Abstract
The prophylactic administration of ceftiofur to newly hatched chicks is a common practice in some hatcheries worldwide to mitigate early gastrointestinal infections caused by Enterobacteriaceae. In spite of the crucial role of the gut microbiome for the broiler's health, there is still limited information on how the microbial composition is affected by such procedure. We investigated the effects of posthatch prophylactic application of ceftiofur on the cecal microbiota of 14-day-old broilers fed regular or sanguinarine-supplemented diets. DNA samples were extracted from cecal contents, amplified for the V3-V4 regions of the microbial 16S rRNA gene, and sequenced in a high-throughput sequencing platform (Illumina MiSeq). After downstream bioinformatics and statistical analyses, our results demonstrated that both ceftiofur and sanguinarine treatments similarly increased the proportions of the phylum Bacteroidetes and the genera Bacteroides and Megamonas, whereas reduced the relative abundances of Firmicutes and Lachnospiraceae in the ceca of the birds. Such changes are probably associated with increased carbohydrate fermentation processes favoring the production of short-chain fatty acids. This was also corroborated by the functional prediction findings, which suggest an increase in some metabolic pathways associated with digestibility in broilers receiving ceftiofur. Considering that antimicrobial stewardship in animal production systems is strongly needed to mitigate the threat of antimicrobial resistance, our findings show that supplementation with a phytogenic feed additive can lead to a similar microbial composition in the ceca of commercial broiler chickens, suggesting that the use of alternative products could lead to functional modifications without increasing pressure for antimicrobial resistance.
Key words: broiler, cecal microbiota, ceftiofur, sanguinarine, 16S rRNA
Introduction
The poultry industry supports the increasing global demand for affordable and high-quality protein and plays a strategic role in the economy of leading chicken meat exporter countries, such as the United States and Brazil (Wen et al., 2019). Improved food efficiency, lower production costs, and more accessible prices to consumers are some competitive advantages that ensure the success of the poultry industry over the production of other animal proteins such as beef (OECD/FAO, 2019).
For more than half a century, antibiotics have significantly contributed to the improvement of animal productivity either as growth promoters that enhance performance or astherapeutic, prophylactic, and metaphylactic drugs to treat or prevent infectious diseases that compromise production (Singh et al., 2012; Cox et al., 2014; Brown et al., 2017). Efficient antibiotic therapy is and will continue to be a crucial resource for the broiler industry to treat infectious diseases (Sharma et al., 2016). On the other hand, the emergence of antimicrobial-resistant avian pathogens has imposed a challenge for the poultry industry (Bortolaia et al., 2016). In recent decades, there has been an intense global debate about how public health is actually impacted by the use of antibiotics in intensive animal production. The drivers for this are not restricted to the emergence and spread of zoonotic resistant pathogens (Bortolaia et al., 2016; Bueno et al., 2018) but also include putative risks associated with the spread of resistance genes to humans through the consumption of foods, such as chicken meat, or some direct and indirect contact with environmental sources contaminated by waste of farming systems, where antimicrobials are intensively used (Maron et al., 2013; Panzenhagen et al., 2016; Wall et al., 2016; Costa et al., 2017; Xiong et al., 2018). The presence of commensal bacteria in foods represents a public health concern, as they can serve as vehicles carrying resistance genes that could be ultimately transferred to the human gut bacteria by horizontal transfer mechanisms (Lerner et al., 2017).
Epidemiologic studies indicate a direct positive association between the use of some classes of antibiotics in animal production and increased antimicrobial resistance among pathogens causing infections in humans (Tang et al., 2017). The use of antimicrobial substances in intensive animal production has been restricted by regulations in several countries, including the United States and Brazil, whereas in other regions, such as the European Union, antimicrobial growth promoters have been banned for almost 2 decades (Maron et al., 2013; Costa et al., 2017).
In some poultry hatcheries, the Marek's disease vaccine is mixed with ceftiofur, a third-generation cephalosporin belonging to the β-lactam group, to be administered prophylactic in a single dose to posthatch chicks (Baron et al., 2014). The mechanisms underlying the beneficial effects of this practice are unknown. The gut commensal microbiome is responsible for the rapid development of the intestinal epithelium, as well as for its proper functionality. Modulation of specific commensal microbial species can favor animal performance by improving feed conversion (Kogut, 2019). On the other hand, the recurrent use of antibiotics can cause imbalances in the bacterial community and, as a consequence, predisposition to various conditions, mainly when they occur in the early stages of development (Yassour et al., 2016).
Moreover, cephalosporins are known to induce the bacterial conjugation process of resistance genes, a phenomenon observed in vitro among several pathogenic microorganisms, for example, Salmonella enterica and Escherichia coli originating from commercial poultry (Mo et al., 2017; Campos et al., 2018). Resistance to cephalosporins represents a major public health problem, as they are listed among the Highest Priority Critically Important Antimicrobials classes of drugs by the World Health Organization (Scott et al., 2019).
Considering the major challenges represented by antimicrobial resistance and the consequent limitation of the use of antibiotics, the animal industry has been exploring alternatives to conventional drugs, such as digestive enzymes, probiotics, prebiotics, organic acids, and phytogenic compounds, to reduce losses in productivity and improve animal health (Gadde et al., 2017). These substances can act directly modulating the intestinal microbiota and improving performance (Liu et al., 2017; Salaheen et al., 2017; Mehdi et al., 2018). Among these alternative compounds, sanguinarine, a bioactive phytogenic alkaloid, has been used as an alternative feed additive in commercial broiler production (Hassan et al., 2018). It is a benzophenanthridine alkaloid with anti-inflammatory (Wang et al., 2017), antibacterial (Miao et al., 2011), antitumoral (Gaziano, 2016), and antihelminthic properties (Huang et al., 2020). However, as in the case of the prophylactic administration of ceftiofur, there is not much information available on the potential effects of sanguinarine on the gut microbial community of broiler chickens. Furthermore, there is no information on the potential effect of sanguinarine as a modulator of the microbiota in case of changes caused by a previous antibiotic treatment.
The aim of this study was to investigate putative changes in the cecal microbiota of 14-day-old chicks triggered by the posthatch administration of ceftiofur. In addition, we investigated possible changes in the microbiota resulting from the dietary supplementation of sanguinarine alone or in combination with posthatch administration of ceftiofur. To investigate that, we used 16S rRNA sequencing, that is, high-throughput sequencing of amplified hypervariable regions of the microbial 16S rRNA gene, as it has been shown to be an efficient method for microbial ecology studies (Gill et al., 2006).
Material and methods
Experimental Design
The experimental proposal was submitted and approved (protocol 6513240218) by the Animal Use Ethics Committee of the Federal University of Paraiba, accredited by the Brazilian Council for Animal Experimentation Control.
The experiment was carried out in accordance with a completely randomized design, with 4 treatments and 3 repetitions per treatment with 11 birds (n = 132): negative control (NC) (subcutaneous injection of Marek's disease [MD] vaccine resuspended in 0.2 mL sterile saline solution at the first day after hatch); PHYTO (birds receiving only MD vaccine and fed the sanguinarine-based commercial product Sangrovit, 50 g/ton); ATB (birds given subcutaneous injection of 0.2 mL of MD vaccine resuspended in 1 mg/mL sodium ceftiofur solution); and MIXED (birds given subcutaneous MD vaccine plus sodium ceftiofur and also fed the sanguinarine-supplemented diet). The animals were fed antibiotic-free corn–soybean meal diets (22% CP; 2,950 kcal ME/kg; 1.31% digestible lysine; 0.852% digestible threonine; 0.94% digestible methionine + cysteine), according to Rostagno et al. (2017). Water and food were provided ad libitum. The animals were reared on floor pens with wood shavings as litter material, and temperature was monitored twice daily. After 14 D of hatch, 5 birds per treatment were randomly chosen and euthanized, and samples of the mucosa and contents of the left cecum were scraped. Samplings were performed at 14 D after hatch because this is the age in which the gut microbiota of commercial broilers presents maximum richness and evenness when exposed to antibiotics (Ocejo et al., 2019).
DNA Extraction and Sequencing Library Preparation
Total DNA was extracted using a commercial kit (PowerSoil DNA Isolation Kit, Qiagen) as per the manufacturer's protocols. The V3-V4 region of the microbial 16S rRNA gene was amplified by PCR (95°C for 3 min, followed by 25 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s and a final extension to 72°C for 5 min) using the primers 341F: 5'-TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG-3′ and 785R: 5′-GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGA CTA CHV GGG TAT CTA ATC C-3'. Library preparation was performed as per the standard Illumina 16S rRNA gene protocol (Illumina). The amplification products were visualized in 1.5% agarose gel for integrity analysis before being purified using AMPure beads (Beckman Coulter). The purified PCR products were then quantified by fluorometry (Qubit2.0, Life Invitrogen), and quality was assessed using a capillary electrophoresis system (Fragment Analyzer, Agilent). Sequencing was performed with the Illumina V2 kit (2 × 250 cycles) using the Illumina MiSeq.
Sequencing Data Processing
The raw demultiplexed paired-end sequences were downstream processed using QIIME 2 platform v.19.10 (Bolyen et al., 2019). Reads from 200 to 500 bp were selected, joined, quality filtered (minimum Phred score of 20) and rereplicated through VSEARCH (Rognes et al., 2016). Chimeric sequences were removed using UCHIME (Edgar et al., 2011). Operational taxonomic unit (OTU) identification was performed by the de novo clusterization method with 99% similarity between the centroid groups. The sequences were aligned by MAFFT (Katoh, 2002) and then used for the construction of the phylogenetic tree by FastTree2 (Price et al., 2010). The visualization of taxonomic compositions at their different levels, relative abundances of OTU, and alpha diversity were performed using phyloseq v.1.8.2 (McMurdie and Holmes, 2013) in R v.3.5.7. Taxonomic classifications were attributed using the Naïve Bayes method on the trained database of SILVA, version 132, with 99% for region V3-V4 (Quast et al., 2012).
The indexes Chao1 and Shannon, which estimate richness and evenness of the microbial communities, respectively, were chosen for the assessment of alpha diversity. For beta diversity analysis, the distance matrix was determined considering all pairs of samples by the UniFrac method (Lozupone and Knight, 2005) in its qualitative unweighted metric variant. Visualization was performed by principal component analysis using the QIIME 2 visualization platform. We also performed a functional prediction for the intestinal microbial community based on the taxonomic information by means of the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (Langille et al., 2013).
Statistical Analysis
Statistics for alpha diversity indices were performed by paired Kruskall-Wallis test, whereas the dissimilarity between treatments was assessed via permutational multivariate ANOVA at 5% probability. Differential abundance analysis to identify putative differences regarding specific OTU between treatments was performed by means of linear discriminant analysis effect size (Segata et al., 2011).
The statistical analysis of taxonomy and functional profiles (Parks et al., 2014) was used for visualization and significance testing of Phylogenetic Investigation of Communities by Reconstruction of Unobserved States gene prediction.
Results and discussion
16S rRNA Sequencing Products
A total of 24,500 reads rarefaction per sample were performed before downstream analyses. The mean length of reads was 448.95 bp, ranging from 207 to 497 bp. It was possible to detect 84,000 OTU after de novo centroid-based clustering using a 99% cutoff, corresponding to 322 different taxa as per SILVA v. 132 classifier.
Microbial Diversity Analyses
In terms of alpha diversity, the NC group showed the highest values for Shannon's diversity index (Figure 1A) and the lowest for the Chao1 index (Figure 1B). The opposite pattern was observed in the groups PHYTO, ATB, and MIXED. However, no statistical differences were observed (P > 0.05).
Figure 1.
Boxplots showing alpha diversity measured by Shannon (A) and Chao1 (B) diversity indexes for each treatment group: NC (negative control), PHYTO (sanguinarine supplementation), ATB (prophylactic use of ceftiofur), and MIXED (sanguinarine and ceftiofur). Three-dimensional PCoA plot from an unweighted UniFrac distance matrix showing the dissimilarities (beta diversity) across the different groups (C): NC (red), ATB (blue), PHYTO (green), MIXED (purple). Abbreviation: PCoA, principal component analysis.
In accordance with the beta diversity analysis, the different treatment groups were clustered apart, as shown in the principal component analysis plot (Figure 1C), where the NC group was the most dissimilar one to MIXED. Less dissimilarity was observed between NC and PHYTO. Our findings corroborate previous studies revealing increased dissimilarities between antimicrobial-exposed birds and their respective control (nonexposed) groups (Costa et al., 2017). This microbial shift caused by ceftiofur use has already been observed in other species, such as steers (Foster et al., 2019) and piglets (Ruczizka et al., 2019), but not in chickens. Considering that antibiotic-driven shifts in the microbiota might be associated with a decreased adaptive immune system stimulation, compromising some basic sanitary protocols, such as vaccination (Yitbarek et al., 2019), the practice of administering ceftiofur with MD vaccine should be revisited.
Microbial Compositional Analyses
The compositional structure of the cecal microbiota of 14-day-old broiler chicks (Figure 2A) evidenced microbial shifts in the most abundant taxa between the treatment groups. The phyla Firmicutes (83.4%/NC, 55.5%/PHYTO, 57.26%/ATB, and 50.77%/MIXED) and Bacteroidetes (12.40%/NC, 43.50%/PHYTO, 40.98%/ATB, and 47.14%/MIXED) comprised the main taxa at this taxonomic level. In comparison with the control group, all treatments led to a reduction in the Firmicutes abundance while increased Bacteroidetes. Increased Bacteroidetes abundance was also observed in birds given antibiotics as growth promoters (Mancabelli et al., 2016). In contrast, non–antibiotic-exposed free-range chickens presented higher Firmicutes abundance (Ocejo et al., 2019). Changes in the Firmicutes/Bacteroidetes ratio in the gut microbiota could lead to dysbiosisand therefore predispose to gastrointestinal diseases (Le Roy et al., 2019).
Figure 2.
Relative abundances of the 7 most prevalent phyla (A), the 10 most prevalent families (B), and the 15 most prevalent genera (C) across the 4 treatment groups: NC (negative control), PHYTO (sanguinarine supplementation), ATB (prophylactic use of ceftiofur), and MIXED (sanguinarine and ceftiofur).
Major bacterial families (Figure 2B) included Lachnospiraceae (42.62%/NC, 34.37%/PHYTO, 26.40%/ATB and 28.78%/MIXED), Ruminococcaceae (28.11%/NC, 13.63%/PHYTO, 25.75%/ATB and 14.83%/MIXED), Bacteroidaceae (12.64%/NC, 43.04%/PHYTO, 41.68%/ATB and 47.90%/MIXED), and Enterobacteriaceae (4.04%/NC, 0.95%/PHYTO, 1.72%/ATB and 1.91%/MIXED). The decrease in Lachnospiraceae triggered by both sanguinarine and ceftiofur could be considered as an undesirable result because this family is associated with BW gain and improved digestion (Torok et al., 2011; Crisol-Martínez et al., 2017), as well as an indicator for healthy gut microbiota in broiler chickens (Apajalahti and Vienola, 2016).
Bacteroides (19.37%/NC, 53/11%/PHYTO, 52.91%/ATB, and 59.69%/MIXED) and Ruminococcus (27.66%/NC, 23.91%/PHYTO, 13.34%/ATB, and 14.63%/MIXED) comprised the main microbial genera (Figure 2-C). According to Johnson et al. (2017), higher Bacteroides abundance is associated with increased polysaccharide fermentation and production of short-chain fatty acids, which contribute to the rapid development in broiler chickens (Zheng et al., 2019).
Our findings indicate that sanguinarine supplementation in the diet (PHYTO), ceftiofur administration (ATB), or both (MIXED) led to similar effects in terms of structural composition of the cecal microbiome (Figure 2) at the end of the initial production phase (14 D), corroborating a previous study about the use of different antibiotics in broilers (Chen et al., 2019). The most significant advantage of the phytobiotics relies on the fact that no pressure for antimicrobial resistance has been expected by this product (Yadav and Jha, 2019), and it is associated with the prevention of gastrointestinal diseases, such as necrotic enteritis related to gut dysbiosis in broilers (Xue et al., 2017). Furthermore, no negative collateral effects have been observed in chickens fed sanguinarine, which is partially absorbed in the intestines and metabolized in the liver (Hu et al., 2019).
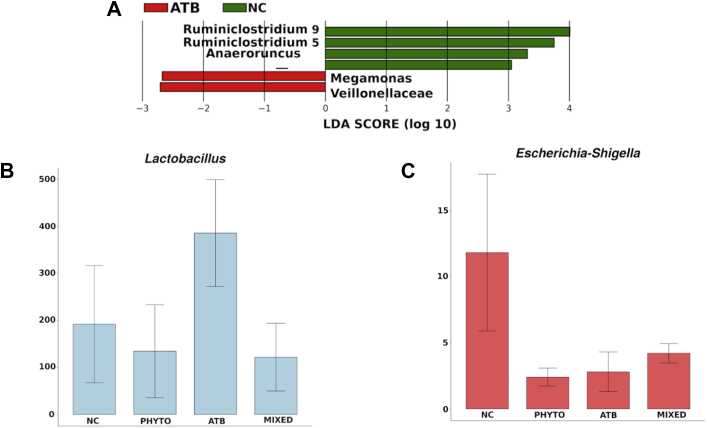
There were significantly higher abundances of Ruminoclostridium and Anaerotruncus in NC, whereas increased abundances of Megamonas and Veillonellaceae were observed in ATB (Figure 3A). Interestingly, these differences were not observed in birds treated with ceftiofur and supplemented with sanguinarine, indicating that the phytogenic additive mitigated the microbial shifts associated with the use of antibiotic only. The higher abundance of Ruminiclostridium in NC suggests that the cecal microbiota of nonexposed birds could be more efficient in terms of fibrous material digestion because some organisms belonging to this genus, such as Ruminiclostridium cellulolyticum, are able to metabolize cellulose (Ravachol et al., 2016). The higher abundance of Anaerotruncus in NC may suggest a high short-chain fatty acid production because the main byproducts of its metabolism are acetic and butyric acids (Lawson, 2015). In this case, the absence of antimicrobial drugs may favor not only fiber digesting bacteria such as Ruminococcus but also organisms involved in carbohydrate fermentation. On the other hand, it is possible that the higher abundance of Megamonas observed in ATB may be associated with improved carbohydrate digestion and short-chain fatty acid production, similar to Bacteroides (Polansky et al., 2016). These changes could lead to augmented carbohydrate fermentation in birds fed antimicrobials and thus contributing to an improved performance.
Figure 3.
Operational taxonomic units (OTU) showing statistically significant (P < 0.05) differential abundances between NC (control group) and ATB (broilers receiving ceftiofur after hatch) assessed by LEfSe (A). Relative abundance of Lactobacillus-associated OTU among the different treatment groups (B). Relative abundance of Enterobacteriaceae-associated OTU among the different treatment groups (C). Abbreviation: LEfSe, linear discriminant analysis effect size
Interestingly, a higher abundance of Veillonellaceae was detected in ceftiofur-treated birds. These organisms seem to be responsive to dietary calcium as they are more abundant in animals under calcium supplementation (Tilocca et al., 2016) and involved in bone formation disorders in broiler chickens, such as tibial dyschondroplasia (Tong et al., 2018).
To better compare the results of the present study with those of previous reports using conventional microbial analyses, the relative abundance of OTU counts related to Escherichia-Shigella and Lactobacillus taxa were selected and compared across the groups (Figures 3B, 3C). The highest abundance of Lactobacillus was observed in the ATB group, which is associated with a better protection against gut pathogens (Clavijo and Flórez, 2018). Although this genus is not very abundant in chickens cecum (Johnson et al., 2018), our findings suggest that the prophylactic use of ceftiofur favored Lactobacillus growth at the end of the initial production phase, which can possibly contribute to improve performance in antibiotic-treated commercial birds (Pereira et al., 2019).
Lower abundances of Enterobacteriaceae in response to both ceftiofur and sanguinarine treatments might have been caused by their bactericidal effects. A massive reduction in Enterobacteriaceae groups within 24 h after the administration of β-lactam antibiotic compounds has already been reported (Schokker et al., 2017). Our findings indicate that such reduction caused by the administration of ceftiofur can last until 14 D in broiler chickens. Although a decreased Enterobacteriaceae population is considered a positive effect of the antimicrobial treatment, the prophylactic use of ceftiofur could be a driver for selection of antibiotic-resistant bacteria. Indeed, the increased number of extended-spectrum beta-lactamase E.coli strains from the chicken gut has been associated with the posthatch administration of ceftiofur (Saraiva et al., 2018). Further studies on the dynamics of resistance determinants in the microbiota of broiler chickens under supplementation of phytogenic feed additives are warranted.
Gene Prediction
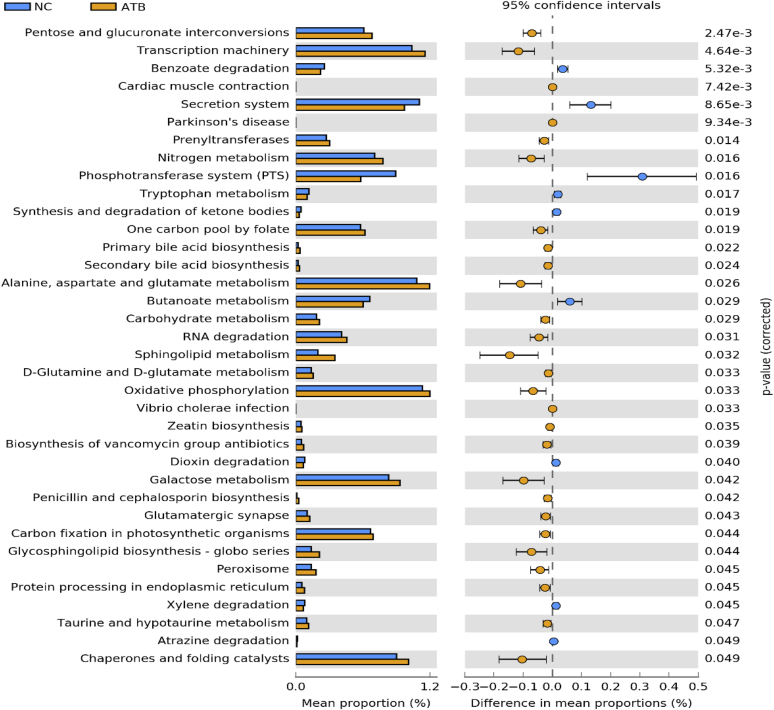
It was possible to detect significant differences (P < 0.05) between NC and ATB in terms of functional prediction performed by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (Figure 4). The prophylactic use of ceftiofur seems to increase the metabolism of alanine, aspartate, glutamine, and glutamate, as well as the transcription machinery that indicates intense protein metabolic routes. The interaction between the gut microbiota and the host is crucial to guarantee appropriate digestion and promote BW gain (Pan and Yu, 2014). The findings observed in this study indicate that ceftiofur treatment is associated with increased metabolic pathways related to protein synthesis, which corroborates the augmented stimulation of enterocyte development and the increased nutrient absorption in birds receiving antibiotics (Qi et al., 2018; Xue et al., 2018).
Figure 4.
Gene prediction results from PICRUSt showing KEGG metabolic pathways at the third hierarchical level between ATB (ceftiofur) and NC (negative control). Abbreviation: PICRUSt, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States.
The difference between NC and ATB groups for butanoate production by the phosphotransferase system is probably due to the higher abundances of fiber-degrading bacteria such as Ruminococcus and Ruminiclostridium, which are associated with the ability to degrade fiber and the establishment of healthy gut commensal bacteria (Hou et al., 2016).
Conclusion
Microbial shifts in the cecum of broiler chickens at the end of the first production phase (14 D) caused by sanguinarine added to feed were similar to those triggered by the off-label administration of ceftiofur after hatch. In view of the challenges imposed by the emergence of antimicrobial resistance and the need to reduce the use of highly critically important antimicrobial in livestock, the use of sanguinarine could be an alternative to modulate the microbiota of commercial broiler chickens under intensive production systems. Our findings warrant further investigation on the mechanisms associated with intestinal microbial modulation.
Acknowledgments
Funding: This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES, financing code 001).
Conflict of Interest Statement: The authors declare that there is no conflict of interest.
References
- Apajalahti J., Vienola K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Tech. 2016;221:323–330. [Google Scholar]
- Baron S., Jouy E., Larvor E., Eono F., Bougeard S., Kempf I. Impact of third-generation-cephalosporin administration in hatcheries on fecal Escherichia coli antimicrobial resistance in broilers and layers. Antimicrob. Agents Chemother. 2014;58:5428–5434. doi: 10.1128/AAC.03106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodríguez A.M., Chase J., Cope E., Da Silva R., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G., Janssen S., Jarmusch A.K., Jiang L., Kaehler B., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G., Lee J., Ley R., Liu Y.-X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., II, Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft J.J., Vargas F., Vázquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Qiime 2: reproducible, interactive, scalable, and extensible microbiome data science. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolaia V., Espinosa-Gongora C., Guardabassi L. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect. 2016;22:130–140. doi: 10.1016/j.cmi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Brown K., Uwiera R.R.E., Kalmokoff M.L., Brooks S.P.J., Inglis G.D. Antimicrobial growth promoter use in livestock: a requirement to understand their modes of action to develop effective alternatives. Int. J. Antimicrob. Agents. 2017;49:12–24. doi: 10.1016/j.ijantimicag.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Bueno I., Williams-Nguyen J., Hwang H., Sargeant J.M., Nault A.J., Singer R.S. Systematic Review: impact of point sources on antibiotic-resistant bacteria in the natural environment. Zoonoses Public Health. 2018;65:e162–e184. doi: 10.1111/zph.12426. [DOI] [PubMed] [Google Scholar]
- Campos J., Mourão J., Silveira L., Saraiva M., Correia C.B., Maçãs A.P., Peixe L., Antunes P. Imported poultry meat as a source of extended-spectrum cephalosporin-resistant CMY-2-producing Salmonella Heidelberg and Salmonella Minnesota in the European Union, 2014–2015. Int. J. Antimicrob. Agents. 2018;51:151–154. doi: 10.1016/j.ijantimicag.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Chen Y., Ni J., Li H. Effect of green tea and mulberry leaf powders on the gut microbiota of chicken. BMC Vet. Res. 2019;15:77. doi: 10.1186/s12917-019-1822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V., Flórez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M.C., Bessegatto J.A., Alfieri A.A., Weese J.S., Filho J.A.B., Oba A. Different antibiotic growth promoters induce specific changes in the cecal microbiota membership of broiler chicken. PLoS One. 2017;12:e0171642. doi: 10.1371/journal.pone.0171642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L.M., Yamanishi S., Sohn J., Alekseyenko A.V., Leung J.M., Cho I., Kim S.G., Li H., Gao Z., Mahana D., Zárate Rodriguez J.G., Rogers A.B., Robine N., Loke P., Blaser M.J. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisol-Martínez E., Stanley D., Geier M.S., Hughes R.J., Moore R.J. Sorghum and wheat differentially affect caecal microbiota and associated performance characteristics of meat chickens. PeerJ. 2017;5:e3071. doi: 10.7717/peerj.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D.M., Jacob M.E., Farmer K.A., Callahan B.J., Theriot C.M., Kathariou S., Cernicchiaro N., Prange T., Papich M.G. Ceftiofur formulation differentially affects the intestinal drug concentration, resistance of fecal Escherichia coli, and the microbiome of steers (K Mühldorfer, Ed.) PLoS One. 2019;14:e0223378. doi: 10.1371/journal.pone.0223378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gaziano R. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J. Gastrointest. Oncol. 2016;8:30. doi: 10.4251/wjgo.v8.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.R., Pop M., DeBoy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., Gordon J.I., Relman D.A., Fraser-Liggett C.M., Nelson K.E. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H.M.A., Samy A., Youssef A.W., Mohamed M.A. Using different feed additives as alternative to antibiotic growth promoter to improve growth performance and carcass traits of broilers. Int. J. Poult. Sci. 2018;17:255–261. [Google Scholar]
- Hou Q., Kwok L.-Y., Zheng Y., Wang L., Guo Z., Zhang J., Huang W., Wang Y., Leng L., Li H., Zhang H. Differential fecal microbiota are retained in broiler chicken lines divergently selected for fatness traits. Sci. Rep. 2016;6:37376. doi: 10.1038/srep37376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N.-X., Chen M., Liu Y.-S., Shi Q., Yang B., Zhang H.-C., Cheng P., Tang Q., Liu Z.-Y., Zeng J.-G. Pharmacokinetics of sanguinarine, chelerythrine, and their metabolites in broiler chickens following oral and intravenous administration. J. Vet. Pharmacol. Therap. 2019;42:197–206. doi: 10.1111/jvp.12729. [DOI] [PubMed] [Google Scholar]
- Huang H., Yao J., Liu K., Yang W., Wang G., Shi C., Jiang Y., Wang J., Kang Y., Wang D., Wang C., Yang G. Sanguinarine has anthelmintic activity against the enteral and parenteral phases of Trichinella infection in experimentally infected mice. Acta Trop. 2020;201:105226. doi: 10.1016/j.actatropica.2019.105226. [DOI] [PubMed] [Google Scholar]
- Johnson E.L., Heaver S.L., Walters W.A., Ley R.E. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017;95:1–8. doi: 10.1007/s00109-016-1492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.J., Youmans B.P., Noll S., Cardona C., Evans N.P., Karnezos T.P., Ngunjiri J.M., Abundo M.C., Lee C.-W. A consistent and predictable commercial broiler chicken bacterial microbiota in antibiotic-free production displays strong correlations with performance (CA Elkins, Ed.) Appl. Environ. Microbiol. 2018;84:e00362-18. doi: 10.1128/AEM.00362-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Scie. Tech. 2019;250:32–40. [Google Scholar]
- Langille M.G.I., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R., Beiko R.G., Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson P.A. Anaerotruncus. In: Whitman W.B., Rainey F., Kämpfer P., Trujillo M., Chun J., DeVos P., Hedlund B., Dedysh S., editors. Bergey’s Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Ltd; Chichester, UK: 2015. pp. 1–f4. [Google Scholar]
- Le Roy C.I., Woodward M.J., Ellis R.J., La Ragione R.M., Claus S.P. Antibiotic treatment triggers gut dysbiosis and modulates metabolism in a chicken model of gastrointestinal infection. BMC Vet. Res. 2019;15:37. doi: 10.1186/s12917-018-1761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A., Matthias T., Aminov R. Potential effects of horizontal gene exchange in the human gut. Front. Immunol. 2017;8:1630. doi: 10.3389/fimmu.2017.01630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y., Yang X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancabelli L., Ferrario C., Milani C., Mangifesta M., Turroni F., Duranti S., Lugli G.A., Viappiani A., Ossiprandi M.C., van Sinderen D., Ventura M. Insights into the biodiversity of the gut microbiota of broiler chickens: the gut microbiota of broiler chickens. Environ. Microbiol. 2016;18:4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- Maron D., Smith T.J., Nachman K.E. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Glob. Health. 2013;9:48. doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi Y., Létourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Côté C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao F., Yang X.-J., Zhou L., Hu H.-J., Zheng F., Ding X.-D., Sun D.-M., Zhou C.-D., Sun W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011;25:863–875. doi: 10.1080/14786419.2010.482055. [DOI] [PubMed] [Google Scholar]
- Mo S.S., Sunde M., Ilag H.K., Langsrud S., Heir E. Transfer potential of plasmids conferring extended-spectrum-cephalosporin resistance in Escherichia coli from poultry. (DW Schaffner, Ed.) Appl. Environ. Microbiol. 2017;83:e00654-17. doi: 10.1128/AEM.00654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocejo M., Oporto B., Hurtado A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019;9:2506. doi: 10.1038/s41598-019-39323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD/FAO. 2019. OECD-FAO agricultural outlook 2019-2028, OECD Publishing, Paris, France/Food and Agriculture Organization of the United Nations, Rome, Italy. Accessed Aug. 2020. 10.1787/agr_outlook-2019-en. [DOI]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzenhagen P.H.N., Aguiar W.S., Frasão B.S., Pereira V.L.A., Costa Abreu D.L., Rodrigues D.P., Nascimento E.R., Aquino M.H.C. Prevalence and fluoroquinolones resistance of Campylobacter and Salmonella isolates from poultry carcasses in Rio de Janeiro, Brazil. Food Control. 2016;61:243–247. [Google Scholar]
- Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R., Bortoluzzi C., Durrer A., Fagundes N.S., Pedroso A.A., Rafael J.M., de Lima Perim J.E., Zavarize K.C., Napty G.S., Andreote F.D., Costa D.P., Menten J.F.M. Performance and intestinal microbiota of chickens receiving probiotic in the feed and submitted to antibiotic therapy. J. Anim. Physiol. Anim. Nutr. 2019;103:72–86. doi: 10.1111/jpn.13004. [DOI] [PubMed] [Google Scholar]
- Polansky O., Sekelova Z., Faldynova M., Sebkova A., Sisak F., Rychlik I. Important metabolic pathways and Biological processes Expressed by chicken cecal microbiota (CM Dozois, Ed.) Appl. Environ. Microbiol. 2016;82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M.N., Dehal P.S., Arkin A.P. FastTree 2 – Approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi B., Wang J., Ma Y., Wu S., Qi G., Zhang H. Effect of dietary β-alanine supplementation on growth performance, meat quality, carnosine content, and gene expression of carnosine-related enzymes in broilers. Poult. Sci. 2018;97:1220–1228. doi: 10.3382/ps/pex410. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravachol J., de Philip P., Borne R., Mansuelle P., Maté M.J., Perret S., Fierobe H.-P. Mechanisms involved in xyloglucan catabolism by the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Sci. Rep. 2016;6:22770. doi: 10.1038/srep22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes T., Flouri T., Nichols B., Quince C., Mahé F. VSEARCH: a versatile open source tool for metagenomics. Peerj Prep. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostagno H.S., Albino L.F.T., Hannas M.I., Donzele J.L., Sakomura N.K., Perazzo F.G., Saraiva A., Teixeira M.V., Rodrigues P.B., Oliveira R.F., Barreto S.L.T. Departamento de Zootecnia, Universidade Federal de Viçosa; Viçosa, MG: 2017. C. O. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 4a edição; p. 488p. [Google Scholar]
- Ruczizka U., Metzler-Zebeli B., Unterweger C., Mann E., Schwarz L., Knecht C., Hennig-Pauka I. Early parenteral administration of ceftiofur has gender-specific short- and long-term effects on the fecal microbiota and growth in pigs from the suckling to growing phase. Animals. 2019;10:17. doi: 10.3390/ani10010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaheen S., Kim S.-W., Haley B.J., Van Kessel J.A.S., Biswas D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 2017;8:2088. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M.M.S., Moreira Filho A.L.B., Freitas Neto O.C., Silva N.M.V., Givisiez P.E.N., Gebreyes W.A., Oliveira C.J.B. Off-label use of ceftiofur in one-day chicks triggers a short-term increase of ESBL-producing E. coli in the gut. PLoS One. 2018;13:e0203158. doi: 10.1371/journal.pone.0203158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker D., Jansman A.J.M., Veninga G., de Bruin N., Vastenhouw S.A., de Bree F.M., Bossers A., Rebel J.M.J., Smits M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genomics. 2017;18:241. doi: 10.1186/s12864-017-3625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H.M., Acuff G., Bergeron G., Bourassa M.W., Gill J., Graham D.W., Kahn L.H., Morley P.S., Salois M.J., Simjee S., Singer R.S., Smith T.C., Storrs C., Wittum T.E. Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N.Y. Acad. Sci. 2019;1441:8–16. doi: 10.1111/nyas.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.K., Johnson N., Cizmas L., McDonald T.J., Kim H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere. 2016;150:702–714. doi: 10.1016/j.chemosphere.2015.12.084. [DOI] [PubMed] [Google Scholar]
- Singh K.M., Shah T., Deshpande S., Jakhesara S.J., Koringa P.G., Rank D.N., Joshi C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012;39:10595–10602. doi: 10.1007/s11033-012-1947-7. [DOI] [PubMed] [Google Scholar]
- Tang K.L., Caffrey N.P., Nóbrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., Polachek A.J., Ganshorn H., Sharma N., Kellner J.D., Ghali W.A. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet. Health. 2017;1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilocca B., Witzig M., Rodehutscord M., Seifert J. Variations of phosphorous accessibility causing changes in microbiome functions in the gastrointestinal tract of chickens. PLoS One. 2016;11:e0164735. doi: 10.1371/journal.pone.0164735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X., Rehman M.U., Huang S., Jiang X., Zhang H., Li J. Comparative analysis of gut microbial community in healthy and tibial dyschondroplasia affected chickens by high throughput sequencing. Microb. Pathog. 2018;118:133–139. doi: 10.1016/j.micpath.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Torok V.A., Allison G.E., Percy N.J., Ophel-Keller K., Hughes R.J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. Environ. Microbiol. 2011;77:3380–3390. doi: 10.1128/AEM.02300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall B.A., Mateus A., Marshall L., Pfeiffer D., Lubroth J., Ormel H.J., Otto P., Patriarchi A. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production. FAO - Food and Agriculture Organization of the United Nations; Rome, Italy: 2016. Mechanisms of spread of antimicrobial resistance between animals and humans; pp. 28–36. [Google Scholar]
- Wang Q., Dai P., Bao H., Liang P., Wang W., Xing A., Sun J. Anti-inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp. Ther. Med. 2017;13:263–268. doi: 10.3892/etm.2016.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Li L., Sun S., He Q., Tsai F.-S. The Contribution of chicken products’ export to economic growth: evidence from China, the United States, and Brazil. Sustainability. 2019;11:5253. [Google Scholar]
- Xiong W., Wang Y., Sun Y., Ma L., Zeng Q., Jiang X., Li A., Zeng Z., Zhang T. Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome. 2018;6:34. doi: 10.1186/s40168-018-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G.D., Barekatain R., Wu S.B., Choct M., Swick R.A. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult. Sci. 2018;97:1334–1341. doi: 10.3382/ps/pex444. [DOI] [PubMed] [Google Scholar]
- Xue G.D., Wu S.B., Choct M., Pastor A., Steiner T., Swick R.A. Impact of a Macleaya cordata-derived alkaloid extract on necrotic enteritis in broilers. Poult. Sci. 2017;96:3581–3585. doi: 10.3382/ps/pex164. [DOI] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M., Vatanen T., Siljander H., Hämäläinen A.-M., Härkönen T., Ryhänen S.J., Franzosa E.A., Vlamakis H., Huttenhower C., Gevers D., Lander E.S., Knip M., on behalf of the DIABIMMUNE Study Group. Xavier R.J. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016;8:343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitbarek A., Astill J., Hodgins D.C., Parkinson J., Nagy É., Sharif S. Commensal gut microbiota can modulate adaptive immune responses in chickens vaccinated with whole inactivated avian influenza virus subtype H9N2. Vaccine. 2019;37:6640–6647. doi: 10.1016/j.vaccine.2019.09.046. [DOI] [PubMed] [Google Scholar]
- Zheng M., Mao P., Tian X., Guo Q., Meng L. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult. Sci. 2019;98:2250–2259. doi: 10.3382/ps/pey550. [DOI] [PubMed] [Google Scholar]