Abstract
The anticoccidial activity of thymol, carvacrol, and saponins was assessed in an in vitro model of coccidiosis. Eimeria spp. sporozoites were collected from field samples, characterized, and used for 2 different invasion assays on Madin-Darby Bovine Kidney cells (MDBK). The cells were challenged with 5 × 104 sporozoites without (control) or with various treatments: saponins (10 ppm), thymol, and carvacrol (7 ppm each) or a combination of saponins, thymol, and carvacrol at 2 doses; MIX 1 (saponins 5 ppm, thymol 3.5 ppm, and carvacrol 3.5 ppm) and MIX 2 (saponins 10 ppm, thymol 7 ppm, and carvacrol 7 ppm). The treated cells were incubated at 37°C for 24 h (invasion assay 1) and for 2, 24, and 48 h (invasion assay 2). The efficiency of invasion was determined by counting the sporozoites left in the supernatant that were not able to invade the cells, whereas intracellular Eimeria DNA was detected by qPCR to confirm the data. Data were analyzed with ANOVA, and differences were considered significant when P value was ≤0.05. Data from invasion assay 1 showed that the thymol and carvacrol-containing blends significantly reduced invasion, especially in combination with saponins at the highest dose. Saponins alone did not have a strong inhibiting activity but acted synergistically with the other molecules. Interestingly, in invasion assay 2, it was found that the effect of the highest dose of the blend of saponins, thymol, and carvacrol was already visible at 2 h postinfection, whereas the other treatments were significantly successful at 24 h postinfection. The invasion assay protocol was designed to screen molecules in vitro starting from field fecal samples, and it can represent a potential tool in Eimeria research. Moreover, this study shows that invasion in MDBK cells by Eimeria sporozoites is inhibited in presence of thymol, carvacrol, and saponins, thus highlighting the anticoccidial potential of these compounds.
Key words: Eimeria, in vitro, botanical, MDBK
Introduction
Eimeria is an important avian parasite which causes severe enteritis, leading to relevant economic losses in poultry industry, estimated to be more than 3 billion US dollars per year (Cobaxin-Cardenas, 2016). Five are the species of Eimeria mainly involved in the disease onset: Eimeria acervulina, Eimeria maxima, Eimeria brunetti, Eimeria necatrix, and Eimeria tenella, with the latter 3 associated with the highest mortality rate and majority of symptoms, whereas the others lead to subclinical signs, which are often hard to recognize (Quiroz-Castañeda and Dantán-González, 2015). In addition, coccidia can contribute to the outbreak of secondary infections, such as clostridiosis, responsible for severe necrotic enteritis (Moore, 2016). Methods for controlling the disease include the use of ionophores and synthetic anticoccidial drugs applied with rotation programs or vaccination with live Eimeria oocysts. However, vaccines can trigger undesired reactions that affect the birds' performance, and recently, many cases of resistance to anticoccidial drugs have been documented (Abbas et al., 2012). As a consequence, research is now focusing on finding new cost-effective alternatives to control these pathogens (Peek and Landman, 2011). Botanicals and nature identical compounds are well renowned for their antimicrobial and antiparasitic activity, so they can represent a valuable tool against Eimeria (Cobaxin-Cardenas, 2016). The mechanisms of action of these molecules include degradation of cell wall, cytoplasm damaging, ion loss with reduction of proton motive force, and also induction of oxidative stress, that lead to inhibition of invasion as well as impairment of Eimeria spp. development (Abbas et al., 2012; Nazzaro et al., 2013). These compounds are often tested in vivo, but ethical concerns for animal welfare and high cost are pushing toward the assessment of standardized in vitro methods to screen new molecules (Singh et al., 2016). Among botanicals, thymol, carvacrol, and saponins are promising molecules because they can interfere with the membrane permeability of pathogens, causing a cascade of reactions that involve the entire cell and eventually leads to its death (Nazzaro et al., 2013). These compounds are naturally found in plants: thymol and carvacrol are major constituents of oregano, thyme, and basil (Sakkas and Papadopoulou, 2017), whereas Quillaja spp. and Yucca spp. are common sources of saponins, amphipathic glycosides used as defense mechanisms (Francis et al., 2002). The aim of this study was to evaluate the antiparasitic effect of different blends of thymol, carvacrol, and saponins on the invasion efficiency of Eimeria sporozoites in vitro. The protocol was designed to test alleged anticoccidial compounds starting from field samples, thus respecting animal welfare and without animal sacrifice, in agreement to the “3 Rs” guidelines (Russel and Burch, 1959).
Materials and methods
Eimeria Sporozoites Recovery From Field Samples
Eimeria spp. oocysts were collected from fecal samples of nonvaccinated animals showing coccidiosis symptoms. The samples were processed as indicated in Guidelines on techniques in coccidiosis research (Shirley, 1995) with some changes. Oocysts were resuspended in potassium dichromate 2% (Cat.#P5271, Sigma-Aldrich, St. Louis, MO) to allow sporulation. The oocyst samples were cleaned with Dulbecco's modified phosphate buffered saline (DPBS, Cat.#D8537, Sigma-Aldrich) from potassium dichromate, and then, they were resuspended in lysis buffer T1 (Cat.# 740952.240 C, MACHEREY-NAGEL Inc., Bethlehem, PA) and stored at −80°C until qPCR analysis was performed as described below. After sporulation, the oocysts were washed and resuspended in sodium hypochlorite for sterilization, and then, they were washed and lysed with glass beads (0.5 mm) for 1 min with Disruptor Genie (Cat.# SI-D258, Scientific Industries, Bohemia, NY) to obtain sporocysts. Those were washed and resuspended in excystation medium, containing 2.5 g/L trypsin (Cat.#T4049, Sigma-Aldrich), 5 g/L bile salts (Cat.#B3301, Sigma-Aldrich), 2 g/L pancreatin (Cat.#P1750, Sigma-Aldrich), and 2 g/L MgCl2 (Cat.# 459337, Carlo Erba Reagents, Milan, Italy). The suspension was incubated for 90 min at 39°C. Afterward, the obtained sporozoites were washed and resuspended in cell medium to initiate the invasion assay.
Cell Culture
Madin-Darby Bovine Kidney (MDBK, Cat.# CCL-22, ATCC, Manassas, VA) cells were seeded (1 × 105 cells/well) on 24-well plates (Cat.#353047, Corning Incorporated, Corning, NY) and grown until confluency for 48 h in basal medium containing Dulbecco's Modified Eagle's Medium (Cat.#D1145, Sigma-Aldrich), 10% fetal bovine serum (Cat.#F7524, Sigma-Aldrich), 1x Penicillin-Streptomycin (Cat.#P4333, Sigma-Aldrich), and 10 mM L-glutamine (Cat.#G7513, Sigma-Aldrich). Cells were incubated at 37°C and 5% CO2.
Invasion Assay 1
Confluent cells were infected with 5 × 104 sporozoites per well and treated with one of the treatments based on thymol (Cat.#T0501, Sigma-Aldrich), carvacrol (Cat.#W224511, Sigma-Aldrich), and saponins (Vetagro S.p.A., Reggio Emilia, Italy). The treatment groups were negative control (no sporozoite and no treatment), infected control (C+), saponins 10 ppm (SAP), thymol and carvacrol (7 ppm each) (THY:CAR), saponins 5 ppm + thymol 3.5 ppm + carvacrol 3.5 ppm (MIX1), or saponins 10 ppm + thymol 7 ppm + carvacrol 7 ppm (MIX2). The efficacy of the treatments was studied at 24 h postinfection (hpi). After the invasion assay, cells were stained with Giemsa to observe the actual internalization of the processed sporozoites. Moreover, the cells were accurately washed with DPBS until most of the residual debris was removed. Then cells were detached with trypsin 0.25%, washed with DPBS, resuspended in lysis buffer T1, and stored at −80°C until analysis. To measure the efficiency of invasion, the noninvading sporozoites found in the supernatant of 18 wells per group (n = 18) were counted on 4 squares of a Burker chamber under inverted microscope (Nikon Eclipse TS100, Nikon corporation, Tokyo, Japan), and the resulting number was used to estimate invasion efficiency with the following formula:
Invasion Assay 2
Confluent cells were infected with 5 × 104 sporozoites per well and treated with one of the treatments described before. The efficiency of invasion was estimated at time points (2 hpi, 24 hpi, and 48 hpi) with sporozoites counts of 12 wells per treatment (n = 12), as defined above.
DNA Extraction and qPCR
DNA extraction and qPCR was performed to characterize the oocyst samples and to detect intracellular Eimeria DNA in the cells that were harvested after the assays. NucleoSpin DNA extraction kit (Cat.# 740952.240 C, MACHEREY-NAGEL Inc.) was used according to the manufacture instructions. DNA concentration was measured using Denovix DS-11 Series Spectrophotometer/Fluorometer (Microvolume Mode with Smart Path Technology–Cat.# DS11, Denovix, Hanby Building, Wilmington, NC) at 260 nm, and quality was verified by 260/280 ratio. The PCR reaction was prepared in a final volume of 10 μL, including 5 μL of iTaq Universal SYBR Green Supermix (Cat.# 1725120, Bio-Rad Laboratories, Hercules, CA), 500 nM of forward and reverse primers, and 2 μL of DNA, and the instrument used was the CFX96 TouchTM Real-Time PCR Detection System (Cat.# 1855195, Bio-Rad Laboratories). The primers used to detect Eimeria spp. were supplied by Sigma-Aldrich and are listed in Table 1. Cycling reaction was carried out under the following conditions: 3 min at 95°C, followed by 40 cycles of 10 s at 95°C and 30 s at 60°C. Nonspecific product formation was monitored with a melting curve analysis. The melting cycle included of 0.5°C increments from 55°C to 95°C for 5 s. A relative quantification method (2−ΔΔCt) was used to verify the efficiency of invasion, using as reference the gene of Bovine cytochrome B (Livak and Schmittgen, 2001).
Table 1.
Primers used to detect the Eimeria spp.
| Target | Primer sequence (5′→3′) | Product size (bp) | Accession number | Reference | |
|---|---|---|---|---|---|
| E. brunetti | F | TTGCGTAAATAGAGCCCT | 148 | AF026383 | (Kawahara et al., 2008) |
| R | CATGCAGAAAACTCCAAAAG | ||||
| E. maxima | F | GTTGCGTAAATAGAGCCCTCT | 152 | AF065094 | (You, 2014) |
| R | ACCAATGCAGAACGCTCCAG | ||||
| E. necatrix | F | GCAGTCGTTCTTGGGTGT | 148 | AF026385 | (Kawahara et al., 2008) |
| R | TGCTCACGCCCATACTAC | ||||
| E. tenella | F | TGGAGGGGATTATGAGAGGA | 147 | AF026388 | (Kawahara et al., 2008) |
| R | CAAGCAGCATGTAACGGAGA | ||||
| E. acervulina | F | GCAGTCCGATGAAAGGTATTTG | 103 | Ac-AD18-953 | (Siddiki et al., 2014) |
| R | GAAGCGAAATGTTAGGCCATCT | ||||
| Bovine Cytochrome B | F | CGGAGTAATCCTTCTGCTCACAGT | 116 | D34635 | (Dooley et al., 2004) |
| R | GGATTGCTGATAAGAGGTTGGTG |
DNA in the samples and inside the cells after the invasion assays. Forward primer (F), reverse primer (R), base pair (bp).
Statistical Analysis
GraphPad Prism 8.1.1 was used to perform statistical analysis. Descriptive analysis of data was done, and normality was checked with Shapiro-Wilk test. Normally distributed data were analyzed with a parametric one-way ANOVA test, whereas non-normal data were analyzed with Kruskal-Wallis tests. For the timepoint invasion assay, the comparison was done with two-way ANOVA. Post-hoc multiple comparison was done with Tukey's test, and differences were considered significant when P value was ≤0.05.
Results
Sample Characterization
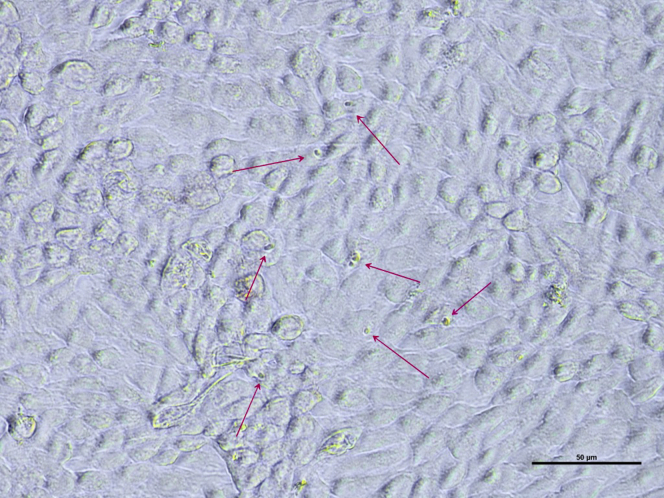
Different samples were characterized with qPCR to detect the species of Eimeria inside the processed samples. E. tenella, E. brunetti, and E. acervulina were detected. Then, the actual invading capability of the processed sporozoites was visualized by Giemsa staining (Figure 1).
Figure 1.
Eimeria sporozoites inside MDBK cells, Giemsa stained. The arrows indicate intracellular sporozoites after 24 h of invasion.
Invasion Assay 1
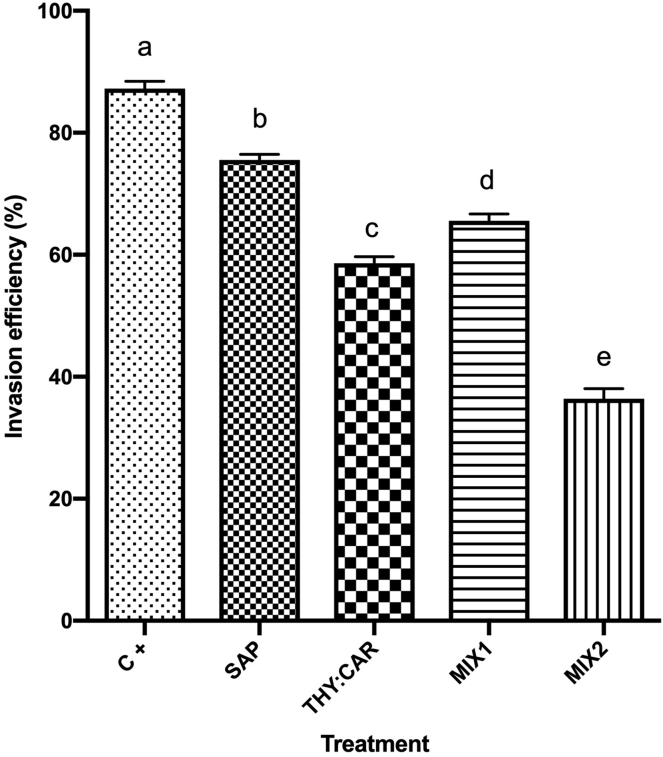
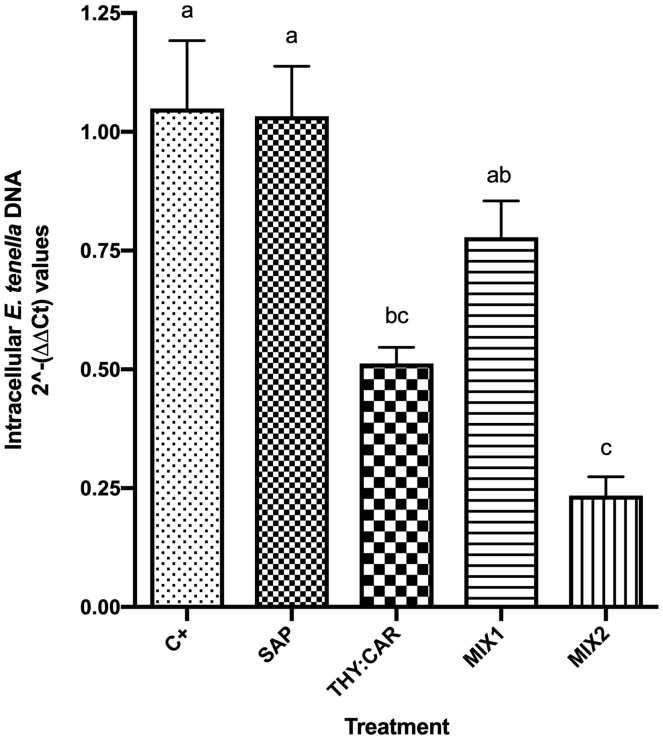
The results of the first invasion assay are reported in Figure 2. The counts highlighted significant decreases of the invasion efficiencies among all the thymol and carvacrol-based treatments (P ≤ 0.0002) compared with C+, especially THY:CAR and MIX2, whereas saponins effect was not significant (P = 0.409). The qPCR results confirmed the counts, as the same inhibition trend was visible (data are shown in Figure 3). THY:CAR and MIX2 significantly reduced E. tenella DNA quantity inside the cells compared with C+ (P values are respectively 0.022 and <0.0001), whereas SAP and MIX1 did not differ from it. The DNA of other Eimeria spp. was not detected inside the cells.
Figure 2.
Invasion efficiency determined by sporozoites count. Percentual values are presented as means, and SEM is symbolized with a bar; n = 18. Statistical analysis was performed with one-way ANOVA, letters above the columns represent significant differences among treatments (P ≤ 0.05). The treatments were infected control (C+), saponins 10 ppm (SAP), thymol 7 ppm + carvacrol 7 ppm (THY:CAR), saponins 5 ppm + thymol 3.5 ppm + carvacrol 3.5 ppm (MIX1), and saponins 10 ppm + thymol 7 ppm + carvacrol 7 ppm (MIX2).
Figure 3.
Intracellular E. tenella DNA relative quantity. Mean 2−(ΔΔCt) values are represented in the graph, and SEM is symbolized with a bar; n = 6. Statistical analysis was performed with one-way ANOVA, letters above the columns represent significant differences among treatments (P ≤ 0.05). The treatments were infected control (C+), saponins 10 ppm (SAP), thymol 7 ppm + carvacrol 7 ppm (THY:CAR), saponins 5 ppm + thymol 3.5 ppm + carvacrol 3.5 ppm (MIX1), and saponins 10 ppm + thymol 7 ppm + carvacrol 7 ppm (MIX2).
Invasion Assay 2 (Timepoints)
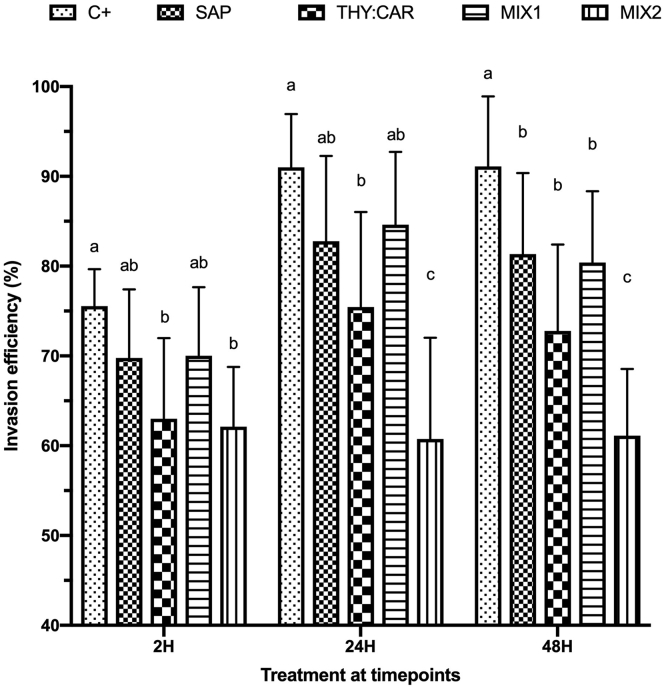
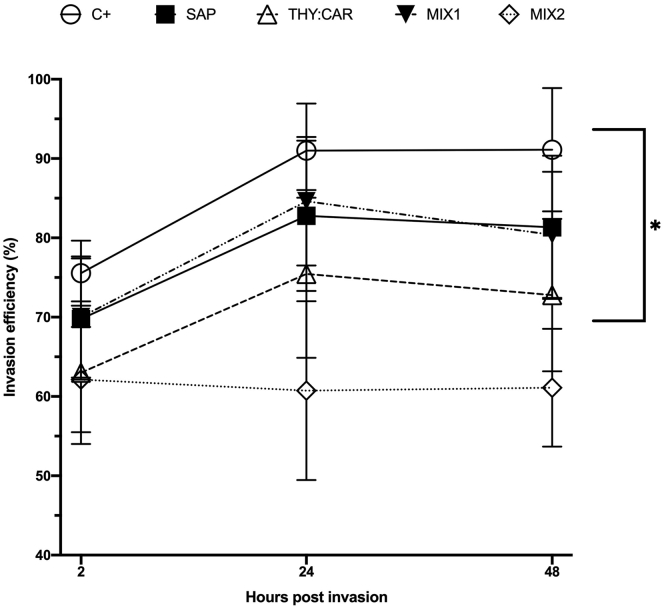
In the second invasion assay, supernatant counts were carried out at timepoints. The inhibition trend was visible at 2 hpi: SAP and MIX1 did not differ from C+, whereas THY:CAR and MIX2 were significantly reduced compared with C+ (P = 0.003 and 0.001, respectively). At 24 hpi, THY:CAR and MIX2 invasion efficiency was significantly lower than C+ (P < 0.0001); at 48 hpi, all of the treatments reduced invasion efficiency compared with C+ (P < 0.05) (Figure 4).
Figure 4.
Invasion efficiency determined by sporozoites count. Percentual values are presented as means, and SEM is symbolized with a bar; n = 12. Statistical analysis was performed with two-way ANOVA, letters above the columns represent significant differences among treatments within the same timepoint (P ≤ 0.05). The treatments were infected control (C+), saponins 10 ppm (SAP), thymol 7 ppm + carvacrol 7 ppm (THY:CAR), saponins 5 ppm + thymol 3.5 ppm + carvacrol 3.5 ppm (MIX1), and saponins 10 ppm + thymol 7 ppm + carvacrol 7 ppm (MIX2).
In this assay, changes in invasion efficiency over time were also compared within each treatment (Figure 5). Over time, MIX2 was the only treatment able to inhibit invasion within 2 hpi, whereas all the other treatments reached a plateau after 24 hpi.
Figure 5.
Overtime changes in invasion efficiency determined by sporozoites count. Percentual values are presented as means and SEM is symbolized with a bar; n = 12. Statistical analysis was performed with two-way ANOVA. The asterisk means that 24hpi and 48hpi values are significantly different from the 2 hpi values for C+, SAP, THY:CAR, MIX1 (P ≤ 0.05). The treatments were: infected control (C+), saponins 10 ppm (SAP), thymol 7 ppm + carvacrol 7 ppm (THY:CAR), saponins 5 ppm + thymol 3.5 ppm + carvacrol 3.5 ppm (MIX1), and saponins 10 ppm + thymol 7 ppm + carvacrol 7 ppm (MIX2). Abbreviation: hpi, hours postinfection.
Discussion
Recently documented cases of resistance to the classic anticoccidial treatments and restrictions to the use of antibiotics for livestock have increased the need of screening new compounds to control Eimeria (Giannenas et al., 2003). In vivo anticoccidial efficacy tests represent a routine tool but problematic issues, like high costs and ethical concerns are pushing toward the assessment of new methods to screen substances in vitro (Thabet et al., 2017). The aim of this study was to replicate an in vitro invasion model for Eimeria with a suitable method to estimate invasion efficiency on MDBK and to use it to test botanical molecules.
Eimeria sporozoites were extracted from multispecies field fecal samples to use them in an in vitro assay. Field samples are a valid source for in vitro experiments because they are more representative of the disease; indeed, field cases of coccidiosis are usually characterized by multiple strains of Eimeria. Despite this, only E. tenella DNA was detected inside the cells, suggesting that there might be a specific mechanism for internalization; in fact, there is little evidence that other Eimeria spp. sporozoites can be internalized by MDBK, and this aspect should be further investigated (Burt et al., 2013; Alnassan et al., 2015; Jitviriyanon et al., 2016). The natural target of avian Eimeria is the intestinal epithelia, but a chicken intestinal epithelial cell model is not available yet. Other cell lines are used to study Eimeria, but some are more permissive than others (Augustine, 2001a). Tierney and Mulcahy (2003) demonstrated that E. tenella sporozoites can be internalized by different cell models, especially baby hamster kidney cells, MDBK, and rabbit kidney cells, with different efficiencies (Tierney and Mulcahy, 2003). In another study, Augustine (2001) demonstrated that different species of Eimeria diverged in their ability to invade cells because of both sporozoital and cellular factors (Augustine, 2001b). Actually, invasion seems to occur by recognition of receptive molecules; in this study, the conditions might have allowed only E. tenella to successfully invade the cells.
To estimate invasion efficiency, supernatant sporozoites counts and qPCR were applied. Both are cheap, fast, and reliable. Former studies applied different quantification methods, but most of these rely on the use of expensive items and dangerous reagents such as antibodies or radioactive compounds. qPCR was also previously used by Alnassan et al. (2015) and Thabet et al. (2017) to quantify E. tenella in MDBK, but they applied an absolute quantification method instead, using internal transcribed spacer 1 gene (ITS-1) of E. tenella from a pSCA-amp/kan plasmid (Alnassan et al., 2015; Thabet et al., 2017).
In the present study, bovine cytochrome B was used as a housekeeping gene to detect the quantity of Eimeria DNA in the invaded cells, thus applying a relative quantification method. Supernatant sporozoites count was a newly assessed method of quantification, and we are not aware of other studies where it was used. It was a robust and precise tool, and it allowed fast estimation of invasion. However, by comparison of the results obtained with qPCR and sporozoites count, some divergences came out; even though the trend on inhibition is maintained for all treatments, the values were quite different between the 2 methods. This might be explained by the fact that for sporozoites counts, the percentage of inhibition is calculated in relation to all the free sporozoites, which are of various species, whereas in the case of qPCR, it is calculated on the DNA of one specific Eimeria spp. (in this case it was E. tenella only). This is probably why the 2 methods were not fully comparable, so sporozoites counts were chosen as the best marker for this study.
Among the tested treatments, thymol and carvacrol–based blends were the most effective. Also other studies have demonstrated that thymol and carvacrol exert an antiparasitic activity on Eimeria spp. and their mode of action is linked to the destruction of sporozoites' membrane and consequent loss of calcium ions from the parasite, essential for invasion in E. tenella (Sárközi et al., 2007; Bozkurt et al., 2013). Studies on saponins reveal that these compounds may interact with cholesterol on the sporozoites' membrane, thus hindering Eimeria lifecycle (Bozkurt et al., 2013). In the present study, the results suggest that thymol and carvacrol exert the main inhibiting effect on sporozoites and saponins act as adjuvants, but they do not have a strong inhibiting action by themselves. Saponins might instead facilitate the activity of thymol and carvacrol on the sporozoites. In fact, data from the second invasion assay highlight that sporozoites take 24 h to complete the invasion process. The action of thymol, carvacrol, and saponins was visible at 2 hpi already in THY:CAR and MIX2 groups. However, only MIX2 managed to stop invasion at 2 hpi, whereas in all the other treatments, the process went on for longer. This suggests that saponins might increase the anticoccidial power of thymol and carvacrol allowing a very rapid action of these compounds.
The activity of thymol and carvacrol against Eimeria was previously discussed by Giannenas et al. (2003) and Küçükyilmaz et al. (2012) in in vivo trials. Both found that oregano essential oils, rich in thymol and carvacrol, contribute to improve animal's health during a coccidia challenge and reduce the number of oocysts shed in feces. Giannenas et al. detected an increase in body weight gain similar to the uninfected group in chickens treated oregano EO. Küçükyilmaz et al. also found an improvement in immunity linked to oregano EO. Burt et al. analyzed the effect of carvacrol containing blends on MDBK in vitro and found that carvacrol significantly inhibited MDBK invasion by E. tenella. In these studies, the composition of the mixtures was variable, and other methods of detections were used, so it is difficult to compare the results (Giannenas et al., 2003; Küçükyilmaz et al., 2012; Burt et al., 2013). However, the anticoccidial efficacy of these molecules has been confirmed again by the outcomes of this study.
Scientific literature lacks studies on Eimeria spp., and those available are very different and hard to compare to one another. The protocol we applied can be used to screen fast and successfully other compounds at various doses to replace and reduce animals sacrifice. A fine and universal method for Eimeria research that respects the “3 Rs” guidelines should be assessed, and this study is one of the first to use field samples in a successful way to screen substances in vitro. We also found that thymol and carvacrol blends are interesting compounds to treat coccidiosis; the actual modes of action need to be elucidated in future, by investigating sporozoites' metabolic pathway and life cycle.
Acknowledgments
This work was supported by a grant from Vetagro S.p.A. (Reggio Emilia, Italy).
Conflict of Interest Statement: Andrea Piva serves as a professor at the University of Bologna and is a member of the board of directors of Vetagro S.p.A. (Reggio Emilia, Italy). Ester Grilli serves as an advisor of Vetagro S.p.A.
References
- Abbas R.Z., Colwell D.D., Gilleard J. Botanicals: an alternative approach for the control of avian coccidiosis. Worlds Poult. Sci. J. 2012;68:203–215. [Google Scholar]
- Alnassan A.A., Thabet A., Daugschies A., Bangoura B. In vitro efficacy of allicin on chicken Eimeria tenella sporozoites. Parasitol. Res. 2015;114:3913–3915. doi: 10.1007/s00436-015-4637-2. [DOI] [PubMed] [Google Scholar]
- Augustine P.C. Cell: sporozoite interactions and invasion by apicomplexan parasites of the genus Eimeria. Int. J. Parasitol. 2001;31:1–8. doi: 10.1016/s0020-7519(00)00150-8. [DOI] [PubMed] [Google Scholar]
- Augustine P.C. Invasion of different cell types by sporozoites of Eimeria species and effects of Monoclonal Antibodv 1209-C2 on invasion of cells bv sporozoites of several apicomplexan parasites. J. Eukaryot. Microbiol. 2001;48:177–181. doi: 10.1111/j.1550-7408.2001.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Giannenas I., Küçükyilmaz K., Paneri E., P-Florou C. An update on approaches to controlling coccidia in poultry using botanical extracts. Br. Poult. Sci. 2013;54:713–727. doi: 10.1080/00071668.2013.849795. [DOI] [PubMed] [Google Scholar]
- Burt S.A., Tersteeg-Zijderveld M.H.G., Jongerius-Gortemaker B.G.M., Vervelde L., Vernooij J.C.M. In vitro inhibition of Eimeria tenella invasion of epithelial cells by phytochemicals. Vet. Parasitol. 2013;191:374–378. doi: 10.1016/j.vetpar.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Cobaxin-Cárdenas M.E. Natural compounds as an alternative to control farm diseases: avian coccidiosis. In: Quiroz-Castañeda R.E., editor. Farm Animals Disease. Recent Omic Trends and New Strategies of Treatment. IntechOpen; London, UK: 2018. pp. 135–149. [Google Scholar]
- Dooley J.J., Paine K.E., Garrett S.D., Brown H.M. Detection of meat species using TaqMan real-time PCR assays. Meat Sci. 2004;68:431–438. doi: 10.1016/j.meatsci.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Francis G., Kerem Z., Makkar H.P.S., Becker K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Giannenas I., Florou-Paneri P., Papazahariadou M., Christaki E., Botsoglou N.A., Spais A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003;57:99–106. doi: 10.1080/0003942031000107299. [DOI] [PubMed] [Google Scholar]
- Jitviriyanon S., Phanthong P., Lomarat P., Bunyapraphatsara N., Porntrakulpipat S., Paraksa N. In vitro study of anti-coccidial activity of essential oils from indigenous plants against Eimeria tenella. Vet. Parasitol. 2016;228:96–102. doi: 10.1016/j.vetpar.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Kawahara F., Taira K., Nagai S., Onaga H., Onuma M., Nunoya T. Detection of five avian Eimeria species by species-specific real-time Polymerase Chain reaction assay. Avian Dis. 2008;52:652–656. doi: 10.1637/8351-050908-Reg.1. [DOI] [PubMed] [Google Scholar]
- Küçükyilmaz K., Bozkurt M., Selek N., Güven E., Eren H., Atasever A., Bintaş E., Çatli A.U., Çinar M. Effects of vaccination against coccidiosis, with and without a specific herbal essential oil blend, on performance, oocyst excretion and serum IBD titers of broilers reared on litter. Ital. J. Anim. Sci. 2012;11:1–8. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- Nazzaro F., Fratianni F., de Martino L., Coppola R. Effect of essential oils on pathogenic Bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Quiroz-Castañeda R.E., Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. Biomed. Res. Int. 2015;2015:430610. doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel W.M.S., Burch R. Methuen; London: 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- Sakkas H., Papadopoulou C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017;27:429–438. doi: 10.4014/jmb.1608.08024. [DOI] [PubMed] [Google Scholar]
- Sárközi S., Almássy J., Lukács B., Dobrosi N., Nagy G., Jóna I. Effect of natural phenol derivatives on skeletal type sarcoplasmic reticulum Ca2+-ATPase and ryanodine receptor. J. Muscle Res. Cell Motil. 2007;28:167–174. doi: 10.1007/s10974-007-9113-x. [DOI] [PubMed] [Google Scholar]
- Shirley M. Eimeria species and strains of chickens. In: Eckert J., Braun R., Shirley M.W., Coudert P., editors. Guidelines on Techniques in Coccidiosis Research. European Commission; Luxembourg City, Luxemburg: 1995. pp. 1–51. [Google Scholar]
- Siddiki A.Z., Mina S., Anayet Hasan M., Touaha Akbar M., Alam R., Ashraful Islam M., Zahan Ira I., Ayesa B. Molecular characterization of Eimeria spp. from chicken by Polymerase Chain Reaction based on species-specific SCAR markers. IOSR J. Agric. Vet. Sci. 2014;7:13–17. [Google Scholar]
- Singh V.P., Pratap K., Sinha J., Desiraju K., Bahal D., Kukreti R. Critical evaluation of challenges and future use of animals in experimentation for biomedical research. Int. J. Immunopathol. Pharmacol. 2016;29:551–561. doi: 10.1177/0394632016671728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabet A., Zhang R., Alnassan A.A., Daugschies A., Bangoura B. Anticoccidial efficacy testing: in vitro Eimeria tenella assays as replacement for animal experiments. Vet. Parasitol. 2017;233:86–96. doi: 10.1016/j.vetpar.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Tierney J., Mulcahy G. Comparative development of Eimeria tenella (Apicomplexa) in host cells in vitro. Parasitol. Res. 2003;90:301–304. doi: 10.1007/s00436-003-0846-1. [DOI] [PubMed] [Google Scholar]
- You M.J. Detection of four important Eimeria species by multiplex PCR in a single assay. Parasitol. Int. 2014;63:527–532. doi: 10.1016/j.parint.2014.01.006. [DOI] [PubMed] [Google Scholar]