Abstract
The yolk sac tissue (YST) is a multifunctional metabolic organ supporting chicken embryonic development. This study examined whether incubation temperatures (ITs) affect YST functions. For this purpose, 300 eggs were assigned to 3 groups and incubated at control IT of 37.8°C, at 1.5°C below, 36.3°C (cold IT), and at 1.5°C above, 39.3°C (hot IT). For each group, 6 embryos' whole body mass and residual yolk (RSY) weights were recorded during incubation, and YST was sampled for both histology and gene expression analysis. YST functionality during incubation was examined by regression analysis, comparing changes in expression patterns of genes involved in lipid uptake and metabolism (LRP2, ApoA1), oligopeptides uptake (PepT1), gluconeogenesis (FBP1), glycogenesis (GYS2), and thyroid hormones regulation (TTR, DIO1, DIO2). Results show that hot and cold ITs affected YST gene expression and yolk utilization. PepT1 expression decreased towards hatch, in both hot and cold ITs, while in the Control IT, it reached a plateau. ApoA1 and DIO2 expression showed a moderate linear fit compared to polynomial fit in the control. GYS2 expression had no change along incubation, while in the control IT, it showed a polynomial fit. Expression of LRP2, FBP1, and DIO1 genes was affected by either cold or hot IT's. TTR expression patterns were similar in all IT groups. The variations in gene expression patterns observed in the 3 ITs can explain the changes in yolk utilization, an important parameter for hatchling quality. While the control IT showed optimal utilization, with an RSY value of 11.12% at the day of hatch, the cold and hot IT groups exhibited lower utilization with an RSY value of 18.18 and 29.99%, respectively. These findings are the first to show that ITs change the expression of key YST genes, leading to variations in yolk utilization by the embryo.
Key words: incubation, broiler embryo, yolk sac, gene expression
Introduction
During the period of incubation, the egg compartments (shell, albumen, and yolk) serve as a source of essential maternal nourishment, supporting the chicken embryo's development. The yolk, which constitutes nearly 60% of the egg's dry weight, is a primary source of nutrients and is comprised of 55.02% water, 26.71% lipids, 15.5% proteins, and 1.68% essential micronutrients including vitamins and minerals and contains a small amount (1.08%) of carbohydrates (Romanoff, 1967; Noble and Cocchi, 1990; Anton, 2007; Bauer et al., 2013; Réhault-Godbert et al., 2019). During incubation, most of the yolk nutrients are used via the yolk sac tissue (YST), an extra-embryonic tissue that envelopes the yolk (Romanoff, 1960).
The development of the YST is initiated at the onset of gastrulation, forming out of the developing gut of the embryo (Bellairs, 1953; Palis et al., 1995; Bauer et al., 2013). It is comprised of 3 morphological regions: the area vitellina, the nonvascularized region of the YST and the most distant from the embryo; the vitellina to vasculosa transition zone; and the area vasculosa, the region closest to the embryo and the major vascularized key functioning part of the YST (Duval, 1889; Mobbs and McMillan, 1981; Sheng, 2010). During its development, the YST projects villi structures into the yolk content which increases the surface area available for absorption, similar to the villi of the digestive tract. The villi are comprised of endodermal epithelial cells (EECs) that mediate the transport of nutrients from the yolk content to the blood circulation of the developing embryo (Mobbs and McMillan, 1979,1981; Noble and Cocchi, 1990; Yoshizaki et al., 2004; Sheng, 2010).
The YST serves as the main site of digestion, absorption, and nutrient transfer from the yolk content to the developing embryo. Previous research showed that the YST perform “intestine-like” functions by producing digestive enzymes and expressing nutrient transporters (Yadgary et al., 2011; Speier et al., 2012; Bauer et al., 2013). Furthermore, studies are pointing toward the conclusion that the YST is a metabolic organ capable of multiple functionalities because it synthesizes bile acids which are critical for yolk lipid digestion (Yadgary et al., 2013). It accomplishes “liver-like” functions such as glucose synthesis from noncarbohydrate precursors (gluconeogenesis) as well as glycogen synthesis (Yadgary and Uni, 2012) and regulation of thyroid hormones (THs) (Too et al., 2017). The YST also demonstrates roles in hematopoiesis and bone marrow development, as it serves as the only source of cells for primitive erythropoiesis and the main niche and source of cells for early definitive erythropoiesis (Romanoff, 1960; Freeman and Vince, 1974; Sheng, 2010). A whole transcriptome analysis (Yadgary et al., 2014) showed temporal large-scale expression of genes associated with the metabolic processes that are occurring in the YST from E13 to the day of hatch (DOH; E21) and demonstrated the various functions of the YST that are mentioned previously.
The YST along with its remaining yolk content are referred as the residual yolk (RSY) sac. From embryonic day 19 (E19), the RSY begins to be internalized into the embryo's body cavity and provide the perihatch embryos and hatchling with nourishment (Romanoff, 1960; Noble and Ogunyemi, 1989). Appropriate utilization of yolk (i.e., low RSY weight relative to embryo weight) and also proper amount of nutrients stored in the yolk sac (i.e., glycogen, glucose, fatty acids, and peptides) are key factors for high-quality embryos and hatchlings (Yadgary and Uni, 2012; Yadgary et al., 2013; Araujo et al., 2016; Van der Wagt et al., 2020). These factors may possibly be affected by incubation conditions. It is well documented that inadequate temperature reduces yolk utilization and leads to low-quality embryos and hatchlings which impact broiler performance (Freeman and Vince, 1974; Brunham et al., 2001; Joseph et al., 2006; Moran, 2007; Nangsuay et al., 2016; Lin et al., 2017).
As the YST plays a key role in yolk nutrients utilization, in glucose and glycogen synthesis, and in THs regulation, it will be interesting to examine the development and functionality of the YST in different incubation temperatures (ITs). Therefore, the aim of our work was to determine the effect of altered ITs (1.5°C below or above the control 37.8°C) on YST morphology and function from E5 to the DOH (E21). This was performed by examining the parameters of 1) embryo and RSY weights, 2) YST EEC diameters, and 3) expression levels of genes involved in lipid uptake and metabolism (LRP2, ApoA1), in oligopeptides uptake (PepT1), gluconeogenesis (FBP1), glycogen synthesis (GYS2), and THs regulators (TTR, DIO1, DIO2).
Materials and methods
Eggs and YST Sampling
Three hundred fertile eggs (mean weight = 57.7 g, SD = 1.6 g) from 31-week-old broiler breeder Cobb 500 hens were purchased from a commercial breeder farm (Y. Brown and Sons Ltd., Hod Hasharon, Israel). Eggs were randomly divided into 3 groups (control; cold; hot), stored for 3 d at 19 to 21°C and 60 to 65% relative humidity and later incubated in separate incubators (Rcom-Maru 190 Deluxe, Republic of Korea). Each group was incubated from day 0 (day of set) at different temperatures: 37.8°C in the control group, 36.3°C in the cold group, and 39.3°C in the hot group. Relative humidity was set to 65% in all groups. At E10, eggs were candled, and unfertilized eggs and dead embryos eggs were removed. At E18, all eggs were moved to hatching trays at 37.8°C for all groups. At each age, E5, E7, E9, E11, E15, E18, E19, and E21 (DOH), 6 embryos from each group were randomly selected and euthanized by cervical dislocation. Embryo whole body mass (WBM) and RSY weights were recorded, and YST samples were dissected from the area vasculosa, for gene expression analysis and histology. Samples for gene expression were stored at −20°C in RNAsave (BI, Beit-HaEmek, Israel), and samples for histology were placed in a fixative (0.1% GA–3.7% FA [vol/vol] in PBS), until further processing. While hatchability was 91.6% in the control IT group and 75% in the cold IT group, the hot IT group embryos did not complete their hatching process, as the majority died between E19 and E21 and the rest died during the hatching process (hatchability 8.4%). For this reason, hot IT group tissues were not sampled at E21, and measurements for RSY weight were taken only from externally piped dead embryos.
Morphological Examinations by Light Microscopy
YST samples (taken from 3 embryos from each IT group) were dehydrated, cleared, and embedded in paraffin. Serial sections were then cut at 4- to 6-μm thickness, deparaffinized in HistoChoice clearing agent (Sigma-Aldrich, St. Louis, MO), rehydrated, and stained with hematoxylin and eosin. After drying, samples were mounted on cover glass using fluka glue. Tissues were visualized using an Olympus BX40 microscope and Olympus DP73 camera (Japan), and cell sizes were measured using ImageJ software (Schindelin et al., 2012). In total, perimeters of 12 EECs from each embryo sample were measured (i.e., 36 cells per IT group).
Yolk Utilization Calculations
To track yolk utilization between ages E15 and E21, WBM (i.e., embryo and its extraembryonic membranes) and RSY weights values were recorded (n = 6 embryos per age and per IT group). RSY percent relative to WBM was calculated as follows:
Total RNA Isolation and cDNA Synthesis
Total RNA was isolated from 80 mg of YST using TRI-Reagent (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's protocol. RNA concentration was determined using a Nano Drop ND-1000 instrument (Thermo Fisher Scientific, Wilmington, DE). In order to avoid genomic DNA contamination, the total RNA was treated with DNase using a Turbo DNA-free Kit according to the manufacturer's protocol (Ambion; Thermo Fisher Scientific, Wilmington, DE). cDNA was created from 1 μg of DNA-free RNA using the qPCRBIO cDNA synthesis kit according to manufacturer's protocol (PCRBIOSYSTEMS, London, UK).
Determination of mRNA Abundance by Real-Time PCR
To assess the relative mRNA abundance of LRP2, ApoA1, PepT1, FBP1, GYS2, TTR, DIO1, DIO2, and the housekeeping gene β-Actin, gene-specific primers were used. Primer sequences (Table 1) were designed using Primer-BLAST software (Ye et al., 2012) based on published cDNA sequences when available and purchased from Sigma-Aldrich (Park Rabin, Rehovot, Israel). PCR products were validated by gel electrophoresis in 1.5% agarose gel. Real-time qPCR reactions were conducted in a Roche Light cycler 96 (Roche Molecular Systems, Inc., Pleasanton, CA). Each reaction (20 μL total) was composed of 3 μL of cDNA sample diluted 1:20, 1 μL of each primer (4 μmol/L), 5 μL UPW (Biological Industries, Beit Haemek, Israel), and 10 μL of Platinum SYBR Green qPCR super mix-UDG (Thermo Fisher Scientific, Wilmington, DE). Reaction conditions: preincubation at 95°C for 60 s, followed by 40 cycles of a 2-step amplification cycle of 95°C for 10 s and 60°C for 30 s, ended with a melting curve generated by the following conditions: 95°C for 60 s, 65°C for 60 s, and 97°C for 1 s. Samples were analyzed in triplicates in 96-well PCR microplates sealed with optically clear sealing film (Axygen Scientific, Inc., Union City, CA). To avoid false positives, a nontemplate control was run for each template and primer pair. Relative expression was calculated using the delta-delta CT method. Briefly, cycle threshold (Ct) values from the Roche LightCycler 96 program per sample were subtracted from the mean of the control treatment and corrected using β-Actin as the reference gene (Pfaffl, 2007).
Table 1.
Primers used for real-time PCR analysis of yolk sac tissue gene expression.
| Target1 | Accession no. | Primer F (5′-3′) | Primer R (5′-3′) | Ref. |
|---|---|---|---|---|
| LRP2 | XM_025152621 | GGAGACTCTGCTATAGACAGAGC | CCACACTACCAGCTCCTGTTA | Bauer et al., 2013 |
| ApoA1 | NM_205525.4 | GACCGCATTCGGGATATGGT | GGCCGGATCTTCTCCTTCAC | |
| PepT1 | NM_204365.1 | AAAACAGGTTTCGGCATCGC | CATCTCGATCCCTGCTGGTC | Yadgary and Uni, 2012 |
| FBP1 | NM_001278048.1 | TTCCATTGGGACCATATTTGG | ACCCGCTGCCACAAGATTAC | |
| GYS2 | XM_015291547.2 | CATCTGTACACTGTGCCCATGTG | TTTGGAGTGACAACATCAGGATTT | |
| TTR | NM_001281498.1 | CTCTTCTTGTTTTCTTAGCTGGAC | GACTGCATCCAGCACTTTCAC | Too et al., 2017 |
| DIO1 | NM_001097614.1 | TCAGGGTGCTCCTACACAAAC | TTTCGCCCAGCTTTAGGATGT | |
| DIO2 | NM_204114.4 | CGCCTACAAGCAGGTCAAA | ACTTGGTTCCACACTTGCCA | |
| β-Actin | NM_205518.1 | AATGGCTCCGGTATGTGCAA | GGCCCATACCAACCATCACA |
LRP2, LDL receptor; ApoA1, the major protein component of HDL; PepT1, oligopeptides transporter; FBP1, gluconeogenesis regulatory enzyme, catalyzes the hydrolysis of fructose; GYS2, catalyzes the rate-limiting step in the synthesis of glycogen; TTR, THs carrier protein in the plasma; DIO1, THs activating and deactivating enzyme; DIO2, THs activating enzyme; β-Actin, housekeeping gene.
Statistical Analysis
In order to assess differences in RSY percent between IT groups, at each age (E15, E18, E19, and E21), data were analyzed by one-way ANOVA. Tukey HSD test was used to separate means when P values (P) were significant (P ≤ 0.05).
In order to assess differences in the YST EEC perimeters between IT groups, at each age (E5-E21), data were analyzed by one-way ANOVA. Student t test was used to separate means of each pair when P values were significant (P ≤ 0.05).
For gene expression, according to heterogeneity of variance of embryonic exponential growth, logarithmic (ln) transformation was carried out for the data (Bartlett, 1947). For each IT group, regression analysis of expression over time was performed to assess gene expression patterns throughout the incubation period (E5-E21). A line of best-fit regression was added, and R2 values of these lines were recorded. Regressions were considered significant at P ≤ 0.05.
All data were analyzed using JMP-pro 15 software (SAS Institute Inc., Cary, NC).
Results and discussion
This study is the first to show that ITs have an effect on gene expression in the YST, thereby affecting yolk utilization and yolk sac nutrients resources. YST functionality during incubation was examined by regression analysis, comparing changes in expression patterns of genes involved in lipid uptake and metabolism (LRP2, ApoA1), oligopeptides uptake (PepT1), gluconeogenesis (FBP1), glycogenesis (GYS2), and THs regulation (TTR, DIO1, DIO2), as well as by EEC perimeters and RSY percentage.
Yolk Utilization Is Affected by Incubation Temperature
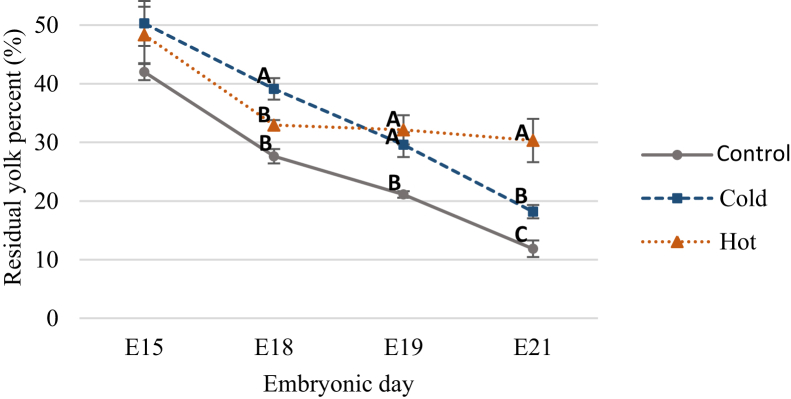
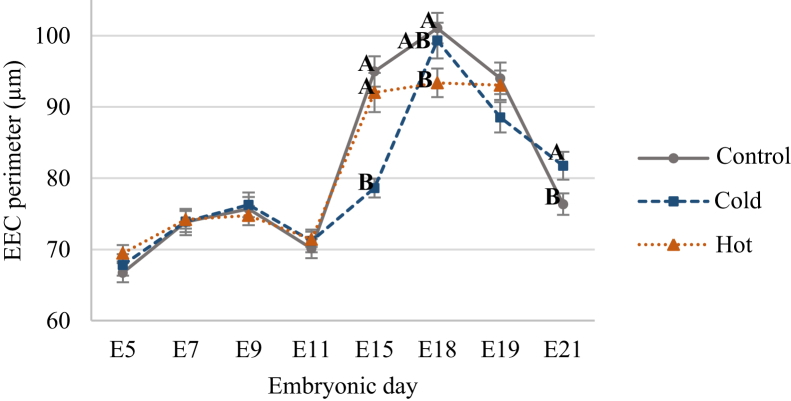
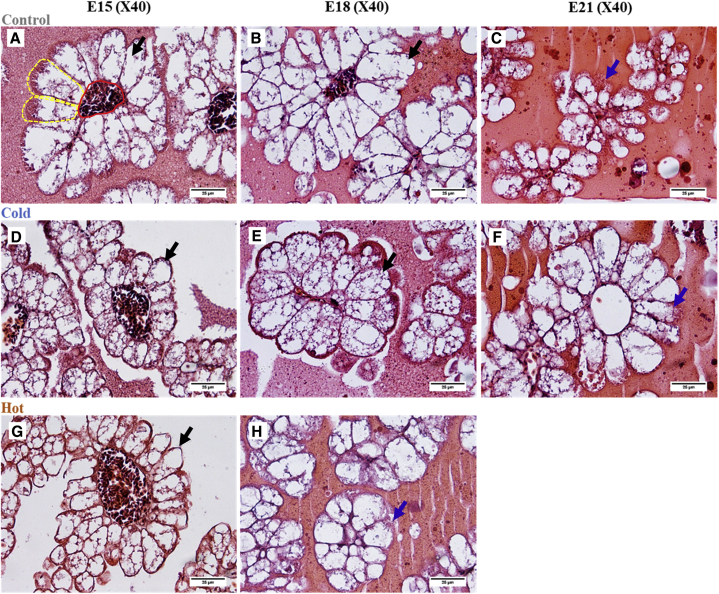
RSY percentage toward hatch provides an indication for embryonic development and its nutritional status, as a lower RSY percent during the perihatching period is indicative of better yolk utilization by the chicken embryo. RSY percentage measurements showed significantly reduced yolk utilization when eggs were incubated in both cold and hot ITs (Figure 1). The average RSY percent at E21 was 11.12% in the control IT and 18.18% and 29.99% in the cold and hot IT groups, respectively. Furthermore, while the control IT group showed a 71.7% decrease in RSY percent from E15 to E21, the cold and hot groups showed a lower decrease of 63.8% and 37.2%, respectively. This points on lower absorption and utilization of egg nutrients by the embryo which leads to lower quality embryos, hatchlings, and growing chickens in both layers (Romanoff, 1935) and broilers (Joseph et al., 2006; Sahan et al., 2014; Hamidu et al., 2018). No change was seen in RSY percent between E18 and E19 (32.94 and 32.14%) for embryos of the hot IT group, suggesting these embryos stopped using their yolk content. In contrast, the cold IT group embryos showed similar pattern of yolk utilization to the control but with higher values of RSY percent suggesting delayed growth. Another indicator for reduced yolk utilization in cold and hot IT groups is the decrease in size of the absorptive EECs (Figure 2). While no difference in EEC perimeter was observed between groups before E15, at E15, the cold IT group had significantly smaller EEC perimeters (78.61 μm) than the control IT (94.98 μm) and hot IT (92.02 μm) groups (P < 0.0001). This suggest lower lipid absorption in the cold IT group at E15. From E15 onwards, the EEC perimeters in the cold IT group had reached similar values to those of the control IT. In contrast, the hot IT group showed almost no change—ranging from 92.02 μm to 93.38 μm and 93.05 μm, at E15, E18, and E19, respectively (see representative images of H&E sections from E15, E18, and E21 in Figure 3). In addition, lipid vacuole breakdown in EECs is observed in the hot IT group on E18, while in the control and cold IT groups, this is evident at E19 (data not shown) and at E21, DOH (Figure 3). This may indicate early start of the apoptotic stage in response to hot IT which probably impairs YST competence to use yolk. However, it is not yet clear what leads to the initiation of YST apoptosis (Yadgary et al., 2013, 2014).
Figure 1.
The percent of residual yolk (RSY) weight of whole embryo body mass (WBM) toward hatch (E15-E21), in 3 IT groups; control IT 37.8°C (round dots), cold IT 36.3°C (square dots), and hot IT 39.3°C (triangular dots). Data were analyzed by one-way ANOVA, Tukey HSD test. Means with different letters differ significantly between IT groups at each age (P ≤ 0.05). n = 6 per age and IT group.
Figure 2.
The average perimeters of yolk sac tissue endodermal epithelial cells (EECs) throughout incubation in 3 IT groups; control 37.8°C (round dots), cold 36.3°C (square dots), and hot 39.3°C (triangular dots). Cross-section perimeters were measured on histological sections (shown in Figure 3). Data were analyzed by one-way ANOVA, Student t test. Means with different letters differ significantly between groups at each age (P ≤ 0.05). n = 36 cells per age (12 cells × 3 tissues).
Figure 3.
Histology sections of the yolk sac tissue (YST) (E15, E18, and E21). Hematoxylin and eosin sections were used for endodermal epithelial cell (EEC) perimeter measurements, in 3 IT groups: upper panel (A–C), control IT (37.8°C); middle panel (D–F), cold IT (36.3°C); lower panel (G, H) hot IT (39.3°C). (scale bar size: 25 μm). The YST villi are comprised of the EECs (see dashed yellow frames in a), which are surrounding a central blood vessel (see red frame in a). Black arrows, EECs are bloated containing lipid vacuoles (A, B, D, E, G). Blue arrows, EECs are smaller, lipid vacuole breakdown is evident (C, F, H).
Taken together with the RSY percent measurements, these results suggest that the cold IT reduced the rate of yolk utilization causing delayed yolk nutritional support to the developing embryo which leads low-quality hatchlings while the hot IT had even greater effect causing halted development of the embryos at E18, subsequently causing their mortality before hatch.
Mortality in the Hot Incubation Temperature Group Embryos
As mentioned in the “Materials and methods” section, the effect of the hot IT resulted in embryonic mortality, making it impossible to collect YST samples at E21 for gene expression analysis and for histological measurement data (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8). However, measurements for RSY weight were possible and taken from externally piped dead embryos, that had developed well enough to be ready to hatch (Figure 1). Piestun et al. (2008) published similar findings that are demonstrating a prominent reduction in hatchability in response to a high IT. This “mortality before hatch” phenomenon was explained by the increase in the embryos' body heat production during the second half of incubation combined with the heat of the incubator which resulted in an acute heat stress to the embryos (Yahav, 2014). Additional explanation was provided by Christensen et al. (2001) who suggested that embryos did not complete their hatching process because of upset of glycogen levels in the complexus muscle (the “pipping muscle”), which together with the liver and yolk sac are the main energy sources for pipping.
Figure 4.
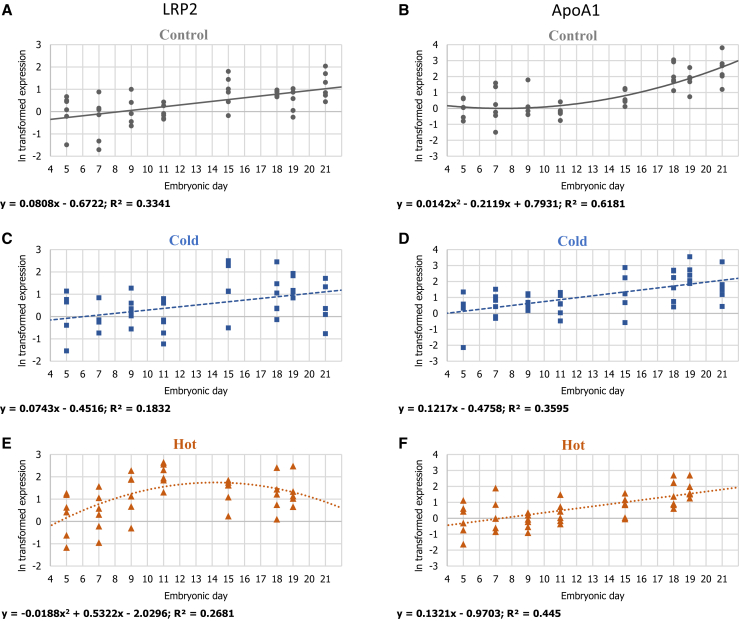
Gene-expression patterns of LRP2 and ApoA1 in 3 IT groups: (A, B) control IT 37.8°C; (C, D) cold IT 36.3°C; and (E, F) hot IT 39.3°C. For each ln transformed relative expression, a regression was fitted. Regression considered significant at P ≤ 0.05. n = 6 per age in each IT group.
Figure 5.
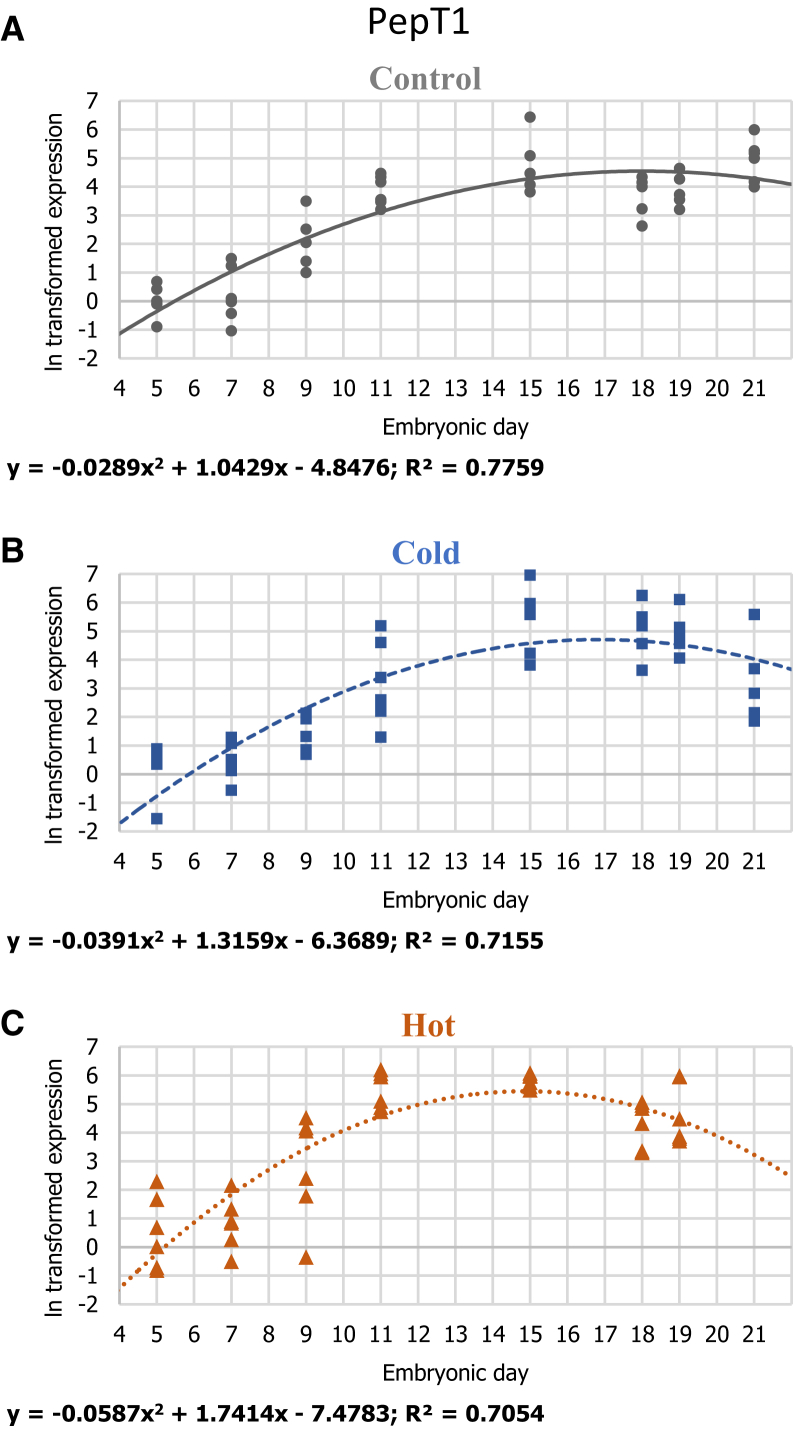
Gene-expression patterns of PepT1 in 3 IT groups: (A) control 37.8°C, (B) cold 36.3°C, and (C) hot 39.3°C. For each ln transformed relative expression, a regression was fitted. Regression considered significant at P ≤ 0.05. n = 6 per age in each IT group.
Figure 6.
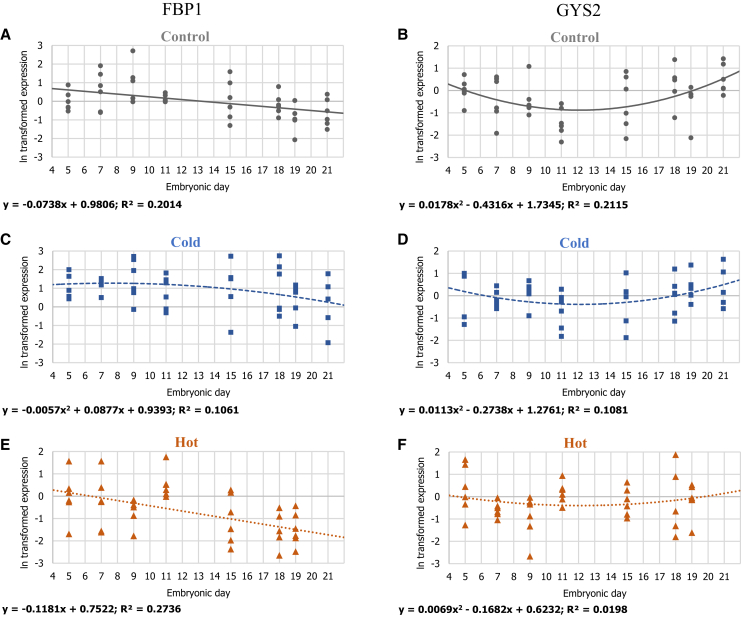
Gene-expression patterns of FBP1 and GYS2 in 3 IT groups: (A, B) control 37.8°C, (C, D) cold 36.3°C, and (E, F) hot 39.3°C. For each ln transformed relative expression, a regression was fitted. Regression considered significant at P ≤ 0.05. n = 6 per age in each IT group.
Figure 7.
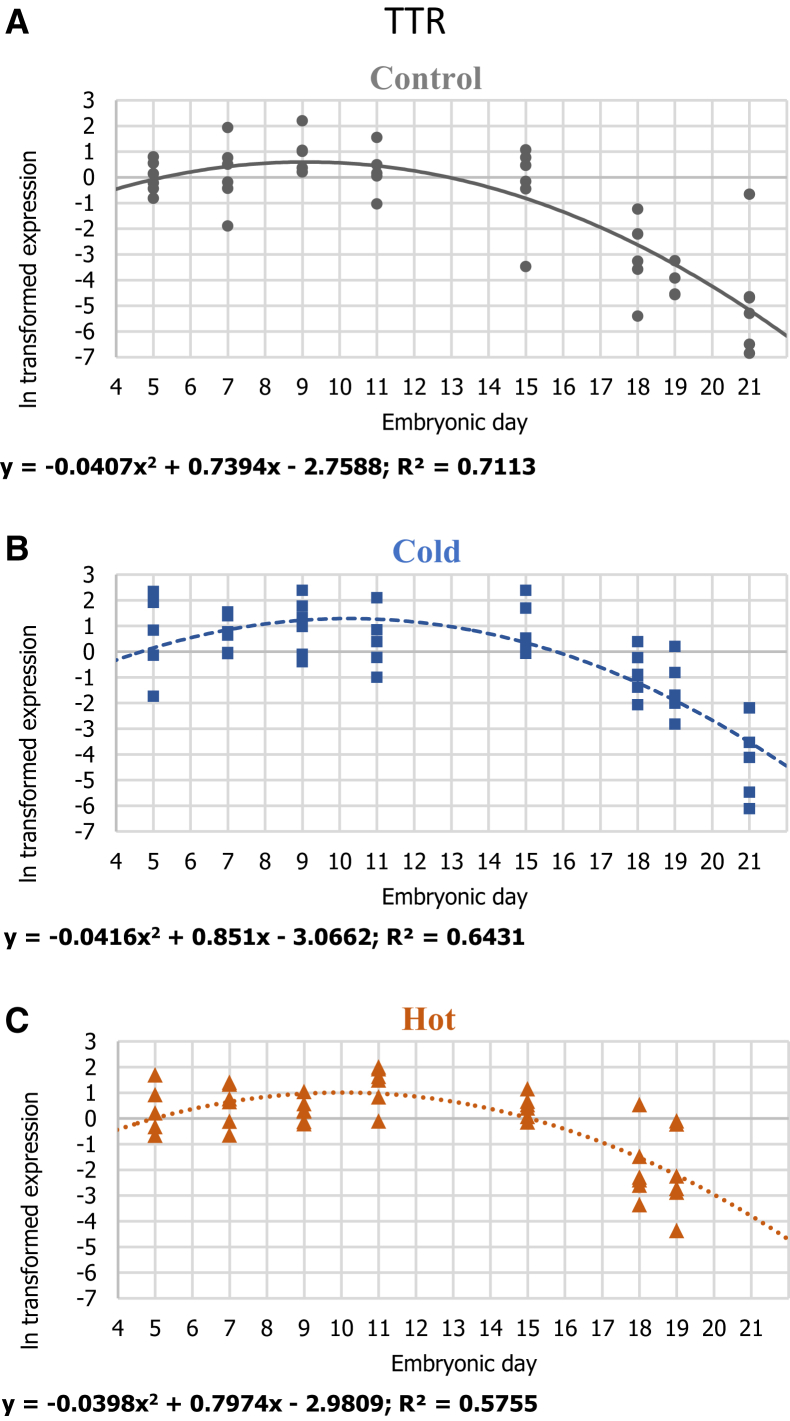
Gene-expression patterns of TTR in 3 IT groups: (A) control 37.8°C, (B) cold 36.3°C, and (C) hot 39.3°C. For each ln transformed relative expression, a regression was fitted. Regression considered significant at P ≤ 0.05. n = 6 per age in each IT group.
Figure 8.
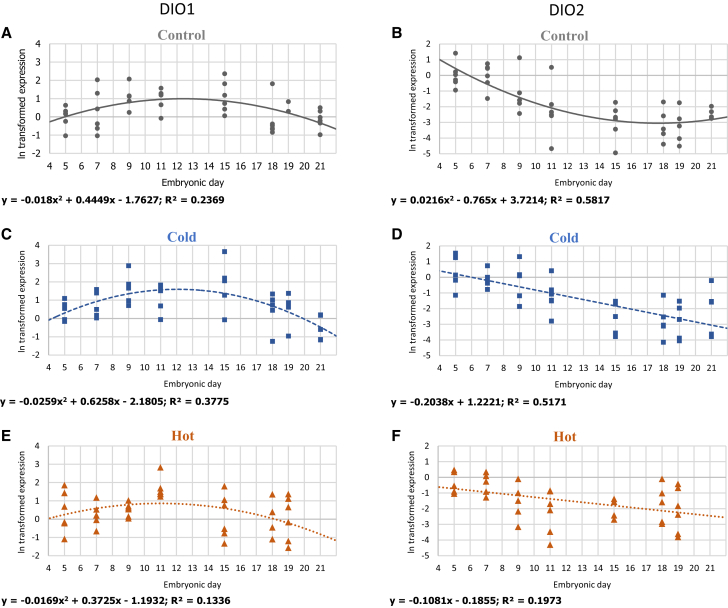
Gene-expression patterns of DIO1 and DIO2 in 3 IT groups: (A, B) control 37.8°C, (C, D) cold 36.3°C, and (E, F) hot 39.3°C. For each ln transformed relative expression, a regression was fitted. Regression considered significant at P ≤ 0.05. n = 6 per age in each IT group.
YST Genes Involved in Nutrients Uptake and Metabolism Are Affected by Incubation Temperature
During the last week of incubation, the main source of energy for the developing embryo is yolk lipids (Noble and Cocchi, 1990, Yadgary et al., 2010). Accordingly, the expression pattern of LRP2, a receptor for a range of lipoproteins (Kozyraki and Gofflot, 2007), increased linearly toward hatch in the YST of embryos from the control and cold IT groups (P < 0.0001 and P = 0.0037, respectively) (Figure 4). In contrast, the hot IT group exhibited a different expression pattern of a quadratic polynomial regression fit (P = 0.015), with an increase in expression between E5 and E11 and a decrease in expression toward hatch. The reduction in expression of LRP2 from E11 onward can explain the reduced yolk utilization that is seen in the hot IT group at E21 (Figure 1). Willemsen et al. (2010) pointed toward a similar pattern of downward shift in carbohydrates and lipid metabolism in response to high IT. This is probably related to the selective absorption of fatty acids from the yolk content exhibited in heat-induced embryos (Yalçin et al., 2008).
After their absorption by the EECs, yolk lipoproteins are intracellularly processed, and newly synthesized lipoproteins are formed. An integral part of these newly synthesized lipoproteins is apolipoproteins, such as ApoA1, that serve as structural protein and a cell surface receptor. Its expression in the YST is a marker for lipoprotein metabolism (Bauer et al., 2013). Significant increased expression of ApoA1 was observed in all IT groups (Figure 4). However, expression in both hot and cold IT groups exhibited a linear fit pattern (P < 0.0001), compared to the quadratic polynomial fit of the control IT group (P = 0.0068), which showed a sharp increase in expression from E18 to E21. These results suggest that the hot and cold ITs perturbed lipid absorption and led to a reduced expression of downstream proteins, such as ApoA1, necessary for further lipid mobilization.
In order to absorb other yolk nutrients, the YST expresses specific nutrient transporters such as PepT1, enabling the uptake of oligopeptides (Yadgary et al., 2011; Speier et al., 2012). In the control IT group, expression of the gene encoding for PepT1 (Figure 5) showed an increase until E15, remaining at similar levels thereafter. Both the hot and cold IT groups showed a similar increase in PepT1 expression peaking at E15. However, unlike the control IT group, they both show a decrease in PepT1 expression toward hatch. In fact, PepT1 expression pattern in the hot IT group simulates LRP2 expression pattern. Barri et al. (2011) showed that intestinal PepT1 expression in chicks incubated at elevated temperature was also lower at DOH. This may relate to overall negative effect of the high IT on protein, carbohydrate, and lipid metabolism during the last days of incubation (Willemsen et al., 2010). Taken together, these results suggest that reduced expression of transporters essential for proteins mobilization into the YST and in the EECs may cause the observed decrease in yolk nutrient utilization (Figure 1).
YST Genes Involved in Gluconeogenesis and Glycogenesis Are Affected by Incubation Temperature
Inside the YST, amino acids and fatty acids absorbed from the yolk can be used immediately for energy through gluconeogenesis (by FBP1) or converted into glycogen stores through glycogenesis (by GYS2) for future use by the embryo and hatchling (Matthews and Holde, 1990; Christensen et al., 2001; Yadgary and Uni, 2012). In the control IT group, the expression pattern of glycogen synthase (GYS2) showed higher levels at E5, and this correlates with the establishment of germ phase, characterized by supply of energy to the embryo via anaerobic glycolysis (Moran, 2007; De Oliveira et al., 2008). Then, a decrease was observed toward E11 and an increase during the second half of incubation (P = 0.0038) with a best-fit polynomial regression (Figure 6). This is in agreement with the findings of Yadgary and Uni (2012), showing low glycogen accumulation at E11 and increased expression at E19 (1.2 mg/g at E11 and 23.2 mg/g at E19), correlating with characteristics of embryo completion and emergence phases (Moran, 2007; De Oliveira et al., 2008).
On the contrary, in the hot and cold IT groups, no significant regression of GYS2 expression was found, indicating that not as in the control IT group, no change in GYS2 expression was found along incubation. This may suggest altered glycogen stores in the yolk and in the YST of embryos and hatchlings from hot and cold IT groups.
Expression of FBP1, which is the key enzyme controlling the overall rate of gluconeogenesis (Pilkis and Granner, 1992), showed the highest expression on E5, followed by a steady decrease in expression toward hatch in the control IT group (Figure 6). These results complement data from our laboratory (Yadgary and Uni, 2012) which showed that the chick embryo's liver exhibited a reverse pattern of FBP1 expression (i.e., increase toward hatch). This suggests that during the first half of incubation (E5 to E11), gluconeogenesis occurs mainly in the YST and not the liver of the embryo. While the hot IT group exhibited a similar expression pattern to the control IT (P = 0.0004), FBP1 expression in the cold IT group showed a mild decrease over time, presenting a better fit to a quadratic polynomial regression (P = 0.0435). This suggests delayed liver development in embryos from the cold IT group.
YST Genes Involved in Thyroid Hormone Regulation Are Affected by Incubation Temperature
From early stages of development, THs (triiodothyronine, T3, and thyroxine, T4) are essential for numerous physiological processes, including regulation of heat production, mobilization of glycogen reserves, and development of almost all embryonic body structures (McNabb and Wilson, 1997a,b). In chicken, potent T3 is the main metabolism-stimulating hormone and was found to be associated with body temperature regulation (McNabb and King, 1993; Gabarrou et al., 1997; Yahav, 2000). Recent work has shown that the YST regulates levels of THs for the embryo (Too et al., 2017). Thus, YST functionality at high or low optimal IT can lead to poorer TH supply that may affect embryonic development and embryo response to different ITs. We, therefore, examined the effect of ITs on the expression of genes encoding for TH regulators, TTR, DIO1, and DIO2 (Figures 7 and 8).
The expression of the gene encoding for TTR, a major TH carrier protein in the plasma, indicates the role of the YST in mediating TH transfer from the yolk to the embryo via the extraembryonic bloodstream. Our results show that TTR was expressed highly in the YST at the first half of incubation (E5-E11), then gradually decreases until DOH (E21), showing best fit with quadratic polynomial regression in all 3 IT groups (Figure 7).
The THs activating and inactivating enzymes DIO1 and DIO2 are also expressed in the YST, indicating its role not only in TH transfer but also in their metabolism. In our experiment, DIO1 showed a slight increase in expression toward mid incubation and a slight decrease thereafter in the control and cold IT groups (P = 0.0027 and 0.0001, respectively), while the hot IT group exhibited almost a constant level of expression with no significant change during the incubation period.
Measuring the expression of DIO2, the main enzyme for local TH activation, that is, T4 to T3 conversion (McNabb and Darras, 2014), showed that DIO2 expression in the control IT group was higher during the first half of incubation (E5–E11), then decreased toward hatch. The cold and hot IT groups showed a slower decrease in expression having a better fit to a linear regression (P = 0.0052 and <0.0001, respectively), rather than the quadratic polynomial fit of the control group (P = 0.0067).
These results indicate the “liver-like” role during incubation of the YST in TH metabolism before the maturation and activity of the liver after hatch.
It should be noticed that data published by Too et al. (2017), who also examined the expression of TTR, DIO1, and DIO2 in the YST of Hy-Line embryos (layer strain), are not similar to the expression patterns data in our study. This can be explained by the physiological differences between the meat-type and egg-type strains as the broiler strains have an increased metabolic rate starting as early as the embryonic stage (Tona et al., 2004; Everaert et al., 2008; Druyan, 2010).
In conclusion, the present study emphasizes the importance of the YST for yolk utilization and for metabolic processes that affect embryos' and hatchlings' quality. The results point toward the YST as a multifunctional metabolic organ with “liver-like” and “intestine-like” functions, which are responsive to IT. High-quality embryos and hatchlings are defined by optimal utilization of yolk, namely, low RSY weight relative to whole body weight (RSY percent of WBM) and also by the nutrient reserves stored in the yolk sac, including glycogen, glucose, fatty acids, and peptides (Yadgary and Uni, 2012; Yadgary et al., 2013; Araujo et al., 2016; Van der Wagt et al., 2020). This study showed that deviation from the optimal IT (e.g., 1.5°C below or above 37.8°C) resulted in altered expression of YST genes encoding for LRP2 (a lipoprotein receptor), ApoA1 (an apolipoprotein, indicator of lipoprotein metabolism), PepT1 (an oligopeptide transporter), GYS2 (glycogen synthase), and FBP1 (a gluconeogenesis regulator gene) which are responsible for glucose and glycogen synthesis and for absorption and digestion of yolk lipids and peptides. Accordingly, RSY weight toward hatch (RSY%) is changed. In addition, this study also showed altered expression of YST genes encoding for THs regulators (TTR, DIO1, and DIO2) during incubation period. As THs are responsible for various processes, including regulation of heat production and mobilization of glycogen reserves (McNabb and Wilson, 1997a,b), changes in their expression may also affect embryo and hatchling quality.
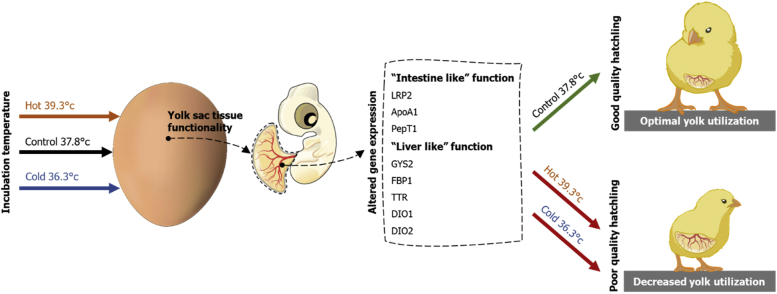
This study is the first to show that ITs have an effect on gene expression in the YST, thereby affecting yolk utilization and yolk sac nutrients resources leading to either poor or improved quality of embryos and hatchlings (Figure 9). Based on these new findings, it is proposed to find the environmental and nutritional factors that can optimize YST functionality during incubation period and will lead to improved hatchery practice and production of better-quality embryos, hatchlings, and growing chickens.
Figure 9.
Graphical illustration demonstrating the effect of incubation temperature on yolk sac tissue functionality, thereby affecting yolk utilization leading to either poor or improved quality of embryos and hatchlings.
Acknowledgements
The authors would like to acknowledge Avigdor Cahaner, Emeritus Professor, the Faculty of Agriculture, Hebrew University of Jerusalem, for his assistance with the statistical analysis and Dana Beutler design studio for the graphical illustration of Figure 9.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Anton M. Composition and structure of hen egg yolk. In: Huopalahti R., López-Fandiño R., Anton M., Schade R., editors. Bioactive Egg Compounds. Springer-Verlag; Heidelberg, Germany: 2007. pp. 1–6. [Google Scholar]
- Araujo I.C.S., Leandro N.S.M., Mesquita M.A., Café M.B., Mello H.H.C., Gonzales E. Effect of incubator type and broiler breeder age on hatchability and chick quality. Braz. J. Poult. Sci. 2016;18:17–25. [Google Scholar]
- Barri A., Honaker C.F., Sottosanti J.R., Hulet R.M., McElroy A.P. Effect of incubation temperature on nutrient transporters and small intestine morphology of broiler chickens. Poult. Sci. 2011;90:118–125. doi: 10.3382/ps.2010-00908. [DOI] [PubMed] [Google Scholar]
- Bartlett M.S. The use of transformations published by: International Biometric Society stable. Society. 1947;3:39–52. [Google Scholar]
- Bauer R., Plieschnig J.A., Finkes T., Riegler B., Hermann M., Schneider W.J. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. J. Biol. Chem. 2013;288:1088–1098. doi: 10.1074/jbc.M112.393090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs R. Studies on the development of the foregut in the chick blastoderm: The presumptive foregut area. 1:115- 124. J. Embryol. Exp. Morphol. 1953 [Google Scholar]
- Brunham M.R., Peebles E.D., Gardner C.W., Brake J., Bruzual J.J., Gerard P.D. Effects of incubator humidity and hen age on yolk composition in broiler hatching eggs from young breeders. Poult. Sci. 2001;80:1444–1450. doi: 10.1093/ps/80.10.1444. [DOI] [PubMed] [Google Scholar]
- Christensen V.L., Wineland M.J., Fasenko G.M., Donaldson W.E. Egg storage effects on plasma glucose and supply and demand tissue glycogen concentrations of broiler embryos. Poult. Sci. 2001;80:1729–1735. doi: 10.1093/ps/80.12.1729. [DOI] [PubMed] [Google Scholar]
- De Oliveira J.E., Uni Z., Ferket P.R. Important metabolic pathways in poultry embryos prior to hatch. Worlds Poult. Sci. J. 2008;64:488–499. [Google Scholar]
- Druyan S. The effects of genetic line (broilers vs. layers) on embryo development. Poult. Sci. 2010;89:1457–1467. doi: 10.3382/ps.2009-00304. [DOI] [PubMed] [Google Scholar]
- Duval M. 1889. Atlas D'embryologie. Paris, France: G. Masson. [Google Scholar]
- Everaert N., Willemsen H., De Smit L., Witters A., De Baerdemaeker J., Decuypere E., Bruggeman V. Comparison of a modern broiler and layer strain during embryonic development and the hatching process. Br. Poult. Sci. 2008;49:574–582. doi: 10.1080/00071660802357025. [DOI] [PubMed] [Google Scholar]
- Freeman B.M., Vince M.A. Chapman and Hall; London, UK: 1974. Development of the Avian Embryo. [Google Scholar]
- Gabarrou J.F., Duchamp C., Williams J., Géraert P.A. A role for thyroid hormones in the regulation of diet-induced thermogenesis in birds. Br. J. Nutr. 1997;78:963–973. doi: 10.1079/bjn19970212. [DOI] [PubMed] [Google Scholar]
- Hamidu J.A., Torres C.A., Johnson-Dahl M.L., Korver D.R. Physiological response of broiler embryos to different incubator temperature profiles and maternal flock age during incubation. 1. Embryonic metabolism and day-old chick quality. Poult. Sci. 2018;97:2934–2946. doi: 10.3382/ps/pey089. [DOI] [PubMed] [Google Scholar]
- Joseph N.S., Lourens A., Moran E.T., Jr. The effects of suboptimal eggshell temperature during incubation on broiler chick quality, live performance, and further processing yield. Poult. Sci. 2006;85:932–938. doi: 10.1093/ps/85.5.932. [DOI] [PubMed] [Google Scholar]
- Kozyraki R., Gofflot F. Multiligand endocytosis and congenital defects: roles of cubilin, megalin and amnionless. Curr. Pharm. Des. 2007;13:3038–3046. doi: 10.2174/138161207782110507. [DOI] [PubMed] [Google Scholar]
- Lin Y.M., Druyan S., Yahav S., Brake J. Thermal treatments prior to and during the beginning of incubation affects development of the broiler embryo and yolk sac membranes, and live performance and carcass characteristics. Poult. Sci. 2017;96:1939–1947. doi: 10.3382/ps/pew467. [DOI] [PubMed] [Google Scholar]
- Matthews C.K., Holde K.E. Biochemistry. The Benjamin/Cummings Publishing Company, Inc.; Redwood City, CA: 1990. Carbohydrate metabolism I: anaerobic processes in generating metabolic energy; pp. 670–703. [Google Scholar]
- McNabb F.M.A., Darras V.M. Thyroids. In: Scanes C.G., editor. Sturkie's Avian Physiology. 6th ed. Academic Press; Cambridge, MA: 2014. pp. 535–547. [Google Scholar]
- McNabb F.A., King D.B. Thyroid hormone effects on growth, development, and metabolism. In: Schreibman M.P., Scanes C.G., Pang P.K.T., editors. The Endocrinology of Growth Development and Metabolism in Vertebrates. Academic Press; Cambridge, MA: 1993. pp. 873–885. [Google Scholar]
- McNabb F.M.A., Wilson C.M. Thyroid hormone deposition in avian eggs and effects on embryonic development. Am. Zool. 1997;37:553–560. [Google Scholar]
- McNabb F.M.A., Wilson C.M. Thyroid hormones in quail eggs and their effects on embryonic development. Adv. Comp. Endocrinol. 1997;165:1079–1081. doi: 10.1006/gcen.1997.6906. [DOI] [PubMed] [Google Scholar]
- Mobbs I.G., McMillan D.B. Structure of the endodermal epithelium of the chick yolk sac during early stages of development. Am. J. Anat. 1979;155:287–309. doi: 10.1002/aja.1001550302. [DOI] [PubMed] [Google Scholar]
- Mobbs I.G., McMillan D.B. Transport across endodermal cells of the chick yolk sac during early stages of development. Am. J. Anat. 1981;160:285–308. doi: 10.1002/aja.1001600307. [DOI] [PubMed] [Google Scholar]
- Moran E., Jr. Nutrition of developing embryo and hatching. Poult. Sci. 2007;86:1043–1049. doi: 10.1093/ps/86.5.1043. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., van den Anker I., Heetkamp M.J.W., De Souza Morita V., Kemp B., van den Brand H. Effects of breeder age, strain, and eggshell temperature on nutrient metabolism of broiler embryos. Poult. Sci. 2016;95:1666–1679. doi: 10.3382/ps/pew417. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Cocchi M. Lipid metabolism and the neonatal chicken. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-c. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Ogunyemi D. Lipid changes in the residual yolk and liver of the chick immediately after hatching. Biol. Neonate. 1989;56:228–236. doi: 10.1159/000243127. [DOI] [PubMed] [Google Scholar]
- Palis J.,K., McGrath E., Kingsley P.D. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- Pfaffl M.W. Taylor and Francis; Abingdon, UK: 2007. Relative Quantification. Pages 89–108 in Real-Time PCR. [Google Scholar]
- Piestun Y., Shinder D., Ruzal M., Halevy O., Brake J., Yahav S. Thermal manipulations during broiler embryogenesis: effect on the acquisition of thermotolerance. Poult. Sci. 2008;87:1516–1525. doi: 10.3382/ps.2008-00030. [DOI] [PubMed] [Google Scholar]
- Pilkis S.J., Granner D.K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- Réhault-Godbert S., Nicolas G., Nys Y. The golden egg: nutritional value, bioactivities, and emerging benefits for human health. Nutrients. 2019;11:684. doi: 10.3390/nu11030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanoff A.L. Effects of different temperatures in the incubator on the prenatal and postnatal development of the chick. Poult. Sci. 1935;15:311–315. [Google Scholar]
- Romanoff A.L. The Avian Embryo: Structural and Functional Development. The Macmillan Company; New York, NY: 1960. The extraembryonic membranes; pp. 1041–1140. [Google Scholar]
- Romanoff A.L. Wiley; New York, NY: 1967. Biochemistry of the Avian Embryo. [Google Scholar]
- Sahan U., Ipek A., Sozcu A. Yolk sac fatty acid composition, yolk absorption, embryo development, and chick quality during incubation in eggs from young and old broiler breeders. Poult. Sci. 2014;93:2069–2077. doi: 10.3382/ps.2013-03850. [DOI] [PubMed] [Google Scholar]
- Schindelin J.I., Arganda-Carreras E., Frise V., Kaynig M., Longair T., Pietzsch S., Preibisch C., Rueden S., Saalfeld B., Schmid J., Tinevez D.J., White V., Hartenstein K., Eliceiri P., Eliceiri P. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G. Primitive and definitive erythropoiesis in the yolk sac: a bird’s eye view. Int. J. Dev. Biol. 2010;54:1033–1043. doi: 10.1387/ijdb.103105gs. [DOI] [PubMed] [Google Scholar]
- Speier J.S., Yadgary L., Uni Z., Wong E.A. Gene expression of nutrient transporters and digestive enzymes in the yolk sac membrane and small intestine of the developing embryonic chick. Poult. Sci. 2012;91:1941–1949. doi: 10.3382/ps.2011-02092. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O.M., Jego Y., Kamers B., Decuypere E., Bruggeman V. Comparison of embryo physiological parameters during incubation, chick quality, and growth performance of three lines of broiler breeders differing in genetic composition and growth rate. Poult. Sci. 2004;83:507–513. doi: 10.1093/ps/83.3.507. [DOI] [PubMed] [Google Scholar]
- Too H.C., Shibata M., Yayota M., Darras V.M., Iwasawa A. Expression of thyroid hormone regulator genes in the yolk sac membrane of the developing chicken embryo. J. Reprod. Dev. 2017;63:463–472. doi: 10.1262/jrd.2017-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2161–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Kamers B., Dahlke F., Han H., Song Z., Ansari Pirsaraei Z., Tona K., Decuypere E., Everaert N. High-and low-temperature manipulation during late incubation: effects on embryonic development, the hatching process, and metabolism in broilers. Poult. Sci. 2010;89:2678–2690. doi: 10.3382/ps.2010-00853. [DOI] [PubMed] [Google Scholar]
- Yadgary L.,A., Cahaner O., Kedar and Z., Uni Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010;89:2441–2452. doi: 10.3382/ps.2010-00681. [DOI] [PubMed] [Google Scholar]
- Yadgary L.,O., Kedar O., Adepeju and Z., Uni Changes in yolk sac membrane absorptive area and fat digestion during chick embryonic development. Poult. Sci. 2013;92:1634–1640. doi: 10.3382/ps.2012-02886. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Uni Z. Yolk sac carbohydrate levels and gene expression of key gluconeogenic and glycogenic enzymes during chick embryonic development. Poult. Sci. 2012;91:444–453. doi: 10.3382/ps.2011-01669. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Wong E.A., Uni Z. Temporal transcriptome analysis of the chicken embryo yolk sac. BMC Genomics. 2014;15:690. doi: 10.1186/1471-2164-15-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadgary L., Yair R., Uni Z. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult. Sci. 2011;90:410–416. doi: 10.3382/ps.2010-01075. [DOI] [PubMed] [Google Scholar]
- Yahav S. Domestic fowl-strategies to confront environmental conditions. Poult. Avian Biol. Rev. 2000;11:81–95. [Google Scholar]
- Yahav S. Body temperature: strategies and mechanisms. In: Scanes C.G., editor. Sturkie's Avian Physiology. 6th ed. Academic Press; Cambridge, MA: 2014. pp. 869–905. [Google Scholar]
- Yalçin S., Çabuk M., Bruggeman V., Babacanoğlu E., Buyse J., Decuypere E., Siegel P.B. Acclimation to heat during incubation. 2. Embryo composition and residual egg yolk sac fatty acid profiles in chicks. Poult. Sci. 2008;87:1229–1236. doi: 10.3382/ps.2007-00436. [DOI] [PubMed] [Google Scholar]
- Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki N., Soga M., Ito Y., Mao K.M., Sultana F., Yonezawa S. Two-step consumption of yolk granules during the development of quail embryos. Dev. Growth Differ. 2004;46:229–238. doi: 10.1111/j.1440-169X.2004.00740.x. [DOI] [PubMed] [Google Scholar]