Abstract
Mercuric chloride (HgCl2) is a serious environmental toxicant. So far, the toxicity mechanism of HgCl2 in chicken embryonic kidney (CEK) cells is not still fully understood. In this study, the possible molecular mechanisms of HgCl2 on apoptosis of CEK cells were investigated. Results showed that the cell morphology changed, and cell viability was significantly decreased (P < 0.05) after HgCl2 exposure. Besides, apoptosis rate was significantly increased after HgCl2 exposure (P < 0.05). The gene and protein expressions of B-cell lymphoma-2 associate X/B-cell lymphoma-2 (P < 0.05), caspase-3 (P < 0.05), and caspase-9 (P < 0.05) were significantly enhanced by HgCl2 in CEK cells. We also found that intracellular reactive oxygen species level was significantly enhanced (P < 0.05), and the flux of calcium ion to mitochondria occurred after HgCl2 exposure. In terms of molecular mechanisms, the mRNA and protein expressions associated with endoplasmic reticulum (ER) stress were significantly increased after HgCl2 exposure (P < 0.05), including glucose regulated protein 78, protein kinase RNA-like endoplasmic reticulum kinase (PERK), activating transcription factor 4 (ATF4), and C/EBP homologous protein (CHOP). However, pretreated with 1-μmol/L 4-phenylbutyrate (ER stress inhibitor) alleviated the apoptosis and downregulated PERK-ATF4-CHOP pathway in CEK cells. Taken together, upregulation of PERK-ATF4-CHOP pathway of ER stress induced by HgCl2 is associated with apoptosis in CEK cells.
Key words: apoptosis, chicken embryonic kidney cell, endoplasmic reticulum stress, mercuric chloride, PERK-ATF4-CHOP pathway
Introduction
In recent years, the extensive applications of metals have led to a sharp increase of environmental pollution worldwide. Mercury (Hg) is a well-known hazardous metal. It can be deposited into rivers, lakes, oceans, and soils, which are the sources of Hg contamination in feedstuff of livestock (Caban and Rasmussen, 1994). Accumulated evidence indicates that Hg exposure induces a number of problems in animals, including neurotoxicity, cardiotoxicity, reproduction toxicity, respiratory toxicity, hepatotoxicity, and nephrotoxicity (Azevedo et al., 2012; Jaishankar et al., 2014). Even through Hg exposure causes toxic effects on several tissues, the kidneys are the primary target organ where Hg accumulates and damages (Zalups, 2000). Previous study reported that mercuric chloride (HgCl2) reduced laying performance and egg quality and induced renal damages in laying hens (Ma et al., 2018a).
When the renal cells were stimulated by exogenous HgCl2, apoptosis was the fundamental cause of HgCl2-induced nephrotoxicity and occurred as a defense mechanism (Carranza-Rosales et al., 2005). A previous study reported that there was a close correlation between unfold protein response (UPR) and apoptosis after methylmercury exposure (Senft and Ronai, 2015; Hiraoka et al., 2017). Under a variety of pathological stimuli, UPR regulates intracellular signaling pathways to prevent the deposition of misfolded proteins in endoplasmic reticulum (ER) lumen (Malhotra and Kaufman, 2011). When the ER stress is activated, accumulation of unfold proteins induces dissociation of glucose-regulated protein 78 (GRP78) from inositol-requiring enzyme 1, protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6), which are the 3 transmembrane receptors in ER (Logue et al., 2013). Activated inositol-requiring enzyme 1 combines with X-box protein 1 (XBP-1) to synthesize mature XBP-1s. Activation of PERK restrains protein synthesis by means of phosphorylating eukaryotic initiation factor 2a (eIF2a) followed by activating transcription factor 4 (ATF4). The activation form of ATF6 upregulates a number of ER chaperones, including C/EBP homologous protein (CHOP). When the intracellular homeostasis is unable to be restored, the 3 ER receptors act together to enhance CHOP expression and further trigger apoptosis (Gorman et al., 2012; Schneider et al., 2012). Based on aforementioned studies, we speculated that there might be some connections between Hg-induced apoptosis and the PERK-ATF4-CHOP pathway of ER stress in chicken embryonic kidney (CEK) cells. Besides, at the early step of apoptotic pathway, the B-cell lymphoma-2 (Bcl-2) family members are activated in mitochondrial membrane, including proapoptotic B-cell lymphoma-2 associate X (Bax) and antiapoptotic Bcl-2. Meanwhile, the process of apoptosis is accompanied by the fluctuations of intracellular reactive oxygen species (ROS) and calcium ion (Ca2+) (Gross et al., 1999; Malhotra and Kaufman, 2011). In addition, both caspase-9 and caspase-3 are the vital molecules at the later step of apoptotic pathway (Green and Reed, 1998).
In this study, we investigated the effect of HgCl2 exposure on apoptosis in CEK cells. Furthermore, PERK-ATF4-CHOP signal pathway, mitochondrial membrane potential, intracellular ROS, and Ca2+ levels were studied for exploring the molecular mechanism of Hg-induced cytotoxicity.
Materials and methods
Reagents and Cell Culture
Mercuric chloride (HgCl2, purity > 99.5%; Sigma-Aldrich, St. Louis, MO) was dissolved in 0.1 mol sterile phosphate buffered saline (PBS) and added to mediums to provide Hg concentrations of 0 (Control), 5, 10, and 15 μmol/L. 4-Phenyl butyric acid (4-PBA, ER stress inhibitor) was obtained from Sigma Chemical Co. (St. Louis, MO).
Antibodies of PERK, p-PERK, CHOP, ATF4, GRP78, Pro-caspase-3, Cleaved caspase-3, Pro-caspase-9, Cleaved caspase-9, Bcl-2, Bax, and glyceraldehyde-3-phosphate dehydrogenase were from Abcam (Cambridge, England). The CEK cells were purchased from the Cell Biology Institution (Otwo, Shenzhen, China). They were cultured in high-glucose Dulbecco's Modified Eagle Medium (DMEM, Gibco, Pittsburgh, PA) containing 15% fetal bovine serum (Hyclone, Logan, UT) and incubated in a humidified 5% CO2 and 95% air incubator (Thermo Fisher Scientific, Grand Island, Maldives) at 37°C. Four independent experiments were performed in a parallel manner (n = 4). Each experiment had 4 concentrations, and each concentration had 4 parallel wells.
Cell Morphological Assays
After giving PBS or different dosages of Hg for 24 h, the CEK cells were observed under an optical microscope (Olympus, Melville, NY). The cells were fixed in 4% paraformaldehyde, washed with PBS, and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) reagent (KeyGen Biotech, Nanjing, China) to visualize nuclei for observing under a fluorescence microscope (IX70; Olympus, Tokyo, Japan). Besides, the CEK cells treated with PBS or different concentrations of Hg for 24 h were harvested and fixed in 2.5% glutaraldehyde. The cells were fixed in 1% osmium tetroxide and dehydrated with ethanol. The cells were embedded in epoxy resin, and ultrathin sections were prepared and photographed under a transmission electron microscope (TEM) (JEM-120EX; Jeol, Tokyo, Japan).
Cell Viability Assay
The CEK cells viability was quantitated using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Then the cells in 96-well plants were treated with PBS or different dosages of Hg for 24 h. In parallel, the CEK cells were exposed to the 15-μmol/L Hg for 24 h in the presence or absence of 1-μmol/L 4-PBA. Ten microliters of MTT reagent (Beyotime Institute of Biotechnology, Wuhan, China) was added to each well, and the cells were incubated for another 4h at 37°C followed by adding dimethyl sulfoxide solution to dissolve formazan crystals. Subsequently, the absorbance was determined at 570-nm wavelength by an enzyme-linked immunosorbent assay reader (Bio-tek ELX800; Winooski, VT). The calculation of cell activity was based on a previous study (Ma et al., 2018b).
Annexin V/PI Staining Assay
The CEK cells were treated with PBS or different dosages of Hg for 24 h. In parallel, the CEK cells were exposed to the 15-μmol/L Hg for 24 h in the presence or absence of 1-μmol/L 4-PBA. The CEK cells were harvested and treated with Annexin V-FITC binding and propidum iodide (PI) staining (KeyGen Biotech, Nanjing, China). Results were measured by using a flow cytometer (Becton-Dickinson Biosciences, Franklin Lakes, NJ). The apoptosis rate of CEK cells was calculated using the following formula:
Apoptosis rate (%) = (Number of early apoptotic cells + Number of late apoptotic cells)/Total number of cells × 100%.
Mitochondrial Membrane Potential Assay
Mitochondrial membrane potential was measured by the 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine fluorescent probe (Beyotime Institute of Biotechnology, Wuhan, China). The CEK cells treated with PBS or HgCl2 for 24 h were harvested and resuspended in DMEM. In parallel, the CEK cells were exposed to the 15-μmol/L Hg for 24 h in the presence or absence of 1-μmol/L 4-PBA. The cells were incubated with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine for 30 min at 37°C in the dark. Resuspending with PBS, the fluorescent intensity of cells was quantitated by using a flow cytometer (Becton-Dickinson Biosciences, Franklin Lakes, NJ). The mitochondrial membrane potential of CEK cells was calculated using the following formula:
Percentage of mitochondrial membrane potential decrease (%) = Number of mitochondrial membrane potential decreased cells/Total number of cells × 100%.
Determination of Intracellular ROS Level
The CEK cells treated with PBS or different dosages of Hg for 24 h were incubated with 10-μmol/L ROS-specific fluorescent dye 2, 7-dichlorofluorescein-diacetate (Beyotime Institute of Biotechnology, Wuhan, China) for 30 min at 37°C. The cells were washed twice with PBS. We measured the fluorescence intensity of cells using a flow cytometer (Becton-Dickinson Biosciences, Franklin Lakes, NJ); the excitation and emission wavelengths were 488 and 525 nm.
Determination of Intracellular Ca2+ Level
The CEK cells treated with PBS or different dosages of Hg for 24 h were loaded with Ca2+-specific fluorescent probe Fluo-3 AM (Beyotime Institute of Biotechnology, Wuhan, China) for 30 min at 37°C in the dark. Intracellular mitochondria were isolated by using a cell mitochondrial separation kit (Beyotime Institute of Biotechnology, Wuhan, China). Both the cells and mitochondria were washed with PBS, and the fluorescence intensities of them were quantitated using a flow cytometer (Becton-Dickinson Biosciences, Franklin Lakes, NJ). The excitation and emission wavelengths of flow cytometry were 488 and 525 nm, respectively.
Western Blot
The CEK cells were treated with PBS or different dosages of Hg for 24 h. In parallel, the CEK cells were exposed to the 15-μmol/L Hg for 24 h in the presence or absence of 1-μmol/L 4-PBA. The CEK cells were collected followed by cell lysis with lysis buffer (Beyotime Institute of Biotechnology, Wuhan, China). At 4°C, cell lysis suspension was centrifuged at 13,000 × g for 25 min. Total protein concentrations were measured using the BCA protein assay kit (Beyotime Institute of Biotechnology, Wuhan, China). Equally denatured protein samples were separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA) and blocked. The membranes were probed with specific primary antibody for 2 h at room temperature followed by adding secondary antibody. The immune-relative bands were visible with a chemiluminescence reagent (Biological Industries, Beit Haemek, Israel), and the data were analyzed by densitometry. Glyceraldehyde-3-phosphate dehydrogenase was the protein-loading control.
Quantitative Reverse Transcription-Polymerase Chain Reaction
The CEK cells were treated with PBS or different dosages of Hg for 24 h. In parallel, the CEK cells were exposed to the 15-μmol/L Hg for 24 h in the presence or absence of 1-μmol/L 4-PBA. The CEK cells were collected, followed by cell lysis with lysis buffer (Beyotime Institute of Biotechnology, Wuhan, China). Intracellular total RNA was extracted by using the TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA was reverse-transcribed to cDNA using a reverse transcription kit (TaKaRa, Dalian, China). The PCR assays were performed in a BioRad CFX96 (Bio-Rad, Hercules, CA) and carried out on a 96-well qPCR plant with a 10-μL reaction volume including 5 μL of 2× SYBR Premix Ex Taq (TaKaRa, Dalian, China), 0.5 μL of diluted cDNA template, 4.1 μL of RNase-free water, and forward and reverse primers. The PCR amplification was followed: initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, 60°C for 30 s, and 72°C for 30 s. The relative quantitative data of each gene were analyzed by the 2−ΔΔCt method according to a previous study (Livak and Schmittgen, 2002). The oligonucleotide primer sequences from 5′ to 3′ were referenced from a previous study and showed in Supplementary Data 1 (Zhang et al., 2016).
Statistical Analysis
All statistical analyses were performed using one-way analysis of variance by SPSS 20.0 (SPSS Inc., Chicago, IL). Results were showed as means ± SE of 4 independent experiments performed in a parallel manner. When the analysis of variance showed significant difference among groups (P < 0.05), multiple comparisons were performed with Tukey's multiple range test.
Results
The Effect of HgCl2 on Cell Morphology in CEK Cells
With the increase of Hg dosage, the number of adherent cells was decreased, while the number of floating CEK cells was increased (Figures 1A–1D). After DAPI staining, observation under a fluorescence microscope showed that CEK cells were similar to round shapes in the control group. However, the nucleus in CEK cells became more and more condensate as the dosage of Hg increased. Meanwhile, the number of condensate CEK cells was increased in a dose-dependent manner (Figures 1E–1H). In addition, CEK cells were observed by TEM (Figures 1I–1L). The results showed that CEK cells of the control group displayed normal cell morphology with uniformly dispersed chromatin in cytoplasm. After treatment with HgCl2, the shape of nuclei was irregular. Besides, the condensation of nuclei and chromatin displayed intensification as the dosage of Hg increased.
Figure 1.
Mercuric chloride affected cell morphology in chicken embryonic kidney (CEK) cells. (A–D) The morphological changes of CEK cells were observed under an optical microscope (400×). (E–H) The nucleolus morphologic changes of CEK cells were observed under a fluorescent microscope (400×). (I–L) The morphologic changes of CEK cells were observed under a transmission electron microscope (10,000×). The condensation of nuclei displayed intensification after Hg exposure is indicated by white arrows.
The Effect of HgCl2 on Cell Viability in CEK Cells
The effect of HgCl2 on CEK cell viability is presented in Figure 2A. Compared with control group, the CEK cell viability was significantly decreased in 10-μmol/L and 15-μmol/L groups (P < 0.05). As shown in Figure 2B, compared with the 15-μmol/L Hg group, the CEK cell viability was significantly increased after pretreated with 1-μmol/L 4-PBA (P < 0.05).
Figure 2.
Mercuric chloride affected cell viability in CEK cells. (A) The cell viability of CEK cells was measured by MTT assay. (B) The cell viability of CEK cells was measured by MTT assay after pretreated with 1 μmol/L 4-PBA. Values represent means ± SE (n = 4). Mean values with different superscript lowercase letters differ significantly among different treatment groups (P < 0.05). Abbreviations: 4-PBA, 4-phenylbutyrate; CEK, chicken embryonic kidney; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide.
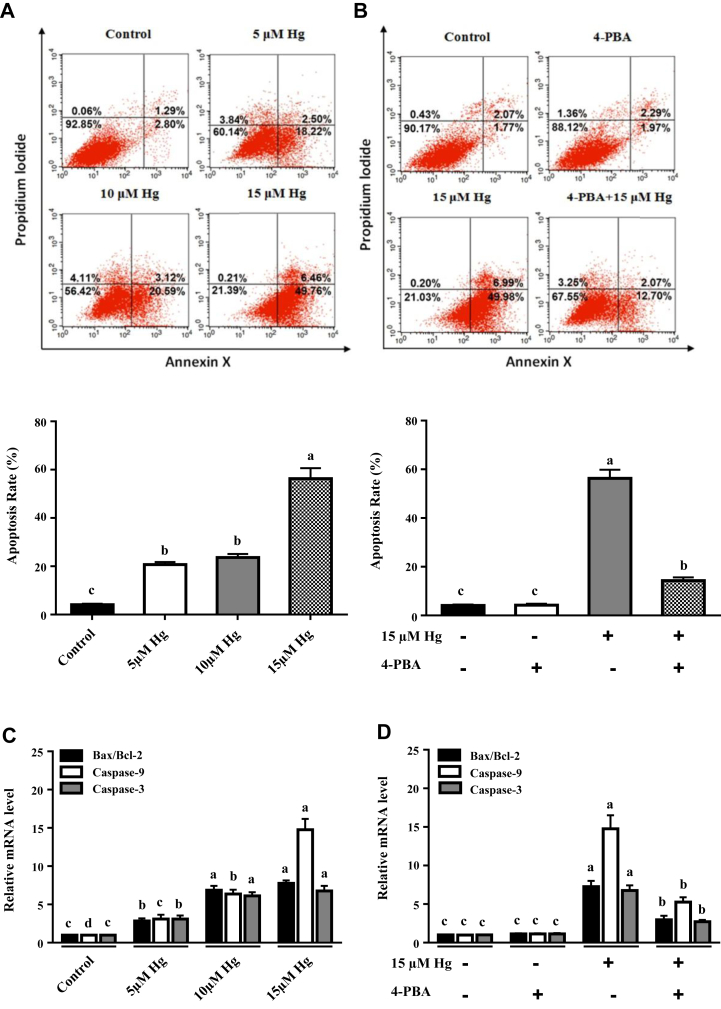
The Effects of HgCl2 on Apoptosis and Apoptosis-Related Gene and Protein Expressions in CEK Cells
The effect of HgCl2 on apoptosis of CEK cells is present in Figure 3A. Compared with the control group, apoptosis rate was significantly increased in the 5-μmol/L Hg group (P < 0.05), followed by a plateau in the 10-μmol/L Hg group, and then sharply increased in the 15-μmol/L Hg group (P < 0.05). As shown in Figure 3B, compared with the 15-μmol/L Hg group, apoptosis rate was significantly decreased after pretreated with 1-μmol/L 4-PBA (P < 0.05).
Figure 3.
Mercuric chloride induced apoptosis in CEK cells. (A) The apoptotic rates of CEK cells were detected by Annexin V/PI double-staining assay. (B) The apoptotic rates of CEK cells were detected by Annexin V/PI double-staining assay after pretreated with 1-μmol/L 4-PBA. (C) The mRNA expressions of Bax/Bcl-2, caspase-9, and caspase-3 were determined in CEK cells. (D) The mRNA expressions of Bax/Bcl-2, caspase-9, and caspase-3 were determined in CEK cells after pretreated with 1-μmol/L 4-PBA. (E) The protein expressions of pro-caspase-3, cleaved caspase-3, pro-caspase-9, cleaved caspase-9, Bcl-2, and Bax were determined by Western blot. (F) The protein expressions of pro-caspase-3, cleaved caspase-3, pro-caspase-9, cleaved caspase-9, Bcl-2, and Bax were determined by Western blot after pretreated with 1-μmol/L 4-PBA. Values represent means ± SE (n = 4). Mean values with different superscript lowercase letters differ significantly among different treatment groups (P < 0.05). Abbreviations: 4-PBA, 4-phenylbutyrate; CEK, chicken embryonic kidney; GAPHD, glyceraldehyde-3-phosphate dehydrogenase.
The effect of HgCl2 on apoptosis-related gene expression in CEK cells is present in Figure 3C. Compared with the control group, the gene expressions of Bax/Bcl-2 and caspase-3 were significantly increased as the dosage of Hg increased to 10 μmol/L (P < 0.05), followed by a plateau in the 15-μmol/L Hg group. Meanwhile, compared with the control group, the caspase-9 gene expression was significantly increased as the dosage of Hg increased up to 15 μmol/L (P < 0.05). As shown in Figure 3D, compared with the 15-μmol/L Hg group, the gene expressions of Bax/Bcl-2, caspase-9, and caspase-3 were significantly decreased after pretreated with 1-μmol/L 4-PBA (P < 0.05).
The effect of HgCl2 on apoptosis-related protein expression in CEK cells is present in Figure 3E. Compared with the control group, the cleaved caspase-3/pro-caspase-3 and cleaved caspase-9/pro-caspase-9 protein expressions were significantly increased as the dosage of Hg increased up to 15 μmol/L (P < 0.05). Meanwhile, compared with the control group, the Bax/Bcl-2 protein expression was significantly decreased in the 10-μmol/L and 15-μmol/L groups (P < 0.05). As shown in Figure 3F, compared with the 15-μmol/L Hg group, the protein expressions of cleaved caspase-3/pro-caspase-3, cleaved caspase-9/pro-caspase-9, and Bax/Bcl-2 were significantly decreased after pretreated with 1-μmol/L 4-PBA (P < 0.05).
The Effects of HgCl2 on Mitochondrial Membrane Potential and ROS Level in CEK Cells
The effect of HgCl2 on mitochondrial membrane potential of CEK cells is present in Figure 4A. The percentage of mitochondrial potential decrease was significantly increased as the dosage of Hg increased up to 15 μmol/L (P < 0.05). As shown in Figure 4B, pretreated with 1-μmol/L 4-PBA significantly prevented the mitochondrial potential decrease induced by HgCl2 exposure (P < 0.05).
Figure 4.
Mercuric chloride decreased mitochondrial potential and accelerated the generation of ROS in CEK cells. (A) The change of mitochondrial potential was detected by using JC-1 staining and analyzed by flow cytometry. (B) The change of mitochondrial potential was detected by using JC-1 staining and analyzed by flow cytometry after pretreated with 1-μmol/L 4-PBA. (C) The level of intracellular ROS was measured by flow cytometry. Values represent means ± SE (n = 4). Mean values with different superscript lowercase letters differ significantly among different treatment groups (P < 0.05). Abbreviations: 4-PBA, 4-phenylbutyrate; CEK, chicken embryonic kidney; FL1-H, CEK cells dyed by the dye A-V in the flow cytometry detection; JC-1, 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine; ROS, reactive oxygen species.
The effect of HgCl2 on ROS level in CEK cells is present in Figure 4C. Compared with the control group, the ROS level was significantly increased in the 10-μmol/L and 15-μmol/L Hg groups (P < 0.05).
The Effects of HgCl2 on Ca2+ Levels in Mitochondria and CEK Cells
The effects of HgCl2 on Ca2+ levels in mitochondria and CEK cells are present in Figure 5. Compared with the control group, 10- and 15-μmol/L Hg groups had significantly enhanced Ca2+ levels in mitochondria and the whole cells (P < 0.05).
Figure 5.
Mercuric chloride affects Ca2+ level in chicken embryonic kidney (CEK) cells. (A) The change of Ca2+ level in CEK cells was detected by Fluo-3 AM and analyzed by flow cytometry. (B) The change of Ca2+ level in mitochondria was detected by Fluo-3 AM and analyzed by flow cytometry. Values represent means ± SE (n = 4). Mean values with different superscript lowercase letters differ significantly among different treatment groups (P < 0.05). Abbreviation: FL1-H, CEK cells dyed by the dye A-V in the flow cytometry detection.
The Effect of HgCl2 on PERK-ATF4-CHOP Pathway in CEK Cells
As shown in Figure 6A, GRP78 mRNA expression was significantly increased as the dosage of Hg increased up to 10 μmol/L (P < 0.05), followed by a plateau in the 15-μmol/L Hg group. Besides, compared with the control group, ATF4 mRNA expressions were significantly increased in the 10-μmol/L and 15-μmol/L Hg groups (P < 0.05). Meanwhile, CHOP mRNA expression was significantly increased as the dosage of Hg increased up to 15 μmol/L (P < 0.05). As shown in Figure 6B, compared with the 15-μmol/L Hg group, the mRNA expressions of GRP78, ATF4, and CHOP were significantly decreased after pretreated with 1-μmol/L 4-PBA (P < 0.05).
Figure 6.
Mercuric chloride initiated ER stress by means of PERK-ATF4-CHOP pathway. (A) The mRNA expressions of GRP78, ATF4, and CHOP were determined in CEK cells. (B) The mRNA expressions of GRP78, ATF4, and CHOP were determined in CEK cells after pretreated with 1-μmol/L 4-PBA. (C) The protein expressions of GRP78, PERK, p-PERK, ATF4, and CHOP were determined by Western blot. (D) The protein expressions of GRP78, PERK, p-PERK, ATF4, and CHOP were determined by Western blot after pretreated with 1-μmol/L 4-PBA. Values represent means ± SE (n = 4). Mean values with different superscript lowercase letters differ significantly among different treatment groups (P < 0.05). Abbreviations: 4-PBA, 4-phenylbutyrate; ATF4, activating transcription factor 4; CHOP, C/EBP homologous protein; GAPHD, glyceraldehyde-3-phosphate dehydrogenase; GRP78, glucose-regulated protein 78; PERK, protein kinase RNA-like endoplasmic reticulum kinase.
As shown in Figure 6C, compared with the control group, the GRP78 protein expressions were significantly increased in 5-μmol/L, 10-μmol/L, and 15-μmol/L Hg groups (P < 0.05). Meanwhile, compared with the control group, the p-PERK/PERK protein expressions were significantly increased in the 10-μmol/L and 15-μmol/L Hg groups (P < 0.05). Besides, the ATF4 protein expression was significantly increased as the dosage of Hg to 10 μmol/L (P < 0.05) followed by a plateau in the 15-μmol/L Hg group. In addition, compared with the control group, the CHOP protein expression was significantly increased in the 5-μmol/L Hg group (P < 0.05) followed by a plateau as the dosage of Hg increased up to 15 μmol/L. As shown in Figure 6D, pretreatment with 1-μmol/L 4-PBA significantly decreased the protein expressions of GRP78, p-PERK/PERK, ATF4, and CHOP (P < 0.05).
Discussion
Accumulation evidences proved that HgCl2 is a well-known environmental toxicant, which activates numerous intracellular signal pathways leading to the induction of programmed apoptosis (Duncan-Achanzar et al., 1996). Ma et al. (2018a) reported that dietary HgCl2 exposure induced kidney damages in laying hens. A previous study found that dietary HgCl2 exposure induced renal apoptosis of laying hens in our laboratory. In this study, we investigated the molecular mechanism of HgCl2-induced apoptosis in CEK cells. We found that cell viability was significantly decreased after HgCl2 exposure. After DAPI staining, the nucleus of CEK cells became more and more condensate as the dosage of Hg increased. Besides, under TEM observation, the nuclei shape of CEK cells was irregular. Meanwhile, the condensation of nuclei and chromatin became more and more intense as the concentration of Hg increased. The morphological changes of CEK cells indicated that the cells were in the apoptosis program after HgCl2 exposure (Carranza-Rosales et al., 2005). In addition, many studies have demonstrated that caspases family plays an important role in cell death, mainly including caspase-3 and caspase-9 (Thornberry, 1998; Salvesen, 2002). Besides, Bcl-2 family proteins in mitochondrial membrane can either promote or inhibit apoptosis. The proapoptosis factors mainly contain Bax and Bak, and the antiapoptosis factors include Bcl-2 and Bcl-XL. Based on a previous study, HgCl2 induced apoptosis by means of enhancing Bax expression and suppressing Bcl-2 expression accompanied with mitochondrial membrane potential dissipation (Venkatesan and Sadiq, 2017). When the cells are in the apoptosis program, caspases-9 is activated by cytochrome C and this leads to activation of caspases-3. In the present study, we found that HgCl2 exposure induced apoptosis in CEK cells. Meanwhile, HgCl2 exposure reduced mitochondrial membrane potential and enhanced the gene and protein expressions of Bax/Bcl-2, caspase-3, and caspase-9 in CEK cells. A previous study reported that copper exposure induced apoptosis associated with enhanced mitochondrial dysfunction, collapse of mitochondrial membrane potential, and induction of mitochondrial permeability transition in hepatic cells, which was similar with this study (Roy et al., 2009).
A previous study reported that ER stress played an important role in apoptosis induced by many toxic metals (Lu et al., 2011; Wang et al., 2017). Once ER stress is activated, accumulation of unfold proteins induces the dissociation of GRP78, which is the most abundant ER chaperone in cells (Zhang et al., 2009). Meanwhile, the PERK-dependent transcription factor ATF4 is implicated in apoptosis (Malhotra and Kaufman, 2011). Besides, CHOP is an inducible transcription factor of ER stress and is regarded as an important trigger of cell death. Choi et al. (2016) reported that the expressions of UPR-related proteins were significantly increased in ethylmercury-treated cells, including CHOP, GRP78, and spliced XBP1. As we know, 4-PBA was a chemical chaperone that alleviated the ER stress in apoptosis progress. After 4-PBA pretreatment, apoptosis induced by HgCl2 was alleviated in this study, which meant that HgCl2-induced apoptosis was associated with ER stress in CEK cells. Furthermore, we found that HgCl2 exposure significantly enhanced the mRNA expressions of GRP78, ATF4, and CHOP and the protein expressions of GRP78, ATF4, p-PERK/PERK, and CHOP in the present study. However, the gene and protein expressions of them were downregulated after 4-PBA pretreatment in CEK cells. These results indicated that HgCl2 might induce apoptosis by means of enhancing PERK-ATF4-CHOP pathway associated with ER stress in CEK cells. Previous studies demonstrated that activation of CHOP induced the proapoptotic protein Bax expression and inhibited the antiapoptotic protein Bcl-2 expression, thereby leading to apoptosis, which was identified with this study (McCullough et al., 2001; Puthalakath et al., 2007).
There are mounting evidences demonstrating that ROS emerges as a crucial regulator of ER function and UPR activation in apoptosis (Malhotra and Kaufman, 2007; Kitamura and Hiramatsu, 2010). After HgCl2 exposure, intracellular ROS level was significantly increased in this study, which was consistent with human bronchial epithelial cells (Park and Park, 2007). As we know, intracellular Ca2+ homeostasis plays a key role for maintaining normal physiological function of cells. When ER stress-induced apoptosis is activated, intracellular endogenous ROS can recruit Ca2+ mobilizing messengers, inducing the flux of Ca2+ from ER to mitochondria (Nutt et al., 2002). There are 2 major associations between intracellular Ca2+ and apoptosis. On the one hand, excessive uptake of calcium in mitochondria might trigger the mitochondrial permeability transition, resulting in apoptosis and then contributing to pathogenesis (Baumgartner et al., 2009). On the other hand, deprivation of calcium in ER disturbs ER function and leads to the deposition of unfold or misfolded proteins in ER, thereby triggering ER stress-mediated apoptotic program via the mitochondrial pathway (Deniaud et al., 2008). In our study, we found that HgCl2 induced Ca2+ releasing from ER to mitochondria. These meant that the transfer of Ca2+ is also an accomplice of HgCl2-induced apoptosis in CEK cells. A previous study indicated that exposure of HK-2 cells to a high dose of ethylmercury enhanced intracellular Ca2+ levels quickly and induced cell death (Choi et al., 2016), which was similar with the present study. In view of the present results, this study provides some new evidences for ER stress-induced apoptosis under HgCl2 exposure in CEK cells for the first time. In addition, a previous study showed dietary Hg exposure decreased laying performance, egg quality, and renal histopathology damages in laying hens (Ma et al., 2018a). The ER stress-induced apoptosis presented the molecular mechanism of Hg on renal injury in laying hens.
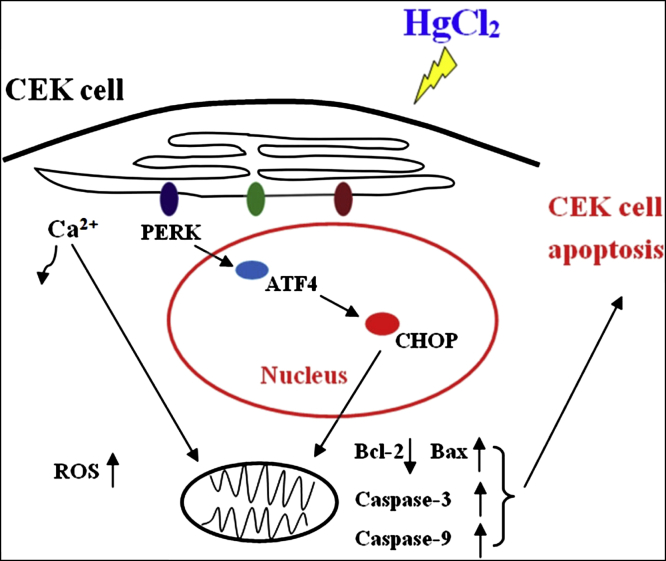
In summary, HgCl2 exposure induced apoptosis by means of upregulating the PERK-ATF4-CHOP pathway in CEK cells. Meanwhile, we found that HgCl2 exposure induced the flow of Ca2+ to mitochondria and enhanced intracellular ROS level in CEK cells (Figure 7). Taken together, we provide the first evidence that the PERK-ATF4-CHOP pathway of ER stress plays an important role in HgCl2-induced apoptosis in CEK cells.
Figure 7.
Proposed mechanism of mercuric chloride inducing apoptosis in CEK cells. Mercuric chloride enhanced intracellular ROS level and induced Ca2+ transferring to mitochondria. Mercuric chloride induced apoptosis by means of upregulating PERK-ATF4-CHOP pathway in CEK cells. Abbreviations: ATF4, activating transcription factor 4; CEK, chicken embryonic kidney; CHOP, C/EBP homologous protein; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ROS, reactive oxygen species.
Acknowledgments
This study was supported by the earmarked fund for PhD research start-up fund of Henan University of Science and Technology (No.13480086).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.06.084.
Supplementary data
References
- Azevedo B.F., Furieri L.B., Peçanha F.M., Wiggers G.A., Vassallo P.F., Simões M.R., Fiorim J., de Batista P.R., Fioresi M., Rossoni L., Stefanon I., Alonso M.J., Salaices M., Vassallo D.V. Toxic effect of mercury on the cardiovascular and central nervous system. J. Biomed. Biotechnol. 2012;2012:949048. doi: 10.1155/2012/949048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner H.K., Gerasimenko J.V., Thorne C., Ferdek P., Pozzan T., Tepikin A.V., Petersen O.H., Sutton R., Watson A.J.M., Gerasimenko O.V. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J. Biol. Chem. 2009;284:20796–20803. doi: 10.1074/jbc.M109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caban G., Rasmussen J.B. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature. 1994;372:255–257. [Google Scholar]
- Carranza-Rosales P., Said-Fernández S., Sepúlveda-Saavedra J., Cruz-Vega D.E., Gandolfi A.J. Morphologic and functional alterations induced by low doses of mercuric chloride in the kidney OK cell line: ultrastructural evidence for an apoptotic mechanism of damage. Toxicology. 2005;210:111–121. doi: 10.1016/j.tox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Choi J.T., Won N.H., Park J.D., Jang S., Eom C.Y., Choi Y., Park Y.I., Dong M.S. Ethylmercury-induced oxidative and endoplasmic reticulum stress-mediated autophagic cell death: involvement of autophagosome-lysosome fusion arrest. Toxicol. Sci. 2016;154:27–42. doi: 10.1093/toxsci/kfw155. [DOI] [PubMed] [Google Scholar]
- Deniaud A., Sharaf el dein O., Maillier E., Poncet D., Kroemer G., Lemaire C., Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Duncan-Achanzar K.B., Jones J.T., Burke M.F., Carter D.E., Laird H.E. Inorganic mercury chloride-induced apoptosis in the cultured porcine renal cell line LLC-PK1. J. Pharmacol. Exp. Ther. 1996;277:1726–1732. [PubMed] [Google Scholar]
- Gorman A.M., Healy S.J., Jäger R., Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol. Ther. 2012;134:306–316. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gross A., McDonnell J.M., Korsmeyer S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Hiraoka H., Kengo N., Yuki K., Shiori A., Kosaku O., Takao I., Masatake F., Yoshito K., Nobumasa T., Takashi U. Modulation of unfolded protein response by methylmercury. Biol. Pharm. Bull. 2017;40:1595–1598. doi: 10.1248/bpb.b17-00359. [DOI] [PubMed] [Google Scholar]
- Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M., Hiramatsu M. The oxidative stress: endoplasmic reticulum stress axis in cadmium toxicity. Biometals. 2010;23:941–950. doi: 10.1007/s10534-010-9296-2. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2002;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Logue S.E., Cleary P., Saveljeva S., Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–546. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- Lu T.H., Su C.C., Chen Y.W., Yang C.Y., Wu C.C., Hung D.Z., Chen C.H., Cheng P.W., Liu S.H., Huang C.F. Arsenic induces pancreatic beta-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol. Lett. 2011;201:15–26. doi: 10.1016/j.toxlet.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox. Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Malhotra J.D., Kaufman R.J. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb. Perspect. Biol. 2011;3:1–13. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Shi Y.Z., Lan L.L., Xie C., Zou X.T. Toxicological effects of mercury chloride on laying performance, egg quality, serum biochemistry, and histopathology of liver and kidney in laying hens. Biol. Trace Elem. Res. 2018;185:465–474. doi: 10.1007/s12011-018-1263-8. [DOI] [PubMed] [Google Scholar]
- Ma Y., Gong Y.J., Xu Q.Q., Zou X.T. Molecular mechanism of mercuric chloride inhibiting progesterone secretion in ovarian granulosa cells of laying hens. J. Anim. Physiol. Anim. Nutr. 2018;102:1533–1542. doi: 10.1111/jpn.12955. [DOI] [PubMed] [Google Scholar]
- McCullough K.D., Martindale J.L., Klotz L.O., Aw T.Y., Holbrook N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt L.K., Pataer A., Pahler J., Fang B., Roth J., McConkey D.J., Swisher S.G. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J. Biol. Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- Park E.J., Park K. Induction of reactive oxygen species and apoptosis in BEAS-2B cells by mercuric chloride. Toxicol In Vitro. 2007;21:789–794. doi: 10.1016/j.tiv.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Puthalakath H., O'Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D., Hughes P.D., Michalak E.M., McKimm-Breschkin J., Motoyama N., Gotoh T., Akira S., Bouillet P., Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Roy D.N., Mandal S., Sen G., Biswas T. Superoxide anion mediated mitochondrial dysfunction leads to hepatocyte apoptosis preferentially in the periportal region during copper toxicity in rats. Chem. Biol. Interact. 2009;182:136–147. doi: 10.1016/j.cbi.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Salvesen G. Caspases and apoptosis. Essays Biochem. 2002;38:9–19. doi: 10.1042/bse0380009. [DOI] [PubMed] [Google Scholar]
- Schneider C.C., Ampofo E., Montenarh M. CK2 regulates ATF4 and CHOP transcription within the cellular stress response signalling pathway. Cell Signal. 2012;24:1797–1802. doi: 10.1016/j.cellsig.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Senft D., Ronai Z.A. UPR, autophage, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry N.A. Caspases: key mediators of apoptosis. Cell Chem. Biol. 1998;5:97–103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- Venkatesan R.S., Sadiq A.M. Effect of morin-5’-sulfonic acid sodium salt on the expression of apoptosis related proteins caspase 3, Bax and Bcl 2 due to the mercury induced oxidative stress in albino rats. Biomed. Pharmacother. 2017;85:202–208. doi: 10.1016/j.biopha.2016.09.090. [DOI] [PubMed] [Google Scholar]
- Wang X.P., Guo Q., Tao L., Zhao L., Chen Y., An T., Chen Z., Fu R. E Platinum, a newly synthesized platinum compound, induces apoptosis through ROS-triggered ER stress in gastric carcinoma cells. Mol. Carcinog. 2017;56:218–231. doi: 10.1002/mc.22486. [DOI] [PubMed] [Google Scholar]
- Zalups R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000;52:113–143. [PubMed] [Google Scholar]
- Zhang L.J., Chen S., Wu P., Hu C.S., Thorne R.F., Luo C.M., Hersey P., Zhang X.D. Inhibition of MEK blocks GRP78 up-regulation and enhances apoptosis induced by ER stress in gastric cancer cells. Cancer Lett. 2009;274:40–46. doi: 10.1016/j.canlet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Zhang W.P., Chen L., Shen Y., Xu J. Rifampicin-induced injury in L02 cells is alleviated by 4-PBA via inhibition of the PERK-ATF4-CHOP pathway. Toxicol. In Vitro. 2016;36:186–196. doi: 10.1016/j.tiv.2016.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.