Abstract
The aim of this study was to compare the dynamic changes of egg selenium (Se) deposition and deposition efficiency and to evaluate the efficacy of selenium-enriched yeast (SY) in laying hens over the 84 d feeding period after SY supplementation. A total of one thousand one hundred fifty-two 30-wk-old, Hy-Line Brown hens were randomly assigned to 1 of 6 groups (192 laying hens per group) with 6 replicates of 32 birds each, fed a basal diet (without Se supplementation), basal diet with 0.3 mg/kg of Se from sodium selenite (SS) or basal diets with 0.1, 0.2, 0.3, or 0.4 mg/kg of Se from SY, respectively. The results showed that the Se concentrations in the eggs and breasts from hens fed a SY-supplemented diet were significantly higher than those from hens fed a SS-supplemented diet or a basal diet (P < 0.001). There was a positive linear and quadratic correlation between Se concentrations in the eggs from hens fed a SY-supplemented diet and dietary Se supplementation on days 28, 56, and 84 (r2 = 0.931, 0.932, 0.976, P < 0.001; r2 = 0.946, 0.935, 0.976, P < 0.001), respectively. The Se deposition efficiency in whole eggs from hens fed a basal or SY-supplemented diet weresignificantly higher than those in eggs from hens fed a SS-supplemented diet on days 28, 56, and 84 (P < 0.001), respectively. In addition, there was a positive linear and quadratic correlation between Se concentrations in the eggs from hens fed SY-supplemented diet (r2 = 0.655, 0.779, 0.874, 0.781, P < 0.001; r2 = 0.666, 0.863, 0.944, 0.781, P < 0.001) or SS-supplemented diet (r2 = 0.363, P = 0.002; r2 = 0.440, P = 0.002) and number of feeding days. In conclusion, the organic Se from SY has higher bioavailability and deposition efficiency of Se in whole eggs as compared with inorganic Se from SS. The Se concentrations and Se deposition efficiency in the eggs increased from hens fed a SS- or SY-supplemented diet but decreased from hens fed a basal diet with the extension of the experimental duration. The results indicate that the dietary Se supplementation from SY should be limited to a maximum of 0.1 mg Se/kg complete feed when the eggs and meat produced from hens fed a SY-supplemented diet are used as food for humans directly, whereas up to 0.4 mg/kg organic Se from SY can be used to supplement the diets for laying hens when the products are used as raw materials for producing Se-enriched food.
Key words: selenium-enriched yeast, efficacy evaluation, egg selenium deposition, deposition efficiency, laying hens
Introduction
The importance of selenium (Se) to human health has become a focus in recent years (Danielle and David, 2003; Kieliszek and Błażejak, 2013, 2016). Selenium has antioxidant properties and protects the organism against the actions of free radicals and carcinogenic factors (Danielle and David, 2003; Kieliszek and Błażejak, 2013). It constitutes an integral part of some enzymes, including the glutathione peroxidase, type I iodothyronine deiodinase, and thioredoxin reductase (Berry et al., 1991), which help to control levels of hydrogen peroxide and lipid peroxides that are produced during normal metabolic activities (Rotruck et al., 1973; Kieliszek and Błażejak, 2016). It induces the occurrence of the selenoprotein synthesis process involved in the antioxidant defense mechanism of the organism. In addition, Se is also essential for the activity of virtually all arms of the immune system (Surai, 2000; Kieliszek and Błażejak, 2016). So, it has become increasingly evident that Se has many potential health benefits beyond meeting basic nutritional requirements.
Selenium-enriched food (meat, milk, and egg) can be used to improve the Se status of humans (Kieliszek and Błażejak, 2016). In poultry industry, it is common practice to supplement the diets of laying hens with appropriate Se to enhance the Se concentration of carcass meat and eggs. Traditionally, sodium selenite (SS) is the most common source of Se used in animal feeds, whereas organic forms, such as Se-enriched yeast (SY) and Se–methionine (SM), have been advocated and approved for use as Se additive to maintain poultry health and to increase the Se concentration of carcass meat and eggs in recent years (Federal Register, 2002; European Union, 2006; Ministry of Agriculture, 2008; Kieliszek and Błażejak, 2016). Organic Se possesses antioxidant properties and has higher bioavailability and rates of product accumulation as well as lower toxicities as compared with inorganic form (Payne et al., 2005; Pan et al., 2007; Thiry et al., 2012; Lu et al., 2018). Organic Se from SY is predominantly composed of SM, which is probably created by the yeast cellular biomass from different forms of Se and could be incorporated into carcass meat and eggs as effectively as methionine (Ochoa-Solano and Gitler, 1968; Beilstein and Whanger, 1986). Potential SM can nonspecifically bind with proteins at the methionine site, which may result in high levels of Se accumulation in tissues (Kieliszek and Błażejak, 2013, 2016). It has been reported that Saccharomyces genus yeast can accumulate up to 2.846 mg of Se in 1 g of biomass dry substance (Kieliszek et al., 2016).
Eventhough Se is an element that fulfills an important physiologic function and organic Se from SY has higher bioavailability in comparison with the inorganic form (Kieliszek and Błażejak, 2013, 2016; Lu et al., 2018), food supplementation with Se should be carried out in a careful and controlled way to avoid causing the opposite effect than intended, because selenium is one of the most toxic elements in relatively small quantities being at the same time an essential micronutrient with an important biological role (Kieliszek and Błażejak, 2013, 2016). There is a narrow line between the concentration that still has beneficial effects on an organism and that at which Se begins exerting toxic effects (Spallholz and Hoffman, 2002; Todorović et al., 2004; Kieliszek and Błażejak, 2013). Excessive Se intake may cause oxidative damage, which leads to genome instability (Thiry et al., 2012; Kieliszek and Błażejak, 2016). Therefore, the efficacy of Se source must be evaluated to ensure consumer safety, as the Se-enriched product (meat, eggs, etc.) produced by hens fed a Se-supplemented diet might be used as food or raw materials for producing Se-enriched food (European Food Safety Authority, 2012).
To provide theoretical bases for producing Se-enriched food and to ensure consumer safety from consumption of eggs and meat produced from hens fed a SY-supplemented diet, the dynamic changes in egg Se deposition and deposition efficiency were compared, and the efficacy of SY in laying hens were evaluated over the 84 d feeding period after SY supplementation.
Materials and methods
Study Design
A total of one thousand one hundred fifty-two 30-wk-old, Hy-Line Brown hens were randomly assigned to 1 of 6 groups (192 laying hens per group) with 6 replicates of 32 birds each. All birds were acclimated to a basal diet for 2 wk. At the end of wk 32, the birds were fed diets supplemented with 0 (blank control group), 0.3 mg/kg of Se from SS (analytical grade, 1% Se content, Chelota Co., Ltd., Sichuan, China, SS control group), and 0.1, 0.2, 0.3, or 0.4 mg/kg Se from SY (2,000 mg/kg Se content, Angel Yeast Co., Ltd., Hubei, China, experimental groups) for 12 wk.
Birds, Diet, and Management
This trial was carried out at the Poultry Institute of the Chinese Academy of Agricultural Sciences (Yangzhou City, Jiangsu, China). Two birds were housed in individual 37 × 40-cm wire cages, providing 740 cm2 of living space per bird, under a 16:8-h light-dark cycle at a constant temperature of 20°C ± 3°C and relative humidity of 65–75%. One cage was empty, and a chipboard was inserted into the feeders between the different replicate cages to prevent hens in 1 replicate from eating the other's diet. A corn–soybean meal basal diet (Table 1) was formulated to meet the recommendations for laying hens of the National Research Council (1994) with regard to the requirements of all nutrients except Se. Dietary Se addition was based on calculated levels for each treatment. Water and feed were provided ad libitum during the 84 d experiment. The experiment was conducted in the spring (from March to June). All animal handing protocols were approved by the Animal Care and Use Committee of the Poultry Institute.
Table 1.
Ingredients and nutrient levels of experimental diet.1
| Ingredients | Composition (%) | Nutrient | Nutrient levels |
|---|---|---|---|
| Corn | 62.70 | Metabolizable energy (MJ/kg) | 11.09 |
| Soybean meal | 26.45 | Crude protein (%) | 16.51 |
| Limestone | 8.65 | Calcium (%) | 3.50 |
| Calcium hydrogen phosphate | 1.30 | Available phosphorus (%) | 0.35 |
| DL-Met | 0.10 | Available lysine (%) | 0.85 |
| Sodium chloride | 0.30 | Available methionine (%) | 0.35 |
| Vitamin and trace mineral premix2 | 0.50 | Selenium3 (mg/kg) | 0.146 |
Values are expressed on an air-dry basis (88% DM).
Premix includes (per kilogram of diet): vitamin A, 8,800 IU; vitamin D3, 3,300 IU; vitamin E, 20 IU; cobalamine, 23 μg; riboflavin, 5.5 mg; niacin, 30 mg; pantothenic acid, 8 mg; choline, 500 mg; menadione, 1.2 mg; folic acid, 0.9 mg; pyridoxine, 1.2 mg; thiamine, 1.7 mg; biotin, 55 μg; manganese, 90.0 mg; zinc, 86.0 mg; iron, 90.00 mg; copper, 9.0 mg; and iodine, 0.6 mg.
Se level is measured value; other nutrient levels are calculated values.
Sample Collection and Analytical Determination
Observations
Cage-side observations, which included the recording of any change in clinical condition or behavior, were made at least twice daily throughout the study period.
Laying Performance
Daily egg production and egg weight were monitored during the trial. The laying rate is expressed as the average hen-day production, calculated from the total number of eggs divided by the total number of days. Feed consumption was recorded on a replicate basis at weekly intervals. The feed conversion is expressed as grams of feed consumed per grams of eggs produced.
Egg Quality
Freshly laid eggs were collected after the 84 d feeding period. For each examination, the internal and external characteristics of 36 randomly selected eggs per group (6 eggs/replicate) were recorded. The eggs were stored at room temperature before measurement, and the time interval between the eggs being laid and measured was less than 24 h.
The length and width of the eggs were measured using the FHK egg shape determinator (Fujihira Industry Co., Ltd., Tokyo, Japan), and the egg shape index was calculated. Eggshell color was measured using the EQ Reflectometer (Fujihira Industry Co., Ltd.) at 3 places (blunt, equatorial, and sharp regions) with the average used for analyses. Eggshell strength was evaluated using the EggShell Force Gauge (Robotmation Co., Ltd., Tokyo, Japan). Egg weight, albumen height, Haugh unit, and yolk color were measured using the Egg Multi Tester EMT-5200 (Robotmation Co., Ltd.). Then, the yolk and albumen were separated and individually weighed. The eggshell was weighed with the eggshell membrane intact. Eggshell thickness without the inner membranes was measured at the equatorial region. The ratios of yolk, albumen, and eggshell to egg weight were calculated.
Se Assay
Twelve eggs per replicate from each treatment group (72 eggs per group) were randomly collected on days 28, 56, and 84 and stored until Se analysis. Each individual egg was weighed and broken. Three liquid eggs were homogenized with an electric blender under chilled conditions, collected into a chilled 10-mL plastic tube as 1 sample, and stored at −30°C until determination of Se content (4 samples/replicate). At the end of the experiment (day 84), 4 hens from each replicate were slaughtered at random. Breast meat samples of about 10 g from each hen were collected and stored at −30°C until analysis. From each slaughtered hen, an aliquot (2.0 g wet weight basis) of breast meat was harvested, weighed, and homogenized by the same method as the liquid eggs. The Se deposition efficiency in whole eggs was calculated (daily egg Se deposition/daily Se consumption × 100). All samples were marked with the treatment number, replicate number, and sampling date.
All reagents used for egg preparation and Se determination were of analytical or higher grade. Selenium stock standard solution of SS [GBW(E)08,0215] and the certified Se reference material, breast [GBW 0,8551], were provided by the National Research Center for Standard Materials (Beijing, China) and the Food Detection Science Institute of the Ministry of Commerce (Beijing, China), respectively. The water used in the chemical analyses was of ultrapure grade (resistance 18 MΩ/cm). The Se content assay of diet, egg, and breast was performed following the method of Pan et al. (2007) using an AF-610A atomic fluorescence spectrometer (Beijing Beifen-Ruili Analytical Instrument (Group) Co., Ltd., Yangzhou, China).
Necropsy
All breast meat samples for Se analysis were collected from birds (n = 144) killed by exsanguination and subjected to a full postmortem examination. At necropsy, the heart, liver, spleen, lung, kidney, pancreas, preovulatory follicle, small yellow follicle, big white follicle, ovary, oviduct, and uterus were weighed (paired organs were weighed together). The numbers of preovulatory follicles, small yellow follicles, and big white follicles, as well as oviduct length were measured.
Statistical Analysis
All data were analyzed using SPSS statistical software (SPSS for Windows, version 16.0; SPSS Inc., Chicago, IL). One-way analysis of variance followed by Duncan's multiple comparison test was used to identify differences in means among treatments. Data were assumed to be statistically significant at P < 0.05.
Results
Actual Se Dietary Level of Each Treatment
The Se sources and levels supplemented in diets are shown in Table 2. The actual Se levels were confirmed by analysis. The actual Se concentrations in the blank control, SS control, and 0.1, 0.2, 0.3, and 0.4 mg/kg SY-supplemented diets were 0.146, 0.342, 0.218, 0.302, 0.359, and 0.487 mg/kg, respectively (Table 2). Hence, the results of the present study were not affected by differences between the actual and calculated Se levels.
Table 2.
Se sources and levels supplemented in diets.1
| Group | Diet | Se source | Se level (mg/kg) |
||
|---|---|---|---|---|---|
| Supplemental | Calculated | Actual | |||
| Blank control | Basal diet | — | — | 0.150 | 0.146 ± 0.002 |
| SS control | Basal diet + SS | SS | 0.30 | 0.450 | 0.342 ± 0.006 |
| 0.10 mg/kg SY | Basal diet + SY | SY | 0.10 | 0.250 | 0.218 ± 0.011 |
| 0.20 mg/kg SY | Basal diet + SY | SY | 0.20 | 0.350 | 0.302 ± 0.019 |
| 0.30 mg/kg SY | Basal diet + SY | SY | 0.30 | 0.450 | 0.359 ± 0.004 |
| 0.40 mg/kg SY | Basal diet + SY | SY | 0.40 | 0.550 | 0.487 ± 0.033 |
Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Se contents are expressed on an air-dry basis (88% DM).
Visual Observations and Laying Performance
No treatment-related adverse clinical signs were observed. As shown in Table 3, there were no statistically significant differences in the mean laying rate, average egg weight, average daily egg mass, average daily feed consumption, feed conversion ratio, and mortality rate between the treatment and control groups (P = 0.172, 0.305, 0.641, 0.913, 0.665, and 0.768, respectively).
Table 3.
Laying performance of hens during a study to evaluate SY efficacy.1
| Item | Blank control | SS control | Supplemental Se level (mg/kg of diet, SY) |
SEM | Significance | |||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | |||||
| Laying rate (%) | 93.65 | 93.77 | 93.32 | 93.72 | 93.51 | 93.53 | 0.397 | 0.172 |
| Average egg weight (g) | 60.62 | 60.10 | 60.55 | 60.86 | 59.92 | 60.87 | 0.166 | 0.305 |
| Average daily egg mass (g/bird per day) | 56.77 | 56.36 | 56.51 | 56.98 | 56.03 | 56.93 | 0.304 | 0.641 |
| Average daily feed consumption (g/bird per day) | 118.43 | 118.68 | 118.32 | 118.60 | 117.96 | 118.74 | 0.158 | 0.913 |
| Feed conversion ratio (g of feed/g of egg) | 2.09 | 2.11 | 2.09 | 2.08 | 2.11 | 2.09 | 0.012 | 0.665 |
| Mortality (%) | 4.17 | 0.52 | 1.56 | 2.60 | 1.56 | 1.56 | 0.792 | 0.768 |
Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Values are means of 6 replicates per dietary treatment.
Necropsy
After 84 d of SY treatment, there were no statistically significant changes in visceral and reproductive organ development between the treated and control hens (P > 0.05; Tables 4 and 5).
Table 4.
Visceral organs development of hens during a study to evaluate SY efficacy.1
| Item (%) | Blank control | SS control | Supplemental Se level (mg/kg of diet, SY) |
SEM | Significance | |||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | |||||
| Heart index | 0.320 | 0.356 | 0.325 | 0.308 | 0.313 | 0.325 | 0.007 | 0.689 |
| Liver index | 1.694 | 1.910 | 1.840 | 1.611 | 1.824 | 1.748 | 0.045 | 0.880 |
| Spleen index | 0.099 | 0.112 | 0.089 | 0.094 | 0.089 | 0.098 | 0.003 | 0.657 |
| Lung index | 0.370 | 0.433 | 0.412 | 0.393 | 0.352 | 0.351 | 0.011 | 0.746 |
| Kidney index | 0.693 | 0.656 | 0.638 | 0.632 | 0.644 | 0.659 | 0.011 | 0.435 |
| Pancreas index | 0.164 | 0.197 | 0.177 | 0.170 | 0.180 | 0.173 | 0.005 | 0.848 |
Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Values are means of 6 replicates per dietary treatment.
Table 5.
Reproductive organs development of hens during a study to evaluate SY efficacy.1
| Item | Blank control | SS control | Supplemental Se level (mg/kg of diet, SY) |
SEM | Significance | |||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | |||||
| Preovulatory follicle amount (n) | 5.500 | 5.500 | 5.167 | 5.667 | 5.200 | 5.333 | 0.161 | 0.533 |
| Small yellow follicle amount (n) | 20.833 | 17.167 | 19.667 | 20.333 | 21.400 | 22.000 | 1.061 | 0.970 |
| Big white follicle amount (n) | 27.500 | 37.167 | 37.167 | 35.333 | 35.400 | 26.500 | 1.695 | 0.125 |
| Oviduct length (cm) | 72.750 | 74.583 | 76.117 | 72.267 | 70.100 | 70.583 | 1.227 | 0.449 |
| Oviduct length index (cm/kg) | 42.250 | 45.457 | 44.653 | 41.339 | 41.017 | 44.644 | 0.686 | 0.270 |
| Preovulatory follicle index (%) | 1.829 | 2.045 | 1.772 | 1.823 | 1.895 | 1.872 | 0.068 | 0.549 |
| Small yellow follicle index (%) | 0.137 | 0.127 | 0.119 | 0.122 | 0.126 | 0.144 | 0.007 | 0.741 |
| Big white follicle index (%) | 0.027 | 0.031 | 0.028 | 0.030 | 0.028 | 0.019 | 0.002 | 0.300 |
| Ovary index (%) | 2.155 | 2.430 | 2.167 | 2.210 | 2.272 | 2.278 | 0.067 | 0.318 |
| Oviduct index (%) | 3.665 | 4.106 | 4.086 | 3.567 | 3.805 | 4.239 | 0.088 | 0.049 |
| Uterus index (%) | 0.186 | 0.192 | 0.201 | 0.182 | 0.166 | 0.180 | 0.005 | 0.309 |
Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Values are means of 6 replicates per dietary treatment.
Egg Quality
The external and internal qualities of fresh eggs from hens administered with different sources and doses of Se-supplemented diets are shown in Table 6. There was no significant difference in any of the egg quality traits among dietary groups (P > 0.05).
Table 6.
Egg quality of hens during a study to evaluate SY efficacy.1
| Item | Blank control | SS control | Supplemental Se level (mg/kg of diet, SY) |
SEM | Significance | |||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | |||||
| Eggshell percentage (%) | 10.44 | 10.69 | 9.66 | 9.97 | 10.00 | 10.29 | 0.092 | 0.103 |
| Yolk percentage (%) | 23.32 | 22.70 | 22.52 | 22.12 | 22.96 | 22.37 | 0.180 | 0.224 |
| Albumen percentage (%) | 66.24 | 66.60 | 67.83 | 67.91 | 67.04 | 67.34 | 0.214 | 0.076 |
| Eggshell strength (Kg/cm2) | 4.27 | 4.21 | 3.55 | 4.04 | 4.02 | 4.05 | 0.096 | 0.194 |
| Egg shape index | 1.31 | 1.31 | 1.30 | 1.31 | 1.29 | 1.30 | 0.005 | 0.609 |
| Eggshell thickness (mm) | 0.433 | 0.433 | 0.416 | 0.423 | 0.423 | 0.428 | 0.003 | 0.682 |
| Yolk color | 3.83 | 3.33 | 3.75 | 3.50 | 4.00 | 3.42 | 0.082 | 0.132 |
| Albumen height (mm) | 6.13 | 5.99 | 6.02 | 5.65 | 5.93 | 5.54 | 0.139 | 0.657 |
| Haugh Unit | 77.12 | 76.66 | 76.77 | 73.87 | 77.18 | 74.28 | 1.013 | 0.752 |
| Eggshell color | ||||||||
| L | 57.29 | 57.62 | 56.38 | 56.73 | 57.14 | 56.22 | 0.377 | 0.891 |
| a | 17.89 | 17.73 | 18.48 | 18.41 | 17.88 | 18.66 | 0.194 | 0.634 |
| b | 28.16 | 28.61 | 28.48 | 27.43 | 28.29 | 28.79 | 0.259 | 0.580 |
Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Values are means of 6 replicates per dietary treatment.
Egg Se Concentration
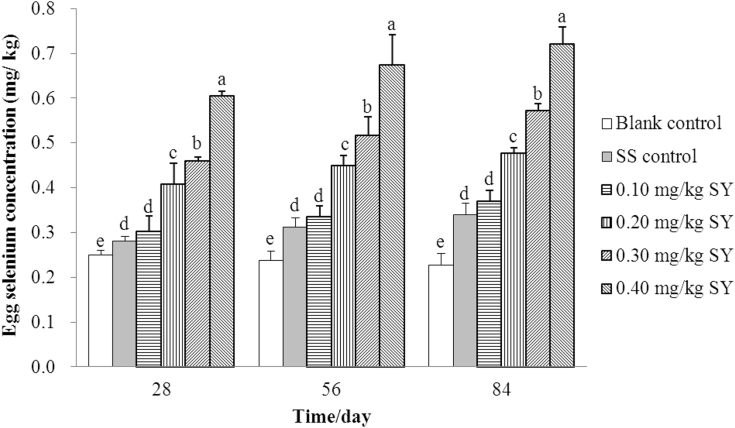
The Se concentrations in the eggs from hens fed a SS- or SY-supplemented diet were significantly higher (P < 0.001) than those in eggs from hens fed a basal diet on days 28, 56, and 84, respectively (Figure 1). The Se concentrations in the eggs from hens fed diets supplemented with 0.3 mg/kg of Se from SS or 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY were 12.42, 21.23, 62.88, 83.91, and 141.86% higher (P < 0.001); 31.04, 40.57, 88.58, 117.17, and 183.39% higher (P < 0.001); and 49.49, 62.39, 109.46, 151.17, and 217.01% higher (P < 0.001) than those in eggs from hens fed a basal diet on days 28, 56, and 84, respectively. The Se concentrations in the eggs from hens fed diets supplemented with 0.2, 0.3, and 0.4 mg/kg of Se from SY were 44.89, 63.60, and 115.14% higher (P < 0.001); 43.90, 65.72, and 116.26% higher (P < 0.001); and 40.12, 68.02, and 112.06% higher (P < 0.001) than those in eggs from hens fed diet supplemented with 0.3 mg/kg of Se from SS on days 28, 56, and 84, respectively. There were no statistically significant differences between the Se concentrations in the eggs from hens fed diets supplemented with 0.1 mg/kg of Se from SY and 0.3 mg/kg of Se from SS on days 28, 56, and 84 (P > 0.05; 0.303 vs. 0.281, 0.334 vs. 0.312, 0.369 vs. 0.340 mg/kg, respectively). In addition, there was a positive linear and quadratic correlation between Se concentrations in the eggs from hens fed a SY-supplemented diet and dietary Se supplementation on days 28 (r2 = 0.931, P < 0.001; r2 = 0.946, P < 0.001), 56 (r2 = 0.932, P < 0.001; r2 = 0.935, P < 0.001), and 84 (r2 = 0.976, P < 0.001; r2 = 0.976, P < 0.001), respectively (Table 7).
Figure 1.
Egg Se concentrations of hens over the 84 d feeding period (mg/kg, wet weight basis). Blank control = a basal diet without Se supplementation, SS control = a basal diet plus 0.3 mg/kg of Se from sodium selenite, 0.1 mg/kg SY = a basal diet plus 0.1 mg/kg of Se from selenium-enriched yeast, 0.2 mg/kg SY = a basal diet plus 0.2 mg/kg of Se from selenium-enriched yeast, 0.3 mg/kg SY = a basal diet plus 0.3 mg/kg of Se from selenium-enriched yeast, 0.4 mg/kg SY = a basal diet plus 0.4 mg/kg of Se from selenium-enriched yeast. Columns with different superscripts (a, b, c, d, e) are significantly different at P < 0.05. Egg Se concentrations for each treatment data at the same collected day are means of 6 replicates of 4 samples each (3 eggs per sample). Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Table 7.
Egg and breast Se concentrations of hens over the 84 d feeding period (mg/kg).1
| Item | Blank control | Supplemental Se level (mg/kg of diet, SY) |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | Se | Linear | Quadratic | |||
| Egg (28 d) | 0.250e | 0.303d | 0.407c | 0.459b | 0.604a | 0.024 | <0.001 | <0.001 | <0.001 |
| Egg (56 d) | 0.238e | 0.334d | 0.449c | 0.517b | 0.674a | 0.029 | <0.001 | <0.001 | <0.001 |
| Egg (84 d) | 0.227e | 0.369d | 0.476c | 0.571b | 0.721a | 0.032 | <0.001 | <0.001 | <0.001 |
| Breast (84 d) | 0.060d | 0.134c | 0.159c | 0.238b | 0.284a | 0.015 | <0.001 | <0.001 | <0.001 |
a–eMeans without a common superscripts with a row differ significantly (P < 0.05).
Abbreviations: Se, Selenium; SY, Se-enriched yeast.
Values are means of 6 replicates per dietary treatment.
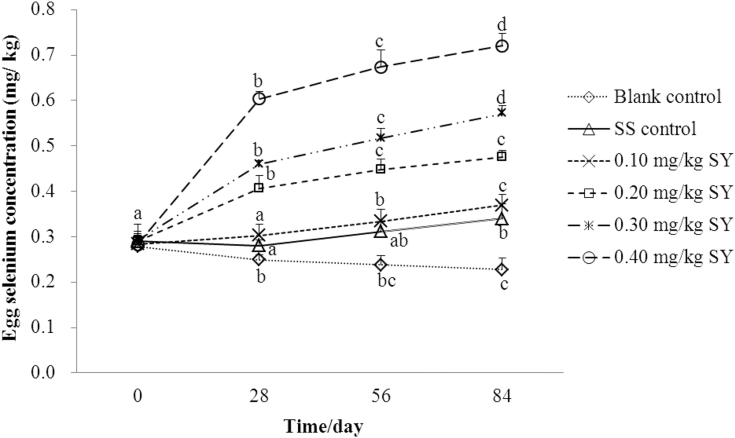
As shown in Figure 2, there was a positive linear and quadratic correlation between Se concentrations in the eggs from hens fed diets supplemented with 0.1 (r2 = 0.655, P < 0.001; r2 = 0.666, P < 0.001), 0.2 (r2 = 0.779, P < 0.001; r2 = 0.863, P < 0.001), 0.3 (r2 = 0.874, P < 0.001; r2 = 0.944, P < 0.001), and 0.4 (r2 = 0.781, P < 0.001; r2 = 0.935, P < 0.001) mg/kg of Se from SY or 0.3 mg/kg of Se from SS (r2 = 0.363, P = 0.002; r2 = 0.440, P = 0.002) and number of feeding days. However, there was a negative linear and quadratic correlation between Se concentrations in the eggs from hens fed a basal diet (r2 = 0.531, P < 0.001; r2 = 0.565, P < 0.001) and number of feeding days. The Se concentrations in the eggs from hens fed diets supplemented with 0.2, 0.3, and 0.4 mg/kg of Se from SY for 28 d were 39.67, 57.43, and 113.62% higher (P < 0.001), whereas the Se concentrations in the eggs from hens fed a basal diet for 28 d were 10.57% lower (P < 0.001) than those in eggs from hens fed the corresponding diets for 0 d, respectively. The Se concentrations in the eggs from hens fed diets supplemented with 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY for 56 d were 17.72, 54.04, 77.09, and 138.44% higher (P < 0.001), whereas the Se concentrations in the eggs from hens fed a basal diet for 56 d were 14.81% lower (P < 0.001) than those in eggs from hens fed the corresponding diets for 0 d, respectively. The Se concentrations in the eggs from hens fed diets supplemented with 0.3 mg/kg of Se from SS or 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY for 84 d were 17.25, 29.99, 63.54, 95.77, and 154.95% higher (P < 0.001), whereas the Se concentrations in the eggs from hens fed a basal diet for 84 d were 18.57% lower (P < 0.001) than those in eggs from hens fed the corresponding diets for 0 d, respectively.
Figure 2.
The dynamic change of egg Se concentrations over the 84 d feeding period (mg/kg, wet weight basis). Blank control = a basal diet without Se supplementation, SS control = a basal diet plus 0.3 mg/kg of Se from sodium selenite, 0.1 mg/kg SY = a basal diet plus 0.1 mg/kg of Se from selenium-enriched yeast, 0.2 mg/kg SY = a basal diet plus 0.2 mg/kg of Se from selenium-enriched yeast, 0.3 mg/kg SY = a basal diet plus 0.3 mg/kg of Se from selenium-enriched yeast, 0.4 mg/kg SY = a basal diet plus 0.4 mg/kg of Se from selenium-enriched yeast. Values on the same line with different superscripts (a, b, c, d) are significantly different at P < 0.05. Egg Se concentrations for each treatment data at the same collected day are means of 6 replicates of 4 samples each (3 eggs per sample). Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Egg Se Deposition Efficiency
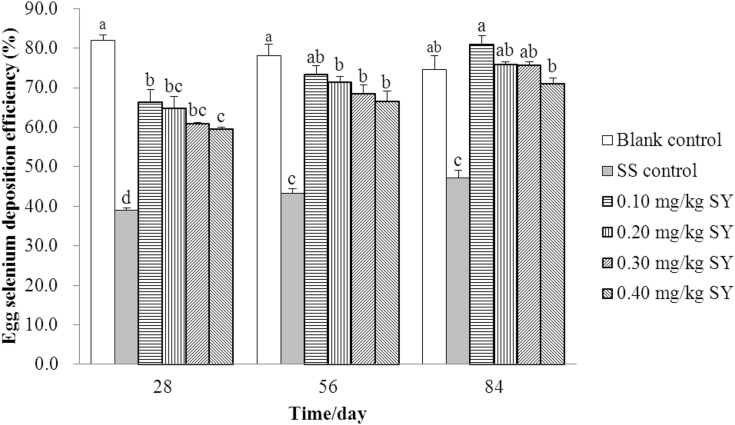
The Se deposition efficiency in whole eggs from hens fed a basal or SY-supplemented diet were significantly higher (P < 0.001) than those in eggs from hens fed a SS-supplemented diet on days 28, 56, and 84, respectively (Figure 3). The Se deposition efficiency in whole eggs from hens fed diets supplemented with 0, 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY were 110.27, 70.14, 65.88, 55.87, and 52.51% higher (P < 0.001); 80.35, 69.20, 64.77, 57.76, and 53.26% higher (P < 0.001); and 58.03, 71.33, 60.45, 59.96, and 50.25% higher (P < 0.001) than those in eggs from hens fed diet supplemented with 0.3 mg/kg of Se from SS on days 28, 56, and 84, respectively. The Se deposition efficiency in whole eggs from hens fed a basal diet were significantly higher (P < 0.001) than those in eggs from hens fed a SY-supplemented diet on days 28 and 56, but no significant difference was found on day 84 (P > 0.05). In addition, there was a negative linear and quadratic correlation between Se deposition efficiency in whole eggs from hens fed a SY-supplemented diet and dietary Se supplementation on days 28 (r2 = 0.585, P < 0.001; r2 = 0.700, P < 0.001) and 56 (r2 = 0.363, P < 0.001; r2 = 0.370, P = 0.002), respectively, whereas there was an increasing and then decreasing quadratic correlation between Se deposition efficiency in whole eggs from hens fed a SY-supplemented diet and dietary Se supplementation on day 84 (r2 = 0.335, P = 0.010; Table 8).
Figure 3.
The Se deposition efficiency in whole eggs over the 84 d feeding period (%). Blank control = a basal diet without Se supplementation, SS control = a basal diet plus 0.3 mg/kg of Se from sodium selenite, 0.1 mg/kg SY = a basal diet plus 0.1 mg/kg of Se from selenium-enriched yeast, 0.2 mg/kg SY = a basal diet plus 0.2 mg/kg of Se from selenium-enriched yeast, 0.3 mg/kg SY = a basal diet plus 0.3 mg/kg of Se from selenium-enriched yeast, 0.4 mg/kg SY = a basal diet plus 0.4 mg/kg of Se from selenium-enriched yeast. Columns with different superscripts (a, b, c, d) are significantly different at P < 0.05. Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Table 8.
The Se deposition efficiency in whole eggs over the 84 d feeding period (%).1
| Item | Blank control | Supplemental Se level (mg/kg of diet, SY) |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | Se | Linear | Quadratic | |||
| 28 d | 81.94a | 66.30b | 64.65b,c | 60.74b,c | 59.44c | 1.736 | <0.001 | <0.001 | <0.001 |
| 56 d | 78.10a | 73.28a,b | 71.36a,b | 68.32b | 66.37b | 1.239 | <0.001 | <0.001 | 0.002 |
| 84 d | 74.61a,b | 80.89a | 75.75a,b | 75.52a,b | 70.94b | 1.033 | 0.036 | 0.082 | 0.010 |
a–cMeans without a common superscripts with a row differ significantly (P < 0.05).
Abbreviations: Se, Selenium; SY, Se-enriched yeast.
Values are means of 6 replicates per dietary treatment.
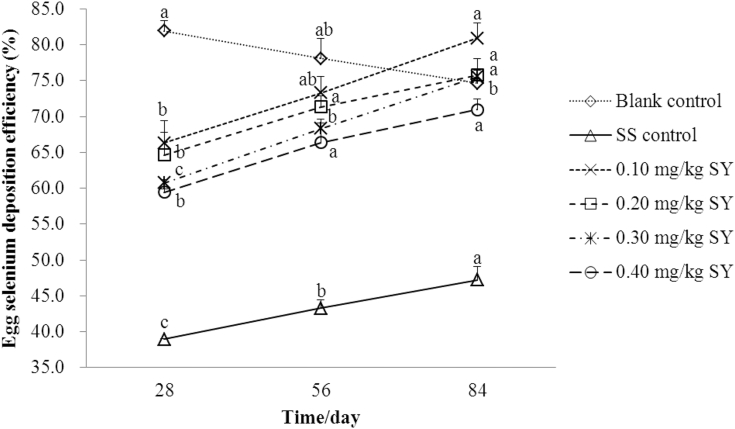
As shown in Figure 4, there was a positive linear and quadratic correlation between Se deposition efficiency in whole eggs from hens fed diets supplemented with 0.1 (r2 = 0.520, P = 0.001; r2 = 0.520, P = 0.004), 0.2 (r2 = 0.481, P = 0.001; r2 = 0.488, P = 0.007), 0.3 (r2 = 0.772, P < 0.001; r2 = 0.772, P < 0.001), and 0.4 (r2 = 0.570, P < 0.001; r2 = 0.578, P = 0.002) mg/kg of Se from SY or 0.3 mg/kg of Se from SS (r2 = 0.580, P < 0.001; r2 = 0.581, P = 0.001) and number of feeding days. However, there was a negative linear and quadratic correlation between Se deposition efficiency in whole eggs from hens fed a basal diet (r2 = 0.397, P = 0.009; r2 = 0.397, P = 0.015) and number of feeding days. The Se deposition efficiency in whole eggs from hens fed diets supplemented with 0.3 mg/kg of Se from SS or 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY for 56 d were 11.13, 10.51, 10.38, 12.48, and 11.67% higher (P < 0.001), whereas the deposition efficiency in whole eggs from hens fed a basal diet for 56 d were 4.68% lower (P < 0.001) than those in eggs from hens fed the corresponding diets for 28 d, respectively. The Se deposition efficiency in whole eggs from hens fed diets supplemented with 0.3 mg/kg of Se from SS, or 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY for 84 d were 21.15, 22.00, 17.18, 24.34, and 19.36% higher (P < 0.001), whereas the deposition efficiency in whole eggs from hens fed a basal diet for 84 d were 8.95% lower (P < 0.001) than those in eggs from hens fed the corresponding diets for 28 d, respectively.
Figure 4.
The dynamic change of Se deposition efficiency in whole eggs over the 84 d feeding period (%). Blank control = a basal diet without Se supplementation, SS control = a basal diet plus 0.3 mg/kg of Se from sodium selenite, 0.1 mg/kg SY = a basal diet plus 0.1 mg/kg of Se from selenium-enriched yeast, 0.2 mg/kg SY = a basal diet plus 0.2 mg/kg of Se from selenium-enriched yeast, 0.3 mg/kg SY = a basal diet plus 0.3 mg/kg of Se from selenium-enriched yeast, 0.4 mg/kg SY = a basal diet plus 0.4 mg/kg of Se from selenium-enriched yeast. Values on the same line with different superscripts (a, b, c) are significantly different at P < 0.05. Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
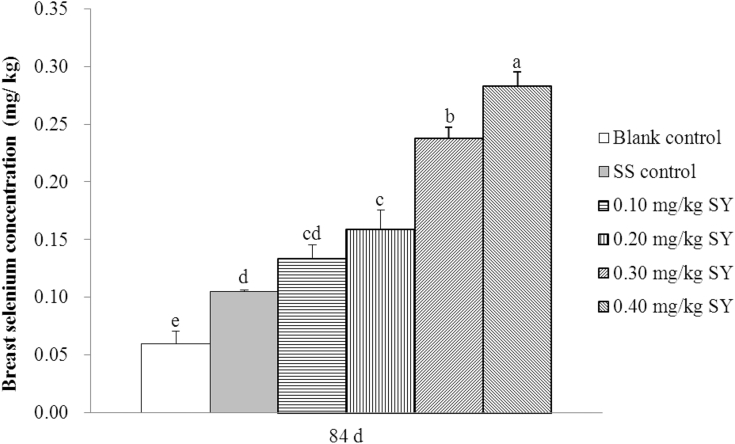
Breast Se Concentration
The Se concentrations in the breasts from hens fed diets supplemented with 0.3 mg/kg of Se from SS or 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY were 75.98, 124.86, 166.48, 299.44, and 375.42% higher (P < 0.001) than those in breasts from hens fed a basal diet after the 84 D feeding period, respectively (Figure 5). The Se concentrations in the breasts from hens fed diets supplemented with 0.2, 0.3, and 0.4 mg/kg of Se from SY were 51.43, 126.98, and 170.16% higher than those in breasts from hens fed diet supplemented with 0.3 mg/kg of Se from SS on day 84 (P < 0.001), respectively. The Se concentrations in the breasts were similar from hens fed diets supplemented with 0.1 mg/kg of Se from SY or 0.3 mg/kg of Se from SS (P > 0.05; 0.134 vs. 0.105 mg/kg). In addition, there was a positive linear (r2 = 0.907, P < 0.001) and quadratic (r2 = 0.907, P < 0.001) correlation between Se concentrations in the breasts from hens fed a SY-supplemented diet and dietary Se supplementation (Table 7).
Figure 5.
Breast Se concentrations of hens after the 84 d feeding period (mg/kg, wet weight basis). Blank control = a basal diet without Se supplementation, SS control = a basal diet plus 0.3 mg/kg of Se from sodium selenite, 0.1 mg/kg SY = a basal diet plus 0.1 mg/kg of Se from selenium-enriched yeast, 0.2 mg/kg SY = a basal diet plus 0.2 mg/kg of Se from selenium-enriched yeast, 0.3 mg/kg SY = a basal diet plus 0.3 mg/kg of Se from selenium-enriched yeast, 0.4 mg/kg SY = a basal diet plus 0.4 mg/kg of Se from selenium-enriched yeast. Columns with different superscripts (a, b, c, d, e) are significantly different at P < 0.05. Breast Se concentrations for each treatment data are means of 6 replicates of 4 samples each (1 bird per sample). Abbreviations: Se, selenium; SS, sodium selenite; SY, Se-enriched yeast.
Discussion
In the present study, no statistically significant differences were found among all laying performance parameters, no macroscopic observations were noted at necropsy, and no histological changes were considered to be related to treatment. In addition, these doses did not adversely affect the visceral and reproductive organ development. According to the 2009 annual report of the European Food Safety Authority, the health status, mortality rate, laying performance, clinical chemistry parameters (glucose, total protein, albumin, total bilirubin, cholesterol, and enzymatic activities of aspartate transaminase, alanine aminotransferase, and alkaline phosphatase) of laying hens (Isa-Brown-Warren, 22 wk of age) were not affected by a basal diet (without Se supplementation) supplemented with 0.4 or 5.7 mg/kg of Se from SS or SY (from Saccharomyces cerevisiae CNCM I-3399) for 56 d (European Food Safety Authority, 2009). However, the laying rate decreased when laying hens were supplemented with more than 7 mg/kg of Se from SS (Arnold et al., 1973; Ort and Latshaw, 1978; Cantor et al., 1984). In addition, no adverse morphological or histological changes were noticed in the liver or kidneys of chickens (Hybro) fed 2.0 or 5.0 mg/kg Se from SS, and 2.0, 5.0, 10.0, or 15.0 mg/kg Se from SY for 42 d, but higher concentrations (10.0 mg/kg Se from SS, 20.0 mg/kg Se from SY, and higher) caused certain alterations in liver and kidney (Todorović et al., 2004). The results of the aforementioned studies might indicate that there is a fine line between the concentration that still has beneficial effects on an organism and that at which Se begins exerting toxic effects. On the one hand, Se deficiency leads primarily to degeneration of many organs and tissues, resulting from decreased expression of selenoproteins, and thereby changes in the biological processes in which it participates (Pedrero and Madrid, 2009). The requirement for Se of laying hens ranges from 0.05 to 0.08 mg/kg depending on daily feed consumption (National Research Council, 1994). In the present study, the actual Se concentration of 0.146 mg/kg in the basal diets met the basic nutritional requirements, which might be a possible explanation for the zootechnical parameters when the birds were fed a basal diet. On the other hand, high levels of Se in the organism cause serious liver damage, decreased triiodothyronine concentration, and the loss of natural killer cells (Navarro-Alarcon and Cabrera-Vique, 2008). The toxic effects of Se on the organism are related to the production of free radicals causing DNA damage. Toxic effects of Se are also associated with affinity toward thiol groups affecting disorder of the integrity of protein functions responsible for DNA repair (Letavayová et al., 2008). Reduced laying rate and feed consumption are the 2 main external signs (Payne et al., 2005), and the occurrence of hematological abnormalities in blood and the damage of organs are the internal signs of Se toxicity in laying hens (Khanal and Knight, 2010; Thiry et al., 2012). The maximum tolerable level of Se for poultry set by the National Research Council (2005) is 3 mg Se/kg dry matter feed. Apparently, the actual concentration of Se in the experimental diets had not exceeded the toxic dose. Based on the results of this study as well as those of the aforementioned studies, it can be concluded that dietary treatment in this trial appear to produce no adverse side-effects or undesirable changes in performance and organs following daily administration to laying hens for 84 d.
The treatments did not affect the egg quality of fresh eggs significantly, and this is in accordance with the results obtained in previous reports (Payne et al., 2005; Pan et al., 2011; Han et al., 2017). However, other studies have reported that supplementation with SY significantly affected shell weight, shell thickness, and Haugh unit positively compared with the selenite and control groups (Pappas et al., 2005; Arpasova et al., 2009; Baylan et al., 2011). In our study, no beneficial or harmful effects on fresh egg quality were found after the 84 d feeding period. The difference in basal Se level, added Se level, genetic factors, temperature and humidity of the environment, and the time of storage might explain the discrepancy among some of these results. The basal diet used in this experiment contained 0.146 mg/kg Se from dietary ingredients as organic form. The majority of the organic Se in plant or SY is in the form of SM, which could be actively absorbed and can be directly incorporated into protein (Ochoa-Solano and Gitler, 1968; Beilstein and Whanger, 1986). Se-methionine is deposited in the egg to a greater extent, and the Se in SM would be incorporated into glutathione peroxidase (Payne et al., 2005; Kieliszek and Błażejak, 2013, 2016). The Se supplementation can increase the glutathione peroxidase activity of eggs, and the extent of increase might be positively correlated to the dietary Se level (Pan et al., 2011). This increase in glutathione peroxidase activity would protect the egg from damage by free radicals, resulting in decreased potential of cellular damage to the shell or fluid egg (Payne et al., 2005; Pan et al., 2011). The Se concentration in the eggs from hens fed a basal diet for 84 d was 0.227 mg/kg, which might be above the requirement for Se, for incorporation into glutathione peroxidase protecting fresh egg from free-radical damage. The bird used in the current experiment was 30-wk-old Hy-Line Brown hens, which was not the same as that used in those experiments. In addition, the discrepancy among some of those results might be related to the temperature and humidity of the environment and the time of storage. Thus, more defined reasons must be further investigated.
In the present study, the Se concentrations in eggs and breasts increased with the Se supplementation from either Se source (SS or SY), and the Se concentrations were significantly higher from hens fed a SY-supplemented diet than those from hens fed a SS-supplemented diet. In addition, the Se concentrations in eggs and breasts were gradually increased with an increase of the dietary organic Se from SY supplementation. The results of this experiment are consistent with those of many studies. The addition of commercial SY or SS significantly increased Se concentrations in the egg and tissue (liver, kidney, spleen, and cardiac and breast muscles) of laying hens in comparison with the control, and SY supplementation increased Se concentrations in egg and tissue of laying hens more than SS supplementation (Payne et al., 2005; Utterback et al., 2005; Pan et al., 2007). The present study proved that the organic Se from SY had higher bioavailability and rates of product accumulation, as compared with inorganic Se from SS. In addition, the results in this trial also suggested that dietary Se was gradually transferred into eggs and the Se deposition efficiency from hens fed a SS- or SY-supplemented diet increased, whereas the Se concentrations in the eggs and Se deposition efficiency from hens fed a basal diet decreased with the extension of the experimental duration. The different absorption pathways might be a possible explanation for the different bioavailabilities and rates of product accumulation when the birds were fed a SY- or SS-supplemented diet (Kieliszek and Błażejak, 2013, 2016). The organic Se in SY is predominantly composed of SM, which could be incorporated into eggs as effectively as methionine (Ochoa-Solano and Gitler, 1968; Beilstein and Whanger, 1986). The organic Se sources, such as SM or SY, are actively absorbed and can be directly incorporated into protein, whereas inorganic Se sources, such as SS, are required for selenocysteine synthesis and passively absorbed by the body (Ochoa-Solano and Gitler, 1968; Latshaw and Biggert, 1981). Consequently, the Se deposition efficiency in whole eggs from hens fed a basal or SY-supplemented diet were much higher than those in eggs from hens fed a SS-supplemented diet.
The breast Se concentrations reflected the increases in dietary Se levels (Se from SY), whereas the egg Se deposition of laying hens administered different doses of Se from SY for 28, 56, and 84 d also responded significantly to SY supplementation. Based on the present study, a 60-g egg from hens fed diets supplemented with 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY for 84 d will provide approximately 22.15 to 43.24 μg of Se, which are about 8.63 to 112.06% higher than those in eggs from hens fed diet supplemented with 0.3 mg/kg of Se from SS, and a 100-g portion of breast muscle from hens fed diets supplemented with 0.1, 0.2, 0.3, and 0.4 mg/kg of Se from SY for 84 d will provide approximately 13.42 to 28.37 μg of Se, which are about 27.78 to 170.16% higher than those in breasts from hens fed diet supplemented with 0.3 mg/kg of Se from SS. Obviously, the eggs and breast muscle produced from hens fed a SY-supplemented diet had higher Se concentrations than those from hens fed a SS-supplemented diet. According to the annual report of the European Food Safety Authority, the daily intake of selenium in the European population is estimated between 20 and 70 μg (European Food Safety Authority, 2008), and the maximal intake of Se for adults is 300 μg/day (European Food Safety Authority, 2012). According to the Referenced Dietary Nutrient Intake of Chinese published by the Nutrition Council of China in 2000, the adequate intake of Se for people >14 yr old is 50 μg/day and the maximal intakes for youths (14–18 yr old) and those >18 yr old are 360 and 400 μg/day, respectively (Nutrition Council of China, 2001). Obviously, it is safe for healthy persons to consume 2 eggs per day or 1 egg and 100 g of breast muscle per day as produced from hens fed diets supplemented with 0.1 mg/kg of Se from SY for 84 d, which would not only provide enough Se for people >14 yr old, but also provide many potential health benefits.
In conclusion, our study findings reveal that the organic Se from SY has higher bioavailability and deposition efficiency of Se in whole eggs as compared with inorganic Se from SS. The Se concentrations and Se deposition efficiency in the eggs increased from hens fed a SS- or SY-supplemented diet but decreased from hens fed a basal diet with the extension of the experimental duration. The results indicate that the dietary Se supplementation from SY should be limited to a maximum of 0.1 mg Se/kg complete feed when the eggs and meat produced from hens fed a SY-supplemented diet are used as food for humans directly, whereas up to 0.4 mg/kg organic Se from SY can be used to supplement the diets for laying hens when the products are used as raw materials for producing Se-enriched food.
Acknowledgments
The work was supported in part by the China Agriculture Research Systems (CARS-40-K01), Natural Science Foundation of Jiangsu Province (BK20190240) P. R. China, Program for the Agricultural Major New Variety of the Jiangsu Province (PZCZ201729) P. R. China, Independent research funds for public welfare research institutes of the Jiangsu Province (BM2018026) P. R. China, Project of Key Laboratory for Poultry Genetics and Breeding of Jiangsu province P. R. China (JQLAB-ZZ-201801), and Yangzhou Talent Cultivation Program ((2018)YZYC-040).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Contributor Information
L. Qu, Email: quliang19820603@163.com.
K.H. Wang, Email: sqbreeding@126.com.
References
- Arnold R.L., Olson O.E., Carlson C.W. Dietary selenium and arsenic additions and their effects on tissue and egg selenium. Poult. Sci. 1973;52:847–854. doi: 10.3382/ps.0520847. [DOI] [PubMed] [Google Scholar]
- Arpasova H., Petrovic V., Mellen M., Kacaniova M., Cobanova K., Leng L. The effects of supplementing sodium selenite and selenized yeast to the diet for laying hens on the quality and mineral content of eggs. J. Anim. Feed Sci. 2009;18:90–100. [Google Scholar]
- Baylan M., Canogullari S., Ayasan T., Copur G. Effects of dietary selenium source, storage time, and temperature on the quality of quail eggs. Biol. Trace. Elem. Res. 2011;143:957–964. doi: 10.1007/s12011-010-8912-x. [DOI] [PubMed] [Google Scholar]
- Beilstein M.A., Whanger P.D. Deposition of dietary organic and inorganic selenium in rat erythrocyte proteins. J. Nutr. 1986;116:1701–1710. doi: 10.1093/jn/116.9.1701. [DOI] [PubMed] [Google Scholar]
- Berry M.J., Banu L., Larsen P.R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991;349:438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Cantor A.H., Nash D.M., Johnson T.H. Toxicity of selenium in drinking water of poultry. Nutr. Rep. Int. 1984;29:683–688. [Google Scholar]
- Danielle R.E., David E.S. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003;6:273–279. doi: 10.1016/s1369-5266(03)00030-x. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority Scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission on selenium-enriched yeast as source for selenium. EFSA J. 2008;766:1–42. [Google Scholar]
- European Food Safety Authority Safety and efficacy of SELSAF (Selenium enriched yeast from Saccharomyces cerevisiae CNCM I-3399) as feed additive for all species. EFSA J. 2009;992:1–24. [Google Scholar]
- European Food Safety Authority Scientific Opinion on safety and efficacy of selenium in the form of organic compounds produced by the selenium-enriched yeast Saccharomyces cerevisiae NCYC R646 (Selemax 1000/2000) as feed additive for all species. EFSA J. 2012;10:2778. [Google Scholar]
- European Union Concerning the authorization of selenomethionine as a feed additive. Off. J. Eur. Union. 2006;330:9–11. [Google Scholar]
- Federal Register Food additives permitted in feed and drinking water: selenium yeast. Fed. Regist. 2002;67:46850–46851. [Google Scholar]
- Han X.J., Qin P., Li W.X., Ma Q.G., Ji C., Zhang J.Y., Zhao L.H. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult. Sci. 2017;96:3973–3980. doi: 10.3382/ps/pex216. [DOI] [PubMed] [Google Scholar]
- Khanal D.R., Knight A.P. Selenium: its role in livestock health and productivity. J. Agric. Environ. 2010;11:101–106. [Google Scholar]
- Kieliszek M., Błażejak S. Selenium: significance and outlook for supplementation. Nutrition. 2013;29:713–718. doi: 10.1016/j.nut.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Kieliszek M., Błażejak S. Current knowledge on the importance of selenium in food for living organisms: a review. Molecules. 2016;21:609. doi: 10.3390/molecules21050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M., Błażejak S., Płaczek M. Spectrophotometric evaluation of selenium binding by Saccharomyces cerevisiae ATCC MYA-2200 and Candida utilis ATCC 9950 yeast. J. Trace Elem. Med. Bio. 2016;35:90–96. doi: 10.1016/j.jtemb.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Latshaw J.D., Biggert M.D. Incorporation of selenium into egg proteins after feeding selenomethionine or sodium selenite. Poult. Sci. 1981;60:1309–1313. [Google Scholar]
- Letavayová L., Vlasáková D., Spallholz J.E., Brozmanová J., Chovanec M. Toxicity and mutagenicity of selenium compounds in Saccharomyces cerevisiae. Mutat. Res. 2008;638:1–10. doi: 10.1016/j.mrfmmm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Lu J., Qu L., Shen M.M., Hu Y.P., Guo J., Dou T.C., Wang K.H. Comparison of dynamic change of egg selenium deposition after feeding sodium selenite or selenium-enriched yeast. Poult. Sci. 2018;97:3102–3108. doi: 10.3382/ps/pey161. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture . Ministry of Agriculture of People’s Republic of China Bulletin; Beijing, China: 2008. Approved Feed Additives. pp. 1224–1226. [Google Scholar]
- Nutrition Council of China Chinese dietary reference intakes. Acta Nutr. Sin. 2001;23:193–196. [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- National Research Council . 2nd rev. ed. Natl. Acad. Press; Washington DC: 2005. Mineral Tolerance of Animals. [Google Scholar]
- Navarro-Alarcon M., Cabrera-Vique C. Selenium in food and the human body: a review. Sci. Total Environ. 2008;400:115–141. doi: 10.1016/j.scitotenv.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Ochoa-Solano A., Gitler C. Incorporation of 75Se-selenomethionine and 35Se-methionine into chicken egg white protein. J. Nutr. 1968;94:243–248. doi: 10.1093/jn/94.2.243. [DOI] [PubMed] [Google Scholar]
- Ort J.F., Latshaw J.D. The toxic level of sodium selenite in the diet of laying chickens. J. Nutr. 1978;108:1114–1120. doi: 10.1093/jn/108.7.1114. [DOI] [PubMed] [Google Scholar]
- Pan C.L., Huang K.H., Zhao Y.X., Qin S.Y., Chen F., Hu Q.H. Effect of selenium source and level in hen’s diet on tissue selenium deposition and egg selenium concentrations. J. Agric. Food Chem. 2007;55:1027–1032. doi: 10.1021/jf062010a. [DOI] [PubMed] [Google Scholar]
- Pan C.L., Zhao Y.X., Liao S.F., Chen F., Qin S.Y., Wu X.S., Zhou H., Huang K.H. Effect of selenium-enriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J. Agric. Food Chem. 2011;59:11424–11431. doi: 10.1021/jf202014k. [DOI] [PubMed] [Google Scholar]
- Pappas A.C., Acamovic T., Sparks N.H., Surai P.F., McDevitt R.M. Effects of supplementing broiler breeder diets with organic selenium and polyunsaturated fatty acids on egg quality during storage. Poult. Sci. 2005;84:865–874. doi: 10.1093/ps/84.6.865. [DOI] [PubMed] [Google Scholar]
- Payne R.L., Lavergne T.K., Southern L.L. Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poult. Sci. 2005;84:232–237. doi: 10.1093/ps/84.2.232. [DOI] [PubMed] [Google Scholar]
- Pedrero Z., Madrid Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: a review. Anal. Chim. Acta. 2009;634:135–152. doi: 10.1016/j.aca.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Spallholz J.E., Hoffman D.J. Selenium toxicity: cause and effects in aquatic birds. Aquat. Toxicol. 2002;57:27–37. doi: 10.1016/s0166-445x(01)00268-5. [DOI] [PubMed] [Google Scholar]
- Surai P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2000;41:235–243. doi: 10.1080/713654909. [DOI] [PubMed] [Google Scholar]
- Thiry C., Ruttens A., De Temmerman L., Schneider Y., Pussemier L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012;130:767–784. [Google Scholar]
- Todorović M., Jovanović M., Jokić Ž., Hristov S., Davidović V. Alterations in liver and kidneys of chickens fed with high levels of sodium selenite or selenized yeast. Acta Vet. (Beograd) 2004;54:191–200. [Google Scholar]
- Utterback P.L., Parsons C.M., Yoon I. Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poult. Sci. 2005;84:1900–1910. doi: 10.1093/ps/84.12.1900. [DOI] [PubMed] [Google Scholar]