Abstract
This research investigated effects of dietary phytosterols supplementation on growth performance and cecal gut microflora in yellow-feather broilers. A total of 360 yellow-feather broilers (1-day-old) were randomly assigned to 3 treatment groups: control group (basal diet), antibiotic group (basal diet supplemented with 200 mg/kg oxytetracycline calcium and 250 mg/kg nosiheptide), and phytosterols groups (basal diet supplemented with 25 mg/kg phytosterols). Each treatment group had 6 replicates, and there were 20 broilers within each replicate. No treatment effects on average daily feed intake, average daily gain, and food conversion rate were observed. The antibiotic group had a lower liver index compared with control group and phytosterols group. Other visceral indexes including bursa of Fabricius, spleen, and heart were not different among the 3 treatment groups. In terms of alpha diversity, no treatment effects on Shannon and Simpson indexes were observed. Supplementation of phytosterols significantly decreased the Chao1 and Ace indexes, indicating lower community richness of the gut microflora. At phylum level, the phytosterols group had a higher abundance of Bacteroidetes compared with the control group. At genus level, no treatment effect was observed on the top 10 genera. Overall, supplementation of phytosterols at 25 mg/kg level did not affect the growth performance of yellow-feather broilers, and its effect on gut microflora was limited.
Key words: phytosterol, broiler, growth performance, microflora
Introduction
Phytosterols are plant sterols and stanols which are widely distributed in a variety of vegetable oils, nuts, and plant seeds. The most frequent phytosterols in nature are campesterol, β-sitosterol, stigmasterol, and brassicasterol (Moreau et al., 2002). Phytosterols act as hypocholesterolemic, immunomodulatory, anti-inflammatory, and antidiabetic agents in animal and humans (Santas et al., 2013). It has been considered as one of the safe feed additives in animal production. Shi et al. (2014) observed that long-term use of high-dose phytosterols (up to 800 mg/kg) did not induce any toxicological effects.
Phytosterols have been supplemented in poultry diet to decrease cholesterol in plasma or product (eggs, muscle) because of the regulatory effects on blood lipids profile and total cholesterol by inhibiting the absorption of cholesterol in the small intestine (Luo et al., 2015). Phytosterol ester (fatty acyl ester of phytosterol) supplementation significantly decreased the serum low-density lipoprotein-cholesterol, triglyceride, total cholesterol, free fatty acids, and some cytokines including TGF-β, IL-6, IL-10, as well as C-reactive protein in rats (Song et al., 2017). Phytosterols can also ameliorate oxidative stress by increasing superoxide dismutase activity and decreasing xanthine oxidase and malondialdehyde (Song et al., 2017). Zhao et al. (2019b) reported that phytosterols supplementation improved antioxidant status and meat quality of Partridge Shank chickens. The optimum level of phytosterols for Partridge chicken was recommended to be 40 mg/kg diet. Baskar et al. (2012) also observed phytosterols could enhance antioxidant enzyme activities such as superoxide dismutase and GSH-Px while decreased malondialdehyde concentration. Phytosterols supplementation in maternal diet could promote muscle development of offsprings in chickens and mice. Wang et al. (2020) reported that dietary phytosterol ester supplementation in broilers promoted bile acid deposition in egg yolk and skeletal muscle growth/development of female offsprings. The authors pointed out that it may be because of the activation of bile acid receptors and increased expression of decorin, MyoD, and Myogenin. In mice, it was also observed that maternal dietary supplementation of phytosterol esters during gestation significantly reduced sterols concentration in the amniotic fluid and promoted the skeletal muscle development in the offspring (Zhao et al., 2019a).
The intestinal bacteria in healthy animals are mutually restricted and interdependent. Together, they establish a balance in their variety and quantity. The occurrence of necrotic enteritis has risen in poultry industry since the use of antibiotics and anticoccidials decreased (Hernandez-Patlan et al., 2019). Alternatives to maintain poultry health include probiotics, prebiotics, organic acids, phytochemicals, enzymes, and novel vector vaccines (Hernandez-Patlan et al., 2019). Therefore, maintaining a balance of intestinal microflora through functional and safe phytochemicals may provide a practical strategy for improving performance and preventing diseases. It was reported that gut microbial communities were highly associated with host cholesterol metabolism (Martinez et al., 2013). Aldini et al. (2014) reported that phytosterols had an antioxidant effect on dextran sodium suphate–induced colonic inflammation in mice by regulating the intestinal microflora. Song et al. (2020) reported that phytosterol ester supplementation had beneficial effect on rats with nonalcoholic fatty liver disease by regulating intestinal microflora and fecal metabolites. Phytosterol ester may improve the intestinal mechanical barrier by increasing the mRNA expression levels of colonic claudin-1 and occludin (Song et al., 2020). The potential effects of phytosterols on intestinal microflora of caged yellow-feather broilers are not yet fully understood. Maintaining gut homeostasis in terms of microflora with phytosterols presents an effective and safe preventative strategy. Therefore, the aim of this study was to investigate the effects of phytosterols supplementation on growth performance and cecal gut microflora of yellow-feather broilers.
Materials and methods
Ethics Statement
This experimental protocol was approved by the Ethical Committee and conducted under the supervision of the Institutional Animal Care and Use Committee of Foshan University (Foshan, China).
Experimental Design and Diets
A total of 360 yellow-feather broilers (1-day-old) were randomly assigned to 3 treatment groups: control group (basal diet), antibiotic group (basal diet supplemented with 200 mg/kg oxytetracycline calcium and 250 mg/kg nosiheptide), and phytosterols groups (basal diet supplemented with 25 mg/kg phytosterols). The phytosterols product contained β-sitosterol, stigmasterol, and campesterol (Guangdong Weilai Biotechnology Co. Ltd., Guangzhou, China). Each treatment group had 6 replicates with 20 birds per replicate. Diets were formulated following the nutrient requirement recommendation of Chinese Yellow Feather Broiler (NY/T33-2004; Table 1). The broilers were fed twice a day in the morning and in the afternoon. All broilers had free access to clean water and feed. The broilers were raised in a temperature-controlled room which was cleaned and disinfected daily. The lighting was provided 24 h per day. The feeding trial lasted 63 D and was divided into 3 periods: starter phase (1–21 D), grower phase (22–42 D), and finisher phase (43–63 D).
Table 1.
Feed ingredients and nutrient composition of the basal diet.
| Feed ingredients (%) | Day 1–21 | Day 22–42 | Day 43–63 | Nutrient composition | Day 1–21 | Day 22–42 | Day 43–63 |
|---|---|---|---|---|---|---|---|
| Corn | 61.0 | 63.26 | 65.52 | ME(kcal/kg) | 2,896 | 2,997 | 3,097 |
| Soybean meal | 32.0 | 28.0 | 24.0 | Crude protein(%) | 19.91 | 18.63 | 17.6 |
| Corn gluten meal | 1.5 | 2.0 | 3.0 | Lysine(%) | 1.09 | 1.0 | 0.92 |
| Soybean oil | 1.4 | 2.5 | 3.5 | Methionine(%) | 0.51 | 0.46 | 0.42 |
| Limestone | 1.41 | 1.41 | 1.35 | Calcium(%) | 0.87 | 0.88 | 0.84 |
| Calcium bicarbonate | 1.33 | 1.33 | 1.33 | Phosphorus(%) | 0.42 | 0.40 | 0.38 |
| Methionine | 0.18 | 0.15 | 0.12 | ||||
| Lysine | 0.18 | 0.18 | 0.18 | ||||
| Wheat bran | 0 | 0.17 | 0 | ||||
| 1% Premix | 1.0 | 1.0 | 1.0 |
1% Premix includes:Vitamin A 6,000IU, Vitamin D3 1,000IU, Vitamin B2 5 mg, Vitamin E 30 mg, Vitamin K 2 mg, Vitamin B1 3 mg,Vitamin B12 1 mg, Vitamin B5 800 mg, Niacin 3g, Folic acid 500 mg, Biotin 0.2 mg, Choline 1,500 mg, Fe 10 mg, Cu 8 mg, Mn 10 mg, I 42 mg and Se 30 mg.
Feed Intake and Growth Performance
The feed intake of broilers within each replicate was recorded daily to calculate the average daily feed intake of each broiler. At the end of each period, the sum of body weight was recorded for each replicate to calculate the average daily gain of each broiler. Feed conversion rate (total feed intake/total body weight gain within each replicate) was also calculated.
Tissue Sampling
At the end of each period, 1 broiler was randomly selected from each replicate. The selected bird was weighed individually and then sacrificed by cervical dislocation and exsanguinated. The liver, bursa of Fabricius, spleen, and heart were removed by trained personnel, and the organ weight was recorded after flushing with cold PBS. Visceral index was calculated and expressed as a percentage of body weight. The digesta from right and left cecum (pooled within broiler) were aseptically collected from each individual broiler and immediately placed into cap vials. The digesta samples were stored at −80°C for later analysis.
DNA Extraction and High-Throughput Sequencing Analysis
Total genome DNA from cecal digesta was extracted using the cetyltrimethyl ammonium bromide method. DNA quality and quantity were monitored using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE). Total digesta DNA was diluted to 1 ng/μL to prepare amplicons for high-throughput sequencing. Conventional PCR was used to amplify the V3-V4 regions of the 16S rRNA genes using primers F341 (5′-CCTAYGGGRBGCASCAG-3′) and R806 (5′-GGACTACNNGGGTATCTAAT-3′). The PCR reaction mix consisted of 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA), 0.2 μmol of forward and reverse primers, and 10 ng template DNA. Reaction condition consisted of initial denaturation at 98°C for 1 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30 s, elongation at 72°C for 30 s, and a final extension at 72°C for 5 min. Sequencing libraries were generated using TruSeq DNA PCR-Free sample preparation kit (Illumina, San Diego, CA) following manufacturer's recommendations and index codes were added. The library quality was assessed on a Qubit @ 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Inc., Santa Clara, CA). The bar-coded amplicons were sequenced on an Illumina NovaSeq system, and 250 bp paired-end reads were generated.
Paired-end reads were merged using Fast Length Adjustment of Short reads software (FLASH; V1.2.7 (Magoc and Salzberg 2011); and quality filtering on the raw sequences was conducted on a quality control pipeline using the Quantitative Insight into Microbial Ecology (QIIME) tool kit to obtain the high-quality clean reads (Caporaso et al., 2010; Bokulich et al., 2013). Chimera sequences were removed by comparing with the Silva database using UCHIME algorithm (Edgar et al., 2011; Haas et al., 2011). The effective tags were retained for analysis. The obtained high-quality reads were assigned to the same operational taxonomic units (OTU) at ≥97% similarity using the QIIME Uclust algorithm (Edgar 2013). Taxonomic analysis was performed at the phylum and genus levels. Operational taxonomic units abundance information was normalized, and subsequent diversity analysis was performed using the normalized data. Alpha diversity analysis (Shannon, Simpson, Chao1, and Ace) was conducted to study the complexity of species diversity using QIIME (V1.9.1). Principal coordinate analysis was performed to get principal coordinates with Bray-Curtis distance algorithm, and the data were displayed by WGCNA and ggplot2 packages in R software (V4.0.0).
Statistical Analysis
All data were analyzed using the PROC GLIMMIX procedure of SAS (SAS Institute, Inc., Cary, NC) with between-subject factor treatment and within-subject factor day in the model. The best fit covariance structure model obtained by choosing the lowest Akaike's Information Criteria and Bayesian Information Criteria fit statistics was used to analyze the repeated measurement data. The significance was declared at P < 0.05 and trends at P < 0.1.
Results and discussion
Feed Intake, Growth Performance, and Visceral Index
Average daily feed intake, average daily gain, and feed conversion rate are presented in Table 2. The treatment effects on average daily feed intake and average daily gain were not significant (P > 0.05). The treatment effect on feed conversion ratio was not observed either (P = 0.89). The day effect on these 3 growth performance parameters was significant. Finisher phase broilers had the highest average daily feed intake and feed conversion ratio, whereas the starter phase broilers had the lowest values. With respect to average daily gain, the grower phase broilers had the highest value, and the starter phase had the lowest value indicating that the birds grew more quickly during grower phase and relatively slowed down during the finisher phase. The phytosterols group broilers had a higher liver index compared with antibiotic group but not different from the control group. The day effects on bursa of Fabricius, liver, and heart indices were observed as that the finisher phase broilers had lowest values, and starter phase broilers had the highest values. There was no treatment effect or day effect observed on spleen index. In this study, no treatment by day interaction effect on any of the variables was observed.
Table 2.
Effects of phytosterol supplementation on performance and visceral index of yellow-feather broilers.
| Item | Treatment |
SEM | Day |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | Anti | Phyto | 21 D | 42 D | 63 D | Trt | Day | Trt∗Day | |||
| Growth performance | |||||||||||
| Average feed intake(g/D) | 57.0 | 56.8 | 56.5 | 0.98 | 25.9a | 68.9b | 75.5c | 1.4 | 0.93 | <0.0001 | 0.31 |
| Average daily gain (g/D) | 25.01 | 24.99 | 24.95 | 0.417 | 16.91a | 32.17b | 25.86c | 0.555 | 0.99 | <0.0001 | 0.59 |
| Feed conversion rate | 2.22 | 2.20 | 2.19 | 0.035 | 1.53a | 2.15b | 2.93c | 0.056 | 0.89 | <0.0001 | 0.64 |
| Visceral index, %BW | |||||||||||
| Bursa of Fabricius | 0.199 | 0.213 | 0.179 | 0.015 | 0.276a | 0.209b | 0.106c | 0.019 | 0.29 | <0.0001 | 0.48 |
| Spleen, | 0.192 | 0.181 | 0.153 | 0.0159 | 0.193 | 0.17 | 0.163 | 0.025 | 0.23 | 0.57 | 0.53 |
| Liver | 2.56a,b | 2.42b | 2.79a | 0.075 | 3.72a | 2.36b | 1.69c | 0.131 | 0.011 | <0.0001 | 0.44 |
| Heart | 0.48 | 0.47 | 0.51 | 0.019 | 0.67a | 0.43b | 0.36c | 0.024 | 0.42 | <0.0001 | 0.10 |
a,b,cValues (sharing the same SEM) within the same row with no common superscript letters differ (P < 0.05).
Abbreviations: Con, control group with basal diet; Anti, basal diet supplemented with 200 mg/kg oxytetracycline calcium and 250 mg/kg nosiheptide (Anti); Phyto, basal diet supplemented with 25 mg/kg phytosterols; Trt, treatment.
The benefits of phytosterols on animals are attributed to improve nutrient digestibility, increase secretion of growth-related hormones, promote protein synthesis, regulate immune system, and increase resistance to disease. Shi et al. (2014) reported that supplementation of different doses of phytosterols (0, 20, and 80 mg/kg) for 12 wk had no effect on feed intake and feed conversion rate in laying hens. The effects of phytosterols on average daily feed intake and overall feed conversion rate of Partridge Shank chickens were not observed (Zhao et al., 2019b), which was consistent with our results. The authors observed that supplementation of phytosterols at 40 mg/kg could significantly increase the average daily gain compared with control (34.3 vs. 32.2 g/D). Studies have reported that phytosterols could accelerate growth rate and improve meat quality of broilers and ducks (Wu et al., 2012; Naji et al., 2013). This beneficial effect was attributed to its ability to promote protein synthesis and disease-resistance effect. However, some studies also reported that phytosterols supplementation had no significant effects on growth performance of piglets and laying hens (Liu et al., 2010; Hu et al., 2017). Animal species, age, feeding management, and composition of the basal diet could all cause this discrepancy. Phytosterol ester supplementation was reported to effectively inhibit the liver and abdominal fat indexes of rats on high fat diet (Song et al., 2020). Shi et al. (2014) observed that liver and spleen indexes of laying hens linearly increased with increasing levels of phytosterols although the numerical difference was small. In that study, the heart index was not affected by treatment which was similar to our study.
OTU Diversity, Similarity Analysis, and Alpha Diversity
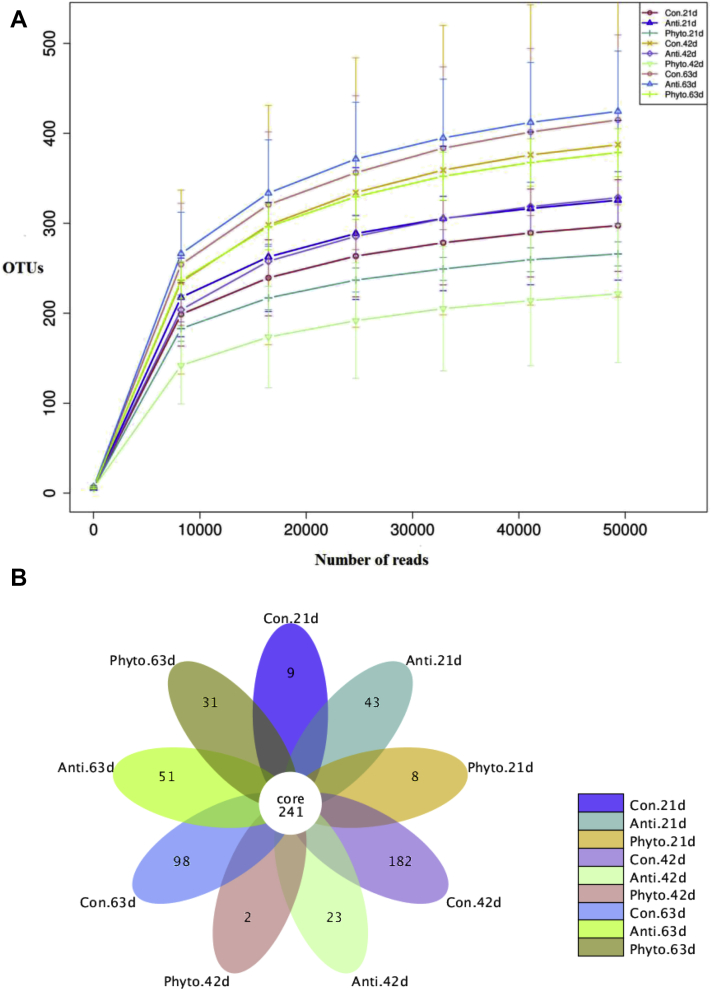
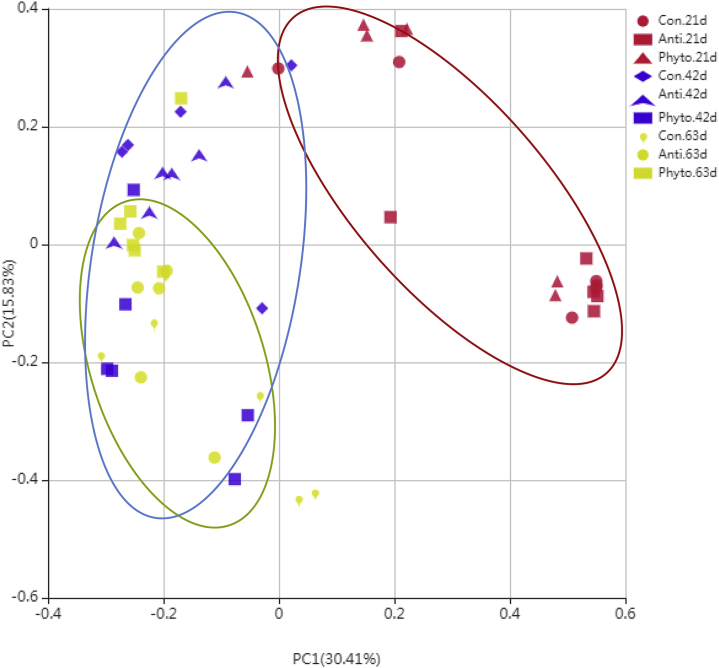
The 16S rRNA gene sequencing of digesta samples was conducted to compare the differences in cecal microbiota among the 3 groups at day 21, 42, and 63. After data filtering, quality control, and removal of chimera sequences, an average of 62,288 effective sequences was obtained for each sample. The length of the sequences ranged between 406 and 424 bp with an average length of 418 bp. Rarefaction curve revealed that there was sufficient OTU coverage to describe the bacterial composition of each group (Figure 1). The overall number of OTU was 688, and 241 shared OTU were detected in all groups (Figure 1). Principal coordinate analysis using the Bray-Curtis similarity method revealed that the first principal component and the second principal component explained 30.41% and 15.83% of the variation among samples, respectively. As shown in Figure 2, the samples from different treatment groups could not be distinctly separated. Samples from day 42 and 63 clustered together and could not be clearly separated either. However, samples from day 21 could be distinctly separated from samples from day 42 and 63. This result indicated that age had more effect on cecal microflora than the treatment applied in our study.
Figure 1.
Number of operational taxonomic units (OTU) in each group by period. Rarefraction curves of OTU (A); Venn diagram of shared OTU (B). Abbreviations: Con, control; Anti, antibiotic group; Phyto, phytosterol group.
Figure 2.
Principle coordinate analysis of the cecal microbiota in different groups by period. Abbreviations: Con, control; Anti, antibiotic group; Phyto, phytosterol group; PC1, first principal component; PC2, second principal component.
The data of alpha diversity indexes are presented in Table 3. The treatment effects on Shannon index and Simpson index were not observed (P > 0.05). The phytosterols group had lower Chao1 and Ace indexes compared with the control and antibiotic groups (P = 0.019; P = 0.013). The Simpson, Chao1, and Ace indexes all increased with age. Song et al. (2020) observed that phytosterol ester group mice had higher Chao1 and Ace indexes than the control group mice. The studies of phytosterols on chicken are limited, and we would not be able to make any comparisons. Chao and Ace indexes identify community richness, whereas Shannon and Simpson identify community diversity (Hernandez-Patlan et al., 2019). Our results indicated that phytosterols supplementation led to a decrease in richness but no effect in diversity of the gut microbiota.
Table 3.
Effects of phytosterol supplementation on alpha diversity indexes of cecal microbiota in yellow-feather broilers.
| Item | Treatment |
SEM | Day |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | Anti | Phyto | 21 D | 42 D | 63 D | Trt | Day | Trt∗Day | |||
| Shannon | 4.32 | 4.25 | 3.90 | 0.163 | 4.03 | 3.97 | 4.47 | 0.203 | 0.18 | 0.061 | 0.74 |
| Simpson | 0.85 | 0.82 | 0.87 | 0.023 | 0.78a | 0.83b,c | 0.87c | 0.028 | 0.46 | 0.025 | 0.34 |
| Chao1 | 449.4a | 451.4a | 360.3b | 25.79 | 365.4a | 383.6a | 512.2b | 31.49 | 0.019 | <0.0001 | 0.55 |
| Ace | 458.6a | 456.9a | 363.9b | 25.56 | 369.9a | 391.4a | 518.1b | 31.84 | 0.013 | <0.0001 | 0.55 |
a,b,cValues (sharing the same SEM) within the same row with no common superscript letters differ (P < 0.05).
Abbreviations: Con, control group with basal diet; Anti, basal diet supplemented with 200 mg/kg oxytetracycline calcium and 250 mg/kg nosiheptide (Anti); Phyto, basal diet supplemented with 25 mg/kg phytosterols; Trt, treatment.
Taxonomic Composition of Cecal Microbiota
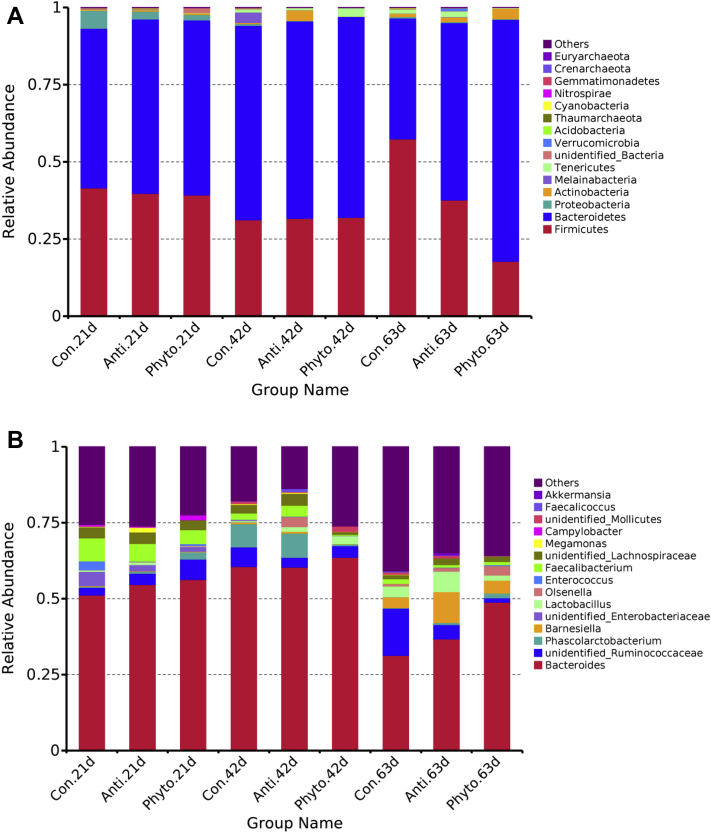
We identified total 34 phyla within the samples. Firmicutes and Bacteroidetes were the dominant phyla in all groups (Figure 3). There were no treatment effect and day effect observed on the relative abundance of Firmicutes (P > 0.05; Table 4). The phytosterols supplementation group had higher relative abundance of Bacteroidetes compared with the control group but not different from the antibiotic group. No treatment effect and day effect were observed on relative abundance of Proteobacteria, Actinobacteria, Melainabacteria, Verrucomicrobia, and Acidobacteria (P > 0.05). The relative abundance of Tenericutes was different among 3 periods, and the relative abundance was significantly lower on day 63 than that on day 21 (P = 0.011). No significant treatment by day interaction effect observed for any of the phyla listed (P > 0.05). The ratio of Firmicutes to Bacteroidetes was not affected by treatment and age. In genus level, there were 340 genera identified, and the relative abundance of top 10 genera was listed in Table 5. Bacteroides and Ruminococcaceae were the dominant genera across all groups. The treatment did not affect any of the genera listed in the table. The relative abundance of Bacteroides, Faecalibacterium, and Lachnospiraceae was significantly lower on day 63 compared with that on day 21. The abundance of Barnesiella on day 63 was significantly higher than that on day 21 and day 42.
Figure 3.
Phylum-level (A) and genus-level (B) taxonomic composition of the cecal bacterial communities in different groups by period. Abbreviations: Con, control; Anti, antibiotic group; Phyto, phytosterol group.
Table 4.
Effects of phytosterol supplementation on phylum level taxonomic compositon of the cecal microbiota in yellow-feather broilers.
| Item | Treatment |
SEM | Day |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | Anti | Phyto | 21 D | 42 D | 63 D | Trt | Day | Trt∗Day | |||
| Firmicutes | 43.41 | 36.39 | 29.69 | 3.929 | 40.21 | 31.63 | 37.65 | 3.649 | 0.065 | 0.17 | 0.083 |
| Bacteroidetes | 51.28a | 59.26a,b | 66.73b | 4.064 | 54.97 | 64.00 | 58.30 | 4.63 | 0.042 | 0.21 | 0.15 |
| Proteobacteria | 2.28 | 1.01 | 0.67 | 0.931 | 3.36 | 0.23 | 0.36 | 1.61 | 0.45 | 0.099 | 0.79 |
| Actinobacteria | 0.54 | 1.78 | 1.28 | 0.97 | 0.38 | 1.27 | 1.96 | 0.82 | 0.68 | 0.19 | 0.37 |
| Melainabacteria | 1.03 | 0.04 | 0.004 | 1.36 | 0.0013 | 1.01 | 0.065 | 0.059 | 0.84 | 0.09 | 0.66 |
| Tenericutes | 0.84 | 0.86 | 1.04 | 0.469 | 0.092 | 1.57a | 1.08a,b | 0.602b | 0.94 | 0.011 | 0.06 |
| Verrucomicrobia | 0.008 | 0.26 | 1.62E-6 | 0.151 | 0.0013 | 0.0018 | 0.271 | 0.261 | 0.39 | 0.57 | 0.26 |
| Acidobacteria | 0.06 | 0.04 | 1.52E-7 | 0.031 | 0.021 | 0.012 | 0.071 | 0.019 | 0.36 | 0.51 | 0.48 |
| unidentified_Bacteria | 0.37 | 0.18 | 0.55 | 0.273 | 0.81 | 0.15 | 0.14 | 0.473 | 0.63 | 0.40 | 0.62 |
| Others | 0.11a | 0.12a | 0.035b | 0.023 | 0.11 | 0.05 | 0.11 | 0.031 | 0.017 | 0.17 | 0.45 |
| Fermicutes:Bacteroidetes | 0.77 | 0.76 | 0.55 | 0.154 | 0.83 | 0.56 | 0.68 | 0.087 | 0.47 | 0.22 | 0.55 |
a,bValues (sharing the same SEM) within the same row with no common superscript letters differ (P < 0.05).
Abbreviations: Con, control group with basal diet; Anti, basal diet supplemented with 200 mg/kg oxytetracycline calcium and 250 mg/kg nosiheptide (Anti); Phyto, basal diet supplemented with 25 mg/kg phytosterols; Trt, treatment.
Table 5.
Effects of phytosterol supplementation on genus level taxonomic compositon of the cecal microbiota in yellow-feather broilers.
| Item | Treatment |
SEM | Day |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | Anti | Phyto | 21 D | 42 D | 63 D | Trt | Day | Trt∗Day | |||
| Bacteroides | 47.90 | 50.62 | 56.32 | 3.995 | 54.1a | 61.72a | 39.01b | 3.983 | 0.32 | 0.001 | 0.81 |
| Ruminococcaceae | 8.08 | 3.88 | 3.91 | 1.590 | 4.27 | 4.44 | 7.17 | 1.794 | 0.12 | 0.32 | 0.15 |
| Phascolarctobacterium | 2.75 | 3.06 | 1.52 | 1.17 | 1.08 | 5.42 | 0.83 | 1.863 | 0.57 | 0.075 | 0.30 |
| Barnesiella | 1.45 | 3.66 | 1.53 | 1.167 | 0.24a | 0.42a | 5.97b | 1.980 | 0.34 | 0.03 | 0.63 |
| Enterobacteriaceae | 1.59 | 0.70 | 0.53 | 0.933 | 2.78 | 0.01 | 0.04 | 1.612 | 0.69 | 0.05 | 0.91 |
| Lactobacillus | 1.50 | 1.47 | 3.04 | 0.949 | 0.49 | 1.53 | 3.98 | 1.530 | 0.43 | 0.05 | 0.46 |
| Olsenella | 0.30 | 1.56 | 1.16 | 0.987 | 0.16 | 1.15 | 1.71 | 0.805 | 0.66 | 0.19 | 0.35 |
| Enterococcus | 1.06 | 0.14 | 0.31 | 0.533 | 1.18 | 0.17 | 0.18 | 0.095 | 0.45 | 0.56 | 0.43 |
| Faecalibacterium | 3.81 | 3.41 | 1.94 | 0.811 | 5.98a | 2.05b | 1.11b | 1.016 | 0.25 | 0.0008 | 0.15 |
| Lachnospiraceae | 2.58 | 3.27 | 1.99 | 0.393 | 3.57a | 2.49a,b | 1.77b | 0.519 | 0.075 | 0.019 | 0.45 |
| Others | 29.05 | 26.66 | 29.32 | 3.754 | 26.13 | 20.68 | 38.22 | 2.463 | 0.85 | 0.005 | 0.35 |
a,bValues (sharing the same SEM) within the same row with no common superscript letters differ (P < 0.05).
Abbreviations: Con, control group with basal diet; Anti, basal diet supplemented with 200 mg/kg oxytetracycline calcium and 250 mg/kg nosiheptide (Anti); Phyto, basal diet supplemented with 25 mg/kg phytosterols; Trt, treatment.
Firmicutes and Bacteroidetes are the dominant bacteria, and these 2 phyla occupy approximately 95% of intestinal flora in the avian gut (Li et al., 2008). In our study, the total relative abundance of these 2 phyla were approximately 94%, 95%, and 95%, respectively. It was observed that rats on a high fat diet had a higher proportion of Firmicutes and a lower abundance of Bacteroidetes (Turnbaugh et al., 2006). Firmicutes is playing an important role to restore the intestinal homeostasis as it can suppress or eliminate Clostridium perfringens growth. Li et al. (2019) observed higher abundance of Proteobacteria in soybean-derived phytosterols–supplementing group, which was similar to our study. Song et al. (2020) reported that supplementation of phytosterol ester (0.1 g per 100 g BW) did not affect the relative abundance of 4 phyla (Firmicutes, Proteobacteria, Actinobacteria, and Verrucomicrobia) and some genera (Faecalibacterium, Akkermansia, etc) in rats with nonalcoholic fatty liver disease. The anaerobic bacteria Faecalibacterium is associated with chronic metabolic diseases such as digestive diseases. It decreased as the broilers grew, indicating a lower occurrence of disease. Although not significant, phytosterols group had a numerically higher abundance of known beneficial Lactobacillus. The genus Lactobacillus plays a crucial role in the homeostasis of the gastrointestinal tract (Hernandez-Patlan et al., 2019). Ayesh et al. (1999) observed that daily consumption of 6.6 g vegetable oil phytosterols by human subjects did not affect the bacterial profile or the metabolic activities of the gut microflora. Phytosterols are reported to have a low absorption in the intestine of both human and experimental animals (4% for sitosterol, 5% for stigmasterol, and 9–10% for campesterol; (Heinemann et al., 1993). We expected that it would exert a huge effect on gut microflora. Limited effect on the gut microflora of broilers observed in our study might be associated with the amount used or health status of the broilers.
Conclusions
Supplementation of phytosterols at 25 mg/kg level did not affect the growth performance of yellow-feather broilers. It decreased the community richness of gut microflora based on the Chao1 and Ace indexes. The abundance of Bacteroidetes was increased with supplementation of phytosterols.
Acknowledgment
The financial support from Guangdong Basic and Applied Basic Research Foundation (2019A1515110780), the Starting Research Fund from Foshan University (CGG07145), the Scientific Research Foundation in the Higher Education Institutions of Educational Commission of Guangdong Province (2017GCZX006), Special Foundation for Key Research Area of Educational Commission of Guangdong Province (2019KZDZX2006), and Guangdong Province Modern Agriculture Poultry Industry technology system innovation team construction project (2019KJ28) were acknowledged.
Conflicts of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Aldini R., Micucci M., Cevenini M., Fato R., Bergamini C., Nanni C., Cont M., Camborata C., Spinozzi S., Montagnani M., Roda G., D'Errico-Grigioni A., Rosini F., Roda A., Mazzella G., Chiarini A., Budriesi R. Antiinflammatory effect of phytosterols in experimental murine colitis model: prevention, induction, remission study. PLoS One. 2014;9:e108112. doi: 10.1371/journal.pone.0108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayesh R., Weststrate J.A., Drewitt P.N., Hepburn P.A. Safety evaluation of phytosterol esters. Part 5. Faecal short-chain fatty acid and microflora content, faecal bacterial enzyme activity and serum female sex hormones in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem Toxicol. 1999;37:1127–1138. doi: 10.1016/s0278-6915(99)00109-x. [DOI] [PubMed] [Google Scholar]
- Baskar A., K S A N A., Paulraj M.G., Alsaif M.A., Muamar M.A., Ignacimuthu S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1, 2-dimethylhydrazine-induced colon cancer. J. Med. Food. 2012;15:335–343. doi: 10.1089/jmf.2011.1780. [DOI] [PubMed] [Google Scholar]
- Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinform. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B.J., Gevers D., Earl A.M., Feldgarden M., Ward D.V., Giannoukos G., Ciulla D., Tabbaa D., Highlander S.K., Sodergren E., Methe B., DeSantis T.Z., Human Microbiome C., Petrosino J.F., Knight R., Birren B.W. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann T., Axtmann G., von Bergmann K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Invest. 1993;23:827–831. doi: 10.1111/j.1365-2362.1993.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Hernandez-Velasco X., Merino-Guzman R., Adhikari B., López-Arellano R., Kwon Y.M., Hargis B.M., Arreguin-Nava M.A., Tellez-Isaias G., Latorre J.D. Impact of a Bacillus Direct-fed microbial on growth performance, intestinal barrier Integrity, necrotic enteritis Lesions, and Ileal microbiota in broiler chickens using a Laboratory Challenge model. Front. Vet. Sci. 2019;6:108. doi: 10.3389/fvets.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Li S., Zhang Y., Zhuo Z., Feng J. Phytosterols on growth performance, antioxidant enzymes and intestinal morphology in weaned piglets. J. Sci. Food Agric. 2017;97:4629–4634. doi: 10.1002/jsfa.8333. [DOI] [PubMed] [Google Scholar]
- Li M., Wang B., Zhang M., Rantalainen M., Wang S., Zhou H., Zhang Y., Shen J., Pang X., Zhang M., Wei H., Chen Y., Lu H., Zuo J., Su M., Qiu Y., Jia W., Xiao C., Smith L.M., Yang S., Holmes E., Tang H., Zhao G., Nicholson J.K., Li L., Zhao L. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. U S A. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang Z., Cheng J., Diao C., Yan Y., Liu D., Wang H., Zheng F. Dietary supplementation of soybean-derived sterols regulates cholesterol metabolism and intestinal microbiota in hamsters. J. Funct. Foods. 2019;59:242–250. [Google Scholar]
- Liu X., Zhao H.L., Thiessen S., House J.D., Jones P.J. Effect of plant sterol-enriched diets on plasma and egg yolk cholesterol concentrations and cholesterol metabolism in laying hens. Poult. Sci. 2010;89:270–275. doi: 10.3382/ps.2009-00249. [DOI] [PubMed] [Google Scholar]
- Luo X., Su P., Zhang W. Advances in Microalgae-derived phytosterols for functional food and Pharmaceutical Applications. Mar. Drugs. 2015;13:4231–4254. doi: 10.3390/md13074231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoc T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinform. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I., Perdicaro D.J., Brown A.W., Hammons S., Carden T.J., Carr T.P., Eskridge K.M., Walter J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl. Environ. Microbiol. 2013;79:516–524. doi: 10.1128/AEM.03046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R.A., Whitaker B.D., Hicks K.B. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting use. Prog. Lipid Res. 2002;41:457–500. doi: 10.1016/s0163-7827(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Naji T.A., Amadou I., Abbas S., Zhao R.Y., Shi Y.H., Le G.W. Phytosterol supplementation improves antioxidant enzymes status and broiler meat quality. Pak. J. Food Sci. 2013;23 [Google Scholar]
- Santas J., Codony R., Rafecas M. Phytosterols: beneficial effects. In: Ramawat K., Mérillon J.M., editors. Natural Products. Springer; Berlin, Heidelberg: 2013. [Google Scholar]
- Shi S., Shen Y., Chang L., Zhou C., Bo Z., Wang Z., Tong H., Zou J. Safety evaluation of phytosterols in laying hens: effects on laying performance, clinical blood parameters, and organ development. Poult. Sci. 2014;93:545–549. doi: 10.3382/ps.2013-03562. [DOI] [PubMed] [Google Scholar]
- Song L., Li Y., Qu D., Ouyang P., Ding X., Wu P., Guan Q., Yang L. The regulatory effects of phytosterol esters (PSEs) on gut flora and faecal metabolites in rats with NAFLD. Food Funct. 2020;11:977–991. doi: 10.1039/c9fo01570a. [DOI] [PubMed] [Google Scholar]
- Song L., Qu D., Zhang Q., Jiang J., Zhou H., Jiang R., Li Y., Zhang Y., Yan H. Phytosterol esters attenuate hepatic steatosis in rats with non-alcoholic fatty liver disease rats fed a high-fat diet. Sci. Rep. 2017;7:41604. doi: 10.1038/srep41604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Wang L., Zuo X., Zhao W., Zhou G., Luo L., Yang K., Shu G., Wang S., Gao P., Zhu X., Jiang Q., Wang L. Effect of maternal dietary supplementation with phytosterol esters on muscle development of broiler offspring. Acta Biochim. Pol. 2020;67:135–141. doi: 10.18388/abp.2020_2884. [DOI] [PubMed] [Google Scholar]
- Wu P., Chen Y.P., Wen C., Tang Z.G., Zhou Y.M. Effects of different phytosterols on growth and meat quality of ducks. J. Chin. Cereals Oils Assoc. 2012;1:75–79. [Google Scholar]
- Zhao W., Su H., Wang L., Sun L., Luo P., Li Y., Wu H., Shu G., Wang S., Gao P., Zhu X., Jiang Q., Wang L. Effects of maternal dietary supplementation of phytosterol esters during gestation on muscle development of offspring in mice. Biochem. Biophys. Res. Commun. 2019;520:479–485. doi: 10.1016/j.bbrc.2019.10.056. [DOI] [PubMed] [Google Scholar]
- Zhao Y.R., Chen Y.P., Cheng Y.F., Qu H.M., Li J., Wen C., Zhou Y.M. Effects of dietary phytosterols on growth performance, antioxidant status, and meat quality in Partridge Shank chickens. Poult. Sci. 2019;98:3715–3721. doi: 10.3382/ps/pez059. [DOI] [PubMed] [Google Scholar]