Abstract
The increased consumption of protein derived from poultry demands greater poultry production, but increased poultry production (meat and eggs) is dependent on the fertility of the parent flocks. Clearly, the fertility of poultry flocks is associated with the fertility of both males and females, but the low numbers of males used for natural or artificial insemination mean that their role is more important. Thus, enhancing the semen volume, sperm concentration, viability, forward motility, and polyunsaturated fatty acids in sperm, as well as protecting against oxidative damage, could help to optimize the sperm membrane functionality, mitochondrial activity, and sperm-egg penetration, and thus fertility. Therefore, this review summarizes the nutritional factors that could improve the fertility of poultry males as well as their associated mechanisms to allow poultry producers to overcome low-fertility problems, especially in aging poultry males, thereby obtaining beneficial impacts on the poultry production industry.
Key words: fertility, nutritional factor, poultry male, semen quality
Introduction
In the industrial production of avian species, one male is responsible for the production of a huge number of fertilized eggs, which can exceed more than 1,000 fertilized eggs per year in some species such as chickens (McGary et al., 2002; Lagares et al., 2017; Wu et al., 2017). The characteristics of semen including the volume; sperm count (total number); the numbers of live sperm, dead sperm, and abnormal sperm; and forward motility are generally tested to evaluate and predict male fertility in poultry (Donoghue, 1999; Cheng et al., 2002; Chelmonska et al., 2008; Liu et al., 2008; Chen et al., 2016; Sun et al., 2019). Various factors can impair the characteristics of semen and reduce the fertility of poultry males, such as the genetic background, stressors, aflatoxicosis, and aging (Rosenstrauch et al., 1994, 1998; Al-Daraji, 2001; Mohan et al., 2011; Hu et al., 2013; Fouad et al., 2016, 2019). In poultry males aged over 45 wk, the testicular weight, testosterone level (Avital-Cohen et al., 2013; Sarabia et al., 2013; Lagares et al., 2017), semen volume, sperm concentration, viability, forward motility, polyunsaturated fatty acids (PUFAs) in sperm (especially n-3 PUFAs), and antioxidant concentrations decrease, whereas seminal plasma lipid peroxidation increases (Douard et al., 2003; Ommati et al., 2013; Iaffaldano et al., 2018; Min et al., 2018; Surai et al., 2019). These alterations are accompanied by reductions in sperm membrane functionality, mitochondrial activity, and sperm-egg penetration, and thus fertility (Cerolini et al., 1997a; Lin et al., 2005; Sarica et al., 2007; Altawash et al., 2017; Bazyar et al., 2019). Nutritional factors can modulate the detrimental impact of aging on the reproductive organs, semen traits, and fertility of poultry males. Therefore, this review summarizes the nutrition factors that can successfully enhance the fertility of poultry males (Figure 1) and their modes of action.
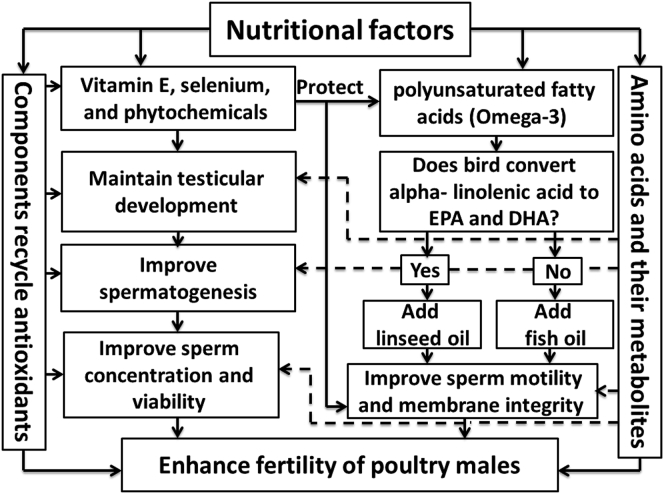
Figure 1.
Impacts of nutritional factors on the fertility of male poultry. Amino acids and their metabolites, oils rich in n-3 polyunsaturated fatty acids, vitamin E, selenium, phytochemicals, and components that regenerate natural antioxidants can maintain testicular evolution and enhance spermatogenesis, semen quality, and fertility. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Fat Types
In avian species, the percentage of n-6 polyunsaturated fatty acids (n-6 PUFAs), especially arachidonic acid (C20:4n-6) and docosatetraenoic acid (C22:4 n-6), is generally high in spermatozoa and seminal plasma (Darin-Bennett et al., 1974; Cerolini et al., 1997b; Surai et al., 1998a, 2001), thereby indicating that they could play important roles in the formation and characteristics of sperm, and thus fertility. It is known that the fatty acid profiles of poultry products (meat and eggs) can change according to the types of lipids added to the diet (Lopez-Ferrer et al.,1999; Fraeye et al., 2012). However, it is unclear whether the inclusion of n-3 PUFAs (present as a low percentage of the fatty acid composition in avian spermatozoa and seminal plasma) in poultry diets can alter the spermatozoa and seminal plasma fatty acid profiles, as well as sperm production and its characteristics, and thus fertility. Adding fish oil (salmon oil) to the diet of Sasso roosters altered the seminal plasma and spermatozoa fatty acid contents, leading to higher eicosapentaenoic acid (EPA; C20:5 n3) and docosahexaenoic acid (DHA; C22:6 n-3) levels, but lower arachidonic acid (C20:4 n-6) and docosatetraenoic acid (C22:4 n-6) levels. Moreover, the fertility increased 4.0% compared with a basal diet containing corn oil (Blesbois et al., 1997). These findings were confirmed in aged Ross roosters (60 wk) fed a diet containing 3% tuna oil as a rich source of DHA (C22:6 n-3) (Surai and Sparks, 2000). Lipids rich in n-3 PUFAs increased the fertility in cockerels fed a diet containing 2% menhaden oil, leading to increased numbers of fertilized eggs when hens were artificially inseminated once each 21 d (Hudson and Wilson, 2003). Increases in the semen volume, spermatozoa number, and forward progressive motility were reported in roosters that consumed a diet containing 2 to 5% fish oil (Surai et al., 2000a; Cerolini et al., 2005). It is well known that a constant volume of semen is used for artificial insemination. Thus, enhancements of the spermatozoa number and forward progressive motility could explain the improved fertility induced by consuming n-3 PUFAs. The forward progressive motility of sperm is linked to energy production (Kamali Sangani et al., 2017). Fish oil enhances fatty acid oxidation by promoting the activity of mitochondria (Asl et al., 2018) and beta oxidation in birds fed a diet containing fish oil (Newman et al., 2002; Poureslami et al., 2010). This could affect the energy production, which may explain why dietary fish oil enhances the forward progressive motility. The high price of fish oil and its sensitivity to heat generated during feed processing and production can affect its quality and negatively influence bird productivity (Tan et al., 2018; Frempong et al., 2019), thereby leading to a preferred use for alternative oil sources. Thus, studies have also examined whether similar results can be achieved using plant oils such as linseed oil. Linseed oil is a rich source of alpha-linolenic acid, which is converted into EPA and DHA in the presence of enzymes via desaturation and elongation processes. Improvements were obtained in the fertility of Cobb breeder hens inseminated by males that consumed diets supplemented with 6% linseed oil (Kelso et al., 1997). Furthermore, the fertility and sperm content of EPA and DHA increased in aged Ross roosters fed a diet supplemented with 2% linseed oil (Zanussi et al., 2019). The enhanced fertility of roosters induced by diets containing linseed oil may be attributable to increased sperm motility due to improved energy production by the activation of beta oxidation (Ferrini et al., 2010). However, adding 2% linseed oil enhanced the semen volume, sperm viability, motility, and total sperm count by enhancing testosterone synthesis via upregulation of the mRNA expression levels of rate-limiting enzymes involved in steroidogenesis, such as steroidogenic acute regulatory protein, to stimulate the entry of cholesterol into the inner mitochondria membrane and cholesterol side-chain cleavage enzyme, which produces pregnenolone from cholesterol (Qi et al., 2019). These findings might partly explain why adding linseed oil to the diets of roosters enhanced their fertility, although these studies did not investigate the relative weights of the testes and testicular histomorphology, including the Sertoli cell number, seminiferous epithelium height, and seminiferous tubule diameter. Therefore, diets containing linseed oil can have positive effects on fertility in roosters in a similar manner to diets containing fish oil. However, it is not clear whether all avian species can efficiently convert the alpha linolenic acid in linseed oil into EPA and DHA because the activities of enzymes involved in desaturation and elongation processes differ among species such as chickens, ducks, turkeys, geese, or quails (Khang et al., 2007; Gregory et al., 2012; Gregory and James, 2014; Osman et al., 2016) and breeds (commercial or native strains) (Rikimaru and Takahashi, 2010; Takahashi, 2018). Feeding the same poultry species with the same level of oil from different sources (linseed or fish) will not lead to the deposition or generation of products containing the same levels of EPA and DHA (Lopez-Ferrer et al., 1999; Baucells et al., 2000; Chen et al., 2017; Hang et al., 2018). Therefore, it not clear whether the semen quality characteristics and fertility will be similar when male birds from the same poultry species or breed are fed with the same level of linseed oil or fish oil. Further research is required to clarify the possible differences.
Vitamin E
In avian species, the PUFA contents of the testes and spermatozoa may indicate their vulnerability to oxidative damage under conditions with a lack of or insufficient antioxidant protection (Cerolini et al., 1997a,b; Surai et al., 1998b, 2000b). Vitamin E (Vit E) is a component of the antioxidant defense system in poultry (Surai et al., 2019). In particular, the testes, semen plasma, and spermatozoa in poultry contain Vit E, and the concentrations present are not negligible (Surai and Sparks, 2000; Surai et al., 1998b, 2000b), thereby indicating that Vit E plays an essential role in protecting the male reproductive system against oxidative damage. Indeed, a deficiency of Vit E retarded the growth of the reproductive organs (cloacal gland and testes) and decreased foam synthesis in quails (Hooda et al., 2007). Adding 150 mg of Vit E/kg diet for quails increased the Vit E content of sperm, reduced sperm lipid peroxidation, and improved the percentage fertility due to increases in the development of the testes and cloacal gland, foam production, semen volume, and the concentration, viability, and percentage motility of sperm, as well as decreasing the percentages of dead and abnormal sperm (Biswas et al., 2007; Adabi et al., 2011). In addition, among local roosters in Taiwan, adding 160 mg of Vit E/kg diet increased the percentage fertility specifically at 49 wk of age because of improved sperm viability and motility (Lin et al., 2005). Feeding Lohmann Brown breeder roosters a diet supplemented with 100 mg of Vit E/kg enhanced the semen volume, sperm concentration, and forward motility percentage (Danikowski et al., 2002), but supplementation with 200 mg of Vit E/kg diet was needed to obtain similar results in terms of the semen quality and to maximize the percentage fertility in Ross breeder roosters fed a diet containing 2.0% linseed oil (Zanussi et al., 2019). Vit E enhances the functions of sperm mitochondria and reduces lipid peroxidation of the sperm membrane to increase the sperm membrane integrity (Asl et al., 2018), which could explain the improved sperm characteristics such as the viability and motility percentages. Male poultry require Vit E to maintain normal testis development (Hooda et al., 2007), and thus spermatogenesis, which could explain the increases in the semen volume and sperm concentration. However, the mechanism by which Vit E affects testis growth is still unknown.
Coenzyme Q10
Coenzyme Q10 (CoQ10) is a unique compound that is required to move electrons and protons in the inner mitochondrial membrane and generate ATP, and it is able to regenerate Vit E (Littarru and Tiano, 2007). In aging roosters, adding 300 mg of CoQ10/kg diet increased sperm production, motility, membrane integrity, and fertility percentage. This could be due to the maintenance of testicular tissues, where the seminiferous tubule diameter, germinal cell layer thickness, and seminal antioxidant status were all enhanced by CoQ10 supplementation (Sharideh et al., 2020). Similar findings in terms of the semen traits and fertility were obtained by adding CoQ10 to semen before freezing (Masoudi et al., 2018, 2019; Sharideh et al., 2019). CoQ10 is one of many poultry diet additives that can activate the regeneration of Vit E and other antioxidants, but previous studies have only investigated the impact of CoQ10 on the fertility of aged roosters.
Selenium
Selenium (Se) is an essential component of the enzyme glutathione peroxidase (GSH-Px), and it is present in all avian seminal tissues and spermatozoa where it is required to protect against oxidative damage (Surai and Fisinin, 2014). Thus, the concentrations of Se and GSH-Px declined, whereas lipid peroxidation increased in the testes of roosters fed a diet deficient in Se (Shi et al., 2014). By contrast, adding 0.3 mg of Se/kg diet elevated the activity levels of GSH-Px in the testes and spermatozoa and decreased lipid peroxidation in the testes and semen of Rhode Island Red roosters (Surai et al., 1998b). The sperm concentration and percentage motility increased in aged toms (male turkeys) that consumed a diet supplemented with 0.3 mg of Se/kg (Słowińska et al., 2011). Ganders fed a diet supplemented with 0.3 mg of Se and 100 mg of Vit E/kg exhibited increases in their ejaculate volume, sperm concentration, and percentage of live normal sperm and a decreased percentage of abnormal sperm (Jerysz and Lukaszewicz, 2013). A diet containing 0.5 mg of Se/kg is recommended to decrease the number of dead sperm, increase the percentage fertility, and upregulate expression of the GSH-Px 4 gene in the testes of pigeons to possibly protect their testes against oxidative damage and enhance the reproductive performance (Wang et al., 2017). Similar findings were obtained in quails where 0.5 mg of Se/kg diet enhanced their percentage fertility as a consequence of increases in the coacal gland size and foam production, which were positively correlated with semen quality characteristics such as the live sperm and motility percentages (Biswas et al., 2017). Increases in semen production and its quality could be due to male reproductive organ development requiring adequate dietary Se to increase the number of Sertoli cells and their survival, as well as reducing the induction of apoptosis in germ cells during spermatogenesis by downregulating the expression of genes related to apoptosis and upregulating expression of the GSH-Px2 and GSH-Px4 genes (Song et al., 2015; Khalid et al., 2016; Gao et al., 2017). The recommended level of Se (0.5 mg of Se/kg) for quails and pigeons greatly increased the fertility compared with the recommended level (0.3 mg of Se/kg) for toms and roosters considering the body size and feed consumption by toms and roosters vs. quails and pigeons. Thus, future studies should test whether Se levels lower than 0.5 mg/kg can achieve similar results in male quails and pigeons.
Amino Acids and Their Metabolities
D-aspartic acid
D-aspartic acid is an endogenous amino acid that is implicated in male reproductive system development because it is present at a high level in the testes during the reproductive season for mature ducks (Di Fiore et al., 2008). In addition, an in vitro study showed that adding d-aspartic acid promoted the secretion of testosterone (Di Fiore et al., 2008). In particular, different levels of d-aspartic acid at 100, 200, and 300 mg/kg body weight were provided orally to aged roosters (Ansari et al., 2017, 2018), and the testosterone levels, semen quality characteristics, including the sperm concentration, membrane integrity, and forward motility, sperm penetration, and fertility all increased at a dose of 200 mg/kg body weight (Giacone et al., 2017). These findings could be attributed to the suppression of sperm lipid peroxidation (Ansari et al., 2017, 2018) and the enhanced development of the testes, where the diameter and thickness of the seminiferous tubules and seminiferous epithelium increased (Ansari et al., 2018).
L-carnitine
L-carnitine is generated from lysine and methionine, and it participates in lipid metabolism by transporting long-chain fatty acids into the mitochondria to initiate fatty acid β-oxidation (Fouad and El-Senousey, 2014), as well as protecting lipids from oxidative damage (Jahanian and Ashnagar, 2018). The sperm concentration increased in White Leghorn roosters when they consumed a diet supplemented with 125 mg of L-carnitine/kg (Zhai et al., 2007), and the same level of dietary L-carnitine increased the sperm concentration, viability, and motility in quails (Ahangari et al., 2014). In male ducks, 150 mg of L-carnitine/kg diet increased the fertility, semen volume, sperm concentration, viability, and motility, as well as decreasing the percentages of defective and dead sperm (Al-Daraji and Tahir, 2014). In aged roosters, dietary supplementation with 150 mg of L-carnitine/kg stimulated testosterone production and maximized fertility by increasing semen production, viability, and motility (Elokil et al., 2019). L-carnitine maintains testicular survival (Sarica et al., 2007), sperm membrane functioning, and sperm mitochondria activity (Fattah et al., 2017a,b) by stimulating the activities of antioxidant enzymes, such as catalase and GSH-Px, to suppress lipid peroxidation (Elokil et al., 2019; Fattah et al., 2017a) and improve the functioning of the testes, including testosterone secretion, spermatogenesis, and sperm features.
Guanidinoacetic acid
Guanidinoacetic acid (GAA) is biosynthesized from arginine, and it is required to produce creatine, which is phosphorylated to yield phosphocreatine with the generation of energy (Ostojic, 2016). The fertility of quail breeders increased by adding 1,200 mg of GAA/kg diet for only 4 wk (Murakami et al., 2014). To test the role of GAA in male fertility, mature cocks were fed diets supplemented with various concentrations of GAA for 25 wk (Tapeh et al., 2017). The diet with 1,200 mg of GAA/kg diet achieved the highest percentage fertility by increasing the sperm production, concentration, and forward motility percentage. The increased sperm production in aged cocks fed diets containing 1,200 mg of GAA/kg could be attributed to the enhanced populations of Sertoli and spermatogonia cells leading to increases in the seminiferous epithelium thickness and spermatogenesis, and thus the sperm concentration (Nasirikhah et al., 2019). The enhanced forward motility of sperm may have been due to the greater availability of energy (ATP) through the phosphorylation of creatine (Fosoul et al., 2018; Zhang et al., 2019).
L-arginine
L-arginine is needed by avian species to produce many essential components such as GAA, which enhances sperm production and forward motility, and nitric oxide (Fouad et al., 2012), which stimulates cellular proliferation and evolution of the digestive system (Gao et al., 2018; Yu et al., 2018) and the reproductive system (Xia et al., 2017). Dietary supplementations with 0.14% L-arginine in aged cocks restored the functioning of their testes in terms of the testicular weight, and the production of testosterone and sperm forward motility were enhanced (Abbaspour et al., 2019). Increases in the diameter of the seminiferous tubules and the populations of Sertoli cells and Leydig cells (Ahangar et al., 2017) could explain the enhanced testicular weight and testosterone production. The availability of energy produced from fatty acid oxidation via nitric oxide (Fouad et al., 2013) or the phosphorylation of creatine (Fosoul et al., 2018; Zhang et al., 2019) could explain the increased sperm forward motility induced by dietary L-arginine. Future studies should determine how limiting amino acids, such as methionine, lysine, and threonine, and other amino acids, as well as the levels of proteins and their sources, might affect the male reproductive system and semen quality.
Probiotics
The intestines of birds contain many different types of bacteria, and thus, semen could be exposed to contamination with these bacteria. Therefore, the semen collected from toms was exposed to different types of bacteria, such as Escherichia coli, Salmonella, Clostridium, Campylobacter, Bifidobacterium animalis, and Lactobacillus acidophilus (Triplett et al., 2016). The results showed that all these bacterial species led to decreases in the sperm quality index and motility, thereby possibly affecting the percentage fertility. These findings indicate that probiotic bacteria such as B. animalis and L. acidophilus species may negatively affect the semen quality and fertility. For this reason, several studies were conducted to investigate whether the probiotics that are commonly added to poultry diets might have negative effects on the semen quality and fertility. Thus, Saccharomyces cerevisiae and Bacillus subtilis were added to rooster diets to test their effects on semen quality (dos Santos et al., 2018a,b), and the results showed that both had no detrimental impacts on the semen characteristics, such as volume, concentration, and numbers of live and dead sperm. By contrast, feeding roosters diets containing Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 increased the sperm concentration and decreased the number of abnormal sperm, as well as increasing fertility (Mazanko et al., 2018). In general, dietary probiotics can boost the absorption and utilization of nutrients by improving the intestinal morphology and antioxidant status and decreasing the abundance of pathogenic bacteria (Forte et al., 2016; Seifi et al., 2017; Zhao et al., 2020), which may have positive effects on male reproductive system development and spermatogenesis. An in vitro study showed that Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 are potential antioxidants that could protect DNA against oxidative damage (Prazdnova et al., 2015). Indeed, adding Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 to the diet of roosters improved the growth of testes and the antioxidant status by elevating the concentrations of Vit E and A as well as reducing DNA damage, thereby decreasing the percentage of abnormal sperm and enhancing the sperm concentration and fertility (Prazdnova et al., 2019). Thus, dos Santos et al. (2018a,b) added probiotics for 8 wk in aged roosters, whereas Mazanko et al. (2018) and Prazdnova et al. (2019) added probiotics to the diets of roosters from 1 d of age until the peak or end of production, respectively, and the results were not similar. These results indicate that in addition to the type of probiotic (yeast or bacteria) and the particular strain used, the application period and time of supplementation with probiotics are factors that can determine their effectiveness as fertility enhancers.
Phytochemicals
Turmeric (Curcuma longa)
Turmeric belongs to the ginger family (Zingiberaceae), and its main active component curcumin is responsible for its antioxidant characteristics (Soleimani et al., 2018). Yan et al. (2017) determined the effects of turmeric on the semen quality in roosters. They found that 0.8 mg of turmeric byproduct/kg diet enhanced the sperm motility, thereby suggesting that the fertility could have been enhanced, although this was not tested. In addition, different levels of curcumin (10, 20, and 30 mg/rooster/d) were added to the diet of aged roosters (Kazemizadeh et al., 2019). The authors found that the use of 30 mg of curcumin/rooster/d led to reductions in the abnormal sperm number, increases in live sperm, and decreased seminal lipid peroxidation. These findings were also accompanied by increases in the sperm membrane integrity, sperm motility, penetration, and fertility. Several studies have indicated that curcumin can enhance the antioxidant status in poultry (Zhang et al., 2015; Ruan et al., 2019) as well as increase the numbers of Leydig cells, spermatogonia cells, and the seminiferous tubule diameter, thereby enhancing the testicular weight and its function (Kazemizadeh et al., 2018). These changes could explain the increases in the sperm membrane integrity and sperm concentration.
Ginger (Zingiber officinale)
Ginger contains gingerol, gingerdiol, and gingerdione, which may promote the functioning of the antioxidant defense system (Baliga et al., 2011). The antioxidant capacity was enhanced in chickens and laying hens when their diet was supplemented with ginger (Zhang et al., 2009; Zhao et al., 2011). These findings suggest that ginger might enhance the fertility of male poultry. Indeed, adding 15 g of ginger root powder/kg diet increased the fertility of aged Cobb male breeders because of the increased number of live sperm, total antioxidant capacity of the seminal plasma, sperm membrane integrity, forward motility, and sperm penetration (Akhlaghi et al., 2014a), while consuming 100 μL of ginger essential oil/kg body weight maximized the fertility of male quails (Herve et al., 2018). Ginger increased sperm production because of improved testes growth by enhancing development of the seminiferous tubules and germ cells and semen quality by suppressing the oxidative damage induced in the testes via the activation of antioxidant enzymes, such as superoxide dismutase and catalase (Saeid et al., 2011; Shanoon, 2011; Herve et al., 2018).
Lycopene
Lycopene is an abundant carotenoid in tomatoes and red fruits, and it can neutralize free radicals and inhibit oxidative damage in cells (Viuda-Martos et al., 2014). Drinking water containing lycopene (5.0 g/L) improved the fertility of roosters by enhancing the semen volume as well as the live sperm content and viability (Mangiagalli et al., 2010). Rosato et al. (2012) recommended adding 0.05 mg of lycopene/mL of tom semen to reduce the oxidative damaged caused by cryopreservation and to enhance the viability of sperm. The semen quality characteristics also improved in roosters fed diets containing 15% dried tomato pomace, with an increase in the live sperm number and decrease in the number of defective sperm (Saemi et al., 2012). Other carotenoids might also affect the fertility of male poultry. Indeed, although the fertility and semen quality did not change after including lutein up to 120 mg/kg diet (Pizzey and Bédécarrats, 2007), a diet containing 6.0 mg of canthaxanthin/kg increased the fertility (Rosa et al., 2012) by improving the antioxidant status (Ren et al., 2016).
Chrysin
Chrysin (5,7-dihydroxyflavone) is a flavonoid and polyphenol with antioxidant characteristics (Mani and Natesan, 2018). Dietary supplementation with 50 mg of chrysin/d in male broiler breeders boosted their fertility and semen traits regardless of whether the collected semen was used fresh or after freezing (Altawash et al., 2017; Zhandi et al., 2017). The fertility of roosters may have been boosted by chrysin decreasing lipid peroxidation and protecting against oxidative damage, thereby increasing the sperm content of PUFAs, specifically n-3 PUFAs (Altawash et al., 2017), as indicated by the enhanced sperm forward motility and plasma membrane integrity. In addition, soybean isoflavones added at 5.0 mg/kg diet that was fed to immature roosters (aged 10 wk) for 9 wk improved the relative weight of the testes because of increases in the diameters of the seminiferous tubules and layers of germ cells (Heng et al., 2017). Thus, the sperm concentration may have increased due to increases in spermatogenesis, sperm motility, and membrane integrity because of the antioxidant effects of soybean isoflavones. Future studies should determine whether improved semen traits, such as the sperm concentration, motility, and membrane integrity, might lead to increases in the percentage fertility in mature roosters fed a diet containing soybean isoflavones.
Other Feedstuffs and Additives Contain Flavonoids and Polyphenols (Rosemary Leaves, Dried Apple Pomace, and Cinnamon)
Supplementation with 5.0 g of rosemary leaf powder/kg diet increased the semen amount, concentration, and quality characteristics, including the live sperm number, forward motility, and sperm penetration (Borghei-Rad et al., 2017), by increasing the diameter of the seminiferous tubules and the thickness of the germinal cell layer. Thus, increases in sperm biosynthesis occurred, as well as the enhanced production of GSH-Px and catalase to protect the testes and semen from oxidative damage (Türk et al., 2016). Dietary supplementation with 0.25 mg of cinnamon bark oil/kg diet reduced lipid peroxidation, maintained the vitality of the testicular tissues, and increased the thickness of the germinal cell layer and sperm production (Türk et al., 2015), but the effects on fertility were not evaluated. A diet containing 20% dried apple pomace increased the PUFA content in sperm and the total antioxidant capacity to reduce lipid peroxidation as well as improve the sperm fluidity and its forward movement to enhance sperm penetration and fertility (Akhlaghi et al., 2014b). Clearly, the concentrations of the active compounds present in different supplements will determine their effects. Table 1 summarizes the nutrients and feed additives that may enhance the fertility of male poultry.
Table 1.
Nutrients and feed additives that may enhance the fertility of male poultry.
| Item | Amount | Bird | Improvement in fertility (%) | Source |
|---|---|---|---|---|
| Fish oil | 20.0 g/kg | Roosters | 6 | Hudson and Wilson, 2003 |
| Linseed oil | 20.0 g/kg 20.0 g/kg plus 200 mg Vit E |
Roosters Roosters |
814 |
Zanussi et al., 2019 Zanussi et al., 2019 |
| Vitamin E | 200 mg/kg 150 mg/kg |
Roosters Quail |
614 |
Zanussi et al., 2019 Biswas et al., 2007 |
| Coenzyme Q10 | 300 mg/kg | Roosters | 5 | Sharideh et al., 2020 |
| Selenium | 0.5 mg/kg 0.5 mg/kg |
Quail Pigeons |
52 |
Biswas et al., 2017 Wang et al., 2017 |
| L-carnitine | 125 mg/kg | Roosters | 15 | Elokil et al., 2019 |
| Guanidinoacetic acid | 1.2 g/kg | Roosters | 13 | Tapeh et al., 2017 |
| Curcumin | 30.0 mg/bird/d | Roosters | 14 | Kazemizadeh et al., 2019 |
| Ginger | 15 g/kg | Roosters | 9 | Akhlaghi et al., 2014a |
| Canthaxanthin | 6.0 mg/kg | Roosters | 1 | Rosa et al., 2012 |
| Chrysin | 50 mg/rooster/d | Roosters | 7 | Altawash et al., 2017 |
| Rosemary | 5.0 g/kg | Roosters | 4 | Borghei-Rad et al., 2017 |
Conclusion
The testicular structures and semen characteristics that lead to high fertility in male poultry in response to the addition of adequate levels of PUFAs (specifically n-3 PUFAs) require further investigation. Adding linseed oil (a source of alpha-linolenic acid) as a precursor to generate long-chain PUFAs (n-3 PUFAs to enhance sperm motility) may have variable effects in different bird species, which should be elucidated. In particular, dietary supplementation with components that have antioxidant characteristics and components with the capacity to recycle and regenerate antioxidants can help to neutralize reactive oxygen species, protect semen against oxidative damage, as well as maintaining the membrane integrity and sperm fluidity to positively enhance fertility. The nutritional factors that improve fertility may have different modes of action, and thus further experiments are required to test whether adding combinations of nutrients with diverse modes of action can improve the fertility of aged poultry males.
Acknowledgments
This work was supported by China Agricultural Research System (CARS-42-K13), National Key Research and Development Program (2018YFD0501504), Key Project of the Science and Technology program of Guangzhou City (201804020091, 201904020001), Science and Technology Program of Guangdong Province (2019A050505007), National Natural Science Foundation of China (3180131540), Natural Science Foundation of Guangdong Province (2018A030310198, 2019A1515012231), and agricultural competitive industry discipline team building project of Guangdong Academy of Agricultural Sciences.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Abbaspour B., Sharifi S.D., Ghazanfari S., Mohammadi-Sangcheshmeh A., Honarbakhsh S. The effect of l-arginine and flaxseed on plasma testosterone concentration, semen quality and some testicular histology parameters in old broiler breeder roosters. Theriogenology. 2019;128:101–109. doi: 10.1016/j.theriogenology.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Adabi S.G., Cooper R.G., Kamali M.A., Hajbabaei A. The influence of inclusions of vitamin E and corn oil on semen traits of Japanese quail (Coturnix coturnix japonica) Anim. Reprod. Sci. 2011;123:119–125. doi: 10.1016/j.anireprosci.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Ahangar M., Asadzadeh S., Rezaeipour V., Shahneh A.Z. Effects of L-Arginine supplementation on semen quality, testosterone concentration and testes histological parameters of Ross 308 breeder roosters. Asian Pac. J. Reprod. 2017;6:133–135. [Google Scholar]
- Ahangari Y.J., Parizadian B., Akhlaghi A., Sardarzadeh A. Effect of dietary l-carnitine supplementation on semen characteristics of male Japanese quail. Comp. Clin. Path. 2014;23:47–51. [Google Scholar]
- Akhlaghi A., Ahangari Y.J., Navidshad B., Pirsaraei Z.A., Zhandi M., Deldar H., Rezvani M.R., Dadpasand M., Hashemi S.R., Poureslami R., Peebles E.D. Improvements in semen quality, sperm fatty acids, and reproductive performance in aged Cobb 500 breeder roosters fed diets containing dried ginger rhizomes (Zingiber officinale) Poult. Sci. 2014;93:1236–1244. doi: 10.3382/ps.2013-03617. [DOI] [PubMed] [Google Scholar]
- Akhlaghi A., Ahangari Y.J., Zhandi M., Peebles E.D. Reproductive performance, semen quality, and fatty acid profile of spermatozoa in senescent broiler breeder roosters as enhanced by the long-term feeding of dried apple pomace. Anim. Reprod. Sci. 2014;147:64–73. doi: 10.1016/j.anireprosci.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Al-Daraji H.J. Sperm-egg penetration in laying breeder flocks: a technique for the prediction of fertility. Br. Poult. Sci. 2001;42:266–270. doi: 10.1080/00071660120048537. [DOI] [PubMed] [Google Scholar]
- Al-Daraji H.J., Tahir A.O. Effect of L-carnitine supplementation on drake semen quality. S. Afr. J. Anim. Sci. 2014;44:18–25. [Google Scholar]
- Altawash A.S.A., Shahneh A.Z., Moravej H., Ansari M. Chrysin-induced sperm parameters and fatty acid profile changes improve reproductive performance of roosters. Theriogenology. 2017;104:72–79. doi: 10.1016/j.theriogenology.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Ansari M., Zhandi M., Kohram H., Zaghari M., Sadeghi M., Gholami M., Deldar H., Di Fiore M.M., Benson A.P. D-Aspartate amends reproductive performance of aged roosters by changing gene expression and testicular histology. Reprod. Fertil. Dev. 2018;30:1038–1048. doi: 10.1071/RD17072. [DOI] [PubMed] [Google Scholar]
- Ansari M., Zhandi M., Kohram H., Zaghari M., Sadeghi M., Sharafi M. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of d-aspartic acid. Theriogenology. 2017;92:69–74. doi: 10.1016/j.theriogenology.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Asl R.S., Shariatmadari F., Sharafi M., Torshizi M.A.K., Shahverdi A. Dietary fish oil supplemented with vitamin E improves quality indicators of rooster cold-stored semen through reducing lipid peroxidation. Cryobiology. 2018;84:15–19. doi: 10.1016/j.cryobiol.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Avital-Cohen N., Heiblum R., Argov-Argaman N., Rosenstrauch A., Chaiseha Y., Mobarkey N., Rozenboim I. Age-related changes in gonadal and serotonergic axes of broiler breeder roosters. Domest. Anim. Eendocrinol. 2013;44:145–150. doi: 10.1016/j.domaniend.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Baliga M.S., Haniadka R., Pereira M.M., D’Souza J.J., Pallaty P.L., Bhat H.P., Popuri S. Update on the chemopreventive effects of ginger and its phytochemicals. Crit. Rev. Food Sci. Nutr. 2011;51:499–523. doi: 10.1080/10408391003698669. [DOI] [PubMed] [Google Scholar]
- Baucells M.D., Crespo N., Barroeta A.C., Lopez-Ferrer S., Grashorn A.M. Incorporation of different polyunsaturated fatty acids into eggs. Poult. Sci. 2000;79:51–59. doi: 10.1093/ps/79.1.51. [DOI] [PubMed] [Google Scholar]
- Bazyar M., Sharafi M., Shahverdi A. Changes in seminal parameters and hormonal profile with use of aromatase inhibitor in management of aging broiler breeder roosters. Poult. Sci. 2019;98:6100–6107. doi: 10.3382/ps/pez325. [DOI] [PubMed] [Google Scholar]
- Biswas A., Mohan J., Mandal A.B., Lal N. Semen characteristics and biochemical composition of cloacal foam of male Japanese quails (Coturnix coturnix Japonica) fed diet incorporated with selenium. J. Anim. Physiol. Anim. Nutr. 2017;101:229–235. doi: 10.1111/jpn.12557. [DOI] [PubMed] [Google Scholar]
- Biswas A., Mohan J., Sastry K.V.H., Tyagi J.S. Effect of dietary vitamin E on the cloacal gland, foam and semen characteristics of male Japanese quail. Theriogenology. 2007;67:259–263. doi: 10.1016/j.theriogenology.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Blesbois E., Lessire M., Grasseau I., Hallouis J.M., Hermier D. Effect of dietary fat on the fatty acid composition and fertilizing ability of fowl semen. Biol. Reprod. 1997;56:1216–1220. doi: 10.1095/biolreprod56.5.1216. [DOI] [PubMed] [Google Scholar]
- Borghei-Rad S.M., Zeinoaldini S., Zhandi M., Moravej H., Ansari M. Feeding rosemary leaves powder ameliorates rooster age-related subfertility. Theriogenology. 2017;101:35–43. doi: 10.1016/j.theriogenology.2017.06.018. [DOI] [PubMed] [Google Scholar]
- Cerolini S., Kelso K.A., Noble R.C., Speake B.K., Pizzi F., Cavalchini L.G. Relationship between spermatozoan lipid composition and fertility during aging of chickens. Biol. Reprod. 1997;57:976–980. doi: 10.1095/biolreprod57.5.976. [DOI] [PubMed] [Google Scholar]
- Cerolini S., Surai P., Maldjian A., Gliozzi T., Noble R. Lipid composition of semen in different fowl breeders. Avian Biol. Rev. 1997;8:141–148. [Google Scholar]
- Cerolini S., Surai P.F., Speake B.K., Sparks N.H.C. Dietary fish and evening primrose oil with vitamin E effects on semen variables in cockerels. Br. Poult. Sci. 2005;46:214–222. doi: 10.1080/00071660500065839. [DOI] [PubMed] [Google Scholar]
- Chelmonska B., Jerysz A., Lukaszewicz E., Kowalczyk A., Malecki I. Semen collection from Japanese Quail (Coturnix japonica) using a teaser female. Turk. J. Vet. Anim. Sci. 2008;32:19–24. [Google Scholar]
- Chen X., Du X., Shen J., Lu L., Wang W. Effect of various dietary fats on fatty acid profile in duck liver: efficient conversion of short-chain to long-chain omega-3 fatty acids. Exp. Biol. Med. 2017;242:80–87. doi: 10.1177/1535370216664031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Liu H.C., Wei L.Y., Huang J.F., Lin C.C., Blesbois E., Chen M.C. Sperm quality parameters and reproductive efficiency in Muscovy duck (Cairina moschata) J. Poult. Sci. 2016;53:223–232. doi: 10.2141/jpsa.0150162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F.P., Guo T.J., Wu J.T., Lin T.E., Ursem P.J., Colenbrander B., Fung H.P. Annual variation in semen characteristics of pigeons (Columba livia) Poult. Sci. 2002;81:1050–1056. doi: 10.1093/ps/81.7.1050. [DOI] [PubMed] [Google Scholar]
- Danikowski S., Sallmann H.P., Halle I., Flachowsky G. Influence of high levels of vitamin E on semen parameters of cocks. J. Anim. Physiol. Anim. Nutr. 2002;86:376–382. doi: 10.1046/j.1439-0396.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- Darin-Bennett A., Poulos A., White I.G. The phospholipids and phospholipid-bound fatty acids and aldehydes of dog and fowl spermatozoa. J. Reprod. Fertil. 1974;41:471–474. doi: 10.1530/jrf.0.0410471. [DOI] [PubMed] [Google Scholar]
- Di Fiore M.M., Lamanna C., Assisi L., Botte V. Opposing effects of D-aspartic acid and nitric oxide on tuning of testosterone production in mallard testis during the reproductive cycle. Reprod. Biol. Endocrinol. 2008;6:28–36. doi: 10.1186/1477-7827-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue A.M. Prospective approaches to avoid flock fertility problems: predictive assessment of sperm function traits in poultry. Poult. Sci. 1999;78:437–443. doi: 10.1093/ps/78.3.437. [DOI] [PubMed] [Google Scholar]
- dos Santos M.N., Ramachandran R., Kiess A.S., Wamsley K.G.S., McDaniel C.D. The impact of dietary yeast fermentation product derived from Saccharomyces cerevisiae on semen quality and semen microbiota of aged white Leghorn roosters. J. Appl. Poult. Res. 2018;27:488–498. [Google Scholar]
- dos Santos M.N., Ramachandran R., Kiess A.S., Wamsley K.G.S., McDaniel C.D. Impact of in vitro inoculation and dietary supplementation with Bacillus subtilis on sperm quality of aged White Leghorn roosters. J. Appl. Poult. Res. 2018;27:304–315. [Google Scholar]
- Douard V., Hermier D., Magistrini M., Blesbois E. Reproductive period affects lipid composition and quality of fresh and stored spermatozoa in turkeys. Theriogenology. 2003;59:753–764. doi: 10.1016/s0093-691x(02)01086-5. [DOI] [PubMed] [Google Scholar]
- Elokil A.A., Bhuiyan A.A., Liu H.Z., Hussein M.N., Ahmed H.I., Azmal S.A., Yang L., Li S. The capability of L-carnitine-mediated antioxidant on cock during aging: evidence for the improved semen quality and enhanced testicular expressions of GnRH1, GnRHR, and melatonin receptors MT 1/2. Poult. Sci. 2019;98:4172–4181. doi: 10.3382/ps/pez201. [DOI] [PubMed] [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V. L-carnitine is a survival factor for chilled storage of rooster semen for a long time. Cryobiology. 2017;74:13–18. doi: 10.1016/j.cryobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V., Najafi A. L-Carnitine in rooster semen cryopreservation: flow cytometric, biochemical and motion findings for frozen-thawed sperm. Cryobiology. 2017;74:148–153. doi: 10.1016/j.cryobiol.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Ferrini G., Manzanilla E.G., Menoyo D., Esteve-Garcia E., Baucells M.D., Barroeta A.C. Effects of dietary n-3 fatty acids in fat metabolism and thyroid hormone levels when compared to dietary saturated fatty acids in chickens. Livest. Sci. 2010;131:287–291. [Google Scholar]
- Forte C., Acuti G., Manuali E., Casagrande Proietti P., Pavone S., Trabalza-Marinucci M., Moscati L., Onofri A., Lorenzetti C., Franciosini M.P. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 2016;95:2528–2535. doi: 10.3382/ps/pew164. [DOI] [PubMed] [Google Scholar]
- Fosoul S.S.A.S., Azarfar A., Gheisari A., Khosravinia H. Energy utilisation of broiler chickens in response to guanidinoacetic acid supplementation in diets with various energy contents. Br. J. Nutr. 2018;120:131–140. doi: 10.1017/S0007114517003701. [DOI] [PubMed] [Google Scholar]
- Fouad A.M., Chen W., Ruan D., Wang S., Xia W.G., Zheng C.T. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: a review. Int. J. Poult. Sci. 2016;15:81–95. [Google Scholar]
- Fouad A.M., El-Senousey H.K. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian Austral. J. Anim. Sci. 2014;27:1057–1068. doi: 10.5713/ajas.2013.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A.M., El-Senousey H.K., Yang X.J., Yao J.H. Role of dietary L-arginine in poultry production. Int. J. Poult. Sci. 2012;11:718–729. [Google Scholar]
- Fouad A.M., El-Senousey H.K., Yang X.J., Yao J.H. Dietary L-arginine supplementation reduces abdominal fat content by modulating lipid metabolism in broiler chickens. Animal. 2013;7:1239–1245. doi: 10.1017/S1751731113000347. [DOI] [PubMed] [Google Scholar]
- Fouad A.M., Ruan D., El-Senousey H.K., Chen W., Jiang S., Zheng C. Harmful effects and control strategies of Aflatoxin B1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry. Toxins. 2019;11:176–197. doi: 10.3390/toxins11030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraeye I., Bruneel C., Lemahieu C., Buyse J., Muylaert K., Foubert I. Dietary enrichment of eggs with omega-3 fatty acids: a review. Food Res. Int. 2012;48:961–969. [Google Scholar]
- Frempong N.S., Nortey T.N., Paulk C., Stark C.R. Evaluating the effect of replacing fish meal in broiler diets with either soybean meal or poultry by-product meal on broiler performance and total feed cost per kilogram of gain. J. Appl. Poult. Res. 2019;28:912–918. [Google Scholar]
- Gao Y., Zhang J., Huang X., Zhang G. Glutathione peroxidase 1, selenoprotein K, and selenoprotein H may play important roles in chicken testes in response to selenium deficiency. Biol. Trace Elem. Res. 2017;179:271–276. doi: 10.1007/s12011-017-0953-y. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M.M., Li Y.J., Zhang L., Li J.L., Yu L.L., Gao F., Zhou G.H. Effects of in ovo feeding of L-arginine on the development of digestive organs, intestinal function and post-hatch performance of broiler embryos and hatchlings. J. Anim. Physiol. Anim. Nutr. 2018;102:e166–e175. doi: 10.1111/jpn.12724. [DOI] [PubMed] [Google Scholar]
- Giacone F., Condorelli R.A., Mongioì L.M., Bullara V., La Vignera S., Calogero A.E. In vitro effects of zinc, D-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine. 2017;56:408–415. doi: 10.1007/s12020-016-1013-7. [DOI] [PubMed] [Google Scholar]
- Gregory M.K., Geier M.S., Gibson R.A., James M.J. Functional characterization of the chicken fatty acid elongases. J. Nutr. 2012;143:12–16. doi: 10.3945/jn.112.170290. [DOI] [PubMed] [Google Scholar]
- Gregory M.K., James M.J. Functional characterization of the duck and turkey fatty acyl elongase enzymes ELOVL5 and ELOVL2. J. Nutr. 2014;144:1234–1239. doi: 10.3945/jn.114.194159. [DOI] [PubMed] [Google Scholar]
- Hang T.T.T., Molee W., Khempaka S. Linseed oil or tuna oil supplementation in slow-growing chicken diets: can their meat reach the threshold of a “high in n-3 polyunsaturated fatty acids” product? J. Appl. Poult. Res. 2018;27:389–400. [Google Scholar]
- Heng D., Zhang T., Tian Y., Yu S., Liu W., Xu K., Liu J., Ding Y., Zhu B., Yang Y., Zhang C. Effects of dietary soybean isoflavones (SI) on reproduction in the young breeder rooster. Anim. Reprod. Sci. 2017;177:124–131. doi: 10.1016/j.anireprosci.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Herve T., Raphaël K.J., Ferdinand N., Vitrice L., Tiwa F., Gaye A., Outman M.M., Marvel W., Moyo N. Growth performance, serum biochemical profile, oxidative status, and fertility traits in male Japanese quail fed on ginger (Zingiber officinale, roscoe) essential oil. Vet. Med. Int. 2018;1:1–8. doi: 10.1155/2018/7682060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooda S., Tyagi P.K., Mohan J., Mandal A.B., Elangovan A.V., Tyagi Pramod K. Effects of supplemental vitamin E in diet of Japanese quail on male reproduction, fertility and hatchability. Br. Poult. Sci. 2007;48:104–110. doi: 10.1080/00071660601157378. [DOI] [PubMed] [Google Scholar]
- Hu J., Chen J.L., Wen J., Zhao G.P., Zheng M.Q., Liu R.R., Liu W.P., Zhao L.H., Liu G.F., Wang Z.W. Estimation of the genetic parameters of semen quality in Beijing-You chickens. Poult. Sci. 2013;92:2606–2612. doi: 10.3382/ps.2013-03328. [DOI] [PubMed] [Google Scholar]
- Hudson B.P., Wilson J.L. Effects of dietary menhaden oil on fertility and sperm quality of broiler breeder males. J. Appl. Poult. Res. 2003;12:341–347. [Google Scholar]
- Iaffaldano N., Di Iorio M., Mannina L., Paventi G., Rosato M.P., Cerolini S., Sobolev A.P. Age-dependent changes in metabolic profile of turkey spermatozoa as assessed by NMR analysis. PLoS One. 2018;13:e0194219. doi: 10.1371/journal.pone.0194219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanian R., Ashnagar M. Effects of dietary supplementation of choline and carnitine on growth performance, meat oxidative stability and carcass composition of broiler chickens fed diets with different metabolisable energy levels. Br. Poult. Sci. 2018;59:470–476. doi: 10.1080/00071668.2018.1476677. [DOI] [PubMed] [Google Scholar]
- Jerysz A., Lukaszewicz E. Effect of dietary selenium and vitamin E on ganders’ response to semen collection and ejaculate characteristics. Biol. Trace Elem. Res. 2013;153:196–204. doi: 10.1007/s12011-013-9652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali Sangani A., Masoudi A.A., Vaez Torshizi R. Association of mitochondrial function and sperm progressivity in slow-and fast-growing roosters. Poult. Sci. 2017;96:211–219. doi: 10.3382/ps/pew273. [DOI] [PubMed] [Google Scholar]
- Kazemizadeh A., Zare S.A., Youssfi A.R., Mehrabani Y.H., Ansar P.Z. The effect of dietary curcumin supplementation on testicular histologyparameters in aged breeder roosters. J. Anim. Prod. 2018;20:487–498. [Google Scholar]
- Kazemizadeh A., Zare Shahneh A., Zeinoaldini S., Yousefi A.R., Mehrabani Yeganeh H., Ansari Pirsaraei Z., Akhlaghi A. Effects of dietary curcumin supplementation on seminal quality indices and fertility rate in broiler breeder roosters. Br. Poult. Sci. 2019;60:256–264. doi: 10.1080/00071668.2019.1571165. [DOI] [PubMed] [Google Scholar]
- Kelso K.A., Cerolini S., Speake B.K., Cavalchini L.G., Noble R.C. Effects of dietary supplementation with α-linolenic acid on the phospholipid fatty acid composition and quality of spermatozoa in cockerel from 24 to 72 weeks of age. J. Reprod. Fertil. 1997;110:53–59. doi: 10.1530/jrf.0.1100053. [DOI] [PubMed] [Google Scholar]
- Khalid A., Khudhair N., He H., Peng Z., Yaguang T., Guixue Z. Effects of dietary selenium supplementation on seminiferous tubules and SelW, GPx4, LHCGR, and ACE expression in chicken testis. Biol. Trace Elem. Res. 2016;173:202–209. doi: 10.1007/s12011-016-0646-y. [DOI] [PubMed] [Google Scholar]
- Khang N.T.K., Jennen D.G., Tholen E., Tesfaye D., Mennicken L., Hoelker M., Schellander K., Ponsuksili S., Murani E., Wimmers K. Association of the FADS2 gene with ω-6 and ω-3 PUFA concentration in the egg yolk of Japanese quail. Anim. Biotechnol. 2007;18:189–201. doi: 10.1080/10495390701201390. [DOI] [PubMed] [Google Scholar]
- Lagares M.A., Ecco R., Martins N.R.S., Lara L.J.C., Rocha J.S.R., Vilela D.A.R., Barbosa V.M., Mantovani P.F., Braga J.F.V., Preis I.S., Gheller V.A. Detecting reproductive system abnormalities of broiler breeder roosters at different ages. Reprod. Domest. Anim. 2017;52:67–75. doi: 10.1111/rda.12804. [DOI] [PubMed] [Google Scholar]
- Lin Y.F., Chang S.J., Yang J.R., Lee Y.P., Hsu A.L. Effects of supplemental vitamin E during the mature period on the reproduction performance of Taiwan native chicken cockerels. Br. Poult. Sci. 2005;46:366–373. doi: 10.1080/00071660500098186. [DOI] [PubMed] [Google Scholar]
- Littarru G.P., Tiano L. Bioenergetic and antioxidant properties of coenzyme Q 10: recent developments. Mol. Biotechnol. 2007;37:31–37. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- Liu S.J., Zheng J.X., Yang N. Semen quality factor as an indicator of fertilizing ability for geese. Poult. Sci. 2008;87:155–159. doi: 10.3382/ps.2007-00300. [DOI] [PubMed] [Google Scholar]
- Lopez-Ferrer S., Baucells M.D., Barroeta A.C., Grashorn M.A. n-3 Enrichment of chicken meat using fish oil: alternative substitution with rapeseed and linseed oils. Poult. Sci. 1999;78:356–365. doi: 10.1093/ps/78.3.356. [DOI] [PubMed] [Google Scholar]
- Mangiagalli M.G., Martino P.A., Smajlovic T., Guidobono Cavalchini L., Marelli S.P. Effect of lycopene on semen quality, fertility and native immunity of broiler breeder. Br. Poult. Sci. 2010;51:152–157. doi: 10.1080/00071660903401540. [DOI] [PubMed] [Google Scholar]
- Mani R., Natesan V. Chyrsin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–196. doi: 10.1016/j.phytochem.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Masoudi R., Sharafi M., Pourazadi L. Improvement of rooster semen quality using coenzyme Q10 during cooling storage in the Lake extender. Cryobiology. 2019;88:87–91. doi: 10.1016/j.cryobiol.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Masoudi R., Sharafi M., Shahneh A.Z., Kohram H., Nejati-Amiri E., Karimi H., Khodaei-Motlagh M., Shahverdi A. Supplementation of extender with coenzyme Q10 improves the function and fertility potential of rooster spermatozoa after cryopreservation. Anim. Reprod. Sci. 2018;198:193–201. doi: 10.1016/j.anireprosci.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Mazanko M.S., Gorlov I.F., Prazdnova E.V., Makarenko M.S., Usatov A.V., Bren A.B., Chistyakov V.A., Tutelyan A.V., Komarova Z.B., Mosolova N.I., Pilipenko D.N. Bacillus probiotic supplementations improve laying performance, egg quality, hatching of laying hens, and sperm quality of roosters. Probiotics Antimicrob. Proteins. 2018;10:367–373. doi: 10.1007/s12602-017-9369-4. [DOI] [PubMed] [Google Scholar]
- McGary S., Estevez I., Bakst M.R., Pollock D.L. Phenotypic traits as reliable indicators of fertility in male broiler breeders. Poult. Sci. 2002;81:102–111. doi: 10.1093/ps/81.1.102. [DOI] [PubMed] [Google Scholar]
- Min Y.N., Niu Z.Y., Sun T.T., Wang Z.P., Jiao P.X., Zi B.B., Chen P.P., Tian D.L., Liu F.Z. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 2018;97:1238–1244. 67. doi: 10.3382/ps/pex417. [DOI] [PubMed] [Google Scholar]
- Mohan J., Singh R.P., Sastry K.V.H., Moudgal R.P., Biswas A., Shit N. Influence of chicken native breeds on some physical and biochemical characteristics and short-term storage of semen. Br. Poult. Sci. 2011;52:395–400. doi: 10.1080/00071668.2011.585145. [DOI] [PubMed] [Google Scholar]
- Murakami A.E., Rodrigueiro R.J.B., Santos T.C., Ospina-Rojas I.C., Rademacher M. Effects of dietary supplementation of meat-type quail breeders with guanidinoacetic acid on their reproductive parameters and progeny performance. Poult. Sci. 2014;93:2237–2244. doi: 10.3382/ps.2014-03894. [DOI] [PubMed] [Google Scholar]
- Nasirikhah A., Zhandi M., Shakeri M., Sadeghi M., Ansari M., Deldar H., Yousefi A.R. Dietary Guanidinoacetic acid modulates testicular histology and expression of c-Kit and STRA8 genes in roosters. Theriogenology. 2019;130:140–145. doi: 10.1016/j.theriogenology.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Newman R.E., Bryden W.L., Fleck E., Ashes J.R., Buttemer W.A., Storlien L.H., Downing J.A. Dietary n-3 and n-6 fatty acids alter avian metabolism: metabolism and abdominal fat deposition. Br. J. Nutr. 2002;88:11–18. doi: 10.1079/BJNBJN2002580. [DOI] [PubMed] [Google Scholar]
- Ommati M.M., Zamiri M.J., Akhlaghi A., Atashi H., Jafarzadeh M.R., Rezvani M.R., Saemi F. Seminal characteristics, sperm fatty acids, and blood biochemical attributes in breeder roosters orally administered with sage (Salvia officinalis) extract. Anim. Prod. Sci. 2013;53:548–554. [Google Scholar]
- Osman R.H., Liu L., Xia L., Zhao X., Wang Q., Sun X., Zhang Y., Yang B., Zheng Y., Gong D., Geng T. Fads1 and 2 are promoted to meet instant need for long-chain polyunsaturated fatty acids in goose fatty liver. Mol. Cell. Biochem. 2016;418:103–117. doi: 10.1007/s11010-016-2737-7. [DOI] [PubMed] [Google Scholar]
- Ostojic S.M. Guanidinoacetic acid as a performance-enhancing agent. Amino Acids. 2016;48:1867–1875. doi: 10.1007/s00726-015-2106-y. [DOI] [PubMed] [Google Scholar]
- Pizzey H., Bédécarrats G.Y. Study of the effects of dietary lutein on reproductive performances in chickens. J. Poult. Sci. 2007;44:409–415. [Google Scholar]
- Poureslami R., Raes K., Turchini G.M., Huyghebaert G., De Smet S. Effect of diet, sex and age on fatty acid metabolism in broiler chickens: n-3 and n-6 PUFA. Br. J. Nutr. 2010;104:189–197. doi: 10.1017/S0007114510000395. [DOI] [PubMed] [Google Scholar]
- Prazdnova E.V., Chistyakov V.A., Churilov M.N., Mazanko M.S., Bren A.B., Volski A., Chikindas M.L. DNA-protection and antioxidant properties of fermentates from Bacillus amyloliquefaciens B-1895 and Bacillus subtilis KATMIRA 1933. Lett. Appl. Microbiol. 2015;61:549–554. doi: 10.1111/lam.12491. [DOI] [PubMed] [Google Scholar]
- Prazdnova E.V., Mazanko M.S., Chistyakov V.A., Denisenko Y.V., Makarenko M.S., Usatov A.V., Bren A.B., Tutelyan A.V., Komarova Z.B., Gorlov I.F., Weeks R. Effect of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 on the productivity, reproductive aging, and physiological characteristics of hens and roosters. Benef. Microbes. 2019;10:395–412. doi: 10.3920/BM2018.0149. [DOI] [PubMed] [Google Scholar]
- Qi X., Shang M., Chen C., Chen Y., Hua J., Sheng X., Wang X., Xing K., Ni H., Guo Y. Dietary supplementation with linseed oil improves semen quality, reproductive hormone, gene and protein expression related to testosterone synthesis in aging layer breeder roosters. Theriogenology. 2019;131:9–15. doi: 10.1016/j.theriogenology.2019.03.016. [DOI] [PubMed] [Google Scholar]
- Ren Z., Jiang S., Zeng Q., Ding X., Bai S., Wang J., Luo Y., Su Z., Xuan Y., Yao B., Cisneros F. Effect of dietary canthaxanthin and 25-hydroxycholecalciferol supplementation on the performance of duck breeders under two different vitamin regimens. J. Anim. Sci. Biotechnol. 2016;7:2–9. doi: 10.1186/s40104-016-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikimaru K., Takahashi H. Evaluation of the meat from Hinai-jidori chickens and broilers: analysis of general biochemical components, free amino acids, inosine 5′-monophosphate, and fatty acids. J. Appl. Poult. Res. 2010;19:327–333. [Google Scholar]
- Rosa A.P., Scher A., Sorbara J.O.B., Boemo L.S., Forgiarini J., Londero A. Effects of canthaxanthin on the productive and reproductive performance of broiler breeders. Poult. Sci. 2012;91:660–666. doi: 10.3382/ps.2011-01582. [DOI] [PubMed] [Google Scholar]
- Rosato M.P., Centoducati G., Santacroce M.P., Iaffaldano N. Effects of lycopene on in vitro quality and lipid peroxidation in refrigerated and cryopreserved turkey spermatozoa. Br. Poult. Sci. 2012;53:545–552. doi: 10.1080/00071668.2012.716508. [DOI] [PubMed] [Google Scholar]
- Rosenstrauch A., Allan Degen A., Friedländer M. Spermatozoa retention by Sertoli cells during the decline in fertility in aging roosters. Biol. Reprod. 1994;50:129–136. doi: 10.1095/biolreprod50.1.129. [DOI] [PubMed] [Google Scholar]
- Rosenstrauch A., Weil S., Degen A.A., Friedländer M. Leydig cell functional structure and plasma androgen level during the decline in fertility in aging roosters. Gen. Comp. Endocrinol. 1998;109:251–258. doi: 10.1006/gcen.1997.7029. [DOI] [PubMed] [Google Scholar]
- Ruan D., Zhu Y.W., Fouad A.M., Yan S.J., Chen W., Zhang Y.N., Xia W.G., Wang S., Jiang S.Q., Yang L., Zheng C.T. Dietary curcumin enhances intestinal antioxidant capacity in ducklings via altering gene expression of antioxidant and key detoxification enzymes. Poult. Sci. 2019;98:3705–3714. doi: 10.3382/ps/pez058. [DOI] [PubMed] [Google Scholar]
- Saeid J.M., Shanoon A.K., Marbut M.M. Effects of Zingiber officinale aqueous extract on semen characteristic and some blood plasma, semen plasma parameters in the broilers breeder male. Int. J. Poult. Sci. 2011;10:629–633. [Google Scholar]
- Saemi F., Zamiri M.J., Akhlaghi A., Niakousari M., Dadpasand M., Ommati M.M. Dietary inclusion of dried tomato pomace improves the seminal characteristics in Iranian native roosters. Poult. Sci. 2012;91:2310–2315. doi: 10.3382/ps.2012-02304. [DOI] [PubMed] [Google Scholar]
- Sarabia F.J., Pizarro Diaz M., Abad Moreno J.C., Casanovas Infesta P., Rodriguez-Bertos A., Barger K. Relationships between fertility and some parameters in male broiler breeders (body and testicular weight, histology and immunohistochemistry of testes, spermatogenesis and hormonal levels) Reprod. Domest. Anim. 2013;48:345–352. doi: 10.1111/j.1439-0531.2012.02161.x. [DOI] [PubMed] [Google Scholar]
- Sarica S., Corduk M., Suicmez M., Cedden F., Yildirim M., Kilinc K. The effects of dietary L-carnitine supplementation on semen traits, reproductive parameters, and testicular histology of Japanese quail breeders. J. Appl. Poult. Res. 2007;16:178–186. [Google Scholar]
- Seifi K., Karimi Torshizi M.A., Rahimi S., Kazemifard M. Efficiency of early, single-dose probiotic administration methods on performance, small intestinal morphology, blood biochemistry, and immune response of Japanese quail. Poult. Sci. 2017;96:2151–2158. doi: 10.3382/ps/pew446. [DOI] [PubMed] [Google Scholar]
- Shanoon A.K. Effects of Zingiber officinale powder on semen characteristic and blood serum sex hormones concentration in broilers breeder male. Int. J. Poult. Sci. 2011;10:863–866. [Google Scholar]
- Sharideh H., Zeinoaldini S., Zhandi M., Zaghari M., Sadeghi M., Akhlaghi A., Peebles E.D. Use of supplemental dietary coenzyme Q10 to improve testicular function and fertilization capacity in aged broiler breeder roosters. Theriogenology. 2020;142:355–362. doi: 10.1016/j.theriogenology.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Sharideh H., Zhandi M., Zenioaldini S., Zaghari M., Sadeghi M. The effect of coenzyme Q10 on rooster semen preservation in cooling condition. Theriogenology. 2019;129:103–109. doi: 10.1016/j.theriogenology.2019.02.028. [DOI] [PubMed] [Google Scholar]
- Shi L., Zhao H., Ren Y., Yao X., Song R., Yue W. Effects of different levels of dietary selenium on the proliferation of spermatogonial stem cells and antioxidant status in testis of roosters. Anim. Reprod. Sci. 2014;149:266–272. doi: 10.1016/j.anireprosci.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Słowińska M., Jankowski J., Dietrich G.J., Karol H., Liszewska E., Glogowski J., Kozłowski K., Sartowska K., Ciereszko A. Effect of organic and inorganic forms of selenium in diets on turkey semen quality. Poult. Sci. 2011;90:181–190. doi: 10.3382/ps.2010-00956. [DOI] [PubMed] [Google Scholar]
- Soleimani V., Sahebkar A., Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances. Phytother. Res. 2018;32:985–995. doi: 10.1002/ptr.6054. [DOI] [PubMed] [Google Scholar]
- Song R., Yao X., Shi L., Ren Y., Zhao H. Effects of dietary selenium on apoptosis of germ cells in the testis during spermatogenesis in roosters. Theriogenology. 2015;84:583–588. doi: 10.1016/j.theriogenology.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Sun Y., Xue F., Li Y., Fu L., Bai H., Ma H., Xu S., Chen J. Differences in semen quality, testicular histomorphology, fertility, reproductive hormone levels, and expression of candidate genes according to sperm motility in Beijing-You chickens. Poult. Sci. 2019;98:4182–4189. doi: 10.3382/ps/pez208. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Blesbois E., Grasseau I., Chalah T., Brillard J.P., Wishart G.J., Cerolini S., Sparks N.H.C. Fatty acid composition, glutathione peroxidase and superoxide dismutase activity and total antioxidant activity of avian semen. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;120:527–533. doi: 10.1016/s0305-0491(98)10039-1. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Brillard J.P., Speake B.K., Blesbois E., Seigneurin F., Sparks N.H.C. Phospholipid fatty acid composition, vitamin E content and susceptibility to lipid peroxidation of duck spermatozoa. Theriogenology. 2000;53:1025–1039. doi: 10.1016/S0093-691X(00)00249-1. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Fisinin V.I. Selenium in poultry breeder nutrition: an update. Anim. Feed Sci. Technol. 2014;191:1–15. [Google Scholar]
- Surai P.F., Fujihara N., Speake B.K., Brillard J.P., Wishart G.J., Sparks N.H.C. Polyunsaturated fatty acids, lipid peroxidation and antioxidant protection in avian semen-review. Asian Austral. J. Anim. Sci. 2001;14:1024–1050. [Google Scholar]
- Surai P.F., Kochish I.I., Romanov M.N., Griffin D.K. Nutritional modulation of the antioxidant capacities in poultry: the case of vitamin E. Poult. Sci. 2019;98:4030–4041. doi: 10.3382/ps/pez072. [DOI] [PubMed] [Google Scholar]
- Surai P., Kostjuk I., Wishart G., Macpherson A., Speake B., Noble R., Ionov I., Kutz E. Effect of vitamin E and selenium supplementation of cockerel diets on glutathione peroxidase activity and lipid peroxidation susceptibility in sperm, testes, and liver. Biol. Trace Elem. Res. 1998;64:119–132. doi: 10.1007/BF02783329. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Noble R.C., Sparks N.H.C., Speake B.K. Effect of long-term supplementation with arachidonic or docosahexaenoic acids on sperm production in the broiler chicken. J. Reprod. Fertil. 2000;120:257–264. [PubMed] [Google Scholar]
- Surai P.F., Sparks N.H.C. Tissue-specific fatty acid and α-tocopherol profiles in male chickens depending on dietary tuna oil and vitamin E provision. Poult. Sci. 2000;79:1132–1142. doi: 10.1093/ps/79.8.1132. [DOI] [PubMed] [Google Scholar]
- Takahashi H. Association between arachidonic acid and chicken meat and egg flavor, and their genetic regulation. J. Poult. Sci. 2018;55:163–171. doi: 10.2141/jpsa.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Rong D., Yang Y., Zhang B. The effect of oxidized fish oils on growth performance, oxidative status, and intestinal barrier function in broiler chickens. J. Appl. Poult. Res. 2018;28:31–41. [Google Scholar]
- Tapeh R.S., Zhandi M., Zaghari M., Akhlaghi A. Effects of guanidinoacetic acid diet supplementation on semen quality and fertility of broiler breeder roosters. Theriogenology. 2017;89:178–182. doi: 10.1016/j.theriogenology.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Triplett M.D., Parker H.M., McDaniel C.D., Kiess A.S. Influence of 6 different intestinal bacteria on Beltsville Small White turkey semen. Poult. Sci. 2016;95:1918–1926. doi: 10.3382/ps/pew119. [DOI] [PubMed] [Google Scholar]
- Türk G., Çeribaşı A.O., Şimşek Ü.G., Çeribaşı S., Güvenç M., Kaya Ş.Ö., Çiftçi M., Sönmez M., Yüce A., Bayrakdar A., Yaman M. Dietary rosemary oil alleviates heat stress-induced structural and functional damage through lipid peroxidation in the testes of growing Japanese quail. Anim. Reprod. Sci. 2016;164:133–143. doi: 10.1016/j.anireprosci.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Türk G., Şimşek Ü.G., Çeribaşı A.O., Çeribaşı S., Kaya Ş.Ö., Güvenç M., Çiftçi M., Sönmez M., Yüce A., Bayrakdar A., Yaman M. Effect of cinnamon (Cinnamomum zeylanicum) bark oil on heat stress-induced changes in sperm production, testicular lipid peroxidation, testicular apoptosis, and androgenic receptor density in developing Japanese quails. Theriogenology. 2015;84:365–376. doi: 10.1016/j.theriogenology.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M., Sanchez-Zapata E., Sayas-Barberá E., Sendra E., Pérez-Álvarez J.A., Fernández-López J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: a review. Crit. Rev. Food Sci. Nutr. 2014;54:1032–1049. doi: 10.1080/10408398.2011.623799. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang H.M., Cao W., Li Y.B. Effect of selenium supplementation on pigeon reproductive performance, selenium concentration and antioxidant status. Poult. Sci. 2017;96:3407–3413. doi: 10.3382/ps/pex121. [DOI] [PubMed] [Google Scholar]
- Wu S.R., Guo W., Li Y.L., Ren X.C., Lei X.Y., Li X.Y., Yao J.H., Yang X.J. miRNA and piRNA expression profiles of breeder cock testes detected by next-generation sequencing. Reprod. Domest. Anim. 2017;52:203–213. doi: 10.1111/rda.12880. [DOI] [PubMed] [Google Scholar]
- Xia W., Fouad A.M., Chen W., Ruan D., Wang S., Fan Q., Wang Y., Cui Y., Zheng C. Estimation of dietary arginine requirements for Longyan laying ducks. Poult. Sci. 2017;96:144–150. doi: 10.3382/ps/pew205. [DOI] [PubMed] [Google Scholar]
- Yan W., Kanno C., Oshima E., Kuzuma Y., Kim S.W., Bai H., Takahashi M., Yanagawa Y., Nagano M., Wakamatsu J.I., Kawahara M. Enhancement of sperm motility and viability by turmeric by-product dietary supplementation in roosters. Anim. Reprod. Sci. 2017;185:195–204. doi: 10.1016/j.anireprosci.2017.08.021. [DOI] [PubMed] [Google Scholar]
- Yu J., Yang H., Wang Z., Dai H., Xu L., Ling C. Effects of arginine on the growth performance, hormones, digestive organ development and intestinal morphology in the early growth stage of layer chickens. Ital. J. Anim. Sci. 2018;17:1077–1082. [Google Scholar]
- Zanussi H.P., Shariatmadari F., Sharafi M., Ahmadi H. Dietary supplementation with flaxseed oil as source of omega-3 fatty acids improves seminal quality and reproductive performance in aged broiler breeder roosters. Theriogenology. 2019;130:41–48. doi: 10.1016/j.theriogenology.2019.02.030. [DOI] [PubMed] [Google Scholar]
- Zhai W., Neuman S.L., Latour M.A., Hester P.Y. The effect of dietary L-carnitine on semen traits of white leghorns. Poult. Sci. 2007;86:2228–2235. doi: 10.1093/ps/86.10.2228. [DOI] [PubMed] [Google Scholar]
- Zhandi M., Ansari M., Roknabadi P., Zare Shahneh A., Sharafi M. Orally administered chrysin improves post-thawed sperm quality and fertility of rooster. Reprod. Domest. Anim. 2017;52:1004–1010. doi: 10.1111/rda.13014. [DOI] [PubMed] [Google Scholar]
- Zhang J., Hu Z., Lu C., Bai K., Zhang L., Wang T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agric. Food Chem. 2015;63:3880–3886. doi: 10.1021/jf505889b. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J.L., Wang X.F., Zhu X.D., Gao F., Zhou G.H. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers. Poult. Sci. 2019;98:3223–3232. doi: 10.3382/ps/pez052. [DOI] [PubMed] [Google Scholar]
- Zhang G.F., Yang Z.B., Wang Y., Yang W.R., Jiang S.Z., Gai G.S. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 2009;88:2159–2166. doi: 10.3382/ps.2009-00165. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang Z.B., Yang W.R., Wang Y., Jiang S.Z., Zhang G.G. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poult. Sci. 2011;90:1720–1727. doi: 10.3382/ps.2010-01280. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zeng D., Wang H., Qing X., Sun N., Xin J., Luo M., Khalique A., Pan K., Shu G., Jing B. Dietary probiotic Bacillus licheniformis H2 enhanced growth performance, morphology of small intestine and liver, and antioxidant capacity of broiler chickens against Clostridium perfringens–induced subclinical necrotic enteritis. Probiotics Antimicrob. Proteins. 2020;12:883–895. doi: 10.1007/s12602-019-09597-8. [DOI] [PubMed] [Google Scholar]