Abstract
We investigated effects of rearing cage type and dietary limestone particle size (LPS) on egg production, egg weight, eggshell, and bone quality in laying hens. The pullets were reared in conventional (CON; 20 chicks/cage, 270 cm2/chick) or furnished (FUR; 30 chicks/cage; 636 cm2/chick) cages and fed 3 LPS (fine, <0.595 mm; medium, 0.595 to <1.68 mm; and 1:1 mixture of F and M wt/wt) to 16 wk of age (woa). Pullets were transitioned to laying furnished cages and retained rearing treatment combination identities (n = 5, 20 hens/cage). Hens had free access to common commercial layer diet and water through to 40 woa. Eggs were recorded daily for calculation of hen day egg production (HDEP). Subsamples of eggs laid on the first day of 24, 28, 32, 36, and 40 woa were used for eggshell quality analyses. Two hens per cage were sacrificed on the last day of 24 and 40 woa for femur and tibia quality assessments. There was no interaction (P > 0.05) between rearing cage type and dietary LPS on response variables. At 19 and 20 woa, HDEP was higher (P < 0.01) for FUR than CON reared hens but was similar (P > 0.05) afterward. At 40 woa, FUR reared hens had higher (P < 0.05) body weight (BW), egg weight (EW), eggshell thickness, and eggshell weight and tended (P < 0.10) to have higher femur and tibia mineral density (BMD) and mineral content (BMC) than CON reared hens. Rearing dietary LPS had no effect (P > 0.05) on HDEP, BW, EW, and eggshell quality. Although, rearing dietary LPS did not affect (P > 0.05) femur and tibia BMD and BMC; at 24 woa, hens reared on medium LPS tended to have higher femur BMD (0.17 vs. 0.14 g/cm2; P = 0.079) and BMC (0.99 vs.0.78 g; P = 0.088) than hens reared on fine LPS. In conclusion, hens reared in furnished cages had better eggshell quality but had marginal effects on femur and tibia quality, whereas rearing dietary LPS had no effects on eggshell and bone attributes in hens.
Key words: bone quality, rearing housing, rearing limestone particle size, egg production, eggshell quality
Introduction
With continuous genetic selection and better management, breeders have succeeded in developing layers with early sexual maturity (Fairfull and Gowe, 1986), early and persistent peak production (McMillan et al., 1990), and higher life time egg mass production (Jones et al., 2001). Another important aspect of modern layers is a long lay cycle without molting (Meng et al., 2013). Persistent long laying cycle has been associated with eggshell and bone quality challenges (Kim et al., 2012; Bain et al., 2016). With molting, reduction of circulating estrogen allows the re-mineralization of structural bones (Bain et al., 2016). As such, the longer laying cycle without molting means the modern hens are prone to structural bone depletion as laying cycle progresses (Akbari Moghaddam Kakhki et al., 2019). Moreover, the structural bone development, both radial and longitudinal, takes place before sexual maturity (during rearing) because of estradiol inhibition once egg production starts (Strickland and Sprinz, 1973; Kidder et al., 1997).
Several studies reported that offering opportunities for load bearing activities in the housing system enhanced the bone quality in pullets through to the laying phase (Casey-Trott et al., 2017a; Eusebio-Balcazar et al., 2018; Neijat et al., 2019). Moreover, the bones of laying hens were found to have higher mineral density and breaking strength when provided with the perches and a spacious cage area (Jendral et al., 2008). There are several findings showing that coarser limestone particle size (LPS) yielded better bone (Fleming et al., 1998) and eggshell quality (Guo and Kim, 2012; Świątkiewicz et al., 2015). Some findings reported that furnished cage housing (Casey-Trott et al., 2017b) and coarser dietary limestone particles (Eusebio-Balcazar et al., 2018) during rearing enhanced the bone quality during the laying. However, little is known on interaction between rearing housing system and dietary LPS on eggshell and bone quality in hens. The present study was a continuation of pullet experiment reported in Khanal et al. (2020) with the objective of investigating the impact of rearing cage types (conventional, CON and furnished, FUR) and dietary LPS (fine, <0.595 mm, F; medium, 0.595–<1.68 mm, M; and 1:1 mixture of F and M wt/wt; FM) on bone and eggshell quality from the onset to 40 wk of age in Lohmann LSL lite hens.
Material and methods
The experimental protocol was reviewed and approved by the University of Guelph Animal Care Committee. This experiment took place at the University of Guelph's Arkell Poultry Research Station in Guelph, ON, Canada, and birds were cared for in accordance with the Canadian Council on Animal Care guidelines (CCAC, 2009).
Birds and Housing
The methodology details of the pullet experiment are described in Khanal et al. (2020). Briefly, the treatments were arranged in a 2 × 3 factorial arrangement, with cage (conventional, CON; and furnished, FUR) and dietary LPS (fine, <0.59 mm, F; medium, 0.59 to 1.19 mm, M; and 1:1 mixture of F and M wt/wt; FM). A total of 900-day-old Lohmann LSL-Lite chicks were placed in CON (20 chicks/cage; 270 cm2/chick) and FUR (30 chicks/cage, 636 cm2/chick) based on body weight. The 3 types of dietary LPS were offered in a three-phase feeding program: starter (0–4 woa), grower (5–8 woa), and developer (9–16 woa) according to breeder's nutrient specifications (Lohmann, 2020). Within cage type, 3 diets were allocated in a completely randomized design to give 6 replicates. To ensure adequate numbers of CON pullets at transition to layer phase experiment, 3 additional CON cages (1 for each of diets) were maintained in the same room and conditions as in the main pullet experiment (Khanal et al., 2020).
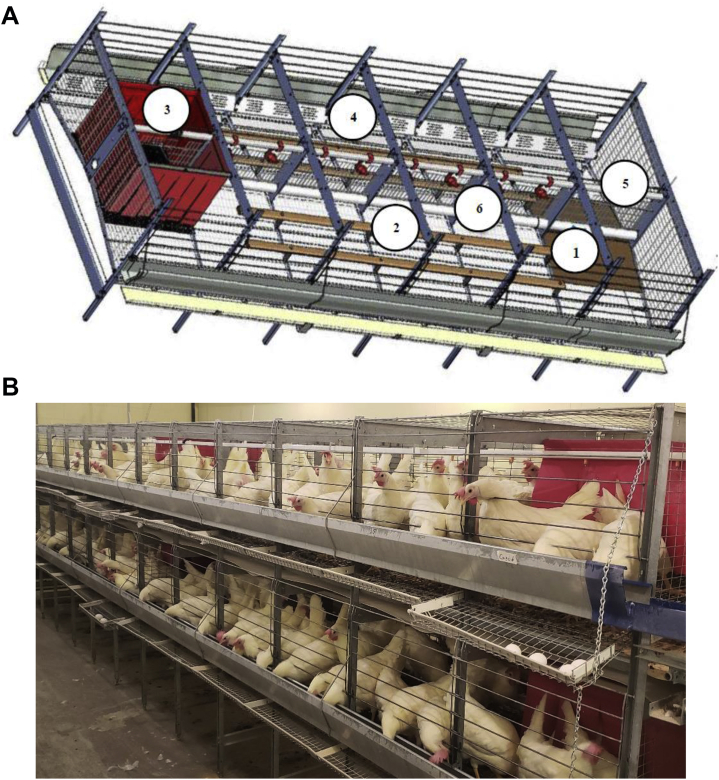
In the present study, pullets were transitioned to laying house at 17 woa and assigned to laying furnished cages based on their pullet treatment combination without any mixing. Each pullet treatment combination was assigned to 5 replicates (cages) with 20 hens per cage. All the cages were similar in all aspects and were furnished with perches, a scratch mat, and a curtained nest (Figure 1, Ford Dickson Inc., Mitchell, ON, Canada). The dimensions of each cage were 365 cm × 65 cm × 55 cm and was equipped with a feeder (365 cm × 8.5 cm × 15 cm) at the front. Two parallel perches, 20 cm apart, of 10 cm in circumference and 240 cm in length were placed in the mid region of cage and installed at 8 cm above the floor (Figure 1). Each cage was equipped with 12 nipple drinkers at equidistant in midline of cage and a nest (60 cm × 30 cm × 55 cm). The average space and total utilizable space for a pullet in a furnished cage was 1,186 cm2 and 23,725 cm2 respectively.
Figure 1.
The design of furnished layer cage (A) (source: Ford Dickson Inc., Mitchell, Ontario, Canada), and hens in the furnished cage during the study (B). Number labeling describes enrichment; 1 = Scratch mat/Litter mat, 2 = Perch, 3 = Curtained nest, 4 = Claw scratcher, 5 = Drinker and 6 = Litter delivery tube.
Experimental Procedures and Sampling
The hens had free access to water and common commercially prepared diet (Floradale Feed Mill Ltd., Floradale, Ontario, Canada). The ingredients and chemical composition of the layer diet is presented in Table 1. The total number of eggs including cracked and shell-less eggs were recorded by cage basis on daily basis (1,000–1,100 h) to calculate hen-day egg production (HDEP, %). From pool pf, all eggs laid on the first day of 24, 28, 32, 36, and 40 woa, 5 eggs per cage were randomly sampled for eggshell thickness (EST), eggshell breaking strength (ESBS), and eggshell weight (ESW) analyses. Two hens per cage were randomly selected on the last day of 24 and 40 woa and sacrificed by cervical dislocation. Right legs were excised, defleshed, and femur and tibia dissected and stored at –20°C until further analyses.
Table 1.
Ingredients and chemical composition of commercial layer diet, as fed basis.
| Ingredients, g/Kg of diet | Amount |
|---|---|
| Corn | 555.0 |
| Soybean meal | 166.0 |
| Pork meal | 70.0 |
| Wheat shorts | 51.0 |
| Corn gluten meal | 25.0 |
| Tallow Pelleter (AV Blend) | 15.0 |
| Tallow mixer (AV Blend) | 10.0 |
| Limestone | 95.0 |
| Mono calcium phosphate | 5.5 |
| Salt (NaCl) | 3.0 |
| Vitamin and trace minerals premix1 | 3.0 |
| Alimet | 1.0 |
| Choline chloride 70% | 0.5 |
| Calculated composition | |
| Dry matter, % | 89.38 |
| ME, Kcal/kg | 2,886.0 |
| Crude protein, % | 18.07 |
| Lysine, % | 0.89 |
| Methionine, % | 0.38 |
| Methionine + cysteine, % | 0.64 |
| Crude fat, % | 5.77 |
| Linoleic acid, % | 1.97 |
| Crude fiber, % | 1.83 |
| Calcium, % | 4.22 |
| Total phosphorous, % | 0.65 |
| Available phosphorous, % | 0.44 |
| Vitamin D3, IU/Kg | 4,500 |
| Determined composition | |
| Dry matter, % | 90.05 |
| Ash, % | 16.52 |
| Calcium, % | 4.20 |
| Total phosphorous, % | 0.58 |
Provided per kilogram of premix: Vitamin A = 1,000,000 IU, Vitamin D3 = 300,000 IU, Vitamin E = 40,000 IU, Vitamin B12 = 25,000 mcg, Biotin = 150,000 mcg, Menadione = 2,500 mg, Thiamine = 2,500 mg, Riboflavin = 9,500 mg, Pantothenic acid = 16,000 mg, Pyridoxine = 4,500 mg, Niacin = 50,000 mg, Folic acid = 2,500 mg, Iron = 50,000 mg, Copper = 8,000 mg, Manganese = 75,000 mg, Zinc = 75,000 mg, Iodine = 1,000 mg.
Sample Processing and Laboratory Analyses
The EST and ESBS were measured according to Mwaniki et al. (2018). The EST (mm) was measured using a high-resolution nondestructive device with precision ultrasound (Model: Ti-Pvx, ORKA Food Technology Ltd., Ramat HaSharon, Israel), and ESBS (N) was measured by Force Reader (ORKA Food Technology Ltd., ORKA Food Technology Ltd.). Eggs were then cracked open, albumen and yolk discarded, albumen adhering to eggshell removed by paper towel, and eggshell dried overnight at room temperature and weighed. The femur and tibia bone mineral density (BMD, g/cm2), bone mineral content (BMC, g), bone breaking strength (BBS, N), and ash concentration (%) were analyzed as described by Khanal et al. (2019). The BMD and BMC were analyzed using Prodigy dual-energy X-ray absorptiometry (GE Healthcare, Madison, WI) equipped with enCORE software (version 14.0). The bones were scanned twice, and the mean values were used for data analyses. Before the BBS measurements, femur and tibia samples were thawed at room temperature for 2 h. A three-point bending test with an Instron material tester (Model: Instron crop, Canton, MA) automated with the material test system (software BlueHill 3.0, version 3.7.7) was used to measure BBS. Briefly, the maximum load of the compressor was set at 500 N with a cross head speed of 5 mm/s. The distance between upper and lower anvil was set to be 27 mm for all bones, and spans were fixed between 4 and 6 mm from center of the bone. Femurs were kept medial side up, and tibia were kept anterior side up. The BBS was determined in Newton as provided by the apical point in the breaking strength curve. Following BBS determination, femur and tibia samples were used for ash determination. Briefly, both femur and tibia were oven dried to constant weight at 100°C for 24 h and ashed in a muffle furnace at 600°C for 12 h (Khanal et al., 2019).
Calculation and Statistical Analysis
The HDEP was calculated by dividing the number of the eggs produced per cage by numbers of the hens and expressed in percentage. The proportion (%) of eggshell was calculated by dividing the weight of dried eggshell by egg weight. The interaction and main effects of rearing cage type and LPS were analyzed using PROC GLIMMIX procedures of SAS 9.4 (SAS Institute, 2014). The model was Yijk = μ + ai + bj + ab ij + εijk, where Yijk = response variable, μ = mean, ai = cage type (FUR or CON), bj = LPS in pullet diet (fine, <0.595 mm, F; medium, 0.595 to <1.68 mm, M; and 1:1 mixture of F and M wt/wt; FM), ab ij = interaction of cage type and LPS on response variable, and εijk = error. The body weight was the function of rearing cage type, so was not used as a covariate in bone quality data. Similarly, the egg weight was found to be a function of rearing cage type and was not used as a covariate for eggshell quality parameters. The statistical significance was declared at P < 0.05, and tendency 0.05 ≤ P ≤ 0.1 was discussed.
Results
Body Weight, Egg Production, and Egg Weight
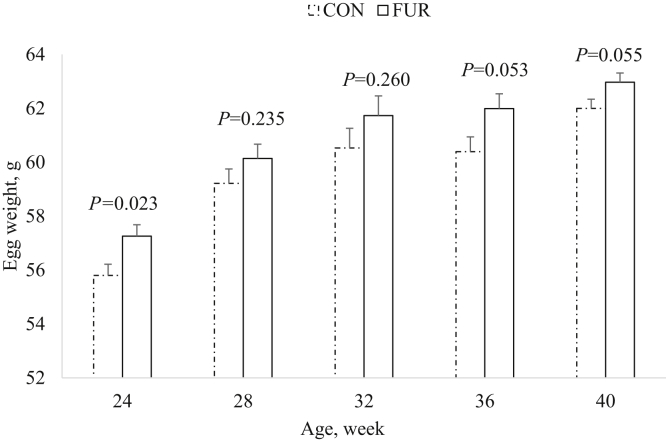
There was no (P > 0.05) interaction between rearing cage type and dietary LPS on BW, egg production and egg weight. The cage type had no (P = 0.911) effect on hen BW at 24 woa; however, the hens reared in FUR were heavier (P = 0.015) than hens reared in CON cages at 40 woa (Figure 2A). The rearing dietary LPS did not (P > 0.05) affect hen BW at 24 and 40 woa (Figure 2B). There was no (P > 0.05) interaction between rearing cage type and dietary LPS on HDEP and egg weight. Pullet cage type affected HDEP in early phase of egg production with hens reared in FUR cages having higher (P < 0.01) HDEP at 19 and 20 woa relative to CON cage pullets (Figure 3). However, pullet cage type did not (P > 0.05) influence HDEP post-20 woa (Figure 3). The HDEP was similar (P > 0.05) for hens reared on F, FM, and M dietary LPS from 19 to 40 woa (Figure 4). Rearing cage type influenced egg weight such that eggs of hens reared in FUR cages were heavier at 24 woa (P = 0.023) and tended to be heavier at 36 and 40 woa (P ≤ 0.055) than for hens reared in CON cages (Figure 5). The rearing dietary LPS feeding program did not (P > 0.05) affect the egg weight from onset of lay to 40 woa. The egg weight for hens reared on F, FM, and M were respectively 56.90, 56.72, and 55.96 g at 24 woa (P = 0.412); 59.16, 60.68, and 59.21 g at 28 woa (P = 0.192); 61.91, 61.73, and 59.76 g at 32 woa (P = 0.196); 61.76, 60.92, and 60.88 g at 36 woa (P = 0.601) and 61.95, 63.10, and 62.41 at 40 woa (P = 0.170).
Figure 2.
Effect of rearing cage type (A) and dietary limestone particle size (B) on the body weight of Lohmann LSL-Lite hens. For cages; conventional (CON) cage 76 cm × 71 cm × 46 cm; furnished (FUR) cage 239 cm × 80 cm × 75 cm outfitted with platforms and terraces. Limestone particle size; F, fine, <0.595 mm, F; medium, 0.595 to <1.68 mm, M; and 1:1 mixture of F and M wt/wt; FM.
Figure 3.
Effect of rearing cage type on hen day egg production in Lohmann LSL-Lite hens. ∗∗ indicates P = <0.01. Conventional (CON) cage 76 cm × 71 cm × 46 cm; furnished (FUR) cage 239 cm × 80 cm × 75 cm outfitted with platforms and terraces.
Figure 4.
Effect of rearing dietary limestone particle size on hen day egg production in Lohmann LSL-Lite hens. Dietary LPS (fine, <0.595 mm, F; medium, 0.595 to <1.68 mm, M; and 1:1 mixture of F and M wt/wt; FM). Abbreviation: LPS, limestone particle size.
Figure 5.
Effect of rearing cage type on egg weight in Lohmann LSL-Lite hens. Conventional (CON) cage 76 cm × 71 cm × 46 cm; furnished (FUR) cage 239 cm × 80 cm × 75 cm outfitted with platforms and terraces.
Eggshell Quality
Parameters, EST, ESBS, ESW, and proportion of eggshell were used to evaluate eggshell quality. There was no (P > 0.05) interaction between rearing cage type and dietary LPS on eggshell response variables at timepoints between onset of lay to 40 woa (Table 2). However, interestingly rearing cage type affected (P < 0.05) the eggshell quality attributes; at 40 woa, hens reared in FUR cages had higher EST, ESW, and proportion of eggshell (Table 2) than hens reared in CON cages. Generally, LPS had no effects (P > 0.05) on eggshell equality attributes at any timepoint, however, at 28 woa hens reared on FM dietary LPS tended (P = 0.060) to have higher ESW (6.19 vs. 5.89 g) than hens reared on F dietary LPS (Table 2).
Table 2.
Effects of rearing cage type and dietary limestone particle size on eggshell quality attributes in Lohmann LSL-lite hens.
| Age, wk: | Cage1 |
LPS2 |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON | FUR | SEM | F | FM | M | Cage | LPS | Cage x LPS | ||
| Eggshell thickness (EST), mm | ||||||||||
| 24 | 0.442 | 0.439 | 0.003 | 0.441 | 0.443 | 0.438 | 0.004 | 0.538 | 0.718 | 0.450 |
| 28 | 0.438 | 0.438 | 0.002 | 0.437 | 0.441 | 0.436 | 0.002 | 0.931 | 0.322 | 0.398 |
| 32 | 0.429 | 0.436 | 0.002 | 0.432 | 0.434 | 0.431 | 0.003 | 0.131 | 0.727 | 0.720 |
| 36 | 0.427 | 0.430 | 0.003 | 0.430 | 0.423 | 0.433 | 0.003 | 0.471 | 0.201 | 0.624 |
| 40 | 0.425b | 0.434a | 0.002 | 0.429 | 0.430 | 0.430 | 0.003 | 0.021 | 0.995 | 0.315 |
| Eggshell breaking strength (ESBS), N | ||||||||||
| 24 | 48.2 | 48.3 | 0.660 | 46.6 | 48.2 | 45.8 | 0.810 | 0.949 | 0.118 | 0.527 |
| 28 | 45.7 | 44.5 | 0.880 | 43.6 | 46.4 | 45.4 | 1.079 | 0.350 | 0.200 | 0.556 |
| 32 | 45.0 | 45.1 | 0.800 | 45.2 | 45.3 | 44.7 | 0.990 | 0.931 | 0.913 | 0.674 |
| 36 | 44.2 | 45.2 | 0.873 | 45.2 | 44.4 | 44.5 | 1.069 | 0.407 | 0.827 | 0.747 |
| 40 | 47.2 | 46.5 | 1.069 | 48.2 | 45.8 | 46.6 | 1.309 | 0.632 | 0.437 | 0.513 |
| Eggshell weight (ESW), g | ||||||||||
| 24 | 5.78 | 5.88 | 0.050 | 5.84 | 5.90 | 5.76 | 0.069 | 0.194 | 0.362 | 0.110 |
| 28 | 6.00 | 6.05 | 0.071 | 5.89 | 6.19 | 5.97 | 0.086 | 0.658 | 0.060 | 0.743 |
| 32 | 6.02 | 6.03 | 0.069 | 6.11 | 6.05 | 5.91 | 0.084 | 0.911 | 0.256 | 0.938 |
| 36 | 5.79 | 5.98 | 0.050 | 5.93 | 5.89 | 5.85 | 0.069 | 0.025 | 0.701 | 0.283 |
| 40 | 6.00b | 6.24a | 0.050 | 6.07 | 6.17 | 6.11 | 0.065 | 0.004 | 0.550 | 0.124 |
| Proportion of eggshell, % | ||||||||||
| 24 | 10.4 | 10.3 | 0.080 | 10.3 | 10.4 | 10.3 | 0.099 | 0.516 | 0.588 | 0.156 |
| 28 | 10.1 | 10.1 | 0.068 | 9.96 | 10.2 | 10.1 | 0.084 | 0.440 | 0.152 | 0.858 |
| 32 | 9.98 | 9.77 | 0.152 | 9.88 | 9.80 | 9.95 | 0.186 | 0.333 | 0.850 | 0.667 |
| 36 | 9.62 | 9.66 | 0.143 | 9.61 | 9.71 | 9.60 | 0.175 | 0.855 | 0.881 | 0.604 |
| 40 | 9.68b | 9.91a | 0.076 | 9.81 | 9.78 | 9.79 | 0.093 | 0.047 | 0.987 | 0.810 |
CON, conventional cage 76 cm × 71 cm × 46 cm; FUR, furnished cage 239 cm × 80 cm × 75 cm outfitted with platforms and terraces to increases opportunities for load bearing exercises (e.g. jumping, perching, flying) (Casey-Trott et al., 2017a; Habinski et al., 2017).
LPS, limestone particle size; F, fine, <0.595 mm, F; medium, 0.595 to <1.68 mm, M; and 1:1 mixture of F and M wt/wt; FM. Within a column, LSmeans with letter superscripts differs, P < 0.05 (n = 5).
Femur and Tibia Quality
The rearing cage type did not (P > 0.05) interact with dietary LPS on femur and tibia BMD, BMC, BBS, and ash concentration at the end of 24 and 40 woa (Table 3). However, cage type and LPS effects were observed (P < 0.10) for some femur and tibia attributes. The FUR hens tended to have higher femur BMD (0.222 vs. 0.171 g/cm2, P = 0.093), BMC (1.320 vs. 0.953 g, P = 0.093), and BBS (203 vs. 152 N, P = 0.058) than CON hens at 40 woa. Similarly, at 40 woa, FUR reared hens exhibited higher tibia BMD (0.220 vs. 0.184 g/cm2; P = 0.081), BMC (1.626 vs. 1.283 g; P = 0.066), and BBS (237 vs. 199 N; P = 0.041) than CON reared hens (Table 3). However, rearing cage did not have effects (P > 0.10) on femur and tibia ash concentration at 24 or 40 woa (Table 3). With respect to LPS at 24 woa, hens fed M LPS during rearing tended (0.995 vs. 0.780 g; P = 0.088) to show higher femur BMC than hens fed fine LPS, whereas femur BMC of hens fed FM LPS during rearing were intermediate. Interestingly, hens fed F LPS during rearing showed higher (P = 0.035) tibia ash concentration than hens fed FM LPS at 40 woa. Specifically, the tibia ash concentration of hens fed F, FM, and M LPS during rearing were 53.16, 47.71, and 47.93%, respectively (Table 3).
Table 3.
Effects of rearing cage type and dietary limestone particle size on femur and tibia attributes in Lohmann LSL-lite hens.
| Item | Age, wk | Cage1 |
SEM | LPS2 |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | FUR | F | FM | M | Cage | LPS | Cage x LPS | ||||
| Mineral density, g/cm2 | |||||||||||
| Femur | 24 | 0.156 | 0.159 | 0.008 | 0.148 | 0.146 | 0.178 | 0.010 | 0.764 | 0.079 | 0.978 |
| 40 | 0.171 | 0.222 | 0.020 | 0.222 | 0.182 | 0.185 | 0.025 | 0.093 | 0.467 | 0.527 | |
| Tibia | 24 | 0.170 | 0.170 | 0.007 | 0.165 | 0.163 | 0.181 | 0.009 | 0.995 | 0.315 | 0.993 |
| 40 | 0.184 | 0.220 | 0.001 | 0.225 | 0.188 | 0.193 | 0.017 | 0.081 | 0.290 | 0.367 | |
| Mineral content, g | |||||||||||
| Femur | 24 | 0.853 | 0.876 | 0.056 | 0.78 | 0.820 | 0.995 | 0.069 | 0.770 | 0.088 | 0.988 |
| 40 | 0.953 | 1.320 | 0.148 | 1.35 | 1.025 | 1.035 | 0.181 | 0.090 | 0.369 | 0.355 | |
| Tibia | 24 | 1.190 | 1.176 | 0.069 | 1.11 | 1.140 | 1.300 | 0.085 | 0.890 | 0.261 | 0.824 |
| 40 | 1.283 | 1.626 | 0.126 | 1.67 | 1.345 | 1.350 | 0.154 | 0.070 | 0.255 | 0.341 | |
| Bone breaking strength, N | |||||||||||
| Femur | 24 | 174.5 | 186.0 | 9.230 | 171.7 | 177.0 | 191.9 | 11.30 | 0.387 | 0.436 | 0.582 |
| 40 | 152.4 | 203.0 | 17.95 | 190.1 | 183.7 | 159.2 | 21.99 | 0.058 | 0.584 | 0.300 | |
| Tibia | 24 | 202.2 | 225.3 | 10.86 | 195.1 | 215.6 | 230.4 | 13.30 | 0.145 | 0.190 | 0.861 |
| 40 | 198.7b | 236.8a | 12.50 | 217.1 | 226.2 | 209.9 | 15.32 | 0.041 | 0.754 | 0.676 | |
| Bone ash, % | |||||||||||
| Femur | 24 | 46.3 | 46.6 | 0.850 | 46.2 | 45.5 | 47.7 | 1.050 | 0.857 | 0.344 | 0.156 |
| 40 | 49.6 | 51.2 | 1.000 | 52.3 | 48.7 | 50.2 | 1.230 | 0.247 | 0.330 | 0.801 | |
| Tibia | 24 | 44.8 | 44.3 | 0.450 | 45.3 | 43.7 | 44.8 | 0.560 | 0.436 | 0.146 | 0.501 |
| 40 | 50.1 | 49.1 | 1.290 | 53.2a | 47.7b | 47.8b | 1.580 | 0.594 | 0.035 | 0.158 | |
CON, conventional cage 76 cm × 71 cm × 46 cm; FUR, furnished cage 239 cm × 80 cm × 75 cm outfitted with platforms and terraces to increases opportunities for load bearing exercises (e.g. jumping, perching, flying) (Casey-Trott et al., 2017a; Habinski et al., 2017).
LPS, limestone particle size; F, fine, <0.595 mm, F; medium, 0.595 to <1.68 mm, M; and 1:1 mixture of F and M wt/wt; FM. (n = 5).
Discussion
The present study focused the effects of rearing cage type and rearing dietary LPS on egg production, eggshell, and long bone quality attributes of Lohmann LSL Lite hens thorough to 40 wk of age. The hens reared in the FUR cages laid more and heavier eggs in early phase of production, which could be explained by higher pullet BW at the onset of lay (Leeson and Summers, 1987; Lacin et al., 2008; Eusebio-Balcazar et al., 2018). The higher early HDEP for hens reared in FUR cages was partly because they started laying earlier. Body weight is one of the key determinants of onset lay in broilers breeders (Bornstein et al., 1984) and quail (Reddish et al., 2003). The rearing housing affected the growth pattern and hence influenced threshold BW for first egg. The heavier BW of hens reared in FUR cages at the beginning of egg production could be attributed to higher feed intake in the final 4 wk (13–16 wk) of pullet phase (Khanal et al., 2020). Although feed intake was not measured in the present study, feed intake might have remained higher for hens reared in FUR cages. Valkonen et al. (2008) reported that hens possessed greater live weight in FUR cages linked to higher feed consumption.
Although the ESBS was similar for all treatments, the eggshell at 40 woa was thicker for hens reared in FUR cages. Thicker eggshell might be because of more calcium carbonate deposition and corroborated heavier ESW and higher eggshell proportion. The eggshell is composed of 96% of calcium carbonate (Hinche et al., 2012). Perhaps more feed intake by hens reared in FUR cages supplied more Ca for more calcium carbonate formation leading to heavier shell weight. Also, the higher body and bone mineral density indicated enhanced eggshell formation (Tyler, 1954). The rearing dietary LPS did not affect the eggshell attributes. Given we observed LPS effect on bone attributes before onset of lay (Khanal et al., 2020), the lack of effects of rearing dietary LPS on eggshell suggested bone mineral reserve were not limiting in 40-wk-old hens (Kim et al., 2012). There are several findings on effect of LPS on eggshell quality; however, there is limited information regarding how the rearing dietary LPS and housing affects the eggshell quality. Eusebio-Balcazar et al. (2018) compared effect of rearing dietary LPS in conventional and aviary type cage in 2 breeds of layers. Their data agreed with the present study as aviary enhanced eggshell quality, but dietary LPS had no effect on eggshell quality. Perhaps extending the study to later stages of laying when eggshell quality deteriorates could have given an indication of whether rearing diets impact eggshell attributes.
Once minerals are depleted from structural bone, they are not replenished until the hen undergoes molting or goes out of lay (Whitehead, 2004). At 40 woa, several of femur and tibia attributes were better in hens reared in FUR cages extending previous observations indicating space and provision of load bearing amenities during rearing improved hen bone quality (Casey-Trott et al., 2017a; Eusebio-Balcazar et al., 2018; Neijat et al., 2019). When the BW of hens were similar, the bone quality parameters were also similar (at 24 woa), but when the BW differed (higher for FUR hens than CON hens), the bone parameters also differed accordingly (better in FUR hens) as observed at 40 woa. This suggested that the hen BW is a key parameter to the bone quality parameters. As discussed earlier, the hens reared in the FUR cages laid bigger eggs with heavier eggshell, implying that more Ca for eggshell formation was driven out from body of these hens. However, although more Ca was driven out of body for these hens, femur and tibia BMD and BMC remained higher, which could be explained by a higher mineral density at onset of lay and tendency of possible higher feed intake (Ca intake) (Khanal et al., 2020). The femur and tibia BMD and BMC increased at 40 woa when compared with that of 24 woa. However, the rate of increment was sharp for hens reared in furnished cages. The femur BMD increased by 10 and 40% and that of tibia by 8 and 30%, respectively for hens reared in CON and FUR cages. Similarly, the femur BMC increased by 12 and 51% and that of tibia by 8 and 32.5% respectively for hens reared in CON and FUR. The difference in pattern of bone mineralization in long bones at 24 to 40 woa could have affected BBS observations. The femur and tibia BBS decreased for hens reared in CON; however, it increased for hens reared in FUR cages. Also, the higher long bone (femur and tibia) mineral density could be one of the contributing factors for eggshell attributes observed in hens reared in FUR cages.
The rationale behind the use of various particle size of limestone in pullet diets is to influence retention in ventriculus which eventually influence the fractional passage rate in gastrointestinal tract. It is established that the limestone retention increased with particle size (Zhang and Coon, 1997). The larger LPS creates lower fractional passage rate from gizzard to small intestine because the limestone solubility goes down with the increase in LPS (Khanal et al., 2020). The fractional passage rate of limestone (slow Ca release) might affect the structural bone mineralization during rearing and thus influence bone mineral density during laying. This possibly influences the eggshell and bone quality in laying hens. The eggshell quality in selected hybrid lines such as Lohmann LSL lite is maintained at the expense of bone mineral density (Hocking et al., 2003). Hence, the higher the bone mineral density of hens before the egg lay, the higher the eggshell quality. However, rearing dietary LPS did not show any effect on femur and tibia quality parameters during laying. This suggested the dietary LPS effects observed at 16 woa on femur BMD and tibia BBS (companion paper, Khanal et al., 2020) did not last through to 40 woa. Eusebio-Balcazar et al. (2018) reported an interaction between rearing LPS (fine = 0.431 mm vs. blend = 0.879 mm), housing system (aviary vs. CON cages), and strain (Lohmann Brown-Classic vs. Bovans White) on tibia attributes at 52 woa. However, the authors did not find any interaction between rearing housing and LPS on tibia quality, which was in line with our findings. Also, white hens reared on blended LPS had similar tibia BMD, BMC and area compared with hens reared on fine LPS.
The Ca required for eggshell formation comes from both the diet and the Ca deposited into the medullary bone (Whitehead, 2004). The osteoclasts deplete Ca from the medullary bones for eggshell formation and are inactive during non-eggshell forming time to allow replenishment of medullary bones (Dacke et al., 1993; Kim et al., 2012). However, when the Ca required for eggshell formation is not enough from both medullary bones and the diet, the osteoclasts resorb Ca from inner layer of structural bones (Kim et al., 2012). It has been suggested that optimizing structural bone mineral deposition before sexual maturity may help lessening progressive osteoporosis as laying cycle progressed (Bain et al., 2016). The present study suggested that the rearing cage type and LPS had independent effects on eggshell and bone quality of hens through to 40 woa. The hens reared in furnished cage had higher body weight, egg weight, eggshell thickness, eggshell weight, and had higher mineral density and mineral content of femur and tibia at 40 woa. The variation in rearing dietary LPS did not influence the eggshell and leg bone quality. However, further investigations should be extended to later phases of lay cycle.
Acknowledgments
This work was supported by Natural Sciences and Engineering Research Council of Canada (#401303), Egg Farmers of Ontario (#053056) and Canada (#052940), Canadian Poultry Research Council (#053347), Wallenstein Feed & Supply Ltd. (#047506), Ontario Agri-Food Innovation Alliance (#27313), and Canada First Research Excellence Fund (#499119). Shoshana Verton-Shaw and Peter Smith thanked for technical support with DEXA and ICP-AES, respectively. Appreciation to Monogastric Nutrition lab mates for assistance with animal care and long sampling. T. Khanal is a recipient of Deborah Whale scholarship.
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Akbari Moghaddam Kakhki R., Heuthorst T., Mills A., Neijat M., Kiarie E. Interactive effects of calcium and top-dressed 25-hydroxy vitamin D3 on egg production, eggshell quality and medullary bones attributes in aged Lohmann LSL-lite layers. Poult. Sci. 2019;98:1254–1262. doi: 10.3382/ps/pey446. [DOI] [PubMed] [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S., Plavnik I., Lev Y. Body weight and/or fatness as potential determinants of the onset of egg production in broiler breeder hens. Br. Poult. Sci. 1984;25:323–341. doi: 10.1080/00071668408454873. [DOI] [PubMed] [Google Scholar]
- CCAC . Canadian Council on Animal Care; Ottawa, ON, Canada: 2009. Guidelines on the Care and Use of Farm Animals in Research, Teaching and Testing; pp. 1–168. [Google Scholar]
- Casey-Trott T.M., Korver D.R., Guerin M.T., Sandilands V., Torrey S., Widowski T.M. Opportunities for exercise during pullet rearing, Part I: effect on the musculoskeletal characteristics of pullets. Poult. Sci. 2017;96:2509–2517. doi: 10.3382/ps/pex059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey-Trott T.M., Korver D.R., Guerin M.T., Sandilands V., Torrey S., Widowski T.M. Opportunities for exercise during pullet rearing, Part II: long-term effects on bone characteristics of adult laying hens at the end-of-lay. Poult. Sci. 2017;96:2518–2527. doi: 10.3382/ps/pex060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacke C.G., Arkle S., Cook D.J., Wormstone I.M., Jones S., Zaidi M., Bascal Z.A. Medullary bone and avian calcium regualtion. J. Exp. Biol. 1993;184:63–87. [Google Scholar]
- Eusebio-Balcazar P.E., Purdum S., Hanford K., Beck M.M. Limestone particle size fed to pullets influences subsequent bone integrity of hens. Poult. Sci. 2018;97:1471–1483. doi: 10.3382/ps/pex412. [DOI] [PubMed] [Google Scholar]
- Fairfull R.W., Gowe R.S. Genotypic and phenotypic parameters of spur incidence and length in White Leghorn hens. Poult. Sci. 1986;65:1995–2001. doi: 10.3382/ps.0651995. [DOI] [PubMed] [Google Scholar]
- Fleming R.H., Mccormack H.A., Whitehead C.C. Bone structure and strength at different ages in laying hens and effects of dietary particulate limestone, vitamin K and ascorbic acid. Br. Poult. Sci. 1998;39:434–440. doi: 10.1080/00071669889024. [DOI] [PubMed] [Google Scholar]
- Guo X.Y., Kim I.H. Impacts of limestone multi-particle size on production performance, egg shell quality, and egg quality in laying hens. Asian-australasian J. Anim. Sci. 2012;25:839–844. doi: 10.5713/ajas.2011.11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habinski A.M., Caston L.J., Casey-Trott T.M., Hunniford M.E., Widowski T.M. Development of perching behavior in 3 strains of pullets reared in furnished cages. Poult. Sci. 2017;96:519–529. doi: 10.3382/ps/pew377. [DOI] [PubMed] [Google Scholar]
- Hinche M.T., Nys Y., Gautron J., Mann K., Rodriguez-Navarro A.B., McKee M.D. The eggshell : structure , composition and mineralization. Front. Biosci. 2012;17:1266–1280. doi: 10.2741/3985. [DOI] [PubMed] [Google Scholar]
- Hocking P.M., Bain M., Channing C.E., Fleming R., Wilson S. Genetic variation for egg production, egg quality and bone strength in selected and traditional breeds of laying fowl Genetic variation for egg production, egg quality and bone strength in selected and traditional breeds of laying fowl. Br. Poult. Sci. 2003;44:365–373. doi: 10.1080/0007166031000085535. [DOI] [PubMed] [Google Scholar]
- Jendral M.J., Korver D.R., Church J.S., Feddes J.J.R. Bone mineral density and breaking strength of white leghorns housed in conventional, modified, and commercially available colony battery cages. Poult. Sci. 2008;87:828–837. doi: 10.3382/ps.2007-00192. [DOI] [PubMed] [Google Scholar]
- Jones D.R., Anderson K.E., Davis G.S. The effects of genetic selection on production parameters of single comb white Leghorn hens. Poult. Sci. 2001;80:1139–1143. doi: 10.1093/ps/80.8.1139. [DOI] [PubMed] [Google Scholar]
- Khanal T., Widowski T., Bédécarrats G., Kiarie E. Effects of pre-lay dietary calcium (2.5 vs. 4.0%) and pullet strain (Lohmann Brown vs. Selected Leghorn LSL-Lite) on calcium utilization and femur quality at 1st through to the 50th egg2. Poult. Sci. 2019;98:4919–4928. doi: 10.3382/ps/pez245. [DOI] [PubMed] [Google Scholar]
- Khanal T., Bédécarrats G.Y., Widowski T., Kiarie E.G. Rearing cage type and dietary limestone particle size: I, effects on growth, apparent retention of calcium and long bones attributes in Lohmann selected Leghorn-Lite pullets. Poult. Sci. 2020;99:4454–4465. doi: 10.1016/j.psj.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder L.S., Schmidt I.U., Evans G.L., Turner R.T. Effects of growth hormone and low dose estrogen on bone growth and turnover in long bones of hypophysectomized rats. Calcif. Tissue Int. 1997;61:327–335. doi: 10.1007/s002239900343. [DOI] [PubMed] [Google Scholar]
- Kim W.K., Bloomfield S.A., Sugiyama T., Ricke S.C. Concepts and methods for understanding bone metabolism in laying hens. Worlds Poult. Sci. J. 2012;68:71–82. [Google Scholar]
- Lacin E., Yildiz A., Esenbuga N., Macit M. Effects of differences in the initial body weight of groups on laying performance and egg quality parameters of Lohmann laying hens. Czech J. Anim. Sci. 2008;53:466–471. [Google Scholar]
- Leeson S., Summers J.D. Effect of immature body weight on laying performance. Poult. Sci. 1987;66:1924–1928. doi: 10.3382/ps.0661924. [DOI] [PubMed] [Google Scholar]
- Lohmann Lohmann Brown and LSL-Lite Commercial Management Guide. 2020. http://www.ltz.de/en/downloads/management-guides.php#anchor_0955c6a8_Accordion-1-Cage Accessed Feb. 2020.
- McMillan I., Fairfull R.W., Gowe R.S., Gavora J.S. Evidence for genetic improvement of layer stocks of chickens during 1950-80. Worlds Poult. Sci. J. 1990;46:235–245. [Google Scholar]
- Meng X.T., Hou N.N., Wang X.J., Jiao H.C., Zhao J.P., Song Z.G., Lin H. Increased hepatic yolk precursor synthesis, secretion and facilitated uptake by follicles are involved in the rejuvenation of reproductive performance of molted hens (Gallus gallus domesticus) Gen. Comp. Endocrinol. 2013;194:198–207. doi: 10.1016/j.ygcen.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Mwaniki Z., Neijat M., Kiarie E. Egg production and quality responses of adding up to 7.5% defatted black soldier fly larvae meal in a corn-soybean meal diet fed to Shaver White Leghorns from wk 19 to 27 of age. Poult. Sci. 2018;97:2829–2835. doi: 10.3382/ps/pey118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijat M., Casey-Trott T.M., Robinson S., Widowski T.M., Kiarie E. Effects of rearing and adult laying housing systems on medullary, pneumatic and radius bone attributes in 73-wk old Lohmann LSL lite hens. Poult. Sci. 2019;98:2840–2845. doi: 10.3382/ps/pez086. [DOI] [PubMed] [Google Scholar]
- Reddish J., Nestor K., Lilburn M. Effect of selection for growth on onset of sexual maturity in randombred and growth-selected lines of Japanese quail. Poult. Sci. 2003;82:187–191. doi: 10.1093/ps/82.2.187. [DOI] [PubMed] [Google Scholar]
- SAS institute Inc. SAS 9.4 . SAS Institute Inc; Cary, NC: 2014. Help and Documentation. [Google Scholar]
- Strickland A.L., Sprinz H. Studies of the influence of estradiol and growth hormone on the hypophysectomized immature rat epiphyseal cartilage growth plate. Am. J. Obstet. Gynecol. 1973;115:471–477. doi: 10.1016/0002-9378(73)90393-1. [DOI] [PubMed] [Google Scholar]
- Świątkiewicz S., Arczewska-Włosek A., Krawczyk J., Puchała M., Józefiak D. Effects on performance and eggshell quality of particle size of calcium sources in laying hens’ diets with different Ca concentrations. Arch. Tierzucht. 2015;58:301–307. [Google Scholar]
- Tyler C. Studies on egg shells. IV.—the site of deposition of radioactive calcium and phosphorus. J. Sci. Food Agric. 1954;5:335–339. [Google Scholar]
- Valkonen E., Venalainen E., Rossow L., Valaja J. Effects of dietary energy content on the performance of laying hens in furnished and conventional cages. Poult. Sci. 2008;87:844–852. doi: 10.3382/ps.2007-00237. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Zhang B., Coon C. The relationship of calcium intake, source, size, solubility in vitro and in vivo, and gizzard limestone retention in laying hens. Poult. Sci. 1997;76:1702–1706. doi: 10.1093/ps/76.12.1702. [DOI] [PubMed] [Google Scholar]