed potential probiotic activity in chicks. Moreover, the combination of the lactic acid bacteria and/or yeast strain was found to rapidly reduce SE numbers in the chick gut and showed a prolonged inhibitory effect against SE colonization. We, thus, propose that the administration of these 2 probiotics, as early as possible after hatching, would be considerably effective in controlling SE colonization in the guts of chicks.
Key words: poultry, probiotic, Salmonella Enteritidis, early administration, dietary additive
Introduction
It is estimated that approximately 1.2 million cases of the human disease salmonellosis occur annually in the United States, including 23,000 hospitalizations and 450 deaths (CDC, 2019). A significant proportion of human salmonellosis cases is caused by Salmonella enterica subsp. enterica serovar Enteritidis (S. Enteritidis, SE), a zoonotic pathogen, which is contracted via the consumption of poultry and poultry-derived products contaminated with pathogenic Salmonella spp. (Ao et al., 2015; Lee et al., 2015; Eguale, 2018). Poultry meats and eggs have been considered as the main reservoirs of SE (CDC, 2018; ECDC, 2018). The subtherapeutic use of antibiotics, which have prophylactic and growth-promoting effects in food-producing animals, contributed to meeting the demands for animal proteins and preventing bacterial diseases (Hao et al., 2014; Nhung et al., 2016). However, over the past few decades, restrictions on the prophylactic usage of antibiotics in animal feed have led to reductions in meat production and a concomitant increase in bacterial infections, resulting in economic losses in the poultry industry (Gadde et al., 2017). Moreover, there has been an increase in the frequency with which Salmonella-contaminated meats, eggs, or animal-derived products can enter the food chain, which may potentially have a high economic cost with respect to the outbreak of foodborne diseases (Morley et al., 2011; Lee et al., 2013; McEwen et al., 2018; Tang et al., 2019). In accordance with the latest report published by the USDA Food Safety and Inspection Service, approximately 1,532 to 174,207 pounds of poultry or poultry-derived products from each case have been recalled as a consequence of suspected contamination with Salmonella in 2018 to 2019 (USDA-FSIS, 2019). Consequently, there is an urgent need to identify novel methods of farm management as an alternative to the use of antibiotics that can be used to reduce the pathogen load in the guts of poultry (from neonatal to adult chickens).
Alternatives to antibiotics, including the use of bacteriophages, vaccines, and probiotics, have been widely studied for their ability to reduce or inhibit Salmonella colonization in the guts of chickens (Andreatti et al., 2007; Prado-Rebolledo et al., 2017; McWhorter and Chousalkar, 2018). An ideal alternative for gut pathogen control would entail the selective and effective eradication of foodborne pathogens without any appreciable changes (or with beneficial changes) in gut microbial structure and function. Following the study conducted by Rantala and Nurmi, who demonstrated the effects of the gut microbiota of mature birds on protecting young chicks from Salmonella infection, numerous studies have assessed the usage of probiotics in the poultry industry, with a view toward promoting the gut health of birds and reducing potential human pathogen levels in this organ (Rantala and Nurmi, 1973; Higgins et al., 2007; 2008; De Oliveira et al., 2014; Prado-Rebolledo et al., 2017).
Kefir, a fermented dairy product originated from the Caucasus mountain region, contains multiple species of probiotic bacteria and yeast that has been demonstrated to promote host gut health and immune functions (Irigoyen et al., 2005; Vinderola et al., 2006; Bellikci-Koyu et al., 2019; Kim et al., 2019b). Our earlier studies showed that 2 probiotics isolated from kefir, the lactic acid bacterium Lactobacillus kefiranofaciens DN1 (LKF_DN1) and the yeast Kluyveromyces marxianus KU140723-05 (KMA5), also appeared to have potential probiotic activity through high antibacterial activity, resistance to gastrointestinal environments (e.g., acid and bile tolerance), and adhesion ability to intestinal cells (Kim et al., 2016; Jeong et al., 2017a; Cho et al., 2018; Lim et al., 2019). The studies showed the probiotic strains with excellent performance on high survivability in the host gastrointestinal tract, low pathogenicity, antimicrobial activity against pathogens including Salmonella and Listeria spp., and modulation of the intestinal microbiota in mice (Jeong et al., 2017a; Lim et al., 2019). In accordance with a previous study, supplementation of drinking water with kefir has been found to effectively prevent the colonization of Campylobacter jejuni in chicks (Zacconi et al., 2003). In the current present study, we evaluated the single and mixed probiotic strains with LKF_DN1 and KMA5 for the prospective applicability to reduce the colonization of SE in the gut of young chicks.
Although the production of pathogen-free chicken meats and eggs is theoretically achievable, it is practically unrealistic. Thus, a regimen to reduce pathogens in the gastrointestinal track of chickens during the entire rearing period may be required to ensure safe poultry production and promote intestinal microbial eubiosis. Notably, SE infection can cause more severe and sustained illness in young chicks than in older birds, owing to a higher susceptibility to enteropathogens (Mon et al., 2015) at this age. Unlike antibiotics that can reduce gut microbial diversity involved in a healthy gut, probiotics can potentially be more suitable for enhancing the intestinal mucosal barrier and immune function in chicks. Mon et al. demonstrated that the SE infection in young chicks significantly reduced cecal microbial diversity with expansion of the family Enterobacteriaceae (including many pathogens) and reduction of the family Lachnospiraceae (including butyrate-producing bacteria) when compared with older and noninfected chicks. Consequently, protection against external pathogens in neonatal animals may be more important than that in older animals. Therefore, in the present study, we aimed to assess the feasibility of using LKF_DN1 and KMA5 as dietary additives for chickens and evaluated the optimal effect of early administration of these probiotics on SE colonization in newly hatched chicks based on 3 different experimental approaches.
Materials and methods
Cultures, Diet, and Animals
The 2 probiotic organisms LKF_DN1 (lactic acid bacterium) and KMA5 (yeast), which were previously isolated from kefir (SensorGen Inc., Seoul, Republic of Korea), were grown in de Man, Rogosa, and Sharpe broth (MRS) (Difco Laboratories, Detroit, MI) at 30°C for 72 to 96 h and in potato dextrose broth (PDB) (Difco Laboratories) at 30°C for 48 h, respectively, as previously described (Cho et al., 2018; Jeong et al., 2017a). We also used a commercial probiotic product, IDF-7, which contains a mixed culture of Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactococcus lactis, Bifidobacterium breve, and Bifidobacterium animalis ssp. lactis. (Ildong Foodis Co., Ltd., Seoul, Republic of Korea). The probiotics were incubated anaerobically in a Whitley DG250 Anaerobic Workstation (Don Whitley Scientific Ltd., Shipley, England) with a gas mixture of 90% N2, 5% H2, and 5% CO2. A strain of SE resistant to rifampicin (SERR) (>128 mg/L), obtained from Dr. Richard K. Gast (USDA/ARS Southeast Poultry Research Laboratory, GA), was used as a reference strain and maintained as previously described (Holt et al., 1999). The SERR strain was grown in tryptic soy broth (TSB) (Difco Laboratories) containing 64 μg/mL rifampicin and stored in TSB containing 20% glycerol at −70°C until use. Chicks were fed a diet purchased from AT Immune Inc. (Cheongju, Choongbuk, Republic of Korea). During the experimental period, there was no supplementation of feed with either antibiotic agents or growth promoters. The Hy-Line Brown commercial male layer chicks used in this study were obtained from a commercial hatchery (Korean Poultry TS Co., Ltd., Icheon-si, Gyunggi-do, Republic of Korea).
Experiment 1
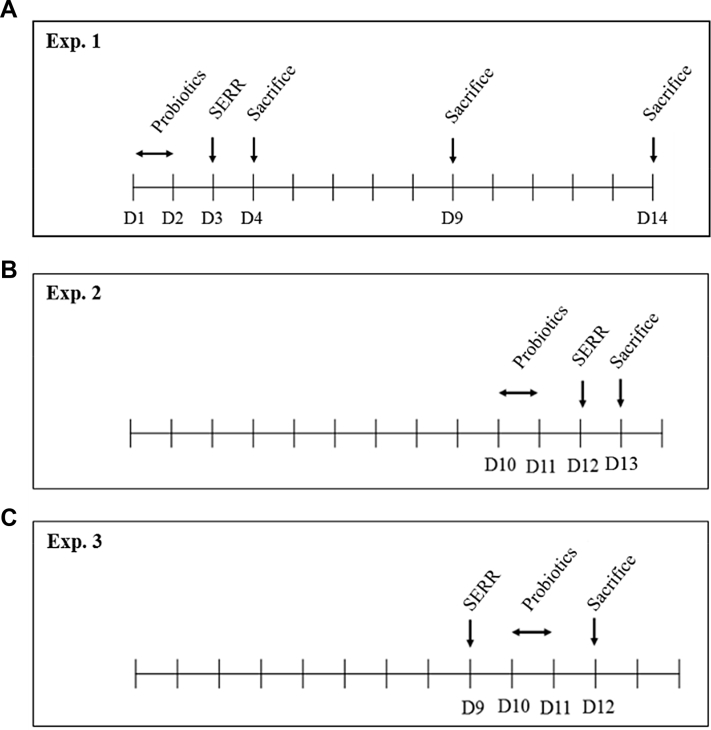
A total of 108 day-of-hatch specific-pathogen-free layer chicks (Hy-Line Brown) were randomly divided into 6 groups, 18 birds/group, as shown in Table 1. On day 1 at the hatchery, chicks in the probiotic-treated groups (PG1, PG2, PG3, and PG4) were orally administrated 0.2 mL of the activated LKF_DN1 (1.22 × 107/chick), 0.2 mL KMA5 (1.23 × 106/chick), 0.1 mL LKF_DN1 and 0.1 mL KMA5 (LKF_DN1 + KMA5), or 0.2 mL IDF-7 (3.24 × 107/chick) cultures before initial feeding and drinking, respectively. The chicks were transported and placed into 1 of the 6 dedicated chicken SK-ISO-600 HBC2 isolators (Three-shine Inc., Daejeon, Republic of Korea) at an experimental station for chickens (Konkuk University, Seoul, Republic of Korea). On day 2, probiotics were again administered to the chicks in the probiotic-treated groups, followed by SERR challenge (3.20 × 107/chick) at 3 d of age. L. kefiranofaciens DN1and KMA5 were cultured in MRS and PDB, respectively, and orally administered. Therefore, in the effort to minimize the potential nutritional impact between groups, 3 of 6 chicks in the negative control (NC) and positive control (PC) groups were orally administered 200 μL of MRS, and the other 3 chicks were orally administered 200 μL of PDB on day 1 and day 2 corresponding probiotics administration. On day 3, the chicks in NC group were orally administered 200 μL of TSB corresponding SERR administration. During the first week of the experiment, the temperature in the chicken isolator was set to 34°C and was thereafter reduced to 30°C until the end of the experiment (day 14). Throughout the 2-wk experimental period, the chicks were illuminated with a 23L:1H schedule. During this period, feed and drinking water were provided ad libitum. The scheme of experiment 1 is shown in Figure 1A. Briefly, 6 chicks in each group were randomly selected, euthanized with CO2 asphyxiation, and sacrificed on day 4, 9, and 14. After sacrifice, cecum samples were collected from the 36 chicks from the 6 isolators (=6 replicates for each group). The cecal digesta obtained from the intestinal segments were used immediately to determine the number of SERR. To obtain plate cell counts, the cecal and external mucosal contents that ranged from 0.1 g to 0.7 g were scraped and placed into 15-mL conical sterile polypropylene centrifuge tubes (SPL Life Sciences Co. Ltd., Pocheon-si, Republic of Korea), serially diluted 10-fold in sterile PBS, and then homogenized using a Vortex-Genie 2 Vortex (Scientific Industries Inc., Bohemia, NY) at a maximum speed for 15 s. The resulting homogenates were serially diluted, and 100 μL of each homogenate dilution was spread plated on xylose lysine deoxycholate agar (Difco Laboratories) containing 64 μg/mL rifampicin. The plates were incubated at 37°C for 24 h, and thereafter, the number of black colonies, which were assumed to be the SERR strain, was determined using the plate count method. All animal procedures used in this study were approved by the Institutional Animal Care and Use Committee at Konkuk University. The approval number is KUIBC-2019-10.
Table 1.
The lactic acid bacterium, yeast, and Salmonella Enteritidis strain administered to experimental groups1.
| Group | Lactobacillus kefiranofaciens DN1 (LKF_DN1) | Kluyveromyces marxianus KU140723-05 (KMA5) | IDF-74 | S. Enteritidis resistant to rifampicin (SERR) |
|---|---|---|---|---|
| 2NC | − | − | − | − |
| 3PC | − | − | − | + |
| PG1 | + | − | − | + |
| PG2 | − | + | − | + |
| PG3 | + | + | − | + |
| PG4 | − | − | + | + |
Three of 6 chicks in the NC and PC groups were orally administered 200 μL of MRS corresponding LKF_DN1 culture medium, and the other 3 chicks were administered 200 μL of PDB corresponding KMA5 culture medium. Chicks in NC group were orally administered 200 μL of TSB corresponding SERR.
NC, negative control.
PC, positive control.
IDF-7, a commercial product containing Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactococcus lactis, Bifidobacterium breve, and Bifidobacterium animalis ssp. lactis.
Figure 1.
In experiment 1, 108 day-of-hatch SPF layer chicks were randomly divided into 6 groups. Chicks were orally administered the probiotics LKF_DN1 and KMA5 on day 1 (D1) and D2, followed by Salmonella Enteritidis resistant to rifampicin (SERR) on D3. Six chicks from each group were sacrificed at D4, D9, and D14 to collect the ceca for viable cell counting of the SERR strain (A). In experiment 2, probiotics were administered consecutively on D10 and D11, followed by administration of the SERR strain on D12. Six chicks from each group were sacrificed on D13 (B). In experiment 3, the SERR strain was administered at D9 before probiotic administration (D10 and D11). Six chicks from each group were sacrificed on D12 (C). Abbreviations: KMA5, Kluyveromyces marxianus KU140723-05; LKF_DN1, Lactobacillus kefiranofaciens DN1; NC, negative control; PC, positive control; SPF, specific-pathogen-free.
Experiment 2
Figure 1B presents the scheme of experiment 2 to provide a comparison for the effects of probiotics exposure period on anti-SE colonization in the gut of neonatal and young chicks. For this experiment, 36 chicks were randomly assigned into 6 groups of 6 chicks/group and similarly housed in the 6 chicken isolators described in experiment 1. Over 2 consecutive days (day 10 and 11), chicks in PG1, PG2, PG3, and PG4 were administered LKF_DN1 (1.53 × 107/chick), KMA5 (7.50 × 106/chick), LKF_DN1+ KMA5, and IDF-7 (3.73 × 107/chick), respectively. Chicks in the NC group were orally administered 200 μL of MRS or PDB as described previoulsy. On day 12 chicks in groups PG1‒PG4 were orally administered the SERR strain (3.00 × 107/chick). Chicks in the NC group were also orally administered 200 μL of TSB as described previoulsy. On day 13, the chicks from each group were sacrificed, and samples of cecal digesta were collected and processed for SERR cell counts using the procedures described in experiment 1.
Experiment 3
Figure 1C shows the scheme of experiment 3 to compare the effects of the order of probiotics and SERR on anti-SE colonization in the gut of young chicks. For this experiment, 36 layer chicks were randomly divided into 6 groups with 6 replicates of 6 chicks/group and similarly housed respectively in the 6 chicken isolators as described previously. On day 9, chicks in PC and PG1–PG4 were orally administered the SERR strain (3.43 × 107/chick). Chicks in the NC group were orally administered 200 μL of TSB. At day 10 and day 11, the chicks in PG1, PG2, PG3, and PG4 groups were then administered LKF_DN1 (1.35 × 107/chick), KMA5 (6.12 × 106/chick), LKF_DN1 and KMA5, and IDF-7 (3.43 × 107/chick), respectively. Three chicks and the other 3 chicks in the NC group were orally administered 200 μL of TSB and PDB, respectively. On day 12, the chicks from each group were sacrificed, and cecal SERR cells were enumerated using xylose lysine deoxycholate agar plates containing rifampicin (64 μg/mL) as described previously.
Statistical Analysis
All experiments performed in the present study were arranged in a completely randomized design. All data sets obtained from the experiments were considered normally distributed. For all groups, data for viable cell numbers of the SERR reference strain were analyzed by one-way ANOVA followed by Tukey's test using SAS (v.9.4; SAS Institute Inc., Cary, NC). Differences between the group means were considered to be statistically significant at a P-value of less than 0.05 (P < 0.05).
Results and discussion
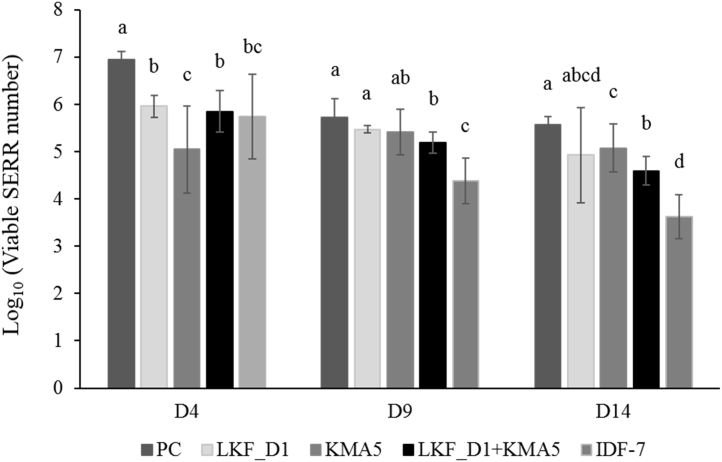
In this study, we evaluated the inhibitory effects of early administration of probiotics on SE colonization of the guts of newly hatched chicks based on 3 independent experiments. L. kefiranofaciens DN1 and/or KMA5 and IDF-7 were orally administered on day 1 and 2. The number of viable SERR cells in the cecal digesta and external mucosal surfaces in chicks from the groups treated with probiotics (LKF_DN1, KMA5, LKF_DN1 + KMA5, and IDF-7) and without probiotics (PC) on day 4, 9, and 14 are shown in Figure 2. On day 1 of after SERR challenge (day 4), we observed a significant reduction in the total number of cecal SERR in chicks in the probiotic-administered groups (P < 0.05) relative to the PC group (Figure 2). We observed a gradual decrease in the numbers of SERR cells in all groups with an increase in the number of days after probiotic administration (P < 0.05), whereas the total number of cecal SERR in the KMA5 group showed no significant difference between time points (day 4, 9, and 14) during the entire experimental period (P = 0.73). The effective reduction in the number of cecal SERR in chicks administered KMA5 for 2 d (day 1 and 2) clearly persisted from the beginning (day 4) to the end (day 14) of experiment 1 (Figure 2). Notably, the KMA5 yeast strain showed the strongest inhibitory effect against SE colonization in the large intestine of chicks on day 4. Six days after SERR challenge (day 9), we detected no significant differences in SERR strain numbers between the PC and the LKF_DN1 and KMA5 groups (Figure 2). In contrast, we observed a significant difference between the number of viable SERR between PC and LKF_DN1+ KMA5 and the commercial product IDF-7 groups. Furthermore, on day 11 of post-SERR challenge (day 14), we noted a significantly lower number of SERR viable cells in the cecum of chicks administered KMA5, LKF_DN1+ KMA5, and IDF-7 than that in the PC group (P < 0.05; Figure 2).
Figure 2.
The number of viable cells of the Salmonella Enteritidis resistant to rifampicin (SERR) strain in the cecal content of chicks in different treatment groups. SERR cell numbers were counted on day 4 (D4), 9, and 14 during the experimental period (D1, D6, and D11 after Salmonella Enteritidis administration, respectively). LKF_DN1 (1.22 × 107/chick), KMA5 (1.23 × 106/chick), and IDF-7 (3.24 × 107/chick) strains were administered on D1 and D2, followed by the SERR strain (3.20 × 107/chick) on D3. Data from experiment 1 were used. Differences in the number of viable SERR cells within each group were considered to be statistically significant at a P-value of less than 0.05 (P < 0.05). Significant differences between groups at the time points are indicated by lowercase letters. Graphs represent the mean of SERR number expressed as log10 cfu/g cecal content. Scale bars represent the SD of the mean. Abbreviations: IDF-7, a commercial product containing Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactococcus lactis, Bifidobacterium breve, and Bifidobacterium animalis ssp. lactis; KMA5, Kluyveromyces marxianus KU140723-05; LKF_DN1, Lactobacillus kefiranofaciens DN1; NC, negative control; PC, positive control.
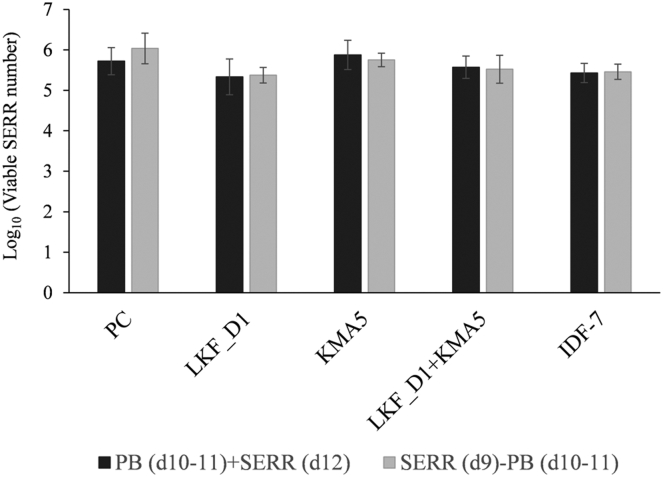
To ensure that we were able to assess the effect of early probiotic administration on inhibiting the colonization of the SERR strain in the guts of chicks, we administered probiotics on day 10 and 11 before (day 9) and after (day 12) SE administration on the assumption that chicks are increasingly becoming contaminated with Salmonella spp. derived from external sources (e.g., feed and water) (Figures 1B, 1C). Data obtained from experiments 2 and 3 revealed that the administration of probiotics before and after potential exposure to an external source Salmonella (day 9–12) was ineffective in reducing the number of gut SERR (Figure 3). The results of experiments 2 and 3 show that there were no significant differences in the number of SERR cells in the guts of chicks from the same groups in each of the 2 experiments (P > 0.05; Figure 3). Regarding the observed data for the ineffectiveness of later probiotic administration on reducing the SERR cecal colonization in young chicks, early probiotic administration in neonatal chicks may be critical to circumvent the cecal colonization of pathogens via the effect of competitive exclusion (CE) using probiotics. Revolledo et al. (2009) demonstrated the efficacy of CE products or a combination product with CE and other products (including probiotics) on reducing Salmonella contamination in only a few day-old chicks. Bolder et al. (1992) also demonstrated that CE cultures can be administered to neonatal chicks after hatching to prevent intestinal Salmonella colonization in poultry. These data including our results may be explained by the protective effect of CE with LKF_DN1 and KMA5 in neonatal chicks against Salmonella spp. introduced from external sources and environment.
Figure 3.
The number of viable cells of the Salmonella Enteritidis resistant to rifampicin (SERR) strain in chicks in each group from experiments 2 and 3 (probiotic administration before and after SERR exposure, respectively). Graphs represent the mean of SERR number expressed as log10 cfu/g cecal content. Scale bars represent the SD of the mean. Abbreviations: IDF-7, a commercial product containing Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactococcus lactis, Bifidobacterium breve, and Bifidobacterium animalis ssp. lactis; KMA5, Kluyveromyces marxianus KU140723-05; LKF_DN1, Lactobacillus kefiranofaciens DN1; NC, negative control; PC, positive control.
Effect of a Combination of Probiotics (LKF_DN1 and KMA5) Isolated From Kefir on the Reduction of SE in Chicks
To evaluate the inhibitory effects of a single lactic acid bacterium, a single yeast, or their combination on Salmonella colonization in the guts of chicks, we attempted to minimize exposure to potential external sources of these bacteria by administering chicks with probiotics soon after they had hatched at a hatchery. Oral administration of the kefir-derived probiotics LKF_DN1 and KMA5 was shown to be considerably effective in reducing SE in the intestine of the chicks at day 4, and then, a significant reduction of SE was shown in the groups with probiotic mixtures until the end of the experimental period (Figure 2). The KMA5 and LKF_DN1 strains lowered the numbers of SERR cells in chicks by up to 1.90 log10 and 0.99 log10, respectively, than that in chicks in the PC group on day 4 (Figure 2). Interestingly, we observed that the administration of KMA5 resulted in a marked reduction in the total number of cecal SERR on day 1 and 11 after SERR administration, indicating that oral administration of the yeast strain to neonatal chicks would be a highly effective approach for inhibiting gut colonization by SE strains. In addition, we found that the synergistic effect of LKF_DN1 and KMA5 strains, with respect to a reduction in the number of viable SERR cells in chicks, persisted up to day 14 (Figure 2). Previously, Higgins et al. (2007) showed that a commercial lactic acid bacterium product administered at day 1 reduced SE in the ceca of chicks by more than 1.5 log10 (Higgins et al., 2007), and consistently, in the present study, we observed that the number of SERR cells in the lactic acid bacterium–treated chicks at day 1, 6, and day 11 after SE administration was reduced by 1.90, 0.53, and 1.18 log10, respectively, when compared with the PC group (Figure 2). Accordingly, these data may indicate that the 2 probiotics LKF_DN1 and KMA5 could be used as the effective dietary additive in the poultry industry.
Effect of Early Probiotic Administration on the Reduction in SE in Chicks
Few studies have compared the effects of administering hatchery chicks with probiotics before and after exposure to feed, water, and the general external environment (Higgins et al., 2007). In experiments 1 and 2 of the present study, we therefore sought to examine the importance of the time point of probiotic administration (1–2 d vs. 10–11 d of age), before SERR administration, in reducing the number of SERR cells in chicks. We found that earlier administration of probiotics at day 1 and 2 resulted in a significantly higher reduction in the number of viable SERR cells in chicks than PC chicks (Figure 2). Specifically, we observed that reductions in the number of SERR cells in chicks orally administered the 2 probiotics, LKF_DN1 and KMA5 isolated from kefir, were 0.99 log10 and 1.90 log10, respectively, relative to the PC group on day 4 (Figure 2), whereas the reduction in the number of SERR cells in chicks administered probiotics at day 10 and 11 was not significantly shown (Figure 3). In addition, in experiment 1, we observed that the reduction in SE colonization in chicks administered probiotics (LKF_DN1 and KMA5) soon after hatching persisted for up to 2 wk (Figure 2), indicating that early probiotic administration could be extremely effective in reducing the number of enteropathogenic bacteria or inhibiting bacterial colonization. Thus, initial exposure of chicks to probiotics immediately after hatching could represent an ideal approach for inhibiting Salmonella gut colonization and thereby enhancing chick health.
Exposure to Probiotics Before Pathogens in Neonatal Chickens May Reduce the Number of Gut SE Strains
In experiments 2 and 3, we examined the changes in the number of viable SE cells in chick guts after exposure to external source Salmonella spp. and evaluated the effects of administering chicks with probiotics before and after exposure to SERR. Figure 3 shows that late administration of the probiotics did not significantly reduce the number of SERR cells between groups. Consequently, a comparison between the results obtained from the 2 experiments revealed that there were no significant differences between the same groups in the 2 studies with respect to the number of viable SERR cells in chicks, regardless of whether probiotics were administered before or after exposure to the SERR strain (Fig. 3). Combined, these findings indicate that the inhibitory effect of probiotic administration on SERR strains in chicks after being exposed to external sources for a sufficient time was somewhat lower than that observed after probiotic administration immediately after hatching.
Here, we observed that strains of both the lactic acid bacterium and yeast derived from kefir can substantially inhibit the colonization of SERR in chick cecum as single probiotic agents and that this effect may be even superior to that obtained in response to administering a commercial multiple probiotic supplement, that is currently used on avian farms. Although countless avian probiotic agents have been developed and used in the field, most of these are used to achieve limited goals, such as improving growth performance and general health conditions, and are not explicitly designed to prevent the colonization of specific pathogens, which, nevertheless, can pose a huge public health risk (Jadhav et al., 2015). However, we found that 2 novel probiotics, LKF_DN1 and KMA5, exerted a potent anti-Salmonella effect in chicks, which might have been expected given our previous findings that LKF_DN1 produces an exopolysaccharide that has a bactericidal effect on SE and is able to survive in simulated gastric and intestinal environments. Moreover, this bacterium was found to have the effect of modulating gut microbiota by reducing populations of bacteria in the family Enterobacteriaceae and increasing those of species belonging to the genus Lactobacillus (Jeong et al., 2017b). Furthermore, we found that a crude metabolite of KMA5 can inhibit the growth of SE (unpublished data) and could contribute to stearic hindrance against Salmonella spp. (i.e., CE) based on its exceptionally larger size than that of bacteria (Czerucka et al., 2007).
However, even considering the aforementioned evidence, it is clearly noteworthy that kefir microorganisms were successfully introduced to chicks as a novel host. Nevertheless, interspecific differences with respect to anatomy, metabolism, and physiology represent an inevitable hurdle in applying probiotic microorganisms to a wide range of different animal species (Smith, 2014). Although the effects of kefir have previously been examined in humans and some animals, including mice and dog (Kim et al., 2019a; Kim et al., 2019b), the present study is, to the best of our knowledge, the first to evaluate and demonstrate the efficacy of single kefir isolates on reducing Salmonella populations in the guts of young chicks. Previously, however, the effects of kefir as a whole have been examined in young chicks, and it was accordingly found that ad libitum consumption of drinking water supplemented with kefir significantly reduced the number of Campylobacter spp. in the chick cecum (Zacconi et al., 2003). The results of the present study are consistent with these findings, although it is particularly notable that we used single isolates of kefir, which are more amenable to scale-up production than that by using whole kefir (Kim et al., 2018). In addition, the chicks in the present study received only a single dose of probiotics, thereby indicating that selected kefir microorganisms could exert a long-term protective effect against Salmonella spp. On the basis of the promising findings of this study, we intend in future studies to focus on elucidating the underlying mechanisms via the use of multiomics technology and by evaluating the effects of kefir microorganisms on subsequent growth and laying performances. In addition, data from field experiments for growth and performance of chicks are needed to overcome the limitations of the present study.
Conclusions
In conclusion, LKF_DN1 and KMA5, recently isolated from kefir, would be effective dietary additives with respect to reducing SE colonization in chicks. Moreover, a combination of a lactic acid bacterial strain (LKF_DN1) and a yeast strain (KMA5) may be more effective in controlling foodborne pathogens in poultry than the use of single isolates. Furthermore, we demonstrated the advantage of an early administration of these 2 probiotics soon after hatching, given that compared with later administration, this was found to result in a considerably more pronounced inhibition of SE colonization in the guts of chicks. Consequently, we believe that these probiotics can be effectively used to enhance host health, particularly during the rearing of chickens.
Acknowledgments
We thank Dr. Jeom-Joo Kim and Ms. Jiwon Kim for critical review of the manuscript; Jinhyun Kim, Yong-Seok Jang, Hye-Young Yoon, Seunghwan Hong for managing the experimental animals and collecting samples; and Yu-Mi Cho and Ji-Hye Kwon for microbiological analysis. This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries [IPET, research project number 119055-02].
Authors’ Contribution: DB designed the study and performed the experiments. DK isolated and characterized Lactobacillus kefiranofaciens DN1 and Kluyveromyces marxianus KU140723-05. DK, JC, and KYS contributed to data interpretation, and DB drafted the manuscript. KHS supervised the study. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate: All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Konkuk University. The approval number is KUIBC-2019-10.
Conflict of Interest Statement: The authors declare they have no competing interests.
References
- Andreatti R.L., Higgins J.P., Higgins S.E., Gaona G., Wolfenden A.D., Tellez G., Hargis B.M. Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar Enteritidis in vitro and in vivo. Poult. Sci. 2007;86:1904–1909. doi: 10.1093/ps/86.9.1904. [DOI] [PubMed] [Google Scholar]
- Ao T.T., Feasey N.A., Gordon M.A., Keddy K.H., Angulo F.J., Crump J.A. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg. Infect. Dis. 2015;21:941–949. doi: 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellikci-Koyu E., Sarer-Yurekli B.P., Akyon Y., Aydin-Kose F., Karagozlu C., Ozgen A.G., Brinkmann A., Nitsche A., Ergunay K., Yilmaz E., Buyuktuncer Z. Effects of regular kefir consumption on gut microbiota in patients with metabolic syndrome: a parallel-group, randomized, controlled study. Nutrients. 2019;11:2089. doi: 10.3390/nu11092089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolder N.M., van Lith L.A., Putirulan F.F., Jacobs-Reitsma W.F., Mulder R.W. Prevention of colonization by Salmonella Enteritidis PT4 in broiler chickens. Int. J. Food Microbiol. 1992;15:313–317. doi: 10.1016/0168-1605(92)90064-a. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Enteric Disease Surveillance: Salmonella Annual report, 2016, 2018. CDC, Atlanta, GA. Accessed Aug. 2020. https://www.cdc.gov/nationalsurveillance/salmonella-surveillance.html.
- CDC. Salmonella. 2019. https://www.cdc.gov/salmonella/index.html
- Cho Y.J., Kim D.H., Jeong D., Seo K.H., Jeong H.S., Lee H.G., Kim H. Characterization of yeasts isolated from kefir as a probiotic and its synergic interaction with the wine byproduct grape seed flour/extract. LWT-Food Sci. Technol. 2018;90:535–539. [Google Scholar]
- Czerucka D., Piche T., Rampal P. Review article: yeast as probiotics - Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- De Oliveira J.E., van der Hoeven-Hangoor E., de Linde I.B.V., Montijn R.C., van der Vossen J.M.B.M. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- ECDC. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2017. EFSA J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguale T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: prevalence and antimicrobial resistance. BMC Vet. Res. 2018;14 doi: 10.1186/s12917-018-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Hao H.H., Cheng G.Y., Iqbal Z., Ai X.H., Hussain H.I., Huang L.L., Dai M.H., Wang Y.L., Liu Z.L., Yuan Z.H. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Higgins S.E., Vicente J.L., Wolfenden A.D., Tellez G., Hargis B.M. Temporal effects of lactic acid bacteria probiotic culture on Salmonella in neonatal broilers. Poult. Sci. 2007;86:1662–1666. doi: 10.1093/ps/86.8.1662. [DOI] [PubMed] [Google Scholar]
- Higgins S.E., Higgins J.P., Wolfenden A.D., Henderson S.N., Torres-Rodriguez A., Tellez G., Hargis B. Evaluation of a Lactobacillus-based probiotic culture for the reduction of Salmonella Enteritidis in neonatal broiler chicks. Poult. Sci. 2008;87:27–31. doi: 10.3382/ps.2007-00210. [DOI] [PubMed] [Google Scholar]
- Holt P.S., Mitchell B.W., Seo K.H., Gast R.K. Use of negative air ionization for reducing airborne levels of Salmonella enterica serovar Enteritidis in a room containing infected caged layers. J. Appl. Poult. Res. 1999;8:440–446. [Google Scholar]
- Irigoyen A., Arana I., Castiella M., Torre P., Ibanez F.C. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005;90:613–620. [Google Scholar]
- Jadhav K., Sharma K.S., Katoch S., Sharma V.K., Mane B.G. Probiotics in broiler poultry feeds: a review. J. Anim. Nutr. Physiol. 2015;1:4–16. [Google Scholar]
- Jeong D., Kim D.H., Kang I.B., Kim H., Song K.Y., Kim H.S., Seo K.H. Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control. 2017;78:436–442. [Google Scholar]
- Jeong D., Kim D.H., Kang I.B., Kim H., Song K.Y., Kim H.S., Seo K.H. Modulation of gut microbiota and increase in fecal water content in mice induced by administration of Lactobacillus kefiranofaciens DN1. Food Funct. 2017;8:680–686. doi: 10.1039/c6fo01559j. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Jeong D., Kang I.B., Lim H.W., Cho Y., Seo K.H. Modulation of the intestinal microbiota of dogs by kefir as a functional dairy product. J. Dairy Sci. 2019;102:3903–3911. doi: 10.3168/jds.2018-15639. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Jeong D., Kim H., Kang I.B., Chon J.W., Song K.Y., Seo K.H. Antimicrobial activity of kefir against various food pathogens and spoilage bacteria. Korean J. Food Sci. 2016;36:787–790. doi: 10.5851/kosfa.2016.36.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Jeong D., Kim H., Seo K.H. Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Crit. Rev. Food Sci. 2019;59:1782–1793. doi: 10.1080/10408398.2018.1428168. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Jeong D., Song K.Y., Seo K.H. Comparison of traditional and backslopping methods for kefir fermentation based on physicochemical and microbiological characteristics. LWT-Food Sci. Technol. 2018;97:503–507. [Google Scholar]
- Lee D.H., Hyeon J.Y., Kim J., Kim J.S., Kim S.J., Jeon S.E., Choi S.W., Hong W.T., Song C.S., Lee S.W. Close genetic relationship between Salmonella enterica serovar Enteritidis isolated from patients with diarrhoea and poultry in the Republic of Korea. Clin. Microbiol. Infect. 2015;21:E68–E70. doi: 10.1016/j.cmi.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Chon J.W., Song K.Y., Hyeon J.Y., Moon J.S., Seo K.H. Prevalence, characterization, and antimicrobial susceptibility of Salmonella Gallinarum isolated from eggs produced in conventional or organic farms in South Korea. Poult. Sci. 2013;92:2789–2797. doi: 10.3382/ps.2013-03175. [DOI] [PubMed] [Google Scholar]
- Lim H.W., Kim D.H., Jeong D., Kang I.B., Kim H., Seo K.H. Biochemical characteristics, virulence traits and antifungal resistance of two major yeast species isolated from kefir: Kluyveromyces marxianus and Saccharomyces unisporus. Int. J. Dairy Technol. 2019;72:275–281. [Google Scholar]
- McEwen S.A., Angulo F.J., Collignon P.J., Conly J.M. Unintended consequences associated with national-level restrictions on antimicrobial use in food-producing animals. Lancet Planet. Health. 2018;2:e279–e282. doi: 10.1016/S2542-5196(18)30138-4. [DOI] [PubMed] [Google Scholar]
- McWhorter A.R., Chousalkar K.K. A long-term efficacy trial of a live, attenuated Salmonella Typhimurium vaccine in layer hens. Front. Microbiol. 2018;9:1380. doi: 10.3389/fmicb.2018.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon K.K., Saelao P., Halstead M.M., Chanthavixay G., Chang H.C., Garas L., Maga E.A., Zhou H. Salmonella enterica serovars Enteritidis infection alters the indigenous microbiota diversity in young layer chicks. Front. Vet. Sci. 2015;2:61. doi: 10.3389/fvets.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley P.S., Dargatz D.A., Hyatt D.R., Dewell G.A., Patterson J.G., Burgess B.A., Wittum T.E. Effects of restricted antimicrobial exposure on antimicrobial resistance in fecal Escherichia coli from feedlot cattle. Foodborne Pathog. Dis. 2011;8:87–98. doi: 10.1089/fpd.2010.0632. [DOI] [PubMed] [Google Scholar]
- Nhung N.T., Cuong N.V., Thwaites G., Carrique-Mas J. Antimicrobial usage and antimicrobial resistance in animal production in Southeast Asia: a review. Antibiotics (Basel) 2016;5:37. doi: 10.3390/antibiotics5040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Rebolledo O.F., Delgado-Machuca J.J., Macedo-Barragan R.J., Garcia-Marquez L.J., Morales-Barrera J.E., Latorre J.D., Hernandez-Velasco X., Tellez G. Evaluation of a selected lactic acid bacteria-based probiotic on Salmonella enterica serovar Enteritidis colonization and intestinal permeability in broiler chickens. Avian Pathol. 2017;46:90–94. doi: 10.1080/03079457.2016.1222808. [DOI] [PubMed] [Google Scholar]
- Rantala M., Nurmi E. Prevention of the growth of Salmonella Infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973;14:627–630. doi: 10.1080/00071667308416073. [DOI] [PubMed] [Google Scholar]
- Revolledo L., Ferreira C.S., Ferreira A.J. Prevention of Salmonella Typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult. Sci. 2009;88:734–743. doi: 10.3382/ps.2008-00410. [DOI] [PubMed] [Google Scholar]
- Smith J.M. A review of avian probiotics. J. Avian Med. Surg. 2014;28:87–94. doi: 10.1647/2012-031. [DOI] [PubMed] [Google Scholar]
- Tang K.L., Caffrey N.P., Nobrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., Polachek A.J., Ganshorn H., Sharma N., Kellner J.D., Checkley S.L., Ghali W.A. Examination of unintended consequences of antibiotic use restrictions in food-producing animals: sub-analysis of a systematic review. One Health. 2019;7:100095. doi: 10.1016/j.onehlt.2019.100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA-FSIS. Current FSIS recalls. 2019. https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/current-recalls-and-alerts/!ut/p/a1/jY_BCoJAGISfxQdY9jdF9CgLlpa7iGS2l1hMTdhWWa1DT5_SyUhy_tPAN_MzmOMccyWeTS2GplVCTp47F0jAMT0CEQv8AEJqBZlLtyYwZwTOM8AzJyBL2J4QcKm1Mr8gH_7loxUPNjomcY15J4YbalTV4rx4aF2qAemyEFL2SKgrErLUQ49PmM87wRxv7EztXUQtYPY38GP0B1he1d2P-etQpWFtGG85uhP2/#consignees Accessed Aug. 2020.
- Vinderola G., Perdigon G., Duarte J., Thangavel D., Farnworth E., Matar C. Effects of kefir fractions on innate immunity. Immunobiology. 2006;211:149–156. doi: 10.1016/j.imbio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Zacconi C., Scolari G., Vescovo M., Sarra P.G. Competitive exclusion of Campylobacter jejuni by kefir fermented milk. Ann. Microbiol. 2003;53:179–187. [Google Scholar]