Abstract
This study investigated the combined efficacy of slightly acidic electrolyzed water (SAEW) and UV light (UV) in improving egg internal quality (weight loss, Haugh unit, yolk index, albumen pH) over a 6-wk storage time at 25°C. Eggs were preserved after immersion for 4 min in SAEW (30 mg/L), irradiation for 4 min under a UV lamp, or a combination of SAEW and UV treatment for 4 min. The combination of SAEW and UV inhibited the deterioration of yolk index over the storage period, as well as reducing the extent of decrease in Haugh unit and of weight loss during storage at 25°C, and it was more effective than SAEW or UV alone in maintaining egg internal quality (P < 0.05). The results highlight the promising use of a SAEW and UV combination treatment to improve egg internal quality during storage.
Key words: slightly acidic electrolyzed water, UV, egg quality, storage
Introduction
Eggs are a valuable food source for humans as they are a complete protein source containing all the essential amino acids (Cherian et al., 2002). However, there are concerns related to the contamination of chicken eggs, which have the potential to become contaminated during their production (De Reu et al., 2006). Contaminated eggs cause a variety of infectious diseases and result in a considerable economic impact on the egg industry (Turtoi and Borda, 2014). Moreover, certain microorganisms can rapidly penetrate the shell and contaminate the internal contents of the egg, resulting in the deterioration of egg quality during storage (Berrang et al., 1998; Caner and Yuceer, 2015). Thus, it is important to decontaminate shell eggs before sale to improve their microbiological safety or to extend storage time. Most disinfectants, such as chlorine and iodine (USDA, 2001), ozone (Yüceer et al., 2016), boiling water (Himathongkham et al., 1999; Geveke et al., 2016), ultrasound (Bi et al., 2020; Yüceer and Caner., 2020), and hydrogen peroxide (Cox et al., 2002), have been used. However, the chemical residues, limited effectiveness, high cost, and adverse environmental impacts make these traditional methods unpopular with consumers (Cao et al., 2009; Turtoi and Borda, 2014). This imposes the search for new disinfectants that do not produce harmful byproducts and are cost-effective.
Slightly acidic electrolyzed water (SAEW) is an attractive option for its disinfection capabilities and because it is environmentally friendly and inexpensive (Sheng et al., 2018; Liang et al., 2019). There are relatively few historical studies of SAEW used as an egg surface disinfectant. In 2004, Bialka et al. (2004) evaluated the efficacy of acidic electrolyzed water (AEW) for the microbial safety of eggshells and reported that a log10 reduction of greater than 2 for Salmonella Enteritidis and Escherichia coli was observed after electrolyzed oxidizing water treatment. However, the high acidity of AEW may cause the corrosion of equipment and consequently limit its practical application (Cao et al., 2009; Nan, 2011). For this reason, Cao et al. (2009) first used SAEW as an egg surface disinfectant and reported that SAEW could be used instead of AEW and sodium hypochlorite (NaCIO) as an effective disinfectant in the shell egg washing process. In 2014, Ni et al. (2014) published an article in which they described that the bactericidal activity of SAEW on shell eggs toward S. Enteritidis was significantly higher than that of chlorine dioxide and NaClO solution at an available chlorine concentration (ACC) of 80 or 100 mg/L. In 2019, we highlighted the promising use of SAEW to enhance the microbial safety and to extend the shelf life of shell eggs (Zang et al., 2019b).
Together, these studies indicate that SAEW is an alternative disinfectant for the control of microorganisms on eggshells. However, it must also be mentioned that single antimicrobial treatments of SAEW require longer washing and treatment times and/or a higher ACC in the poultry industry (Hao et al., 2013; Zang et al., 2015, 2019a). To overcome these drawbacks, Bing et al. (2019) evaluated the synergistic bactericidal efficacy of SAEW and UV-C light (SAEW + UV) for the inactivation of S. Enteritidis on artificially inoculated eggshells with or without manure exposure and found that the SAEW + UV treatment is a novel and more effective method than the SAEW treatment alone to enhance the microbial safety of eggshells. Moreover, some findings indicated that a sterilizing treatment of eggshells could inhibit the decrease in egg quality during storage (Morsy et al., 2015; Yuceer et al., 2016). We hence proposed the hypothesis that the SAEW + UV treatment may lead to a relatively lower loss of eggs internal quality during storage because of its higher inactivation capacity. Notably, another important issue is that SAEW + UV may wash away the cuticle surrounding the egg; it may favor trans-shell contamination with bacteria and moisture loss, and it may shorten the shelf life of the egg. Therefore, it is necessary to thoroughly investigate whether the SAEW + UV method could be used to help to maintain the freshness of eggs.
The objective of our study was to examine the combined effect of SAEW and UV light on the quality (albumen pH, weight loss, yolk index, and Haugh unit) of eggs during storage at 25°C.
Materials and methods
Preparation of SAEW
Slightly acidic electrolyzed water was produced using a nonmembrane generator (made by the Animal Production Laboratory of Jiangxi Agricultural University) at a voltage of 31 V to electrolyze NaCl solution (500 mg/L) containing HCl (0.5 mg/L) for 30 min. Slightly acidic electrolyzed water produced was immediately diluted in sterile deionized water to obtain an ACC concentration of 30 mg/L, a pH of 6.37, and an oxidation–reduction potential (ORP) of 980 mV. The pH, ORP, and ACC were measured immediately before each experiment. The pH and ORP values were measured with a dual-scale pH/ORP meter (PHS-3E; Shanghai Scientific Instruments Co., Shanghai, China). The ACC was determined using a digital chlorine detection system (YXL-1A, RC-2Z; Shanghai Electronic Technology Co., Shanghai, China), which tests the range from 0 to 500 mg/L.
Preparation of UV Light
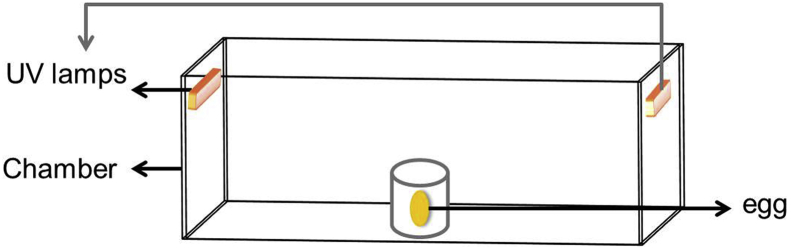
As can be seen from Figure 1, the UV-C treatments were performed in a chamber (85 cm × 75 cm × 45 cm) equipped with 2 sets of 2 unfiltered germicidal emitting lamps (253.7 nm; Philips, Co., Netherlands). One set of lamps was placed on the left and the other one on the right of the radiation cabinet. Each lamp was 40 cm tall. All UV experiments were conducted at a fixed initial UV intensity (10.2 ± 0.3 W/cm2) that was measured by a radiometer (UVX-254; Ultraviolet Products, California). Before each experiment, the UV lamp was turned on for approximately 20 min to achieve a stable irradiation intensity.
Figure 1.
The schematic diagram for UV application. Abbreviation: UV, UV light treatment.
Preparation of Shell Eggs
The freshly laid eggs weighing 55–60 g were provided by a local poultry farm (Nanchang, China) and transported to the laboratory within 5 h. Eggs were first equilibrated at room temperature before testing and then sequentially rinsed in sterile deionized water to completely remove the contamination and air-dried under a biosafety hood (DH-920; Beijing East Union Hall Instrument Manufacturing Co., Ltd., Beijing, China).
Eggs were divided into 4 groups with 50 eggs in each: control (no treatment), SAEW treatment group (30 mg/l), UV light treatment group (UV), and a synergistic group of SAEW and UV light (SAEW + UV). The SAEW treatment was carried out by immersing eggs in prepared SAEW (30 mg/L) for 4 min in a sterile glass beaker (Bing et al.,2019). UV light–treated eggs were aseptically transferred to the base of a sterile glass beaker and placed on a net positioned midway between the UV-C lamps in the aforementioned chamber for 4 min. The SAEW + UV group eggs were immersed in SAEW treatment at an ACC of 30 mg/L in a sterile glass beaker simultaneously with UV treatment for 4 min (Bing et al.,2019).
Three eggs from each group were dried at 37°C in an air blast drying box (DHG-9030A; Shanghai Precision Instruments Co., Ltd. Shanghai, China) and then trimmed into small pieces of 3–5 mm with tweezers. The upper surface of the shell egg was subsequently fixed on the sample table. Then, the morphologies of the surfaces and cross-sections of shell eggs were observed using a scanning electron microscope (JSM-6701F; Japan Electronics Company, Tokyo, Japan) operating at 5.0 kV after platinum sputtering of the samples.
pH Measurement
After the albumen was separated from each egg, it was homogenized in a beaker. The pH values of the albumen were measured using a pH meter (PHS-3E; Shanghai Scientific Instruments Co., Shanghai, China).
Weight Loss
The weight was determined using an analytical balance (FA2248B-220g; Shanghai Jingke Tianmei Electronic Technology Co., Ltd., Shanghai, China) by individually weighing each marked egg to an accuracy of 0.01 g. The weight loss (%) of whole eggs was determined during storage by subtracting the final weight of the egg from the initial weight and then dividing by the initial weight and multiplying by 100, as described in the study by Caner and Cansız (2008).
Yolk Index and Haugh Unit
Eggs were cracked onto a flat glass surface with a spatula to measure various internal parameters. The height of the albumen and yolk was measured with a tripod micrometer (NFN381; Nanjing Mingao Instruments and Equipment Co., Ltd., Nanjing, China), and the diameter of the yolk was measured using a digimatic caliper (500-150-30; Mitutoyo Co., Kawasaki-shi, Japan). The yolk index was determined by the ratio of the yolk height to the yolk diameter (Caner, and Yuceer, 2015).
The Haugh unit was calculated from the egg weight and albumen height using the formula (Haugh, 1937):
| (1) |
where h is the albumen height (mm) and w is the weight of the egg (g).
The parameter h was recorded by averaging three measurements carried out at different points of thick albumen at a distance of 10 mm from the yolk using the tripod micrometer as described previously.
Statistical Analysis
All the parameters were measured on 3 replicates and were expressed as the mean. Statistical analysis was performed using the Origin software package (version 9.0; OriginLab Cor., Hampton). Differences between effects were assessed by the Tukey test (P < 0.05).
Results and discussion
Albumen pH
As seen in Table 1, the pH values of the albumen increased to 9.49 (control), 9.48 (SAEW-treated groups), 9.45 (UV groups), and 9.47 (SAEW + UV–treated groups) owing to the release of CO2 through the pores of the shell from the breakdown of carbonic acid in albumen at the end of the 6-wk storage time. Other studies have reported similar results (Jones and Musgrove, 2005; Morsy et al.; 2015; Zang et al.; 2019a,b). However, this trend was not uniform for all storage experiments. The albumen pH of all groups first increased, then decreased, and then increased again, as shown in Table 1. This result is supported by Yuceer et al (2016), who reported that when fresh eggs were treated with gaseous ozone at concentrations of 2, 4, and 6 ppm with exposure times of 2 and 5 min during storage for 6 wk at 24°C, the albumen pH had a similar change trend. In addition, no significant difference among all groups for albumen pH was observed during the whole storage time.
Table 1.
Effect of disinfects on the albumen pH of eggs.
| 0 wk | 1 wk | 2 wk | 3 wk | 4 wk | 5 wk | 6 wk | |
|---|---|---|---|---|---|---|---|
| Control | 9.10 ± 0.02 | 9.19 ± 0.01 | 9.25 ± 0.02 | 9.15 ± 0.01 | 9.18 ± 0.01 | 9.52 ± 0.02 | 9.49 ± 0.03 |
| SAEW | 9.09 ± 0.02 | 9.22 ± 0.02 | 9.24 ± 0.01 | 9.11 ± 0.02 | 9.13 ± 0.01 | 9.46 ± 0.02 | 9.48 ± 0.02 |
| UV | 9.06 ± 0.01 | 9.19 ± 0.01 | 9.25 ± 0.01 | 9.15 ± 0.03 | 9.13 ± 0.03 | 9.48 ± 0.02 | 9.45 ± 0.01 |
| SAEW + UV | 9.10 ± 0.02 | 9.21 ± 0.01 | 9.27 ± 0.01 | 9.12 ± 0.03 | 9.15 ± 0.02 | 9.49 ± 0.03 | 9.47 ± 0.02 |
Values shown are means ± SD.
Abbreviations: SAEW, slightly acidic electrolyzed water treatment; SAEW + UV, a combination of SAEW and UV treatment; UV, UV light treatment.
Weight Loss
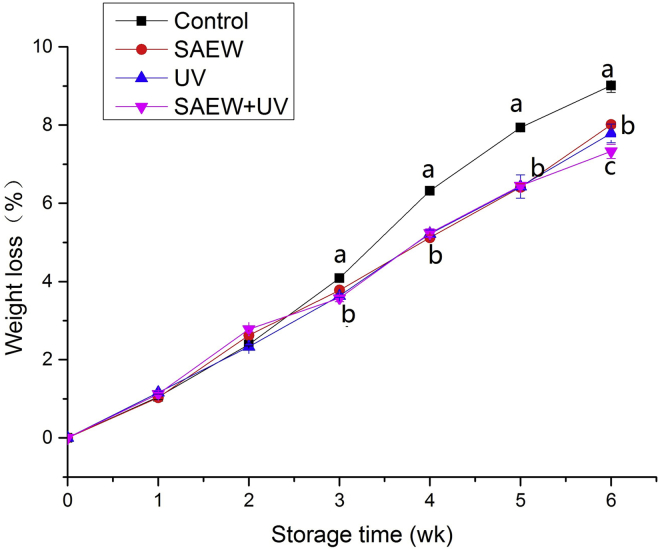
Figure 2 shows an overview of the relative weight loss of all eggs. The weight loss increased as the storage time increased. Many studies have reported similar results (Jones and Musgrove, 2005; Morsy et al.; 2015; Zang et al.; 2019a,b). Yimenu et al reported that eggs stored for longer periods of time lost more of their initial weight because of the evaporation of water and loss of carbon dioxide from the albumen through the pores of the shells (Yimenu et al., 2017). At the end of the storage experiments, the egg weight loss rate was 9.01% for the control, whereas the weight loss was 8.01, 7.79, and 7.33 for the SAEW, UV, and SAEW + UV–treated groups, respectively. As seen in Figure 2, there are significant differences between the control groups and other groups during storage times from 3 wk to 6 wk. In addition, there are significant differences between SAEW + UV–treated groups and other groups at 6 wk (P < 0.05). The different bactericidal effects on eggshells by the SAEW + UV, UV, and SAEW treatments may contribute to this result. Some findings have indicated that sterilizing eggshells could decrease their weight loss during storage. Yuceer et al. (2016) showed that ozone can significantly (P < 0.05) slow down the weight loss of eggs during storage compared with control eggs. Morsy et al. (2015) also confirmed that antimicrobial pullulan-based coated unwashed eggs exhibited slightly higher Haugh unit (HU) than pullulan-based coated eggs. Our previous studies compared the efficacy of different disinfection treatments (SAEW, AEW, NaClO) for inactivation of S. Enteritidis and E. coli on eggshells, as well as shelf life, and found that SAEW has an equivalent or higher bactericidal activity for eggshells than AEW and NaClO solution and could maintain fresh egg quality for a longer storage time than AEW and NaClO solution. Moreover, Bing et al. (2019) reported that simultaneous treatment with SAEW and UV light exhibits higher bactericidal activity for eggshells than UV or SAEW single treatment owing to the formation of •OH. Therefore, we proposed the hypothesis that the higher bactericidal activity of the SAEW + UV treatment may lead to a relatively lower loss of eggs than SAEW single-treatment groups, helping to maintain the freshness of the eggs.
Figure 2.
Effect of disinfects on the weight loss of eggs. Abbreviations: SAEW, slightly acidic electrolyzed water treatment; SAEW + UV, a combination of SAEW and UV treatment; UV, UV light treatment. Values shown are means ± SD. Different letters (a, b, c) denote significant differences between groups.
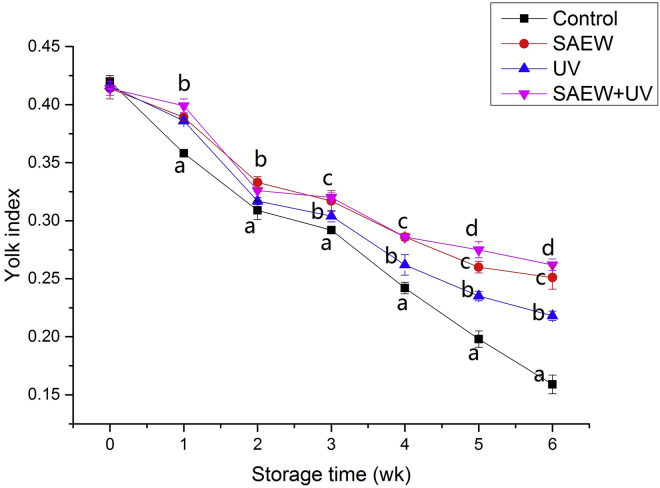
Yolk Index
Yolk index has often been used to reflect yolk quality and egg freshness (Yuceer and Caner, 2016). As seen in Figure 3, deterioration was observed with storage time, and many studies have reported similar results (Jones and Musgrove, 2005; Yuceer et al, 2016; Zang et al., 2019a,b). At the end of the storage time, the yolk index value of the control group was 0.161, whereas the SAEW-treated, UV-treated, and SAEW + UV–treated groups had values of 0.251, 0.218, and 0.262, respectively. The yolk index value of the control group was significantly lower than that of the other groups during storage (P < 0.05). In addition, SAEW-treated groups had a higher yolk index value than the control group during the whole storage time. This is also consistent with the observations from our previous study (Zang et al., 2019b), which demonstrated that SAEW treatment can preserve yolk quality by inhibiting bacterial penetration into the yolk during storage at 25°C. Similarly, UV-treated groups have a higher yolk index value than the control group. Recently, UV as an egg surface decontamination method has been met with increasing interest because UV treatment does not change the barrier properties of the eggshell (Holck et al., 2018). However, UV light does not penetrate well through organic matter, such as protein and other types of organic matter (Holck et al., 2018). Therefore, washing is a necessary treatment before the egg is inactivated with UV because the egg has the potential to become contaminated by organic matter from the surrounding environment. However, the cuticle of the eggs could be damaged by this washing step. Furthermore, the HU index of eggs exposed to the SAEW treatment was significantly higher than those exposed to UV treatment after 3 wk (P < 0.05). It must also be mentioned that the yolk index values of the SAEW + UV–treated groups were better than those of the SAEW-treated groups after 5 wk and better than those of the UV-treated groups during the whole storage time. These results support the previously proposed view that SAEW + UV treatment may lead to a relatively lower loss of eggs internal quality than UV and SAEW treatments because of its higher disinfection activity.
Figure 3.
Effect of disinfects on the yolk index of eggs. Abbreviations: SAEW, slightly acidic electrolyzed water treatment; SAEW + UV, a combination of SAEW and UV treatment; UV, UV light treatment. Values shown are means ± SD. Different letters (a, b, c) denote significant differences between groups.
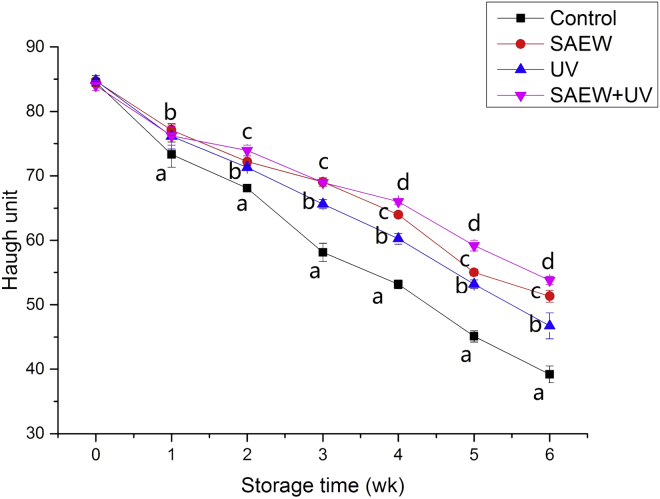
Haugh Unit
Changes in HU for the various disinfection methods of treated and control (untreated) eggs are shown in Figure 4. HU decreased significantly in all groups during storage (P < 0.05). This result is in agreement with many previous publications, which reported that the reduction in HU was induced by the decrease in the thick albumen height, which becomes thinner and loses CO2 during storage (Jin et al., 2013; Yuceer and Caner, 2016). After 6 wk of storage, the HU of the control, SAEW-treated, UV-treated, and SAEW + UV treated groups had values of 39.21, 51.27, 46.72, and 53.78, respectively. All sterilizing treatment eggs exhibited higher HU values than control eggs during 6 wk of storage at 25°C (P < 0.05). This finding also indicates that sterilizing treatment minimized egg white thinning during storage (Caner and Yuceer 2015; Zang et al., 2019b). Moreover, the HU index of eggs exposed to SAEW treatment was significantly higher than that of the UV treatment after 3 wk (P < 0.05). Notably, the HU index of the SAEW + UV–treated groups was significantly higher than that of the UV treatment groups after 2 wk (P < 0.05) and was significantly higher than that of the SAEW treatment groups after 5 wk (P < 0.05). This result also supports our previously proposed view (Zang et al., 2019b; Yuceer and Caner, 2020).
Figure 4.
Effect of disinfects on the Haugh unit of eggs. Abbreviations: SAEW, slightly acidic electrolyzed water treatment; SAEW + UV, a combination of SAEW and UV treatment; UV, UV light treatment. Values shown are means ± SD. Different letters (a, b, c) denote significant differences between groups.
Conclusions
The aim of the present research was to examine the combined efficacy of SAEW and UV treatment to prevent the decrease in egg quality during a 6-wk storage time. All of the egg quality parameters investigated in the present study were significantly (P < 0.05) affected by the storage time. As storage time increased, the yolk index and Haugh unit value decreased and weight loss and albumen pH increased. However, the combination of SAEW and UV inhibited the deterioration of yolk index over the storage period, as well as reduced the extent of decrease in Haugh unit and of weight loss during storage at 25°C, and it was more effective than SAEW or UV alone in maintaining egg internal quality (P < 0.05).
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of Jiangxi Province in China (20192BAB214021) and Key R & D plan of Jiangxi Province in China (20202BBF63009).
Conflict of Interest Statement: No conflicts of interest are declared.
References
- Berrang M.E., Frank J.F., Burh R.J., Bailey J.S., Cox N.A., Mauldin J. Eggshell characteristics and penetration by Salmonella through the productive life of a broiler breeder flock. Poult. Sci. 1998;77:1446–1450. doi: 10.1093/ps/77.9.1446. [DOI] [PubMed] [Google Scholar]
- Bi X., Wang X., Chen Y., Chen L., Xing Y., Che Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020;60:104763. doi: 10.1016/j.ultsonch.2019.104763. [DOI] [PubMed] [Google Scholar]
- Bialka K.L., Demirci A., Knabel S.J., Patterson P.H., Puri V.M. Efficacy of electrolyzed oxidizing water for the microbial safety and quality of eggs. Poult. Sci. 2004;83:2071–2078. doi: 10.1093/ps/83.12.2071. [DOI] [PubMed] [Google Scholar]
- Bing S., Zang Y.T., Li Y.J., Shu D.Q. The synergistic effects of slightly acidic electrolyzed water and UV-C light on the inactivation of Salmonella enteritidis on contaminated eggshells. Poult. Sci. 2019;98:6914–6920. doi: 10.3382/ps/pez454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Zhu Z., Shi Z., Wang C., Li B. Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritidis and its contaminated shell eggs. Int. J. Food Microbiol. 2009;130:88–93. doi: 10.1016/j.ijfoodmicro.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Caner C., Cansiz Ö. Chitosan coating minimises eggshell breakage and improves egg quality. J. Sci. Food Agr. 2008;88:56–61. [Google Scholar]
- Caner C., Yuceer M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015;94:1665–1677. doi: 10.3382/ps/pev102. [DOI] [PubMed] [Google Scholar]
- Cherian G., Holsonbake T.B., Goeger M.P. Fatty acid Composition and egg Components of Specialty eggs. Poult. Sci. 2002;81:30–33. doi: 10.1093/ps/81.1.30. [DOI] [PubMed] [Google Scholar]
- Cox N.A., Bailey J.S., Berrang M.E. Bactericidal treatment of Hatching eggs I. Chemical immersion treatments and Salmonella. J. Appl. Poult. Res. 1998;7:347–350. [Google Scholar]
- De Reu K., Grijspeerdt K., Herman L., Heyndrickx M., Uyttendaele M., Debevere J., Putirulan F.F., Bolder N.M. The effect of a commercial UV disinfection system on the bacterial load of shell eggs. Lett. Appl. Microbiol. 2006;42:144–148. doi: 10.1111/j.1472-765X.2005.01825.x. [DOI] [PubMed] [Google Scholar]
- Geveke D.J., Gurtler J.B., Jones D.R., Bigley A.B. Inactivation of Salmonella in shell eggs by hot water immersion and its effect on quality. J. Food Sci. 2016;81:709–714. doi: 10.1111/1750-3841.13233. [DOI] [PubMed] [Google Scholar]
- Hao X.X., Li B.M., Zhang Q., Lin B.Z., Ge L.P., Wang C.Y., Cao W. Disinfection effectiveness of slightly acidic electrolysed water in swine barns. J. Appl. Microb. 2013;115:703–710. doi: 10.1111/jam.12274. [DOI] [PubMed] [Google Scholar]
- Haugh R.R. The Haugh unit for measuring egg quality. US Poult. Mag. 1937;43:552–573. [Google Scholar]
- Himathongkham S., Riemann H.P., Ernst R.A. Efficacy of disinfection of shell eggs externally contaminated with Salmonella enteritidis: Implications for egg testing. Int. J. Food Microbiol. 1999;49:161–167. doi: 10.1016/s0168-1605(99)00092-6. [DOI] [PubMed] [Google Scholar]
- Holck A.L., Liland K.H., Dromtorp S.M., Carlehog M., Mcleod A. Comparison of UV-C and Pulsed UV light treatments for reduction of Salmonella, Listeria monocytogenes, and Enterohemorrhagic Escherichia coli on eggs. J. Food Prot. 2018;81:6–16. doi: 10.4315/0362-028X.JFP-17-128. [DOI] [PubMed] [Google Scholar]
- Jin T.Z., Gurtler J.B., Li S.Q. Development of antimicrobial Coatings for improving the microbiological safety and quality of shell eggs. J. Food Prot. 2013;76:779–785. doi: 10.4315/0362-028X.JFP-12-460. [DOI] [PubMed] [Google Scholar]
- Jones D.R., Musgrove M.T. Effects of extended storage on egg quality factors. Poult. Sci. 2005;84:1774–1777. doi: 10.1093/ps/84.11.1774. [DOI] [PubMed] [Google Scholar]
- Liang D., Wang Q., Zhao D., Han X., Hao J. Systematic application of slightly acidic electrolyzed water (SAEW) for natural microbial reduction of buckwheat sprouts. LWT. 2019;108:14–20. [Google Scholar]
- Morsy M.K., Sharoba A.M., Khalaf H.H., Eltanahy H.H., Cutter C.N. Efficacy of antimicrobial pullulan-based coating to improve internal quality and shelf-life of chicken eggs during storage. J. Food Sci. 2015;80:1066–1074. doi: 10.1111/1750-3841.12855. [DOI] [PubMed] [Google Scholar]
- Nan S.J. 2011. Disinfection Effect of Slightly Acidic Electrolyzed Water in the Dairy Farm and Application on the Control of Dairy Mastitis. China agricultural university, Ph.D. Thesis. [Google Scholar]
- Ni L., Cao W., Zheng W., Chen H., Li B. Efficacy of slightly acidic electrolyzed water for reduction of Foodborne Pathogens and natural Microflora on shell eggs. Food Sci. Technol. Res. 2014;20:93–100. [Google Scholar]
- Sheng X.W., Shu D., Tang X., Zang Y.T. Effects of slightly acidic electrolyzed water on the microbial quality and shelf life extension of beef during refrigeration. Food Sci. Nutr. 2018;6:1975–1981. doi: 10.1002/fsn3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtoi M., Borda D. Decontamination of egg shells using ultraviolet light treatment. World Poult. Sci. J. 2014;70:265–278. [Google Scholar]
- United States Department of Agriculture. 2001. Agricultural Marketing Service. Agric. Handbook Number 75: Egg-Grading Man. [Google Scholar]
- Yimenu S.M., Kim J., Koo J., Kim B. Predictive modeling for monitoring egg freshness during variable temperature storage conditions. Poult. Sci. 2017;96:2811–2819. doi: 10.3382/ps/pex038. [DOI] [PubMed] [Google Scholar]
- Yuceer M., Aday M.S., Caner C. Ozone treatment of shell eggs to preserve functional quality and enhance shelf life during storage. J. Sci. Food Agr. 2016;96:2755–2763. doi: 10.1002/jsfa.7440. [DOI] [PubMed] [Google Scholar]
- Yüceer M., Caner C. The effects of ozone, ultrasound and coating with shellac and lysozyme–chitosan on fresh egg during storage at ambient temperature. Part II: microbial quality, eggshell breaking strength and FT-NIR spectral analysis. Int. J. Food Sci. Technol. 2020;55:1629–1636. [Google Scholar]
- Zang Y.T., Li B.M., Bing Sh., Cao W. Modeling disinfection of plastic poultry transport cages inoculated with Salmonella enteritids by slightly acidic electrolyzed water using response surface methodology. Poult. Sci. 2015;94:2059–2065. doi: 10.3382/ps/pev188. [DOI] [PubMed] [Google Scholar]
- Zang Y.T., Bing S., Li Y.J., Shu D.Q. Application of slightly acidic electrolyzed water and ultraviolet light for Salmonella enteritidis decontamination of cell suspensions and surfaces of artificially inoculated plastic poultry transport coops and other facility surfaces. Poult. Sci. 2019;98:6445–6451. doi: 10.3382/ps/pez520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y.T., Bing S., Li Y.J., Shu D.Q., Huang A.M., Wu H.X., Lan L.T., Wu H.D. Efficacy of slightly acidic electrolyzed water on the microbial safety and shelf life of shelled eggs. Poult. Sci. 2019;98:5932–5939. doi: 10.3382/ps/pez373. [DOI] [PubMed] [Google Scholar]