Abstract
Broilers are often deprived of feed and water for up to 48 h after hatch. This delayed access to feed (DAF) can inhibit small intestine development. The objective of this study was to determine the effects of DAF on small intestinal morphology, mRNA abundance of the goblet cell marker Muc2 and absorptive cell marker PepT1, and the distribution of goblet cells in young broilers. Cobb 500 chicks, hatching within a 12-h window, were randomly allocated into 3 groups: control with no feed delay (ND), 24-h feed delay (DAF24), and 36-h feed delay (DAF36). Morphology, gene expression, and in situ hybridization analyses were conducted on the duodenum, jejunum, and ileum at 0, 24, 36, 72, 120, and 168 h after hatch. Statistical analysis was performed using a t test for ND and DAF24 at 24 h. A 2-way ANOVA and Tukey's HSD test (P < 0.05) were used for ND, DAF24, and DAF36 from 36 h. At 24 to 36 h, DAF decreased the ratio of villus height/crypt depth (VH/CD) in the duodenum but increased VH/CD in the ileum due to changes in CD, whereas at 72 h, DAF decreased VH/CD due to a decrease in VH. The mRNA abundance of PepT1 was upregulated, while Muc2 mRNA was downregulated in DAF chicks. Cells expressing Muc2 mRNA were present along the villi and in the crypts. The ratio of the number of goblet cells found in the upper half to the lower half of the villus was greater in DAF chicks than in ND chicks, suggesting that DAF affected the appearance of new goblet cells. The number of Muc2 mRNA-expressing cells in the crypt, however, was generally not affected by DAF. In conclusion, DAF transiently affected small intestinal morphology, upregulated PepT1 mRNA, downregulated Muc2 mRNA, and changed the distribution of goblet cells in the villi. By 168 h, however, these parameters were not different between ND, DAF24, and DAF36 chicks.
Key words: delayed access to feed, peptide transporter 1, mucin 2, stem cell, goblet cell
Introduction
As a standard practice by a commercial hatchery, chicks are normally allowed up to a 24-h hatching window before removal from the hatcher and transportation to the brooder. The posthatch chicks are deprived of feed and water during this time, which was found to have negative effects on broiler performance including growth, organ development, digestive enzyme stimulation, and changes in immunological function. A meta-analysis of the effects of posthatch food and water deprivation on development, performance, and welfare of chickens was conducted by de Jong et al. (2017). While posthatch chicks can still obtain nutrients from the residual yolk, it is not sufficient to meet the nutritional requirements of commercial broilers.
Delayed access to feed (DAF) caused morphological changes in the small intestine such as decrease in villus surface area or volume, villus height (VH), crypt depth (CD), or percentage of proliferating cells in posthatch chicks (Yamauchi et al., 1996; Uni et al., 1998; Geyra et al., 2001; Gonzales et al., 2003; Shinde et al., 2015; Ghanem et al., 2018) and posthatch poults (Noy et al., 2001; Potturi et al., 2005). There was also greater density and larger goblet cells in DAF chicks during the first week after hatch (Uni et al., 2003). In most cases, however, intestinal morphology in DAF chicks was restored by market age (Willemsen et al., 2010).
The small intestine of both mammals and birds contains stem cells in the crypt and differentiated cells that line the villi. Stem cells express olfactomedin 4 (Olfm4) and leucine-rich repeat containing G protein–coupled receptor 5 (Carulli et al., 2014; Zhang and Wong, 2018). Epithelial cells that line the villi include enterocytes, goblet cells, and enteroendocrine cells that are involved in absorptive or secretory functions. Chicken enterocytes express a number of transporters for nutrients such as amino acids, peptides, monosaccharides, and minerals, such as the peptide transporter (PepT1) and the sodium glucose transporter (Wong et al., 2018). Goblet cells secrete mucin 2 (Muc2), which is a major component of the mucus layer that protects the epithelial cells from pathogens and mechanical damage (Johansson and Hansson, 2016). In situ hybridization (ISH) has been used to identify chicken intestinal stem cells that express Olfm4 mRNA (Zhang and Wong, 2018), enterocytes that express PepT1 and sodium glucose transporter 1 mRNA (Zhang and Wong, 2017; Zhang et al., 2019), and goblet cells that express Muc2 mRNA (Reynolds et al., 2020).
While much research has been focused on the effect of DAF on chicken small intestinal morphology, there is little known about the impact of DAF on key genes and cell types that are critical to broiler intestinal function and development. Thus, the objectives of this project were to determine the effect of DAF on intestinal morphology, Muc2 and PepT1 mRNA abundance, and the ontogeny of goblet cells in the small intestine of early posthatch chicks.
Materials and methods
Animals and Tissue Collection
Fertile eggs (Cobb 500) were obtained from a local hatchery (George's Hatchery, Harrisonburg, VA) and incubated at 37.5°C with 50% relative humidity (Petersime Incubator Company, Gettysburg, OH). All animal procedures were approved by the Virginia Tech Institutional Animal Care and Use Committee. Chicks hatching within a 12-h window were randomly distributed into 3 experimental groups: control with no delayed access to feed (ND), delay of 24 h before access to feed (DAF24), and delay of 36 h before access to feed (DAF36). Chicks were placed in 3 battery cages (30 cm × 61 cm × 38 cm) per group with 20 chicks per cage. Chicks in all 3 groups received water ad libitum after placement in the cages. Cardboard barriers were set up between different groups of cages to prevent accidental spread of feed during the feed restriction period. A standard corn-soybean meal mash starter diet that was equal to or exceeded the National Research Council (NRC, 1994) nutrient requirement for poultry was provided ad libitum. Body weights of chicks were measured at the day of hatch (doh), which was labeled as 0 h, and at 24, 36, 72, 120, 216, 264, and 360 h after hatch. Body weights were measured to only verify the expected decrease in BW due to DAF. At 0, 24, 36, 72, 120, and 168 h, chicks were euthanized by cervical dislocation, and small intestines were collected. A total of 6 chicks per group were used for tissue sampling at each time point. At 0 h, samples from ND chicks were collected before ND chicks received access to feed. At 24 h, samples from DAF24 chicks were collected before DAF24 chicks received access to feed. At 36 h, samples from DAF36 chicks were collected before DAF36 chicks received access to feed and after DAF24 chicks had access to feed for 12 h. The small intestines were collected; separated into duodenum, jejunum, and ileum; and rinsed with PBS. The middle 1 to 2 cm of each intestinal segment was collected intact for ISH and morphological measurements, and 0.5-cm samples from both ends of the center piece were minced and stored at −80°C for gene expression analysis.
Identification of Stem Cells and Morphological Measurements
Samples for ISH were fixed in 10% neutral buffered formalin at room temperature for 24 h and then stored in 70% ethanol at 4°C until samples were embedded in paraffin (Histo-Scientific Research Lab Inc., Mount Jackson, VA). Paraffin blocks were cut into 5- to 6-μm sections using a microtome (Microm HM 355S; Thermo Fisher Scientific, Waltham, MA) and mounted on Superfrost-Plus glass slides (Electron Microscopy Sciences, Hatfield, PA).
For ISH, the RNAscope method was used following the manufacturer's instructions (Advanced Cell Diagnostics, Newark, CA). Slides were deparaffinized in xylene and washed in 100% ethanol. A singleplex probe for Olfm4 (NM_001040463.1) was detected using the RNAscope 2.5 HD Assay–BROWN detection kit (Advanced Cell Diagnostics). Slides were counterstained with 50% Gill's hematoxylin no. 2 (Sigma-Aldrich, St. Louis, MO), rinsed in distilled water, and placed in 0.02% ammonia water to turn the purple stain to blue. The slides were dehydrated in a graded series of ethanol (70, 95, 95%) and a final xylene wash. Slides were sealed with VectaMount (Vector Lab, Burlingame, CA) and a glass coverslip. Images were captured under bright field using a Nikon Eclipse 80i microscope and a DS-Ri1 digital camera (Nikon Instruments, Inc., Melville, NY).
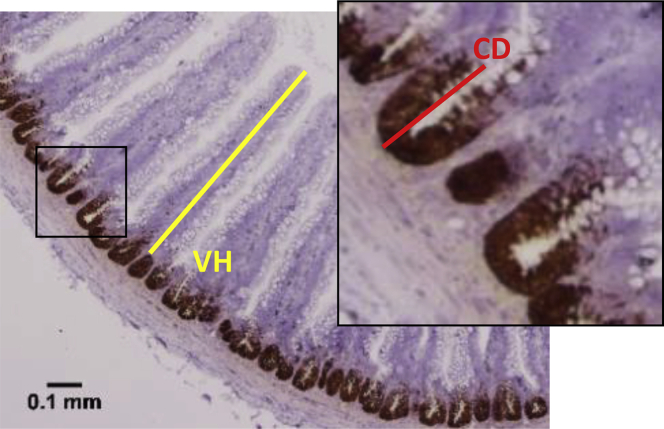
Intestinal VH and CD were measured using ImageJ Software from the National Institutes of Health. Intestinal stem cells in the crypt were identified by staining for Olfm4 mRNA using ISH (Zhang and Wong, 2018). This Olfm4 staining region was defined as the functional crypt. CD was measured from the top to the bottom of the region staining for Olfm4 mRNA (Figure 1). VH was measured from the top of the functional crypt to the tip of the villus.
Figure 1.
Method for measuring villus height (VH) and crypt depth (CD). Formalin-fixed, paraffin embedded tissue sections were processed by in situ hybridization to identify stem cells in the crypt that expressed olfactomedin 4 (Olfm4) mRNA (brown staining). ImageJ software was used to measure the functional CD (red line) and VH (yellow line). Image was taken at 40× magnification. The scale bar represents 0.1 mm.
Identification of Cells Expressing Muc2 mRNA and Mucin Glycoprotein
Cells expressing mucin glycoprotein were identified using a combined alcian blue (AB) and periodic acid Schiff (MiliporeSigma, St. Louis, MO) staining method as described in the study by Layton and Bancroft (2019). Cells expressing Muc2 mRNA were identified using a singleplex probe for Muc2 (NM_001318434.1) and the ISH method as described for Olfm4. The number of Muc2 mRNA–expressing (Muc2 mRNA+) cells in the villi and crypt was manually counted using ImageJ software. Imaged villi were divided into the upper half (VU) and lower half (VL) for goblet cell counting. Ten to 15 random villi and crypts were counted per sample.
RNA Extraction, cDNA Synthesis, and Quantitative PCR
Twenty to 35 mg of intestinal sample (n = 6 per group) were homogenized in TriReagent (Molecular Research Center Inc., Cincinnati, OH) using a TissueLyser II (Qiagen, Hilden, Germany). Total RNA was isolated following the manufacturer's instructions for the Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA). The RNA concentration and purity were determined using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific, Pittsburgh, PA). A 1.0-μg sample of total RNA was reverse transcribed using random primers and the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Grand Island, NY).
The quantitative PCR (qPCR) reaction contained 5 μL of Fast SYBR Green Master Mix (Applied Biosystems), 1 μL of each forward and reverse primers (5 μmol/L), 1.5 μL of DEPC water, and 1.5 μL of cDNA (1:20 dilution). The qPCR reactions were performed in duplicate using an Applied Biosystems 7500 Fast Real-time PCR system (Thermo Fisher Scientific). Cycling conditions for qPCR were 95°C for 20 s, followed by 40 cycles of 90°C for 3 s and 60°C for 30 s. Primers for PepT1 and Muc2 mRNA were designed using Primer Express 3.0 (Applied Biosystems) and are listed in Table 1. Primer efficiency (mean ± SD) was determined using 5 different RNA samples and the ABI 7500 relative standard curve program. Primers for β-actin, glyceraldehyde-3-phosphate dehydrogenase, and 2 ribosomal proteins (RPLP1 and RPL4) were tested as reference genes and are listed in Table 1. GeNorm was used to identify the 2 most stable genes (Vandesompele et al., 2002). The geometric mean of RPLP1 and RPL4 was used as the composite reference gene Ct value to calculate ΔCt. Average ΔCt value of ND for each gene at 0 h was used as the calibrator to calculate ΔΔCt. The 2−ΔΔCt method (Schmittgen and Livak, 2008) was used to determine the relative fold change.
Table 1.
Primers for quantitative PCR.
| Gene name | Forward/Reverse primers (5′–3′) | Amplicon (bp) | Primer efficiency1 (%) | Accession no. |
|---|---|---|---|---|
| Mucin 2 (Muc2) | CTGATTGTCACTCACGCCTTAATC/GCCGGCCACCTGCAT | 147 | 95.9 ± 4.5 | JX284122.1 |
| Peptide transporter 1 (PepT1) | CCCCTGAGGAGGATCACTGTT/CAAAAGAGCAGCAGCAACGA | 66 | 88.1 ± 4.7 | KF366603.1 |
| Ribosomal protein large subunit P1 (RPLP1) | TCTCCACGACGACGAAGTCA/CCGCCGCCTTGATGAG | 55 | 92.6 ± 5.6 | NM_205322.1 |
| Ribosomal protein large subunit 4 (RPL4) | TCAAGGCGCCCATTCG/TGCGCAGGTTGGTGTGAA | 63 | 87.9 ± 5.1 | NM_001007479.1 |
| Beta actin (β-actin) | GTCCACCGCAAATGCTTCTAA/TGCGCATTTATGGGTTTTGTT | 78 | 94.6 ± 6.4 | NM_205518.1 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | GCCGTCCTCTCTGGCAAAG/TGTAAACCATGTAGTTCAGATCGATGA | 73 | 90.8 ± 5.4 | NM_204305.1 |
Primer efficiency is shown as mean ± SD.
Statistical Analysis
Statistical analysis was conducted using JMP Pro 14 (SAS Institute, Inc., Cary, NC). Chick was considered as the experimental unit. Body weights were analyzed by pooled t test at 24 h and one-way ANOVA for 36 h to 360 h. Morphological, gene expression, and goblet cell data were analyzed by pooled t test at 24 h and 2-way ANOVA considering time after hatch and experimental group from 36 h to 168 h (morphology) and 36 h to 120 h (gene expression and goblet cell). When significant ANOVA results were found, Tukey's HSD test was used for mean separation. In all cases, significance was considered to be P < 0.05.
Results
Body Weight and Intestinal Morphology
The changes in BW for ND, DAF24, and DAF36 chicks from 0 to 360 h after hatch are shown in Table 2. At 24 h, there was no difference between the BW of DAF24 and ND chicks. At 36 h, 72 h, and 168 h, DAF36 chicks had lower BW than both ND and DAF24 chicks. At 120 h and 216 h, DAF36 chicks had lower BW than ND chicks but not DAF24 chicks. There was a numerical but nonsignificant difference in BW between groups at 264 h and 360 h.
Table 2.
Effect of delayed access to feed on broiler body weight.
| Groups1 | BW (g) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 36 h | 72 h | 120 h | 168 h | 216 h | 264 h | 360 h | |
| ND | 46.7 | 46.7 | 57.2a | 72.6a | 115.2a | 169.8a | 227.1a | 328.7 | 462.9 |
| DAF24 | - | 42.7 | 57.2a | 68.2a | 109.6a,b | 164.9a | 220.1a,b | 332.1 | 461.8 |
| DAF36 | - | - | 42.8b | 58.6b | 96.1b | 148.9b | 208.5b | 312.2 | 435.9 |
| SEM | - | 1.97 | 1.16 | 2.81 | 3.98 | 4.09 | 4.21 | 7.86 | 9.77 |
| P value | - | 0.06 | <0.001 | 0.005 | 0.020 | 0.004 | 0.007 | 0.14 | 0.07 |
Chicks were weighed at 0 h (n = 60), 72 h (n = 47–48/group), 120 h (n = 40–46/group), 168 h (n = 35–38/group), 216 h (n = 29–31/group), 264 h (n = 23–27/group), and 360 h (n = 23–27/group) after hatch. Only sampled chicks (n = 6) were weighed at 24 h and 36 h posthatch. Significant differences (P < 0.05) between groups within a time point are indicated by different letters in the superscript.
Groups include control with no feed delay (ND), 24 h feed delay (DAF24), and 36 h feed delay (DAF36).
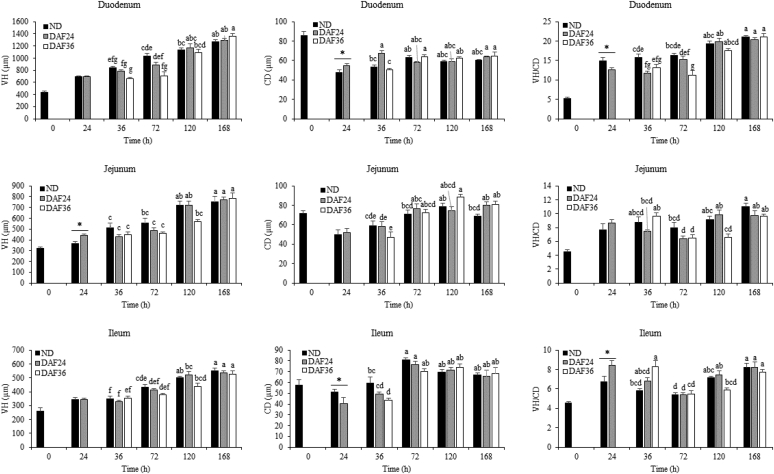
DAF caused changes in VH/CD, which was a result of increases or decreases in VH, CD, or both (Figure 2). At 24 h, VH/CD in the duodenum was lower in DAF24 chicks than in ND chicks because of an increase in CD in DAF24 chicks. In the ileum, there was an effect opposite to that in the duodenum with greater VH/CD in DAF24 chicks than in ND chicks because of a decrease in CD of DAF24 chicks. At 36 h, DAF24 chicks showed a decrease in VH/CD in the duodenum compared with ND chicks because of an increase in CD of DAF24 chicks. In the ileum, VH/CD of DAF36 chicks was greater than that of ND chicks as a result of a decrease in CD in DAF36 chicks. At 72 h, there was a decrease in VH/CD in the duodenum of DAF36 chicks compared with ND chicks because of a decrease in VH of DAF36 chicks. At 120 h, there was a decrease in VH/CD in the jejunum of DAF36 chicks compared with DAF24 chicks because of a numerical decrease in VH of DAF36 chicks. At 168 h, there was no difference in VH, CD, or VH/CD among the ND, DAF24, and DAF36 groups.
Figure 2.
Effect of delayed access to feed on villus height, crypt depth, and the villus height/crypt depth ratio. Villus height (VH) and crypt depth (CD) were measured and VH/CD was calculated for formalin-fixed, paraffin embedded tissue sections stained by in situ hybridization for olfactomedin 4 (Olfm4) mRNA and counterstained with hematoxylin. Ten to 15 random villi and crypts were measured for each of the 6 replicate samples. Groups included control with no feed delay (ND), 24 h feed delay (DAF24), and 36 h feed delay (DAF36). A t-test was used to analyze ND and DAF24 at 24 h. Significant differences (P < 0.05) are indicated by an asterisk. A 2-way ANOVA and Tukey's HSD test were used for analysis of ND, DAF24, and DAF36 from 36 h to 168 h. Significant differences (P < 0.05) are indicated by different letters.
There were age-specific and intestinal segment–specific changes in VH, CD, and VH/CD (Figure 2). In the duodenum, jejunum, and ileum, there was an increase in VH of ND, DAF24, and DAF36 chicks from 36 h to 168 h with an accompanying increase in VH/CD from 36 h to 168 h in the duodenum and from 72 h to 168 h in the jejunum and ileum. There was also an increase in CD from 36 h to 72 h in the duodenum, jejunum, and ileum of DAF36 chicks, in the jejunum and ileum of DAF24 chicks, and in the ileum of ND chicks. From 72 h to 168 h, there was no change in CD.
PepT1 and Muc2 mRNA Abundance
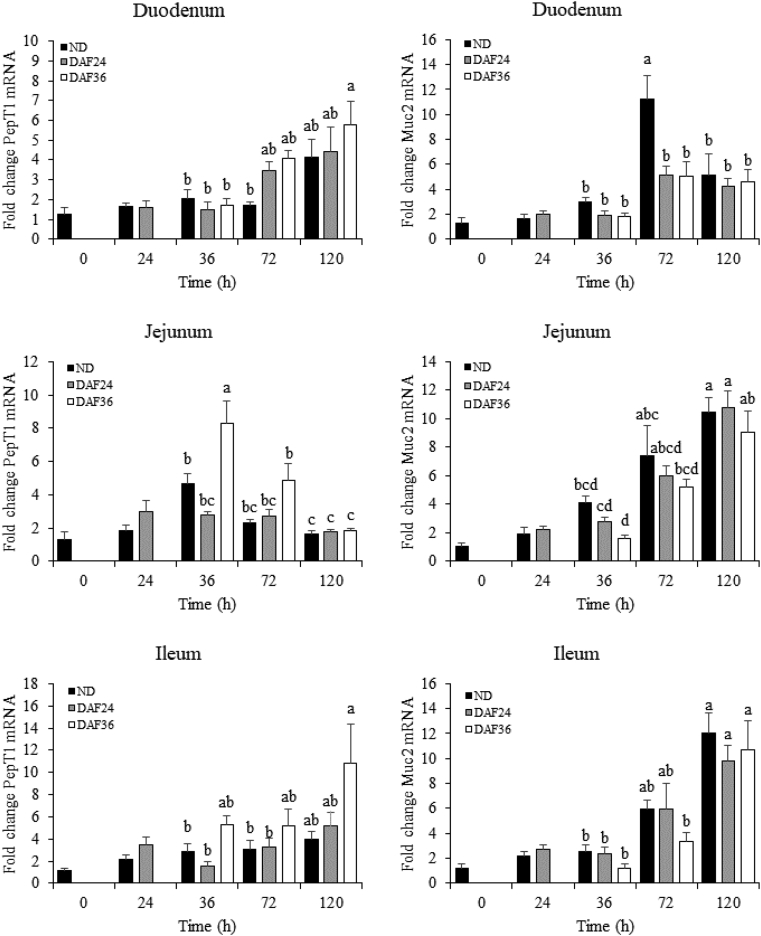
The effect of DAF on PepT1 and Muc2 mRNA abundance in the duodenum, jejunum, and ileum for ND, DAF24, and DAF36 chicks is shown in Figure 3. At 24 h, PepT1 and Muc2 mRNA was the same for ND and DAF24 chicks for all intestinal segments. At 36 h, PepT1 mRNA was upregulated 1.8- and 3.0-fold in the jejunum of DAF36 chicks compared with that in ND and DAF24 chicks, respectively, while Muc2 mRNA showed a numerical decrease in DAF36 chicks compared with ND and DAF24 chicks. At 72 h, Muc2 mRNA was downregulated 2.2-fold in both DAF24 and DAF36 chicks compared with ND chicks in the duodenum, while PepT1 mRNA showed a numerical increase in DAF24 and DAF36 chicks compared with ND chicks. These results suggested that during DAF, there was upregulation of PepT1 mRNA and downregulation of Muc2 mRNA.
Figure 3.
Effect of delayed access to feed on relative abundance of peptide transporter 1 (PepT1) and mucin 2 (Muc2) mRNA. RNA from duodenum, jejunum, and ileum of broilers at 0, 24, 36, 72, and 120 h after hatch were analyzed by relative qPCR (n = 6). Groups include control with no feed delay (ND), 24-h feed delay (DAF24), and 36-h feed delay (DAF36). A t-test was used to analyze ND and DAF24 at 24 h. No significant differences were observed at 24 h. A 2-way ANOVA and Tukey's HSD test were used for analysis of ND, DAF24 and DAF36 from 36 h to 120 h. Significant differences (P < 0.05) are indicated by different letters.
PepT1 and Muc2 mRNA abundance varied with age and intestinal segment (Figure 3). Between 36 h and 120 h, there was an increase in PepT1 mRNA in the duodenum of DAF36 chicks, whereas there was a decrease in PepT1 mRNA in the jejunum of ND and DAF36 chicks. There were no temporal changes in PepT1 mRNA in the ileum. For Muc2 mRNA, there was an increase from 36 h to 72 h followed by a decrease at 120 h in the duodenum of ND chicks. In both the jejunum and ileum, there was an increase in Muc2 mRNA for ND, DAF24, and DAF36 chicks from 36 h to 120 h.
Distribution of Cells Expressing Muc2 mRNA in the Villi and Crypts
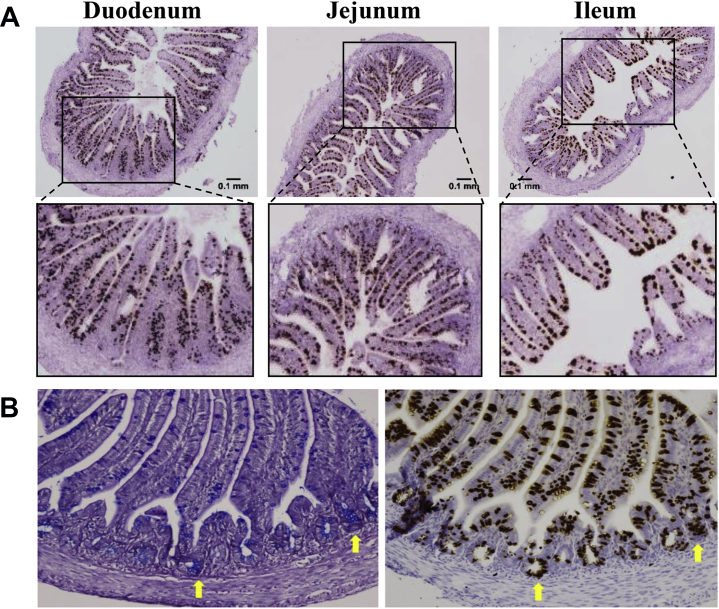
The effects of DAF on Muc2 mRNA+ cells were examined by ISH for the duodenum, jejunum, and ileum of DOH chicks. Cells expressing Muc2 mRNA were found along the intestinal villi and within the crypts (Figure 4A). In the duodenum and jejunum, there was an even distribution of generally uniformly sized goblet cells along the villi. In contrast, in the ileum, there was an uneven distribution with a greater number and larger goblet cells near the tip of the villus than in the bottom of the villus.
Figure 4.
Identification of cells expressing Muc2 mRNA and mucin glycoprotein. Formalin-fixed, paraffin embedded tissue sections were stained for Muc2 mRNA or mucin glycoprotein. (A) Sections from the duodenum, jejunum and ileum of day of hatch chicks were stained for Muc2 mRNA (brown stain). Images were taken at 40x magnification. Scale bar represents 0.1 mm. (B) Serial sections from the jejunum of a ND chick at day 1 were stained for mucin glycoprotein using alcian blue-periodic acid Schiff (left panel, blue stain) and Muc2 mRNA by in situ hybridization (right panel, brown stain). The yellow arrows indicate clusters of cells that stained for Muc2 mRNA and mucin glycoprotein. Images were taken at 200× magnification.
Serial sections were examined for Muc2 mRNA or mucin glycoprotein using ISH or staining with AB and periodic acid Schiff, respectively (Figure 4B). There were more cells that expressed Muc2 mRNA than mucin glycoprotein along the villi, which indicated that ISH was a more sensitive method than staining with AB for detection of goblet cells. In the crypt, Muc2 mRNA+ cells were interspersed with cells that did not express Muc2 mRNA (Figure 4A). Most Muc2 mRNA+ cells in the crypt did not also stain for mucin glycoprotein. Only a subset of crypt cells stained for both Muc2 mRNA and mucin glycoprotein (Figure 4B, yellow arrows).
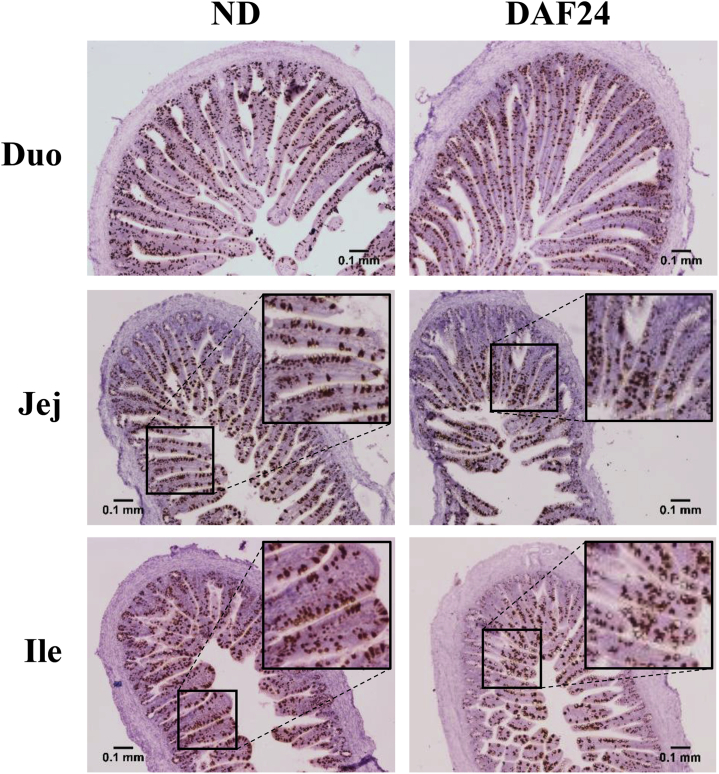
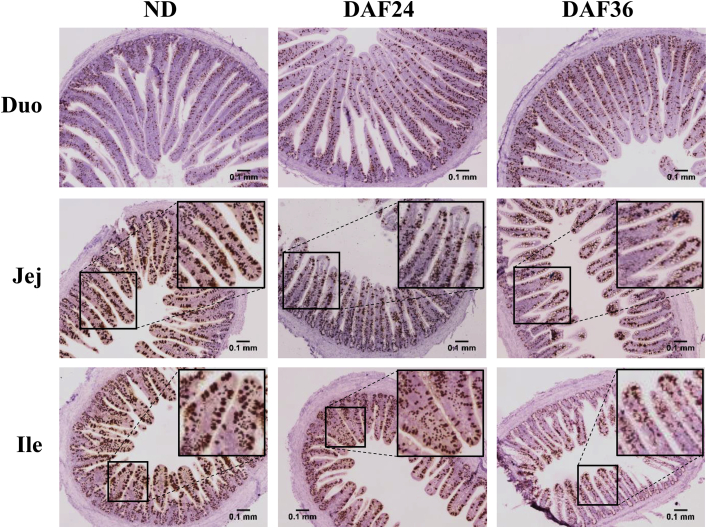
DAF appeared to change the distribution of goblet cells in the villi, with fewer goblet cells present in the lower half than the upper half of the villus. At 24 h, the jejunum and ileum, but not the duodenum, of DAF24 chicks showed an unequal distribution of goblet cells along the villi, with more goblet cells in the upper half vs. the lower half of the villi (Figure 5). The jejunum and ileum of ND chicks showed an even distribution. At 36 h, DAF36 chicks had a similar unequal distribution of goblet cells in the villi of the jejunum and ileum, but not the duodenum, compared to ND chicks (Figure 6). The DAF24 chicks, which had a 24-h delay followed by feed for 12 h, appeared similar to ND chicks. At 72 h and 120 h, the distribution of goblet cells was the same for ND, DAF24, and DAF36 chicks (data not shown).
Figure 5.
Effect of 24 h delayed access to feed on cells expressing Muc2 mRNA in the small intestine. Formalin-fixed, paraffin embedded tissues from the duodenum (Duo), jejunum (Jej), and ileum (Ile) were sectioned and stained for Muc2 mRNA by in situ hybridization (n = 3). Images are from chicks with no feed delay (ND) and chicks with 24 h feed delay (DAF24) sampled at 24 h after hatch. Brown staining represents Muc2 mRNA expression in cells. Tissues were counterstained with hematoxylin. Images were taken at 40× magnification. Scale bar represents 0.1 mm.
Figure 6.
Effect of 36 h delayed access to feed on cells expressing Muc2 mRNA in the small intestine. Formalin-fixed, paraffin embedded tissues from the duodenum (Duo), jejunum (Jej), and ileum (Ile) were sectioned and stained for Muc2 mRNA by in situ hybridization (n = 3). Images are from control chicks with no feed delay (ND), chicks with 24 h feed delay plus 12 h of feeding (DAF24) and chicks with 36 h feed delay (DAF36) sampled at 36 h after hatch. Brown staining represents Muc2 mRNA expression in cells. Tissues were counterstained with hematoxylin. Images were taken at 40x magnification. Scale bar represents 0.1 mm.
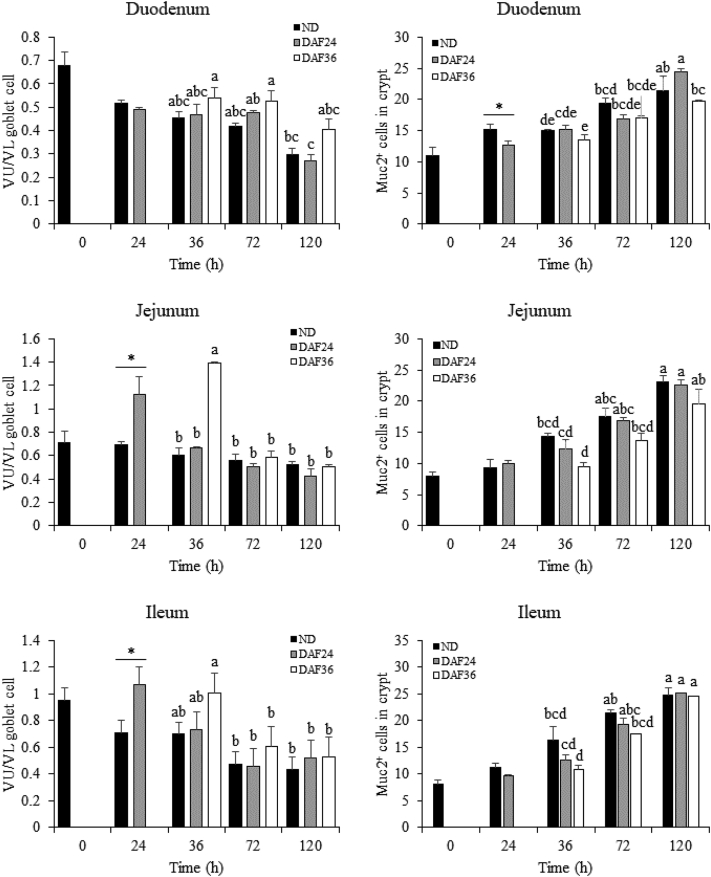
To quantify this apparent change in cellular distribution, goblet cells in the upper half of the villus (VU) and lower half of the villus (VL) were enumerated and expressed as a ratio of VU/VL (Figure 7). At 24 h, VU/VL was greater in DAF24 chicks than that in ND chicks in the jejunum and ileum. At 36 h, VU/VL was greater in DAF36 chicks than that in ND and DAF24 chicks in the jejunum. By 72 h, there was no difference in VU/VL in the jejunum and ileum for ND, DAF24, and DAF36 chicks. The duodenum showed no difference in VU/VL for ND, DAF24, and DAF36 chicks at any time point. These results demonstrated that DAF caused an uneven distribution of goblet cells in the jejunum and ileum but not the duodenum, which returned to normal after feeding. In contrast, the number of Muc2 mRNA+ cells in the crypt was generally not affected by DAF. The 2 exceptions were a decreased number of Muc2 mRNA+ cells in DAF24 chicks compared with ND chicks at 24 h and in DAF36 chicks compared with DAF24 chicks at 120 h in the duodenum.
Figure 7.
Effect of delayed access to feed on the ratio of goblet cells in the upper half to lower half of the villus and number of Muc2 mRNA expressing cells in the crypt. Formalin-fixed, paraffin embedded tissue sections were stained for Muc2 mRNA by in situ hybridization (n = 3). Samples from the duodenum, jejunum, and ileum were collected from chicks at 0, 24, 36, 72, and 120 h after hatch (n = 3). Groups include control chicks with no feed delay (ND), 24-h feed delay (DAF24), and 36-h feed delay (DAF36). The number of goblet cells expressing Muc2 mRNA were counted in the upper half (VU) and lower half (VL) of the villi and expressed as a ratio, VU/VL (left panels). The number of cells expressing Muc2 mRNA in the crypts were also counted (right panels). A t-test was used to analyze ND and DAF24 at 24 h. Significant differences (P < 0.05) are indicated by an asterisk. A 2-way ANOVA and Tukey's HSD test were used for analysis of ND, DAF24 and DAF36 from 36 h to 168 h. Significant differences (P < 0.05) are indicated by different letters.
There were age-specific changes in the distribution of goblet cells along the villi and number of Muc2 mRNA+ cells in the crypt that varied by intestinal segment (Figure 7). In the duodenum of DAF24 chicks, VU/VL decreased from 72 to 120 h, while in the jejunum and ileum of DAF36 chicks, VU/VL decreased from 36 to 72 h. In the crypts of the duodenum, jejunum, and ileum, the number of Muc2 mRNA+ cells increased in ND, DAF24, and DAF36 chicks from 36 h to 120 h.
Discussion
DAF affected BW and intestinal morphology with a more pronounced effect as the duration of feed delay increased. A meta-analysis of the effect of posthatch food and water deprivation found that deprivation for 24 h, which was defined as (≥12–36 h), resulted in significantly lower body weights (de Jong et al., 2017). There appeared, however, to be a difference between DAF for 24 h vs. DAF for 36 h. Broilers subjected to DAF for 24 h did not affect long term growth performance, likely because chicks were still able to derive nutrients from their residual yolk reserves (Juul-Madsen et al., 2004; Saki, 2005; Bhanja et al., 2009). Shinde et al. (2015), however, showed that DAF for 24 h impacted BW at 7 and 42 d. DAF for 34 to 36 h reduced BW from day 4 to day 21 (Noy and Sklan, 1999) and from day 7 to day 42 (Shinde et al., 2015). Similarly, DAF for 32 h decreased BWG from 0 to 7, 0 to 21 and 0 to 35 d (Bhanja et al., 2009). In contrast, Petek et al. (2007) showed that DAF for 36 h resulted in a decrease in BW for the first 4 wk but not at 42 d. The difference in results could be due to the length of the hatching window. A spread of hatch can cause variability in delayed time within a single batch of chicks (Careghi et al., 2005; Willemsen et al., 2010; Wang et al., 2014). In our study, DAF24 chicks showed no difference in BW from ND chicks at 24 h. At 36 h, DAF36 chicks had lower BW compared to ND and DAF24 chicks, but by 264 h, there was a numerical but not a statistically significant difference in BW among groups. With a larger sample size, this difference may have been significant. In addition, in our study the newly hatched chicks had ad libitum access to water but not feed and this also may have impacted BW.
The effects of DAF on intestinal morphology have been examined. Maiorka et al. (2003) showed that either water or feed deprivation increased the number of villi per unit area with a corresponding reduction in villus size in 1 d-old chicks. DAF for 24 h resulted in clumping of microvilli and damaged crypts with an irregular morphology (Uni et al., 1998). Geyra et al. (2001) showed that DAF for 48 h resulted in a decrease in the number of crypts per villi, cells per crypt, and percentage of proliferating cells in the crypt and villi, with a greater effect in the duodenum and jejunum than the ileum. At hatch, the duodenum is the most developed of the 3 small intestinal segments. Because maturation of the duodenum precedes that of the jejunum and ileum, the duodenum is impacted the greatest by DAF (Uni et al., 2000).
VH, CD, and VH/CD are often used as indicators of intestinal function. Longer villi would provide more absorptive area for nutrient uptake, while a deeper crypt would indicate potentially more stem cells. Changes in the calculated VH/CD may result from an increase or decrease in VH, CD or both and therefore, changes in VH/CD need to be evaluated carefully. Villus volume, which was calculated from VH and villus width, and CD increased more rapidly in the duodenum and jejunum than the ileum from DOH to day 7 in chicks (Uni et al., 1998). In poults, there was a similar rapid increase in VH and CD in the duodenum and jejunum and a more gradual increase in the ileum from DOH to day 7 after hatch (Uni et al., 1999). Our results showed a similar increase in VH in the duodenum, jejunum and ileum of ND chicks from 36 h to 168 h. The CD of ND chicks in general did not show a significant increase from 36 h to 168 h with the exception of increases from 36 h to 120 h in the jejunum and 36 h to 72 h in the ileum. This may be due to how the crypt was measured. Our study used ISH staining for Olfm4 mRNA for CD, while Uni et al. (1999) used a morphological measurement of CD. Consistent with the increase in CD observed by Uni et al. (1999), our study showed an increase in the number of Muc2 mRNA+ cells in the crypt of ND chicks from 36 h to 120 h in all 3 small intestinal segments.
DAF affected VH, CD and VH/CD. In the duodenum, jejunum, and ileum, DAF for 36 h caused a decrease in CD (Uni et al., 1998) or a decrease in VH (Yamauchi et al., 1996; Shinde et al., 2015). In these studies, VH/CD was not reported. Ghanem et al. (2018) reported that DAF for 24 h or 48 h reduced both VH and CD in the duodenum at day 7. Tabedian et al. (2010) showed that DAF for 48 h did not affect VH, CD or VH/CD at 48 h, but there was a delayed response with DAF for 48 h resulting in an increase in duodenal CD at day 7 with no effect on VH and VH/CD. Gonzales et al. (2003) reported that DAF for as little as 18 h as well as 36 h resulted in decreased VH in the duodenum, jejunum and ileum, whereas the decrease in CD was mainly observed in the duodenum. In our study, there were both intestinal segment-specific increases and decreases in VH/CD. At 24 and 36 h, VH/CD decreased in the duodenum due to an increase in CD, while VH/CD increased in the ileum due to a decrease in CD. In contrast, VH/CD was decreased in the duodenum at 72 h and in the jejunum and ileum at 120 h, due to a decrease in VH. These results indicate that DAF initially affected the crypts and presumably stem cells and then later affected VH. For DAF24 and DAF36 chicks, there was a parallel increase in both CD and VH in the jejunum and ileum from 36 to 168 h. Because the differentiated cells along the villi arise from the stem cells in the crypt, an effect on the stem cell pool would be expected to impact the villi at a later point.
DAF caused an upregulation of PepT1 mRNA and downregulation of Muc2 mRNA. The upregulation of PepT1 in response to nutrient deprivation, such as DAF in this study, was similar to the upregulation observed in fasted or feed-restricted chickens (Gilbert et al., 2008; Madsen and Wong, 2011). The decrease in Muc2 mRNA would be expected to lead to a decrease in the mucus layer. These results suggest that during times of reduced nutrient availability, the chick prioritizes nutrient uptake over host defense. Consistent with this idea, Ihara et al. (2000) showed that starved rats showed a 179% increase in PepT1 mRNA and a 41% decrease in the mucosal weight, compared to ad libitum fed control rats. Although mucin mRNA abundance was not examined by Ihara et al. (2000), the decrease in mucosal weight, which would include the protective mucus layer, could be a result of a decrease in mucin mRNA and mucus production.
The temporal expression patterns for PepT1 and Muc2 mRNA were altered by DAF. The mRNA abundance of PepT1 (Wong et al., 2018) and Muc2 (Smirnov et al., 2006; Jiang et al., 2013; Zhang et al., 2015; Reynolds et al., 2020) both increased after hatch in the duodenum, jejunum and ileum. The DAF36 chicks showed the expected increase in PepT1 mRNA in the duodenum, but a decrease in the jejunum from 36 h to 72 h. For Muc2 mRNA there was the expected increase in the jejunum and ileum from 36 h to 120 h, but no change in the duodenum. By 120 h, the abundance of PepT1 and Muc2 mRNA abundance was the same between ND, DAF24 and DAF36 chicks.
DAF affected goblet cell density. Chicks fasted for 48 h after hatch showed increased goblet cell density and goblet cell area (Uni et al., 2003). Smirnov et al. (2004) reported that DOH chicks fasted for 72 h had increased Muc-5AC mRNA and mucin glycoprotein in the duodenum and jejunum, with no effect in the ileum. Surprisingly, the increase in mucin glycoprotein in fasted chicks did not result in a thicker mucus adherent layer but a reduction in this layer. Additionally, providing early nutrition to chicks from in ovo feeding resulted in a greater acidic goblet cell density at embryonic day 19 and an upregulation of Muc2 mRNA at 0 h (Smirnov et al., 2006). In our study we observed an increase in VU/VL for goblet cells in DAF chicks, which could be due to reduced number of Muc2 mRNA+ cells exiting the crypt as existing goblet cells migrated up the villi. There was no difference in the number of Muc2 mRNA+ cells in the crypt, which suggested that there was no effect of DAF on the differentiation of Muc2 mRNA+ cells from stem cells. The reduced number of goblet cells in the lower half of the villi of DAF chicks indicated a potential decrease in mucus production, which could decrease the barrier functionality of the mucus layer and expose the underlying epithelia to harmful pathogens.
Cells that expressed Muc2 mRNA were present not only along the villi, but also in the crypt. Most of the goblet cells along the villi stained for both Muc2 mRNA and mucin glycoprotein, whereas only a few Muc2 mRNA+ cells in the crypts also stained for mucin glycoprotein. Uni et al. (2003) had shown that cells staining with AB were present along the villi, but not in the crypt. We similarly had reported little staining for mucin glycoprotein in the crypt even though crypt cells expressed Muc2 mRNA (Reynolds et al., 2020). Although not common, cells in the crypt that expressed Muc2 mRNA but not mucin glycoprotein could represent pre-goblet cells that have started to differentiate from stem cells but have not yet migrated out of the crypt.
In conclusion, the length of DAF can differentially affect BW of early posthatch chicks. DAF inhibited small intestinal development, which was revealed as reduced VH and CD and altered VH/CD. At the molecular level, DAF chicks showed downregulation of Muc2 mRNA and a decrease in goblet cell production, while PepT1 mRNA was upregulated. This suggests that nutrient uptake is prioritized over host defense during DAF and may make a DAF chick more susceptible to pathogens during the early posthatch period. By 168 h, however, all measured parameters were the same for ND, DAF24 and DAF36 chicks.
Acknowledgments
This project was funded in part by the Virginia Agricultural Experiment Station, the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture (NIFA-USDA), and NIFA-USDA grant 2017-67015-26588.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Bhanja S.K., Anjali Devi C., Panda A.K., Shyam Sunder G. Effect of post-hatch feed deprivation on yolk-sac utilization and performance of young broiler chickens. Asian–Australas. J. Anim. Sci. 2009;22:1174–1179. [Google Scholar]
- Careghi C., Tona K., Onagbesan O., Buyse J., Decuypere E., Bruggeman V. The effects of the spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 2005;84:1314–1320. doi: 10.1093/ps/84.8.1314. [DOI] [PubMed] [Google Scholar]
- Carulli A.J., Samuelson L.C., Schnell S. Unraveling intestinal stem cell behavior with models of crypt dynamics. Integr. Biol. 2014;6:243–257. doi: 10.1039/c3ib40163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong I.C., van Riel J., Bracke M.B., van den Brand H. A ‘meta-analysis’ of effects of post-hatch food and water deprivation on development, performance and welfare of chickens. PLoS One. 2017;12:e0189350. doi: 10.1371/journal.pone.0189350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyra A., Uni Z., Sklan D. The effect of fasting at different ages on growth and tissue dynamics in the small intestine of the young chick. Br. J. Nutr. 2001;86:53–61. doi: 10.1079/bjn2001368. [DOI] [PubMed] [Google Scholar]
- Ghanem H.M., Lashen S.E.S., Mahmoud R.E.S., Azzam M. Productive performance and histological evaluation of delayed post hatch feed access broilers fed threonine supplemented diet. Asian J. Anim. Vet. Adv. 2018;13:136–143. [Google Scholar]
- Gilbert E.R., Li H., Emmerson D.A., Webb K.E., Jr., Wong E.A. Dietary protein quality and feed restriction influence abundance of nutrient transporter mRNA in the small intestine of broiler chicks. J. Nutr. 2008;138:262–271. doi: 10.1093/jn/138.2.262. [DOI] [PubMed] [Google Scholar]
- Gonzales E., Kondo N., Saldanha E., Loddy M., Careghi C., Decuypere E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult. Sci. 2003;82:1250–1256. doi: 10.1093/ps/82.8.1250. [DOI] [PubMed] [Google Scholar]
- Ihara T., Tsujikawa T., Fujiyama Y., Bamba T. Regulation of PepT1 peptide transporter expression in the rat small intestine under malnourished conditions. Digestion. 2000;61:59–67. doi: 10.1159/000007736. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Applegate T.J., Lossie A.C. Cloning, annotation and developmental expression of the chicken intestinal MUC2 gene. PLoS One. 2013;8:e53781. doi: 10.1371/journal.pone.0053781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E.V., Hansson G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul-Madsen H.R., Su G., Sorensen P. Influence of early or late start of first feeding on growth and immune phenotype of broilers. Br. Poult. Sci. 2004;45:210–222. doi: 10.1080/00071660410001715812. [DOI] [PubMed] [Google Scholar]
- Layton C., Bancroft J.D. Carbohydrates. In: Suvarna S.K., Layton C.L., Bancroft J.D., editors. Bancroft’s Theory and Practice of Histological Techniques. 8th ed. Elsevier; Amsterdam, The Netherlands: 2019. pp. 185–186. [Google Scholar]
- Madsen S.L., Wong E.A. Expression of the chicken peptide transporter 1 and the peroxisome proliferator-activated receptor α following feed restriction and subsequent refeeding. Poult. Sci. 2011;90:2295–2300. doi: 10.3382/ps.2010-01173. [DOI] [PubMed] [Google Scholar]
- Maiorka A., Santin E., Dahlke F., Boleli I.C., Furlan R.L., Macari M. Posthatching water and feed deprivation affect the gastrointestinal tract and intestinal mucosa development of broiler chicks. J. Appl. Poult. Res. 2003;12:483–492. [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Noy Y., Geyra A., Sklan D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001;80:912–919. doi: 10.1093/ps/80.7.912. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Different types of early feeding and performance in chicks and poults. J. Appl. Poult. Res. 1999;8:16–24. [Google Scholar]
- Petek M., Yilmaz E., Cibik R. Effect of first feed intake time on broiler performance and carcass traits. J. Appl. Anim. Res. 2007;32:203–206. [Google Scholar]
- Potturi P.V.L., Patterson J.A., Applegate T.J. Effects of delayed placement on intestinal characteristics in turkey poults. Poult. Sci. 2005;84:816–824. doi: 10.1093/ps/84.5.816. [DOI] [PubMed] [Google Scholar]
- Reynolds K.L., Cloft S.E., Wong E.A. Changes with age in density of goblet cells in the small intestine of broiler chicks. Poult. Sci. 2020;99:2342–2348. doi: 10.1016/j.psj.2019.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki A.A. Effect of post-hatch feeding on broiler performance. Int. J. Poult. Sci. 2005;4:4–6. [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shinde A.S., Goel A., Mehra M., Rokade J., Bhadauria P., Mandal A.B., Bhanja S.K. Delayed post hatch feeding affects performance, intestinal morphology and expression pattern of nutrient transporter genes in egg type chickens. J. Nutr. Food Sci. 2015;5:1000372. [Google Scholar]
- Smirnov A., Sklan D., Uni Z. Mucin dynamics in the chick small intestine are altered by starvation. J. Nutr. 2004;134:736–742. doi: 10.1093/jn/134.4.736. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Tako E., Ferket P.R., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- Tabedian S.A., Samie A., Pourreza J., Sadeghi G. Effect of fasting or post-hatch diet's type on chick development. J. Anim. Vet. Adv. 2010;9:406–413. [Google Scholar]
- Uni Z., Ganot S., Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poult. Sci. 1998;77:75–82. doi: 10.1093/ps/77.1.75. [DOI] [PubMed] [Google Scholar]
- Uni Z., Geyra A., Ben-Hur H., Sklan D. Small intestinal development in the young chick: crypt formation and enterocyte proliferation and migration. Br. Poult. Sci. 2000;41:544–551. doi: 10.1080/00071660020009054. [DOI] [PubMed] [Google Scholar]
- Uni Z., Noy Y., Sklan D. Posthatch development of small intestinal function in the poult. Poult. Sci. 1999;78:215–222. doi: 10.1093/ps/78.2.215. [DOI] [PubMed] [Google Scholar]
- Uni Z., Smirnov A., Sklan D. Pre- and posthatch development of goblet cells in the broiler small intestine: effect of delayed access to feed. Poult. Sci. 2003;82:320–327. doi: 10.1093/ps/82.2.320. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., Preter K.D., Pattyn F., Poppe B., Roy N.V., Paepe A.D., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y., Willems E., Willemsen H., Franssens L., Koppenol A., Guo X., Tona K., Decuypere E., Buyse J., Everaert N. Spread of hatch and delayed feed access affect post hatch performance of female broiler chicks up to day 5. Animal. 2014;8:610–617. doi: 10.1017/S175173111400007X. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., Decuypere E. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Wong E.A., Gilbert E.R., Miska K.B. Nutrient transporter gene expression in poultry, livestock and fish. In: Scanes C.G., Hill R.A., editors. Biology of Domestic Animals. CRC Press, Taylor and Francis Group; Boca Raton, FL: 2018. pp. 319–344. [Google Scholar]
- Yamauchi K., Kamisoyama H., Isshiki Y. Effects of fasting and re-feeding on structures of the intestinal villi and epithelial cells in White Leghorn hens. Br. Poult. Sci. 1996;37:909–921. doi: 10.1080/00071669608417922. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Eicher S.D., Applegate T.J. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 2015;94:172–180. doi: 10.3382/ps/peu064. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li H., Kidrick J., Wong E.A. Localization of cells expressing SGLT1 mRNA in the yolk sac and small intestine. Poult. Sci. 2019;98:984–990. doi: 10.3382/ps/pey343. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Spatial transcriptional profile of PepT1 mRNA in the yolk sac and small intestine in broiler chickens. Poult. Sci. 2017;96:2871–2876. doi: 10.3382/ps/pex056. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wong E.A. Identification of cells expressing OLFM4 and LGR5 mRNA by in situ hybridization in the yolk sac and small intestine of embryonic and early posthatch chicks. Poult. Sci. 2018;97:628–633. doi: 10.3382/ps/pex328. [DOI] [PubMed] [Google Scholar]