Abstract
In this study, we evaluated the prevalence of Salmonella in retail raw chickens in Shaanxi Province, China, on a monthly basis. In addition, we studied the antibiotic susceptibility, serotype, and genotype of Salmonella isolates and explored their relationships with sampling time, location, market type, and chicken type. The results showed that Salmonella was more prevalent in chickens sampled during the spring and summer than during the autumn and winter. Thirty-nine serotypes were identified from 406 Salmonella isolates, of which Salmonella typhimurium (16.7%) was the most prevalent. Other prevalent serotypes included S. thompson (12.8%), S. essen (9.1%), S. infantis (6.9%), S. rissen (5.7%), and S. enteritidis (5.4%). Approximately 71.4% of the 406 isolates were resistant to 3 or more antibiotics, 11.8% to 12 or more, and 1.7% to all 14 antibiotics tested. The most frequently detected resistance was to trimethoprim/sulfamethoxazole (82.0%), followed by nalidixic acid (71.9%) and tetracycline (59.4%). The frequencies of resistance to ampicillin, chloramphenicol, and amoxicillin/clavulanic acid were moderately high (∼50% each). Resistance to kanamycin, ceftiofur, streptomycin, gentamicin, and ciprofloxacin was less common (<40% each). Serotype distribution and antibiotic susceptibility of Salmonella isolates were related to sampling time, location, chicken type, and market type. Isolates recovered from the same sampling time, market type, location, and chicken type commonly exhibited identical or similar genotypes and antibiotic resistance profiles. However, DNA profiles and antibiotic resistance phenotypes of isolates within some serotypes were diverse. Our results revealed that multiple Salmonella subtypes with antibiotic resistance were prevalent in retail raw chickens in Shaanxi Province. Our study findings provide information for developing preventive measures against contamination of retail foods with Salmonella.
Key words: Salmonella, retail chicken, prevalence, antibiotic resistance, genotype
Introduction
Food safety and public health associated with antibiotic-resistant pathogens represent major challenges in China (Wang et al., 2019a,b). Among the foodborne pathogens, Salmonella causes serious human diseases and disseminates through the food supply, foods, humans, and animals. It has been estimated that ∼1,000,000 Salmonella infections and 378 deaths occur each year in the United States (Scallan et al., 2011). In China, 70%–80% of foodborne bacterial outbreaks are attributed to Salmonella infection (Wang and Zheng, 2007).
Salmonella has been detected in retail foods including chicken, pork, beef, ground meat, ice cream, dumplings, vegetables, powdered infant milk, fish, and ready-to-eat products. Among these foods, retail chicken is the most common carrier of Salmonella (White et al., 2001; Gatto et al., 2006; Nhung et al., 2018). Past studies have reported that chopping boards, butchers' hands, and knives used for retail chicken processing constitute potential sources for Salmonella cross-contamination (Olsen et al., 2003; Suresh et al., 2004).
Antibiotic resistance of Salmonella, which is closely linked to the abuse and misuse of antibiotics in food animal and humans, is increasing worldwide (Van et al., 2003; Gilchrist et al., 2007). Surveillance data have demonstrated a noticeable increase in overall antibiotic resistance of Salmonella from 20% to 30% in the early 1990s to nearly 70% in the 2000s (Su et al., 2004). In recent years, an alarming emergence of multidrug-resistant (MDR) Salmonella has been reported in several European and Asian countries including China (Isenbarger et al., 2002; Cailhol et al., 2006; Xia et al., 2009; Feasey et al., 2015; Wong et al., 2015).
The prevalence and antibiotic susceptibility of Salmonella in retail foods such as meats, dumplings, and cold dishes have been reported in different regions of China (Yang et al., 2011, 2014; Hu et al., 2017; Wang et al., 2017). Moreover, Salmonella isolates recovered from retail meats in Shaanxi Province, China, have been characterized (Yang et al., 2010), but the monthly prevalence of Salmonella in retail raw chickens in this region remains poorly understood. In the present study, we evaluated the prevalence of Salmonella in retail chickens in Shaanxi Province on a monthly basis. In addition, we characterized 406 Salmonella isolates to better understand their serotype distribution and antibiotic susceptibility in retail foods.
Materials and methods
Retail Raw Chicken Sampling
We collected 20 retail raw chicken carcasses each month from April to October of 2011 and from January to March of 2012 in Shaanxi Province, China (Supplementary Table 1). The 20 samples included 10 freshly slaughtered chickens from 4 different wet markets (2 slaughter houses were selected in each wet market in each location, and 1 to 2 chicken carcasses were randomly sampled in each slaughter house); and 10 chickens (5 chilled and 5 frozen) from 4 different supermarkets (one supermarket was selected in each location, and 1 to 2 chilled/frozen chicken carcasses were selected in each supermarket without consideration of the brand of the chicken) across 4 different locations (Yangling, Zhouzhi, Wugong, and Baoji). A total of 200 retail raw chicken carcasses were sampled over a 10-month period. Each sample was aseptically placed in a sterile stomacher bag (Seward, UAC House, London, UK), labeled, and transported in an ice-cooler to the laboratory within 4 h.
Salmonella Isolation and Identification
The prevalence of Salmonella in the samples was quantitatively assessed using the most probable number method developed by the Food Safety and Inspection Service of the United States Department of Agriculture with minor modifications (USDA/FSIS, 2014). Briefly, after the chicken carcass was weighed (ranged from 1.0 kg to 1.8 kg), 400-fold (mL) of sterilized buffered peptone water (BPW; BD, Sparks, MD) of the chicken's weight (kg) was added to the stomacher bag. The carcass was manually rinsed for at least 10 min to ensure BPW was in contact with the external and internal surfaces of the carcass. Subsequently, 1.0 mL of the BPW rinse was transferred to 3 tubes containing 9.0 mL of BPW each, and 0.1 mL of the BPW rinse was transferred to 3 other tubes containing 9.9 mL of BPW each.
The inoculated tubes were incubated at 35 ± 2°C for 20–24 h. Then 10 mL of tetrathionate broth (TT; BD) and 10 mL of modified Rappaport Vassiliadis broth (mRV; BD) were inoculated with 0.5 ± 0.05 mL and 0.1 ± 0.02 mL of the cultures, respectively. The TT and mRV broths were incubated at 42 ± 0.5°C under constant shaking (100 rpm) for 22–24 h. After incubation, a loopful of TT and mRV cultures were streaked onto xylose-lysine-tergitol-4 agar (XLT4; BD) plates. The XLT4 plates were incubated at 35 ± 2°C for 22–24 h. One presumptive Salmonella colony from each XLT4 plate was transferred onto a fresh XLT4 plate. Salmonella colonies were identified and confirmed using the API20 E test kit (bioMérieux, Inc., Hazelwood, MO).
Salmonella Serotyping
Salmonella isolates were serotyped in the Henan Center for Disease Control and Prevention (Zhengzhou, Henan, China). O and H antigens were detected by the slide agglutination method using Salmonella-specific hyperimmune sera (Statens Serum Institute, Artillerivej, Denmark). Serotype was determined following the manufacturer's instructions and White-Kauffmann classification scheme (Kauffmann, 2010).
Antibiotic Susceptibility Test
Antibiotic susceptibility of Salmonella isolates was evaluated by the agar dilution method using Mueller-Hinton agar (Land Bridge Technology Co., Ltd., Beijing, China) according to the protocol reported by the Clinical and Laboratory Standard Institute (CLSI, 2014). Fourteen antibiotics that are monitored by the National Antimicrobial Resistance Monitoring System (http://www.fda.gov/downloads/Animal Veterinary/Safety Health/Antimicrobial Resistance/National Antimicrobial Resistance Monitoring System/UCM334834.pdf) were tested (National Antimicrobial Resistance Monitoring System, 2014). The antibiotics were nalidixic acid, ciprofloxacin, ceftriaxone, cefoxitin, ceftiofur, gentamicin, kanamycin, amikacin, streptomycin, ampicillin, amoxicillin/clavulanic acid, chloramphenicol, trimethoprim/sulfamethoxazole, and tetracycline. Their concentration ranges are listed in Table 1. All antibiotics were acquired from Sigma St. (Louis, MO).
Table 1.
Antibiotic resistance of Salmonella isolates recovered from retail raw chickens in different months.
| Antibiotic | Concentration range (μg/mL) | Total no. (%) | % Resistance to individual antibiotics |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 |
2012 |
|||||||||||
| April (n = 83) | May (n = 94) | June (n = 13) | July (n = 76) | August (n = 32) | September (n = 47) | October (n = 4) | January (n = 3) | February (n = 4) | March (n = 50) | |||
| Trimethoprim/sulfamethoxazole | 0/0–8/152 | 333 (82.0) | 45.8d | 93.6b | 100a | 94.7b | 87.5b | 70.2c | 100a | 100a | 100a | 100a |
| Nalidixic acid | 0–64 | 292 (71.9) | 34.9c | 99a | 100a | 90.8b | 43.8c | 38.3c | 100a | 100a | 98.0a | |
| Tetracycline | 0–32 | 241 (59.4) | 57.8c | 86.2b | 38.5 | 82.9b | 56.3c | 36.2 | 100a | 75.0b | 4.0 | |
| Ampicillin | 0–64 | 219 (53.9) | 25.3 | 88.3b | 30.8 | 76.3c | 75.0c | 36.2 | 100a | 100a | 75.0c | 4.0 |
| Amoxicillin/clavulanic acid | 0/0–64/32 | 212 (52.2) | 25.3 | 86.2b | 38.5 | 69.7c | 71.9c | 34.0 | 100a | 100a | 100a | 4.0 |
| Chloramphenicol | 0–64 | 212 (52.2) | 21.7 | 89.4b | 15.4 | 68.4c | 43.8 | 72.3c | 100a | 75.0c | 2.0 | |
| Kanamycin | 0–128 | 158 (38.9) | 22.9 | 76.6a | 23.1 | 32.9 | 65.6a | 34.0 | 50.0b | |||
| Ceftiofur | 0–128 | 154 (37.9) | 20.5 | 69.1c | 14.5 | 18.8 | 17.0 | 100a | 33.3 | 25.0 | 82.0b | |
| Streptomycin | 0–64 | 136 (33.5) | 18.1c,d | 83b | 15.4d | 27.6c | 21.9c,d | 17.0c,d | 25.0c,d | 100a | 2.0 | |
| Gentamicin | 0–32 | 116 (28.6) | 19.3c | 41.5a,b | 7.7 | 32.9b | 53.1a | 34.0b | 25.0b,c | 2.0 | ||
| Ciprofloxacin | 0–16 | 90 (22.2) | 25.3b,c | 47.9a | 7.7 | 1.3 | 18.8c | 31.9b | 25.0b,c | |||
| Amikacin | 0–128 | 80 (19.7) | 15.7c | 26.6b,c | 13.2c | 53.1a | 29.8b | 2.0 | ||||
| Ceftriaxone | 0–128 | 73 (18.0) | 19.3a,b | 29.9a | 15.8b | 3.1 | 29.8a | 4.0 | ||||
| Cefoxitin | 0–64 | 70 (17.2) | 1.2 | 18.1d | 23.7c,d | 43.8b | 29.8c | 100a | 4.0 | |||
Values sharing the same superscript letter in each row are not significantly different (P > 0.05).
Escherichia coli ATCC 25922 and Enterococcus faecalis ATCC 29212 were used as quality-control microorganisms for the determination of minimum inhibitory concentrations of antibiotics. For the interpretative breakpoints of resistance and susceptibility, we followed CLSI standards (CLSI, 2014) for most antibiotics. An exception was streptomycin, for which we used that in National Antimicrobial Resistance Monitoring System.
Pulsed-Field Gel Electrophoresis
The genetic relatedness of Salmonella isolates was determined using pulsed-field gel electrophoresis (PFGE) combined with restricted enzyme digestion according to the protocol reported by the United States Center for Disease Control and Prevention (Ribot et al., 2006). Briefly, Salmonella cells were embedded in agarose and lysed. The genomic DNAs were digested using XbaI (TaKaRa, Dalian, China) for 1.5–2 h at 37°C in a water bath. DNA digests in 0.5 × Tris-borate-EDTA buffer were subsequently separated at 14°C for 19 h using a Chef Mapper electrophoresis system (Bio-Rad, Hercules, CA) with a pulse time of 2.16–63.8 s. After staining of the PFGE gel with ethidium bromide, the DNA bands were visualized in an UV trans-illumination imaging system (Bio-Rad). The genetic relatedness of the isolates was assessed using the BioNumerics software (version 3.0; Applied-Maths, Kortrijk, Belgium) with different ban coefficients and single linkage clustering. Salmonella Braenderup H9812 was used as a positive standard control for PFGE.
Statistical Analysis
Differences among the detection rates of Salmonella-positive samples, serotypes, and antibiotic-resistant isolates in different months were determined by chi-square test using Minitab v16.2.3 (Minitab Inc., State College, PA). Significant difference and extreme significant difference were set at P < 0.05 and P < 0.01, respectively. The relationship between Salmonella serotype and antibiotic resistance, sampling time, location, market type, and chicken type was determined by redundancy analysis using CANOCO v5.0 (Microcomputer Power, Ithaca, NY). The relationship between antibiotic resistance and the aforementioned factors was also determined by redundancy analysis. Descriptive and comparative analyses of the total number of serotypes and antibiotic resistance phenotypes in each month were performed using Graph Pad Prism v7.0 (GraphPad Software, La Jolla, CA). A Sankey plot was generated to visualize the distribution of Salmonella serotypes in retail raw chickens using the networkD3 library in R v3.6.2 (network D3:D3 JavaScript Network GRAPHS from R; https://rdocumentation.org/).
Results
Salmonella Prevalence and Isolate Recovery
Among the 200 retail raw chicken samples, 93 (46.5%) were Salmonella-positive. In general, Salmonella-positive chickens were not prevalent in October, January, or February but were frequently detected in April and May. The detection rates of Salmonella-positive chickens were higher in April (P < 0.01) and May (P < 0.05) than in June, October, January, and February (Figure 1, Supplementary Table 1). Using the most probable number procedure, a total of 406 Salmonella-positive TT and mRV cultures were obtained from 3,600 TT and mRV tubes. From each TT- and mRV-XLT4 plate, one isolate was selected for further characterization. A total 406 Salmonella isolates were obtained in this study.
Figure 1.
Prevalence of Salmonella in retail raw chickens in Shaanxi province, China (2011 and 2012).
Serotype Distribution
Thirty-nine serotypes were identified from the 406 Salmonella isolates. The 6 most prevalent serotypes were S. typhimurium (16.7%), S. thompson (12.8%), S. essen (9.1%), S. infantis (6.9%), S. rissen (5.7%), and S. enteritidis (5.4%; Figure 2, Supplementary Table 2). S. typhimurium was only detected in retail chickens collected from May through July of 2012. The detection rates of S. typhimurium were higher (P < 0.01) in May (58.5%) and June (61.5%) than in July (6.6%). S. thompson was detected in 6 mo, and the detection rate was higher (P < 0.05) in October (50.0%) than in the other 5 mo. The detection rates of S. essen (32.0%), S. rissen (30.0%), and S. enteritidis (30.0%) were higher (P < 0.01) in March than in the other months. For S. infantis isolates, there were no significant differences in detection rates between July and October; however, the detection rates were higher (P < 0.01) in these 2 mo than in April or May.
Figure 2.
Distribution of 39 Salmonella serotypes identified in retail raw chickens in different months.
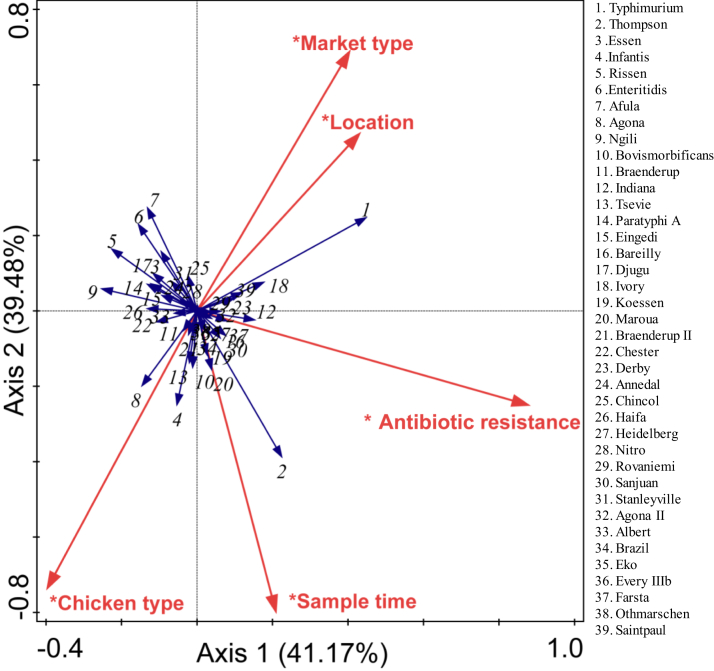
The distribution of Salmonella serotypes was related to location, market type, sampling time, and chicken type. For example, the distribution of S. typhimurium was positively correlated with market type and location, whereas a negative correlation was observed with chicken type. The distribution of S. rhompson was positively correlated with sampling time and chicken type and negatively correlated with market type and location (Figure 3).
Figure 3.
Effects of sampling time, location, market type, and chicken type on Salmonella serotype distribution in retail raw chickens (∗P < 0.01 in “mantel test”).
Antibiotic Susceptibility
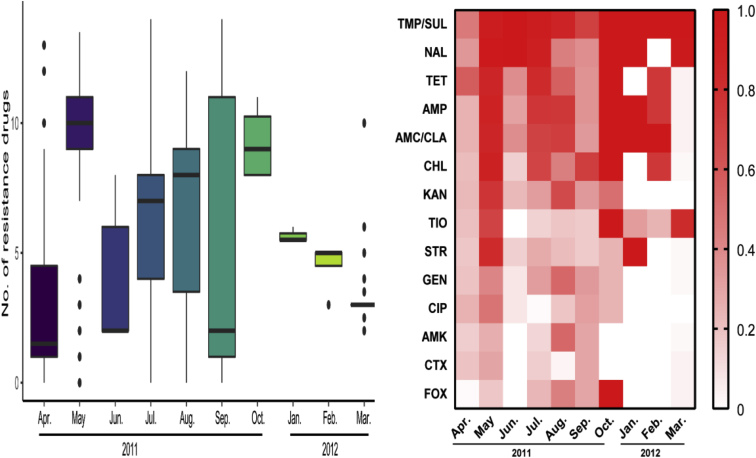
Twenty-nine (7.1%) isolates were susceptible to the 14 antibiotics tested, whereas 377 (92.8%) isolates were resistant to at least one antibiotic, 290 (71.4%) to 3 or more, 48 (11.8%) to 12 or more, and 7 (1.7%) to all 14 antibiotics tested (Table 1). The most frequently detected resistance was to trimethoprim/sulfamethoxazole (82.0%), followed by nalidixic acid (71.9%) and tetracycline (59.4%). Nearly half of the isolates displayed resistance to ampicillin (53.9%), chloramphenicol (52.2%), and amoxicillin/clavulanic acid (52.2%). Resistance to the other antibiotics was less common, ranging from 38.9% (kanamycin) to 17.2% (cefoxitin).
Isolates recovered from May, July, August, and October of 2011 and January and February of 2012 were relatively resistant to multiple antibiotics, whereas those recovered from the other 4 mo were relatively susceptible to the antibiotics tested (Figure 4, Table 1). The detection rates of isolates resistant to ≥3 categories of antibiotics in May (95.7%), July (96.1%), August (75.0%) of 2011 and March (84.0%) of 2012 were higher (P < 0.01) than those in April (27.7%), June (38.5%), and September (42.6%) of 2011 and lower (P < 0.05) than those in October (100%) of 2011 and January (100%) and February (100%) of 2012 (Table 2).
Figure 4.
Antibiotic susceptibility of Salmonella isolates recovered from retail raw chickens in different months. Abbreviations: AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
Table 2.
Multidrug resistance monthly observed among Salmonella isolates recovered from retail raw chickens and their main antibiotic resistance profile.
| Year | Month (isolate no.) | % Resistance to indicated category of antibiotics |
|||
|---|---|---|---|---|---|
| <3 | 3–5 | 6–7 | Total (≥3) | ||
| 2011 | April (n = 83) | 72.3a | 6.0e | 21.7d,e | 27.7e |
| May (n = 94) | 4.3d | 6.4e | 89.4b | 95.7b | |
| June (n = 13) | 61.5a,b | 23.1d | 15.4e | 38.5d,e | |
| July (n = 76) | 3.9d | 51.3c | 44.7c,d | 96.1b | |
| August (n = 32) | 25.0c | 28.1d | 46.9c | 75.0c | |
| September (n = 47) | 57.4b | 10.6e | 31.9d | 42.6d | |
| October (n = 4) | 100a | 100a | |||
| 2012 | January (n = 3) | 100a | 100a | ||
| February (n = 4) | 100a | 100a | |||
| March (n = 50) | 16.0c | 82.0b | 2.0f | 84.0c | |
Values sharing the same superscript letter in each column are not significantly different (P > 0.05).
Resistance to different antibiotics was closely related to various factors. For example, resistance to cefoxitin, amoxicillin/clavulanic acid, ampicillin, chloramphenicol, kanamycin, streptomycin, and tetracycline was most correlated with sampling time and location, followed by chicken type. In addition, resistance to gentamicin, ceftriaxone, amikacin, and ciprofloxacin was most correlated with sampling time, followed by market type. However, resistance to nalidixic acid and ceftiofur was not correlated with any factors (Figure 5).
Figure 5.
Effects of sampling time, location, market type, and chicken type on antibiotic resistance of Salmonella in retail raw chickens (∗P < 0.01 in “mantel test”). Abbreviations: AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
PFGE Subtyping
In total, 203 isolates belonged to the 6 most prevalent Salmonella serotypes, S. typhimurium, S. thompson, S. essen, S. infantis, S. rissen, and S. enteritidis. These 6 serotypes were further subtyped using PFGE. Sixty S. typhimurium isolates, which presented eight different PFGE patterns (DNA profiles), were grouped into 5 clusters (C-1 to C-5) with 96% similarity. Among them, isolates recovered from samples collected at the same location and sampling time were grouped in the same cluster. For example, 32 isolates in C-2, which were derived from samples collected in wet markets from Zhouzhi in May of 2011, shared similar PFGE patterns (Figure 6).
Figure 6.
Dendrogram of PFGE profiles and antibiotic resistance phenotypes of 60 Salmonella typhimurium isolates recovered from retail raw chickens. Abbreviations: Location–Y: Yangling; Z: Zhouzhi. Market type–S: supermarket; F: wet market. Antibiotic–AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; PFGE, pulsed-field gel electrophoresis; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
Even though some S. typhimurium isolates were recovered from samples collected from different locations and market types in the same month, they commonly presented the same or similar DNA profiles. For example, 4 isolates in C-1 were recovered from 2 samples collected from different locations and market types in July; 3 of them showed the same DNA profiles, and the other isolate was similar to the first 3 isolates. Similar findings were obtained for C-5 (Figure 6). Moreover, S. typhimurium isolates with the same or similar DNA profiles commonly exhibited the same or similar antibiotic resistance phenotypes except for some isolates in C-5 (Figure 6). The same or similar PFGE patterns and antibiotic susceptibility of isolates recovered from different locations and months indicated the potential long-term existence or cross-contamination of S. typhimurium in raw chickens and the possibility of foodborne outbreaks.
The same or similar DNA profiles and antibiotic resistance phenotypes were obtained for S. rissen (Figure 7) and S. enteritidis isolates (Figure 8). DNA profiles of S. essen isolates recovered from the same month, location, and market type were also the same or similar, but their antibiotic resistance phenotypes were not consistent (Figure 9). S. thompson and S. infantis isolates (Figures 10 and 11) had more diverse DNA profiles and antibiotic resistance profiles than S. typhimurium, S. rissen, S. enteritidis, and S. essen isolates (Figure 6, Figure 7, Figure 8, Figure 9).
Figure 7.
Dendrogram of PFGE profiles and antibiotic resistance phenotypes of 22 Salmonella rissen isolates recovered from retail raw chickens. Abbreviations: Location–Y: Yangling; Z: Zhouzhi; W: Wugong. Market type–S: supermarket; F: wet market. Antibiotic–AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; PFGE, pulsed-field gel electrophoresis; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
Figure 8.
Dendrogram of PFGE profiles and antibiotic resistance phenotypes of 20 Salmonella enteritidis isolates recovered from retail raw chickens. Abbreviations: Location–Y: Yangling; Z: Zhouzhi; W: Wugong. Market type–S: supermarket; F: wet market. Antibiotic–AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; PFGE, pulsed-field gel electrophoresis; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
Figure 9.
Dendrogram of PFGE profiles and antibiotic resistance phenotypes of 36 Salmonella essen isolates recovered from retail raw chickens. Abbreviations: Location–Y: Yangling; Z: Zhouzhi. Market type–S: supermarket; F: wet market. Antibiotic–AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; PFGE, pulsed-field gel electrophoresis; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
Figure 10.
Dendrogram of PFGE profiles and antibiotic resistance phenotypes of 38 Salmonella thompson isolates recovered from retail raw chickens. Abbreviations: Location–Y: Yangling; Z: Zhouzhi; W: Wugong. Market type–S: supermarket; F: wet market. Antibiotic–AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; PFGE, pulsed-field gel electrophoresis; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
Figure 11.
Dendrogram of PFGE profiles and antibiotic resistance phenotypes of 27 Salmonella infantis isolates recovered from retail raw chickens. Abbreviations: Location–Y: Yangling; Z: Zhouzhi; W: Wugong. Market type–S: supermarket; F: wet market. Antibiotic–AMC/CLA, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; CHL, chloramphenicol; CIP, ciprofloxacin; CTX, ceftriaxone; FOX, cefoxitin; GEN, gentamicin; KAN, kanamycin; NAL, nalidixic acid; PFGE, pulsed-field gel electrophoresis; TET, tetracycline; TIO, ceftiofur; TMP/SUL, trimethoprim/sulfamethoxazole; STR, streptomycin.
Discussion
In this study, we characterized Salmonella isolates recovered from retail raw chickens purchased in wet markets (freshly slaughtered) and supermarkets (chilled and frozen) in Shaanxi Province on a monthly basis. We found that Salmonella was more prevalent in chicken carcasses in spring and summer than during autumn and winter and considered the possible reasons: 1) Salmonella can grow in a wide temperature range (5-47°C), as the temperature gradually reaches to the optimal growth temperature of Salmonella, its reproduction rate will accelerate, and it is easier to survive on the surface of chicken carcass (Oscar, 2009; Smadi et al., 2012). In spring, the temperature in Shaanxi is around 28°C to 35°C; in summer, it is around 35°C to 40°C, that is, the optimal temperature for Salmonella to grow; 2) in Shaanxi, it is often windy in spring and facilitated the prevalence of Salmonella in the environment. Meanwhile, we obtained 406 Salmonella isolates that belonged to 39 serotypes. Among them, S. typhimurium was the most prevalent. This serotype, one of the most important worldwide, contributes to deaths in young broiler chickens and salmonellosis in humans (Padron, 1990; Alban et al., 2002; Loharikar et al., 2013). S. typhimurium has been detected in a wide range of poultry- and animal-derived foods such as retail chickens and pigs from various market types (i.e., wet markets and supermarkets) and animal products stored at various temperatures (i.e., ambient, chilled, and frozen; Humphrey et al., 2000; Yang et al., 2010; Yang et al., 2011; Campos et al., 2012; Yang et al., 2014).
Prior studies conducted in Africa and North America have revealed that S. typhimurium is the most common serotype in cattle and chickens (Thung et al., 2016; Gutema et al., 2019). In our study region, S. typhimurium was also the predominant serotype in retail raw chickens, followed by S. thompson, S. essen, S. infantis, S. riseen, and S. enteritidis. Our results were in accordance with the findings of several studies carried out in Henan and Shannxi, China (Xia et al., 2009; Yang et al., 2011). However, S. thompson (48.7%) has been frequently detected in chickens and giblets in Iran (Sodagari et al., 2015). In Asia, Europe, and Latin America, S. enteritidis is more prevalent than S. typhimurium and other serotypes in chickens (Humphrey et al., 2000; Olsen et al., 2001; Herikstad et al., 2002; Bangtrakulnonth et al., 2004; Galanis et al., 2006). In addition, several serotypes including S. derby and S. heidelberg have been detected in chickens and other foods in several countries of Southeast Asia, while these are rarely identified in the Shaanxi province (Humphrey et al., 2000; Olsen et al., 2001; Herikstad et al., 2002; Bangtrakulnonth et al., 2004; Galanis et al., 2006). It is possible that the lack of agreement between our results and those of previous studies are due to the differences in sample type, location, detection method, sampling time, and sample size.
In recent years, there has been an increasing emergence and spread of antibiotic-resistant Salmonella in several countries, particularly in Asia. This is a global problem caused by numerous interconnected factors, especially the abuse and misuse of antibiotics (Hoge et al., 1998; Davis et al., 1999; Cailhol et al., 2006; Lauderdale et al., 2006; Wang et al., 2019a,b). A high prevalence of antibiotic-resistant Salmonella in retail meats has been reported in Greece, United States, Canada, and China (Daoust et al., 1992; Arvanitidou et al., 1998; Chen et al., 2004). In the present study, 7.1% of 406 Salmonella isolates were susceptible to 14 antibiotics, whereas 92.8% of the isolates resisted at least one antibiotic and 1.7% resisted all 14 antibiotics. The rate of trimethoprim/sulfamethoxazole-resistant Salmonella was significantly higher in our study (82.0%) than that in Shaanxi province, 2007–2008 (58.0%) (Yang et al., 2011). Trimethoprim/sulfamethoxazole, fluoroquinolones, and cephalosporins are frontline therapeutic drugs for most bacterial infections; therefore, the high rates of Salmonella with resistance to these antibiotics are of significant concern (Fey et al., 2000; Bradford, 2001).
When the relationship between sampling time and antibiotic resistance of Salmonella was determined, we found that the rates of MDR Salmonella in March,May, July, and August were substantially higher than those in April, June, and September and lower than those in October, January, and February. Previous studies have reported that March through May and July through September are high-incidence seasons for foodborne Salmonella because of the direct influence of epidemic diseases on pathogen prevalence. The abuse and misuse of antibiotics in animals and humans have therefore prompted the emergence of antibiotic-resistant Salmonella (Xu et al., 2009; Lu et al., 2014). The highest rates of MDR Salmonella in October, January, and February (100%) might be overestimated because of low Salmonella prevalence (10%).
We further subtyped the 6 most prevalent Salmonella serotypes using PFGE and found that some Salmonella isolates within specific serotypes shared identical PFGE patterns. For example, even though the retail chickens were collected from different months, locations, and chicken types, S. typhimurium isolates in clusters C-1, C-3, and C-5 exhibited high genomic similarities. In addition, some S. typhimurium (C-2 and C-5), S. rissen (C-1, C-2, and C-3), S. enteritidis (C-1), S. essen (C-1 and C-3), and S. infantis (C-1 and C-6) isolates from samples collected from the same month and location shared identical or similar PFGE profiles. Prior studies found that Salmonella isolates in certain outbreaks almost shared similar PFGE patterns (Yang et al., 2011; Salwani et al., 2014; Wang et al., 2017; Qi et al., 2019). Taken together, our results revealed that these Salmonella serotypes might be potential risks for salmonellosis outbreaks in the study years because of their identical or similar PFGE profiles, apart from the wide range and long duration of prevalence, even though genomic diversities were found among some of the Salmonella serotypes. According to our PFGE results, the potential risk of S. typhimurium outbreaks in May, S. infantis in July, and S. rissen and S. enteritidis in March could be higher than that of other Salmonella serotypes in Shaanxi province during the sampling time.
In some cases, S. typhimurium in retail raw chickens is more prevalent in wet markets than in supermarkets (Thung et al., 2016; Nhung et al., 2018). In the present study, only 11 of 68 S. typhimurium isolates were obtained from retail chickens in supermarkets in May of 2011; the remaining isolates were recovered from wet markets and exhibited 100% genetic similarity. Outdoor sales, consumer contamination, shared chopping boards, butchers' unclean hands, and unclean knives have contributed to a wide prevalence of S. typhimurium in wet markets (Chen et al., 2011; Nidaullah et al., 2017).
In conclusion, our results strongly suggest that Salmonella contamination in retail raw chickens is common in Shaanxi province, China. Most Salmonella isolates of the same serotype exhibited identical or highly similar genetic relationships. MDR isolates were identified, with specific MDR phenotypes closely associated with Salmonella serotypes and sampling seasons. Preventive measures such as hazard analysis of critical control points and consumer education on the proper handling of raw poultry during preparation and cooking should be implemented to ensure food safety.
Acknowledgements
The study was supported by a grant from the Ministry of Science and Technology of the People's Republic of China (2017YFC1601400) and the Special Fund for Public Projects of Zhejiang Province, China (2016C32073).
Conflicts of interest statement: The authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2020.07.038.
Contributor Information
Yingping Xiao, Email: ypxiao@126.com.
Baowei Yang, Email: ybw090925@163.com.
Supplementary data
References
- Alban L., Olsen A.M., Nielsen B., Sorensen R., Jessen B. Qualitative and quantitative risk assessment for human salmonellosis due to multi-resistant Salmonella Typhimurium DT104 from consumption of Danish dry-cured pork sausages. Prev. Vet. Med. 2002;52:251–265. doi: 10.1016/s0167-5877(01)00258-6. [DOI] [PubMed] [Google Scholar]
- Arvanitidou M., Tsakris A., Sofianou D., Katsouyannopoulos V. Antimicrobial resistance and R-factor transfer of Salmonella isolated from chicken carcasses in Greek hospitals. Int. J. Food Microbiol. 1998;40:197–201. doi: 10.1016/s0168-1605(98)00020-8. [DOI] [PubMed] [Google Scholar]
- Bangtrakulnonth A., Pornreongwong S., Pulsrikarn C., Sawanpanyalert P., Hendriksen R.S., Wong D.M.A.L.F., Aarestrup F.M. Salmonella serovars from humans and other sources in Thailand, 1993–2002. Emerg. Infect Dis. 2004;10:131–136. doi: 10.3201/eid1001.02-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford P.A. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol J., Lailler R., Bouvet P., La Vieille S., Gauchard F., Sanders P., Brisabois A. Trends in antimicrobial resistance phenotypes in non-typhoid Salmonella from human and poultry origins in France. Epidemiol. Infect. 2006;134:171–178. doi: 10.1017/S0950268805004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J., Pichel M., Vaz T.M.I., Tavechio A.T., Fernandes S.A., Munoz N., Rodriguez C., Realpe M.E., Moreno J., Araya P., Fernandez J., Fernandez A., Campos E., Duarte F., Gustafson N.W., Binsztein N., Gutierrez E.P. Building PulseNet Latin America and caribbean Salmonella regional database: first conclusions of genetic subtypes of S. Typhi, S. Typhimurium and S. Enteritidis circulating in six countries of the region. Food Res. Int. 2012;45:1030–1036. [Google Scholar]
- Chen S., Zhao S., White D.G., Schroeder C.M., Lu R., Yang H., Mcdermott P.F., Ayers S., Meng J. Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl. Environ. Microbiol. 2004;70:1–7. doi: 10.1128/AEM.70.1.1-7.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Brown E., Knabel S.J. In Genomics of Foodborne Bacterial Pathogens. 2011. Molecular Epidemiology of Foodborne Pathogens; pp. 403–453. [Google Scholar]
- Clinical and Laboratory Standard Institute. Clinical and Laboratory Standards Institute; Wayne, PA: 2014. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Four Informational Supplement. CLSI Document M100-S24. [Google Scholar]
- Daoust J.Y., Sewell A.M., Daley E., Greco P. Antibiotic resistance of agricultural and foodborne Salmonella isoates in Canada - 1986-1989. J. Food Protect. 1992;55:428–434. doi: 10.4315/0362-028X-55.6.428. [DOI] [PubMed] [Google Scholar]
- Davis M.A., Hancock D.D., Besser T.E., Rice D.H., Gay J.M., Gay C., Gearhart L., Digiacomo R. Changes in antimicrobial resistance among Salmonella enterica serovar Typhimurium isolates from humans and cattle in the Northwestern United States, 1982-1997. Emerg. Infect Dis. 1999;5:802–806. doi: 10.3201/eid0506.990610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feasey N.A., Gaskell K., Wong V., Msefula C., Selemani G., Kumwenda S., Allain T.J., Mallewa J., Kennedy N., Bennett A., Nyirongo J.O., Nyondo P.A., Zulu M.D., Parkhill J., Dougan G., Gordon M.A., Heyderman R.S. Rapid emergence of multidrug resistant, H58-lineage Salmonella Typhi in Blantyre, Malawi. Plos Negl. Trop. Dis. 2015;9:1–13. doi: 10.1371/journal.pntd.0003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey P.D., Safranek T.J., Rupp M.E., Dunne E.F., Ribot E., Iwen P.C., Bradford P.A., Angulo F.J., Hinrichs S.H. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. New Engl. J. Med. 2000;342:1242–1249. doi: 10.1056/NEJM200004273421703. [DOI] [PubMed] [Google Scholar]
- Galanis E., Wong D., Patrick M.E., Binsztein N., Cieslik A., Chalermchaikit T., Aidara-Kane A., Ellis A., Angulo F.J., Wegener H.C., Surv W.H.O.G.S. Web-based surveillance and global Salmonella distribution, 2000-2002. Emerg. Infect Dis. 2006;12:381–388. doi: 10.3201/eid1203.050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto A.J., Peters T.M., Green J., Fisher I.S., Gill O.N., O'Brien S J., Maguire C., Berghold C., Lederer I., Gerner-Smidt P., Torpdahl M., Siitonen A., Lukinmaa S., Tschape H., Prager R., Luzzi I., Dionisi A.M., WK V.D.Z., Heck M., Coia J., Brown D., Usera M., Echeita A., Threlfall E.J. Distribution of molecular subtypes within Salmonella enterica serotype Enteritidis phage type 4 and S. Typhimurium definitive phage type 104 in nine European countries, 2000-2004: results of an international multi-centre study. Epidemiol. Infect. 2006;134:729–736. doi: 10.1017/S0950268805005820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist M.J., Greko C., Wallinga D.B., Beran G.W., Riley D.G., Thorne P.S. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ. Health Perspect. 2007;115:313–316. doi: 10.1289/ehp.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutema F.D., Agga G.E., Abdi R.D., De Zutter L., Duchateau L., Gabriel S. Prevalence and serotype diversity of Salmonella in apparently healthy cattle: Systematic review and meta-analysis of published studies, 2000-2017. Front Vet. Sci. 2019;6:1–11. doi: 10.3389/fvets.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herikstad H., Motarjemi Y., Tauxe R.V. Salmonella surveillance: a global survey of public health serotyping. Epidemiol. Infect. 2002;129:1–8. doi: 10.1017/s0950268802006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C.W., Gambel J.M., Srijan A., Pitarangsi C., Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin. Infect. Dis. 1998;26:341–345. doi: 10.1086/516303. [DOI] [PubMed] [Google Scholar]
- Hu Y.J., He Y.Y., Wang Y.R., Fanning S., Cui S.H., Chen Q., Liu G.H., Chen Q.X., Zhou G., Yang B.W., Huang J.L., Li F.Q. Serovar diversity and antimicrobial resistance of non-typhoidal Salmonella enterica recovered from retail chicken carcasses for sale in different regions of China. Food Control. 2017;81:46–54. [Google Scholar]
- Humphrey T., Wray C., Wray A. Public-health aspects of Salmonella infection. Immunol. Today. 2000;21:83–88. [Google Scholar]
- Isenbarger D.W., Hoge C.W., Srijan A., Pitarangsi C., Vithayasai N., Bodhidatta L., Hickey W.K., Cam P.D. Comparative antibiotic resistance of diarrheal pathogens from Vietnam and Thailand, 1996-1999. Emerg. Infect Dis. 2002;8:175–180. doi: 10.3201/eid0802.010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann F. Supplement to the Kauffmann-White scheme. 8. Acta Pathol. Microbiol. Scand. 2010;52:221–226. doi: 10.1111/j.1699-0463.1961.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Lauderdale T.L., Aarestrup F.M., Chen P.C., Lai J.F., Wang H.Y., Shiau Y.R., Huang I.W., Hung C.L. Multidrug resistance among different serotypes of clinical Salmonella isolates in Taiwan. Diagn. Microbiol. Infect. Dis. 2006;55:149–155. doi: 10.1016/j.diagmicrobio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Loharikar A., Vawter S., Warren K., Deasy M., Moll M., Sandt C., Gilhousen R., Villamil E., Rhorer A., Briere E., Schwensohn C., Trees E., Lafon P., Adams J.K., Le B., Behravesh C.B. Outbreak of human Salmonella Typhimurium infections linked to contact with baby poultry from a single agricultural feed store chain and mail-order hatchery, 2009. Pediatr. Infect. Dis. J. 2013;32:8–12. doi: 10.1097/INF.0b013e3182755e28. [DOI] [PubMed] [Google Scholar]
- Lu Y., Lu A., Zhao H.Y., Hou X.L., Wu G.J. Analysis of antimicrobial resistance among Salmonella enteritidis from chicken. Int. J. Zoonoses. 2014;30:17–22. [Google Scholar]
- National Antimicrobial Resistance Monitoring System. 2014. http://www.fda.gov/downloads/Animal Veterinary/Safety Health/Antimicrobial Resistance/National Antimicrobial Resistance Monitoring System/UCM334834.pdf Accessed Sep. 2020.
- Nhung N.T., Van N.T.B., Cuong N.V., Duong T.T.Q., Nhat T.T., Hang T.T.T., Nhi N.T.H., Kiet B.T., Hien V.B., Ngoc P.T., Campbell J., Thwaites G., Carrique-Mas J. Antimicrobial residues and resistance against critically important antimicrobials in non-typhoidal Salmonella from meat sold at wet markets and supermarkets in Vietnam. Int. J. Food Microbiol. 2018;266:301–309. doi: 10.1016/j.ijfoodmicro.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidaullah H., Abirami N., Shamilasyuhada A.K., Chuah L.O., Nurul H., Tan T.P., Abidin F.W.Z., Rusul G. Prevalence of Salmonella in poultry processing environments in wet markets in Penang and Perlis, Malaysia. Vet. World. 2017;10:286–292. doi: 10.14202/vetworld.2017.286-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S.J., Bishop R., Brenner F.W., Roels T.H., Bean N., Tauxe R.V., Slutsker L. The changing epidemiology of Salmonella: Trends in serotypes isolated from humans in the United States, 1987-1997. J. Infect. Dis. 2001;183:753–761. doi: 10.1086/318832. [DOI] [PubMed] [Google Scholar]
- Olsen J.E., Brown D.J., Madsen M., Bisgaard M. Cross-contamination with Salmonella on a broiler slaughterhouse line demonstrated by use of epidemiological markers. J. Appl. Microbiol. 2003;94:826–835. doi: 10.1046/j.1365-2672.2003.01911.x. [DOI] [PubMed] [Google Scholar]
- Oscar T.P. Predictive model for survival and growth of Salmonella typhimurium DT104 on chicken skin during temperature abuse. J. Food Prot. 2009;72:304–314. doi: 10.4315/0362-028x-72.2.304. [DOI] [PubMed] [Google Scholar]
- Padron M. Salmonella typhimurium outbreak in broiler chicken flocks in Mexico. Avian Dis. 1990;34:221–223. [PubMed] [Google Scholar]
- Qi X., Li P., Xu X., Yuan Y., Bu S., Lin D. Epidemiological and molecular investigations on Salmonella responsible for gastrointestinal infections in the southwest of Shanghai from 1998 to 2017. Front Microbio. 2019;10:2025–2033. doi: 10.3389/fmicb.2019.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot E.M., Fair M.A., Gautom R., Cameron D.N., Hunter S.B., Swaminathan B., Barrett T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- Salwani H., Nuramin A.M., Fauziah M.N., Hani M.H., Wan M.H., Phua K.K., Asma I., Aziah I. Genetic relationship and correspondence of Salmonella Typhi isolated from water samples in typhoid outbreak localities with food handlers and contact by using pulsed field gel electrophoresis (PFGE) Asian Pac. J. Trop. Dis. 2014;4:242. [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadi H., Sargeant J.M., Shannon H.S., Raina P. Growth and inactivation of Salmonella at low refrigerated storage temperatures and thermal inactivation on raw chicken meat and laboratory media: mixed effect meta-analysis. J. Epidemiol. Glob. Health. 2012;2:165–179. doi: 10.1016/j.jegh.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodagari H.R., Mashak Z., Ghadimianazar A. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from retail chicken meat and giblets in Iran. J. Infect Dev. Countr. 2015;9:463–469. doi: 10.3855/jidc.5945. [DOI] [PubMed] [Google Scholar]
- Su L.H., Chiu C.H., Chu C.S., Ou J.T. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin. Infect. Dis. 2004;39:546–551. doi: 10.1086/422726. [DOI] [PubMed] [Google Scholar]
- Suresh T., Hatha A.M., Srinivasan D., Srinivasan S., Lakshmanaperumalsamy P. Salmonella cross-contamination in retail chicken outlets and the efficacy of spice extracts on Salmonella enteritidis growth inhibition on various surfaces. Microbes Environ. 2004;19:286–291. [Google Scholar]
- Thung T.Y., Mahyudin N.A., Basri D.F., Wan Mohamed Radzi C.W., Nakaguchi Y., Nishibuchi M., Radu S. Prevalence and antibiotic resistance of Salmonella Enteritidis and Salmonella Typhimurium in raw chicken meat at retail markets in Malaysia. J. Poult. Sci. 2016;95:1888–1893. doi: 10.3382/ps/pew144. [DOI] [PubMed] [Google Scholar]
- USDA/FSIS. 2014. Isolation and Identification of Salmonella from Meat, Poultry, Pasteurized Egg, and Catfish Products and Carcass and Environmental Sponges.http://www.fsis.usda.gov/PDF/MLG Accessed Sep. 2020. [Google Scholar]
- Van D.E., Wannet W.J., Houwers D.J., Van P.W. Antimicrobial susceptibilities of Salmonella strains isolated from humans, cattle, pigs, and chickens in The Netherlands from 1984 to 2001. J. Clin. Microbiol. 2003;41:3574–3578. doi: 10.1128/JCM.41.8.3574-3578.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zheng R. Risk assessment of Salmonella in annimal derived food. Chin. J. Anim. Quarantine. 2007;24:23–25. [Google Scholar]
- Wang Y., Cao C.Y., Alali W.Q., Cui S.H., Li F.Q., Zhu J.H., Wang X., Meng J.H., Yang B.W. Distribution and antimicrobial susceptibility of foodborne Salmonella serovars in eight provinces in China from 2007 to 2012 (Except 2009) Foodborne Pathog. Dis. 2017;14:393–399. doi: 10.1089/fpd.2016.2237. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang L., Su J., Xu Z. Antibiotic susceptibility, biofilm-forming ability, and incidence of class 1 integron of Salmonella spp., Escherichia coli, and Staphylococcus aureus isolated from various foods in a school canteen in China. Foodborne Pathog. Dis. 2019;17:269–275. doi: 10.1089/fpd.2019.2694. [DOI] [PubMed] [Google Scholar]
- Wang R., Yang Q., Zhang S., Hong Y., Zhang M., Jiang S. Trends and correlation of antibiotic susceptibility and antibiotic consumption at a large teaching hospital in China (2007-2016): a surveillance study. Ther. Clin. Risk Manag. 2019;15:1019–1027. doi: 10.2147/TCRM.S210872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D.G., Zhao S., Sudler R., Ayers S., Friedman S., Chen S., McDermott P.F., McDermott S., Wagner D.D., Meng J. The isolation of antibiotic-resistant Salmonella from retail ground meats. N. Engl. J. Med. 2001;345:1147–1154. doi: 10.1056/NEJMoa010315. [DOI] [PubMed] [Google Scholar]
- Wong V.K., Baker S., Pickard D.J., Parkhill J., Page A.J., Feasey N.A., Kingsley R.A., Thomson N.R., Keanel J.A., Weill F.X., Edwards D.J., Hawkey J., Harris S.R., Mather A.E., Cain A.K., Hadfield J., Hart P.J., Thieu N.T.V., Klemm E.J., Glinos D.A., Breiman R.F., Watson C.H., Kariuki S., Gordon M.A., Heyderman R.S., Okoro C., Jacobs J., Lunguya O., Edmunds W.J., Msefula C., Chabalgoity J.A., Kama M., Jenkins K., Dutta S., Marks F., Campos J., Thompson C., Obaro S., MacLennan C.A., Dolecek C., Keddy K.H., Smith A.M., Parry C.M., Karkey A., Mulholland E.K., Campbell J.I., Dongol S., Basnyat B., Dufour M., Bandaranayake D., Naseri T.T., Singh S.P., Hatta M., Newton P., Onsare R.S., Isaia L., Dance D., Davong V., Thwaites G., Wijedoru L., Crump J.A., De Pinna E., Nair S., Nilles E.J., Thanh D.P., Turner P., Soeng S., Valcanis M., Powling J., Dimovski K., Hogg G., Farrar J., Holt K.E., Dougan G. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat. Genet. 2015;47:632–639. doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S.L., Hendriksen R.S., Xie Z.Q., Huang L.L., Zhang J., Guo W.S., Xu B.L., Ran L., Aarestrup F.M. Molecular characterization and antimicrobial susceptibility of Salmonella isolates from infections in humans in Henan province, China. J. Clin. Microbiol. 2009;47:401–409. doi: 10.1128/JCM.01099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.B., Gu B.K., Ran L., Diao B.W., Chen M., Jin H.M., Xiao W.J., Yuan Z.A. Sourcing reseach on rare serotype Salmonella diarhea in Shanghai. Shanghai J. Prev. Med. 2009;21:7–10. [Google Scholar]
- Yang B.W., Qu D., Zhang X.L., Shen J.L., Cui S.H., Shi Y., Xi M.L., Sheng M., Zhi S., Meng J.H. Prevalence and characterization of Salmonella serovars in retail meats of marketplace in Shaanxi, China. Int. J. Food Microbiol. 2010;141:63–72. doi: 10.1016/j.ijfoodmicro.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Yang B.W., Xi M.L., Wang X., Cui S.H., Yue T.L., Hao H.S., Wang Y., Cui Y., Alali W.Q., Meng J.H., Walls I., Wong D.M.L., Doyle M.P. Prevalence of Salmonella on raw poultry at retail markets in China. J. Food Protect. 2011;74:1724–1728. doi: 10.4315/0362-028X.JFP-11-215. [DOI] [PubMed] [Google Scholar]
- Yang B.W., Cui Y., Shi C., Wang J.Q., Xia X.D., Xi M.L., Wang X., Meng J.H., Alali W.Q., Walls I., Doyle M.P. Counts, serotypes, and antimicrobial resistance of Salmonella isolates on retail raw poultry in the People's Republic of China. J. Food Protect. 2014;77:894–902. doi: 10.4315/0362-028X.JFP-13-439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.