Abstract
The aim of this study was to investigate the effects of selenium (Se)-enriched Saccharomyces cerevisiae (SSC) on meat quality and to elucidate the underlying mechanisms in broilers. A total of 200 one-day-old Arbor Acres broiler chickens were randomly allocated to one of four treatments with 5 replications of 10 chickens each. Group 1 served as a control and was fed a basal diet without Se supplementation, while groups 2, 3, and 4 were fed the basal diet supplemented with S. cerevisiae (SC), sodium selenite (SS), and SSC, respectively. Breast muscle samples were collected to evaluate meat quality, selenium concentration, oxidative stability, and the mRNA levels of antioxidant enzyme genes on day 42. As compared with groups 1 and 2, SS and SSC supplementation increased Se concentration, glutathione peroxidase (GPx) and thioredoxin reductase (TR) activities, total antioxidant capacity, and the mRNA levels of GPx-1, GPx-4, TR-1, and TR-3 (P < 0.05) and decreased drip loss and malondialdehyde (MDA) content (P < 0.05). As compared with group 3, SSC supplementation increased pH, lightness, yellowness, Se concentration, GPx and superoxide dismutase activities, and the mRNA levels of GPx-1 and GPx-4 (P < 0.05) but decreased drip loss and MDA content (P < 0.05). Thus, SSC improved meat quality and oxidative stability by activating the glutathione and thioredoxin systems, which should be attributed to the combined roles of Se and SC.
Key words: selenium, Saccharomyces cerevisiae, meat quality, glutathione, thioredoxin
Introduction
Chicken meat is popular all over the world because of its delicious taste, rich nutrition, and so on. Healthy chicken color is white through red and bright and should feel relatively smooth, as more and more people begin to pay attention to the quality of chicken. As an important trace element, selenium (Se) is an essential micronutrient for the health, growth, reproduction, and immunity of both humans and animals (Rayman, 2000; Hosnedlova et al., 2017; Ying and Zhang, 2019). An increasing body of evidence reinforces the importance of adequate Se intake to maintain normal muscle function (Pappas et al., 2012; Chen et al., 2017; Zoidis et al., 2018), as Se deficiency reduces the expression and activity of several essential selenoproteins, which can lead to myodegenerative diseases, such as Keshan disease, a potentially fatal form of cardiomyopathy, in humans, mulberry heart disease in pigs, and white muscle disease in foals (Delesalle et al., 2017; Hosnedlova et al., 2017).
The bioavailability as well as the pharmacological and toxicological effects of Se in animals was associated with its chemical forms (Han et al., 2017). In comparison with inorganic Se (sodium selenite, SS), organic Se (Se yeast, SY) was an antioxidant that provided greater protection against oxidative damage and was less toxic (Chen et al., 2017; Li et al., 2018). Previous studies had reported that dietary Se supplementation improved biological functions in animals (Han et al., 2017; Khan et al., 2018; Silva et al., 2020). Moreover, dietary supplementation with Se-enriched Saccharomyces cerevisiae (SSC) was useful to maintain the health of broilers (Chen et al., 2017), However, few studies had investigated the effects of SY supplementation on the meat quality of broilers (Pappas et al., 2012; Chen et al., 2014; Markovic et al., 2018), which was regarded as the most important indicator of production performance. Therefore, the aim of the prestent study was to investigate the effects of SSC on the meat quality of broilers and to elucidate the potential underlying mechanisms.
Materials and methods
Chickens and Treatment
A total of 200, one-day-old Arbor Acres, broilers were randomly allocated to one of four experimental groups with 5 replicate pens of 10 chickens each. Group 1 (control group) was fed a basal diet without Se supplementation; group 2 was fed the basal diet supplemented with 1.0 g (108 cfu) of S. cerevisiae (SC) per kg of feed; group 3 was fed the basal diet supplemented with 0.30 mg of Se per kg of feed as SS (analytical grade, >98.0% pure); and group 4 was fed the basal diet supplemented with SSC (1.0 g of Se per kg of feed). The basal diet was formulated in accordance with the dietary guidelines established by the National Research Council. The addition of Se was based on the calculated levels for each source. The basal diet formulation and approximate composition are shown in Table 1. Over the entire experimental period of 42 d, water was provided ad libitum. All chickens were fed a starter diet from day 1 to 21 and then switched to a basal developer diet from day 22 to 42. The animal treatment, housing, and husbandry conditions conformed to the experimental guidelines of the National Laboratory Animal Standardization Technical Committee of China (SAC/TC 281). The experimental protocols were designed in accordance with the regulations for the use of experimental animals and approved by the Ethical Committee for Use of Laboratory Animals of Qingdao Agricultural University (Qingdao, China).
Table 1.
Formulation and proximate composition of experimental diets.
| Ingredient (%) | 1–21 d | 22–42 d |
|---|---|---|
| Corn | 60.00 | 64.50 |
| Corn protein flour | 5.00 | 3.00 |
| Wheat bran | 0.00 | 2.00 |
| Soybean meal | 27.50 | 25.00 |
| Fishmeal (55.5% CP) | 3.40 | 1.60 |
| Stone powder | 1.20 | 1.40 |
| Salt | 0.30 | 0.30 |
| Calcium bicarbonate | 1.50 | 1.20 |
| Methionine | 0.10 | 0.00 |
| Premix1 | 1.00 | 1.00 |
| Chemical composition (g/kg DM) | ||
| Gross energy (MJ/kg) | 12.15 | 13.06 |
| Crude protein | 22.99 | 20.00 |
| Calcium | 1.00 | 0.90 |
| Phosphorus | 0.45 | 0.35 |
| Methionine | 0.50 | 0.38 |
| Lysine | 1.10 | 1.00 |
The vitamins provided (per kg feed): vitamin A, 1,500 IU; vitamin D3, 200 IU; vitamin E, 20 IU; vitamin K, 0.5 mg; vitamin B1, 22 mg; vitamin B2, 8.5 mg; vitamin B12, 0.2 mg; folicin, 0.55 mg; niacin, 0.55 mg; pantothenic acid, 10.0 mg; copper, 8.0 mg; zinc, 40.0 mg; iron, 80.00 mg; iodine, 0.35 mg; manganese, 60.0 mg.
Sampling and Processing
At the end of the experiment, 5 chickens per pen were euthanized with sodium pentobarbitone after feed deprivation overnight. The left pectoralis muscle of each bird was collected for analysis of physical and chemical characteristics. Five grams of fresh pectoralis muscle per bird was placed into a plastic centrifuge tube and frozen in liquid nitrogen for analysis of gene mRNA levels. The right pectoralis muscle was placed in a polyethylene bag and frozen at −70°C for analysis of antioxidase activities and oxidative damage.
Determination of Physical Characteristics of Meat
Immediately after slaughter, the physical characteristics of each muscle sample were assessed. Meat color lightness, redness, and yellowness was measured using a CR410 Chroma Meter (Konica Minolta Sensing Singapore Pte Ltd., Singapore). The pH value was measured using a digital pH meter (pH-STAR; Matthäus GmbH & Co. KG, Eckelsheim, Germany). Shear force was measured with a digital muscle tenderness meter (C-LM3; Tenovo International Co., Ltd., Beijing, China). Drip loss was estimated by the suspension method as the difference between the final and initial weights of a meat sample suspended in a bottle and stored at 4°C for 72 h before and after drip and expressed as the percentage of the initial weight. These procedures were performed in triplicate, and average values were calculated for analysis.
Determination of Chemical Composition of Meat
The chemical composition of the muscle samples was determined, including moisture, crude fat, and crude protein. In brief, 100 g of each sample was minced for 2 times using a meat grinder and mixed well. Then, 5 g of each mixed sample was used to detect the moisture content, while the remaining sample was placed in an electric blast dryer at 105°C to remove moisture for analysis of crude fat and crude protein contents. Moisture content was determined by the heating and drying method in accordance with the Chinese National Standard GB/T 9695.15-2008. Crude fat content was measured by the Soxhlet extractor method in accordance with the Chinese National Standard GB/T 9695.7-2008. Crude protein content was assessed by the Kjeldahl method in accordance with the Chinese National Standard GB/T 5009.5-2016. The percentage of moisture content was calculated as 100 × (initial muscle weight−final muscle weight)/initial muscle weight. The percentage of crude fat was calculated as 100 × (initial muscle weight−final muscle weight)/initial muscle weight. The crude protein content was calculated as the nitrogen content × 6.25. These procedures were performed in duplicate, and average values were calculated for analysis.
Determination of Se Concentration of Meat
The Se concentration of each muscle sample was determined according to the method described by Qin et al. (2015). In brief, 1.0 g of each sample was weighted accurately and placed in a 50-mL triangular flask with 10 mL of acid digest solution (HNO3HClO4 = 41). After 24 h of predigestion at room temperature, the sample was heated to 180°C on an electric heating plate until white fumes appeared. After cooling to room temperature, 10 mL of 5M hydrochloric acid solution was added to the flask, and the mixture was again heated until clarified. Then, the solution digested samples were evaporated to almost 2 mL, transferred to a 25-mL volumetric flask, and mixed with 20 mL of 2M hydrochloric acid solution and 1 mL of 10% potassium ferricyanide solution, to a final volume of 25 mL with 2M hydrochloric acid solution. Ultrapure water and a certified reference material for Se (GBW 08551 pork liver Food Detection Science Institute, Ministry of Commerce, Beijing, China) served as the blank and the standard control which were digested by the same method. Se standard solution at various concentrations was used to construct standard curves for the Se assay. The Se concentrations of the treated samples were detected using an atomic fluorescence spectrometer (AFS-9330; Beijing Titan Instruments Co., Ltd., Beijing, China). These procedures were performed in duplicate, and the average values were calculated for analysis.
Determination of Oxidative Stability of Meat
Glutathione peroxidase (GPX) activity, thioredoxin reductase (TR) activity, total superoxide dismutase (SOD) activity, total antioxidant capacity (T-AOC), malondialdehyde (MDA) content, and total protein content of the muscle samples were determined in accordance with the instructions of a GPx activity assay kit (colorimetric method), TR activity assay kit (colorimetric method), SOD activity assay kit (WST-1 cell proliferation reagent method), T-AOC assay kit (2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid method), MDA assay kit (thiobarbituric acid method), and total protein quantitative assay kit (Bradford method) (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively. The reactive oxygen species (ROS) content of the muscle samples was determined in accordance with the instructions of an ROS assay kit (enzyme-linked immunosorbent assay methods) (Shanghai Jianglai Biological Technology Co., Ltd., Shanghai, China).
Determination of mRNA Levels of Antioxidant Enzyme Genes
Synergy brands (SYBR) green real-time quantitative polymerase chain reaction (RT-PCR) was performed to determine the mRNA levels of 5 antioxidant enzyme genes (GPx-1, GPx-4, TR-1, TR-3, and SOD-1). Total RNA was extracted using TRIzol Reagent (Sangon Biotech [Shanghai] Co, Ltd, Shanghai, China), and cDNA was synthesized in accordance with the instruction of the AMV First Strand cDNA Synthesis Kit (Sangon Biotech Co, Ltd, Shanghai, China). The specific primers for gene expression were designed based on Gallus gallus sequences (Table 2). The β-actin housekeeping gene was used as an internal control. RT-PCR was performed with an ABI Real-time PCR System (StepOnePlus; Applied Biosystems, Waltham, MA). Each 20-μL reaction mixture contained 10 μL of 2 × SybrGreen qPCR Master Mix, 1.0 μL of each primer (10 μmol), 1.0 μL of cDNA template, and 7.0 μL of ddH2O. The PCR procedure consisted of an initial denaturation step at 95°C for 2 min followed by 40 cycles consisting of 95°C for 10 s and 60°C for 40 s. Melting curve analysis showed only one peak for each PCR product.
Table 2.
Primer sequences used for quantitative real-time PCR assay.
| Gene name | Forward primer (5’–3’) | Reverse primer (5’–3’) | Length |
|---|---|---|---|
| β-actin | agtgtctttttgtatcttccgcc | ccacatactggcactttactccta | 147 bp |
| GPx-1 | tctacctggtaactttcgagcaa | cctttattgcagagcctcctt | 147 bp |
| GPx-4 | gccacctccatctacgacttc | ttggtgatgatgcagacgaag | 92 bp |
| SOD-1 | atgcagataggcacgtgg | actgccatcttaagcatttcag | 267 bp |
| TR-1 | tcaagaatgtcaccgcaagtt | cacgcagataacatccccaat | 129 bp |
| TR-3 | tgttttgatagccattggtcg | cataaggcacattggttcgttc | 128 bp |
Abbreviations: GPX-1, cellular glutathione peroxidase; GPX-4, phospholipid hydroperoxide glutathione peroxidase; SOD-1, superoxide dismutase 1; TR-1, thioredoxin reductase1; TR-3, thioredoxin reductase3.
Statistical Analysis
Relative mRNA levels were determined using the 2−ΔΔCt method. The pen was defined as the experimental unit for statistical analysis, and all calculations were generated based on pen averages. All data were expressed the means ± SD and analyzed by one-way analysis of variance with IBM SPSS Statistics for Windows, version 22.0. (IBM Corporation, Armonk, NY). The least significant difference of multiple comparisons was used to determine differences between means. For all tests, a probability (P) value <0.05 was considered statistically significant.
Results
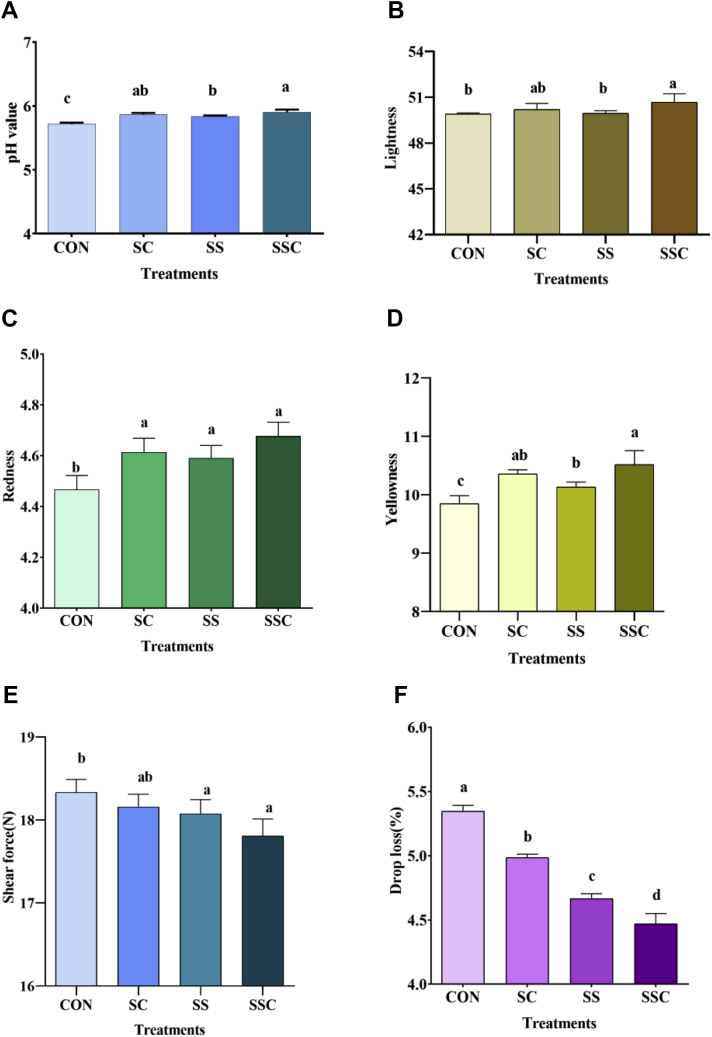
The effects of SSC on the physical characteristics of the muscle samples are shown in Figure 1, including pH value (Figure 1A), lightness (Figure 1B), redness (Figure 1C), yellowness (Figure 1D), drip loss (Figure 1E), and shear force (Figure 1F). As compared with the control group, SC supplementation significantly increased the pH value, redness, and yellowness but decreased drip loss (P < 0.05), while SS supplementation significantly increased the pH value, redness, and yellowness but decreased drip loss and shear force (P < 0.05). As compared with the SC group, SS supplementation significantly decreased drip loss (P < 0.05), while SSC supplementation significantly decreased drip loss and shear force (P < 0.05). As compared with the SS group, SSC supplementation significantly increased the pH, lightness, and yellowness but decreased drip loss (P < 0.05).
Figure 1.
The effects of selenium-enriched Saccharomyces cerevisiae (SSC) on physical characteristics of chicken muscle. (A) pH value, (B) lightness, (C) redness, (D) yellowness, (E) shear force, (F) drop loss. Data are presented as the means ± SD (n = 5). Different superscript letters denote significant differences (P < 0.05). CON refers to a basal diet, Saccharomyces cerevisiae (SC) refers to the basal diet supplemented with SC, sodium selenite (SS) refers to the basal diet supplemented with SS, and SSC refers to the basal diet supplemented with SSC.
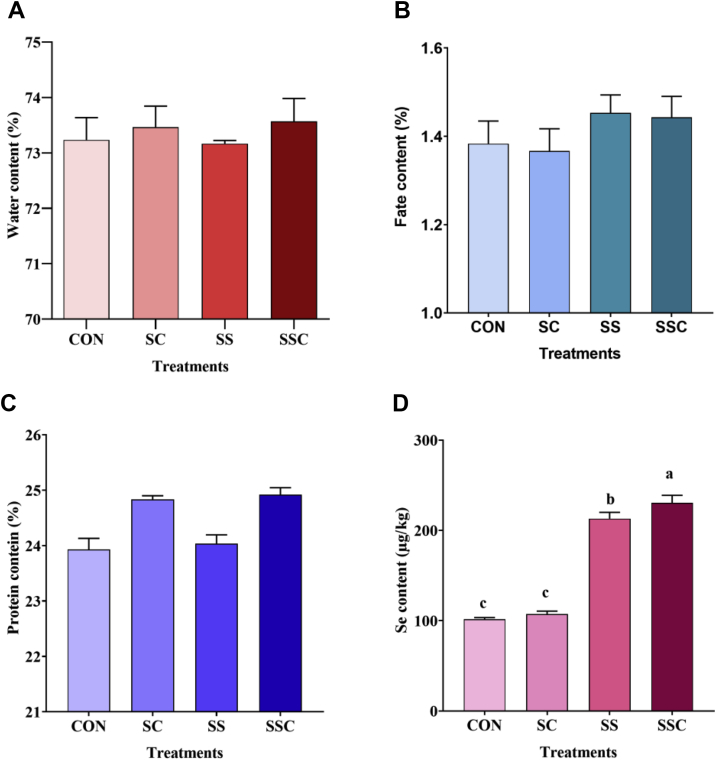
The effects of SSC on chemical composition and Se concentration of muscle samples are shown in Figure 2, including moisture (Figure 2A), crude fat (Figure 2B), crude protein (Figure 2C), and Se content (Figure 2D). As compared with the control and SS groups, SC and SSC supplementation significantly increased crude protein concentration (P < 0.05). Among the 4 groups, there were no significant differences in the moisture and crude fat contents of the muscle samples (P > 0.05). As compared with the control and SC groups, SS and SSC supplementation significantly increased Se concentration (P < 0.05). As compared with the SS group, SSC supplementation significantly increased the Se concentration (P < 0.05).
Figure 2.
The effects of selenium-enriched Saccharomyces cerevisiae (SSC) on the chemical composition of chicken muscle. (A) Water content, (B) fate content, (C) protein content, (D) Se content. Data are presented as the means ± SD (n = 5). Different superscript letters denote significant difference (P < 0.05).
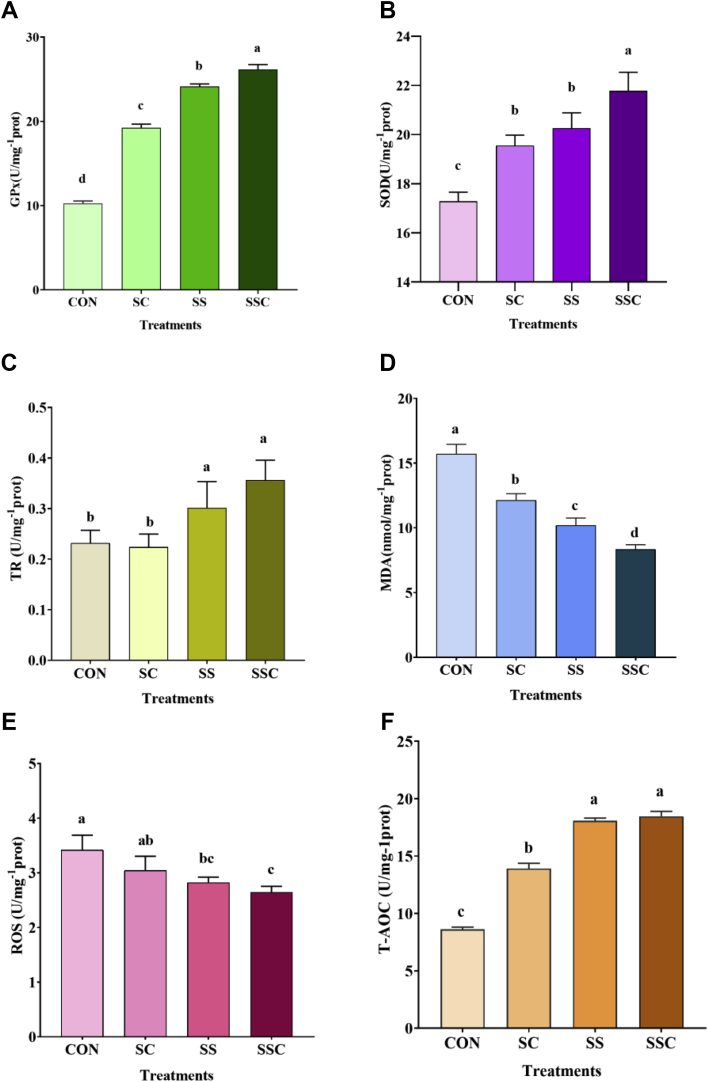
The effects of SSC on the oxidative stability of the muscle samples are shown in Figure 3, including GPx activity (Figure 3A), TR activity (Figure 3B), SOD activity (Figure 3C), T-AOC (Figure 3D), MDA content (Figure 3E), and ROS content (Figure 3F). As compared with the control group, SC supplementation significantly increased T-AOC, GPx activity, and SOD activity but decreased MDA content (P < 0.05), and SS and SSC supplementation significantly increased T-AOC and the activities of GPx, SOD, and TR but decreased MDA and ROS content (P < 0.05). As compared with the SC group, SS and SSC supplementation significantly increased T-AOC, GPx, and TR activity and decreased MDA and ROS content (P < 0.05). As compared with the SS group, SSC supplementation significantly increased GPx activity and SOD activities but decreased MDA content (P < 0.05).
Figure 3.
The effects of selenium-enriched Saccharomyces cerevisiae (SSC) on oxidant stability of chicken muscle. (A) glutathione peroxidase (GPx) activity, (B) superoxide dismutase (SOD) activity, (C) thioredoxin reductase (TR) activity, (D) malondialdehyde (MDA) content, (E) reactive oxygen species (ROS) content, and (F) total antioxidant capacity (T-AOC). Data are shown as means ± SD (n = 5). Different superscript letters denote significant difference (P < 0.05).
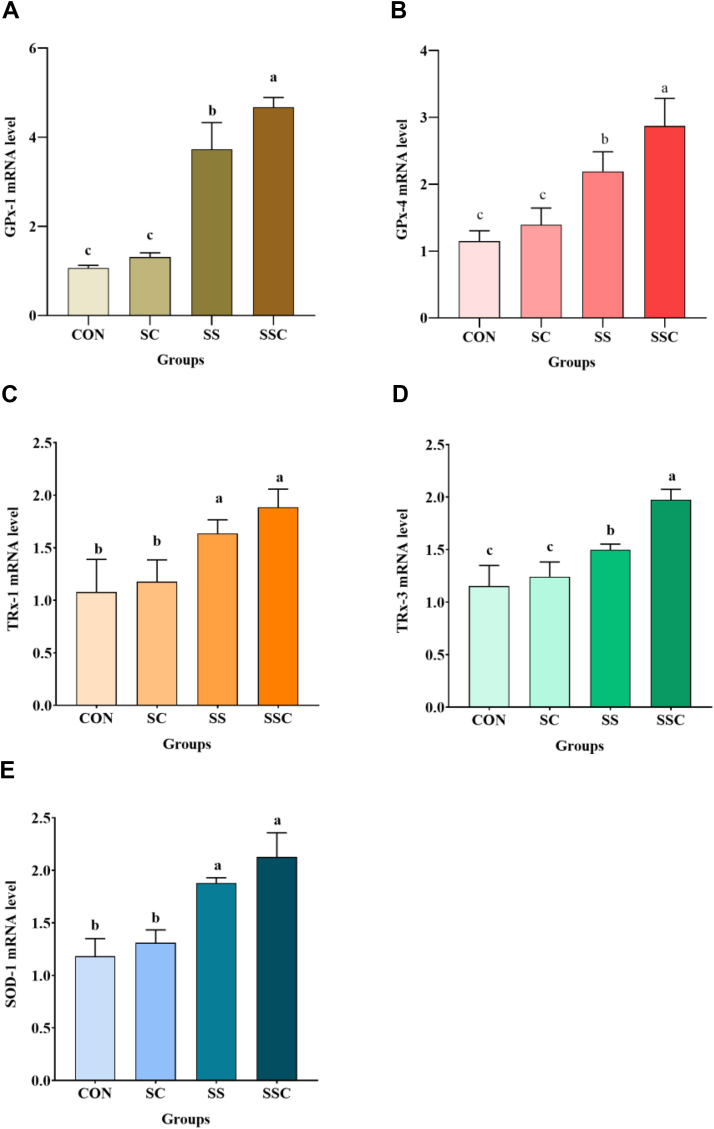
The effects of SSC on the relative mRNA levels of antioxidant enzyme genes in muscle samples are shown in Figure 4: GPx-1 (Figure 4A), GPx-4 (Figure 4B), TRx-1 (Figure 4C), TRx-3 (Figure 4D), and SOD-1 (Figure 4E). As compared with the control and SC groups, SS and SSC supplementation significantly increased GPx-1, GPx-4, TRx-1, TRx-3, and SOD-1 mRNA expression levels (P < 0.05). As compared with the SS group, SSC supplementation significantly increased GPx-1 and GPx-4 mRNA expression levels (P < 0.05).
Figure 4.
The effects of selenium-enriched Saccharomyces cerevisiae (SSC) on the relative mRNA expression levels of antioxidase genes in chicken muscle. (A) glutathione peroxidase (GPx)-1 mRNA level, (B) GPx-4 mRNA level, (C) thioredoxin reductase (TR)-1 mRNA level, (D) TR-3 mRNA level, (E) superoxide dismutase (SOD)-1 mRNA level. Data are presented as the means ± SD (n = 5). Different superscript letters denote significant difference (P < 0.05).
Discussion
Physiological characteristics including appearance and texture directly affect the willingness of consumers to purchase meat products. The pH value, color, drip loss, and shear force were widely used to evaluate the sensory characteristics of meat quality (Shi et al., 2011; Calvo et al., 2017b; Li et al., 2018; Silva et al., 2020). In the present study, SSC and SS supplementation improved physical characteristics of muscle (i.e., pH value, color, drip loss, or shear force), consistent with the increasing amount of evidence indicating the essential involvement of Se on the meat physical characteristics of geese (Baowei et al., 2011), pigs (Calvo et al., 2017a, 2017b), rabbits (Papadomichelakis et al., 2018), and chickens (Jiang et al., 2009; Khan et al., 2018; Li et al., 2018; Yang et al., 2019). However, many studies reported that Se (including SS or SY) supplementation does not affect the meat color of chickens (Chen et al., 2014; Habibian et al., 2016), lambs (Jose et al., 2010), or cattle (Silva et al., 2020) or the pH value of chicken muscle (Kim et al., 2010). Unfortunately, although these studies investigated the effects of inorganic Se (SS) and organic Se (SY) on meat quality, they did not evaluate the effects of yeast on meat quality or compare the effects between yeast as a control and SY. Aristides et al. (2018) reported that different levels of SC fermentation products provided no changes in color, water-holding capacity, cooking loss, or shear force. As the mechanism underlying the effects of Se supplementation on the physical characteristics of meat remains unclear, the aim of the present study was to determine whether supplementation with both Se and SC could improve the physical characteristics of meat, especially the pH value and drip loss, which had been attributed to their antioxidant effects (Habibian et al., 2016; Calvo et al., 2017a; Li et al., 2018). In addition, the benefits of Se and SC supplementation on the physical characteristics of meat differed. The effects of SC supplementation on meat color might be better than those of SS, and the effects of SS supplementation on shear force and drip loss might be better than those of SC. SSC supplementation had better effects on drip loss of meat than SC, while SC supplementation had better effects the pH value, lightness, yellowness, and drip loss than SS. These results indicated that the effects of SSC on the physical characteristics of meat should be attributed to the combined roles of SC and Se.
Nutritional value, referring to the chemical composition of meat, is another important factor affecting the willingness of consumers to purchase meat products. In the present study, SS and SSC supplementation had no effect on the moisture or crude fat content of muscle, but SC and SSC supplementation increased the crude protein content. Similarly, Baowei et al. (2011) reported that SY supplementation had no effect on the crude fat or water content of muscle, while 0.1 and 0.50 mg/kg dietary SY significantly increased the crude protein content of goose muscle. Therefore, SC should be considered as an excellent source of protein, and one of the main factors affecting the crude protein content of meat. In contrast, Geng et al. (2016) reported that supplementation with cultures of SC and SC had no significant effect on the chemical composition of beef. Baowei et al. (2011) also reported that 0.30 mg/kg dietary SY did not affect the crude protein content of goose muscle. Moreover, Mechlaoui et al. (2019) found that Se supplementation had no effect on the lipid and protein content of muscle in seabream juveniles. This discrepancy is likely due to the Se source, the Se concentration, and the experimental animals. Thus, further studies of the effects of Se sources on the chemical composition of meat are warranted.
In addition, Se can increase GPx activity, catalyze the reduction of hydrogen peroxide, protect cell membranes, and reduce oxidative damage. Se supplementation can effectively protect meat quality during storage and prolong the shelf life (Habibian et al., 2016; Calvo et al., 2017b; Delesalle et al., 2017). The oxidant status has become an important indicator of meat quality in consideration of the risk of food deterioration from oxidative stress (Jiang et al., 2009; Habibian et al., 2016; Li et al., 2018). The oxidative stability of meat is dependent on the balance between antioxidants and prooxidants. ROS is a major cause of oxidative stress, MDA is the end product of oxidative stress, and Se and selenoproteins play important roles in protection against oxidative stress (Jiang et al., 2009; Baowei et al., 2011; Li et al., 2018). Therefore, these indicators are widely used in studies of oxidative stress as biomarkers of Se status in various tissues. In the present study, Se (SSC and SS) supplementation not only increased Se concentration but also increased T-AOC and the activities and mRNA levels of GPx, TR, and SOD in muscle and decreased MDA and ROS contents in muscle as compared with the control and SC groups, which were consistent with the results of previous studies (Wang et al., 2011b; Zhou and Wang, 2011; Couloigner et al., 2015), thereby further demonstrating that dietary Se supplementation not only increases Se concentration in meat but also enhances antioxidant status and reduces oxidative damage by upregulating the expression of antioxidant selenoproteins to achieve greater antioxidant status than without Se supplementation (Wu et al., 2010; Yuan et al., 2012; Xiao et al., 2016). In contrast, Payne and Southern (2005) reported that SY supplementation increased muscle and plasma Se concentrations, although plasma GPx-3 activity was not affected by the Se source or concentration. Mechlaoui et al. (2019) reported that dietary Se supplementation increased the muscle content of Se and reduced oxidative stress but had no effect on liver expression of GPx and SOD in gilthead seabream. Wang et al. (2018) reported that SY supplementation increased Se contents and the mRNA levels of 10 selenoproteins in the liver, as well as the Se content, GPx activity, and mRNA levels of 11 selenoproteins in muscle. However, it had no effect on MDA content, or the activities of GPx, SOD, and CAT in the serum and liver, and decreased the activities of CAT and SOD in muscle. The bioavailability of various Se levels and sources differs among tissues and animals, especially absorption, deposition, and metabolism, which leads to alterations in antioxidant stress effects and antioxidant enzyme activities in diverse tissues (Juniper et al., 2009).
Numerous studies have reported that Se efficacy in vivo and in vitro is dependent on its chemical form and demonstrated that organic Se performs a key role in biological processes and has higher bioavailability and accumulates at higher levels in all tissues than inorganic Se (Chen et al., 2014; Habibian et al., 2016; Calvo et al., 2017a; Delesalle et al., 2017). In the present study, SSC supplementation improved meat quality, Se content, and antioxidant status, as compared with SS supplementation. These results further indicated that the effects of organic Se were superior to those of inorganic Se in terms of improving the antioxidant status of animals (Zhang et al., 2014; Delesalle et al., 2017; Wang et al., 2018). However, in contrast, some studies reported different results that organic Se resulted in a highly significant decrease in GPx activity in the plasma, liver, pancreas, breast muscle, and erythrocytes, as compared with inorganic Se (Leeson et al., 2008; Wang et al., 2011a; Sun et al., 2011). In addition, other studies reported that there were no differences in GPx activities in plasma and tissues of broilers fed Se in either an organic or inorganic form (Payne and Southern, 2005; Markovic et al., 2018; Wang et al., 2018). These discrepancies are likely due to the Se sources, the experimental animals, or the lengths of the experiments.
Conclusions
SSC supplementation can potentially improve meat quality and oxidative stability by activating the glutathione and TR systems. In addition, SSC supplementation can improve meat quality better than supplementation with SC or SS, due to the combined roles of SC and Se.
Acknowledgements
The authors are extremely thankful to Mr. Qingjun Gong and laboratory technicians for their assistance with the experimental animals and to the Central Laboratory of Qingdao Agricultural University for providing support for the Se measurements and data analysis presented in this work.
Funding: This work was financially supported by the Shandong Major Science and Technology Innovation Projects, China (grant no.: 2019JZZY020611), Young and Middle-aged Backbone Innovative Talents Training Program of the Higher Educational Institutions of Tianjin and Project of Tianjin one three one innovative talent team, China (grant no.: 20180318), the Shandong Higher Educational Science and Technology Program Project, China (grant no.: J18KA131and J18KA143), and the National Natural Science Foundation, China (grant no.: 31001093 and 31101867).
Conflict of Interest Satatement: The authors did not provide a conflict of interest statement.
Contributor Information
Fu Chen, Email: cf507@sohu.com.
Shunyi Qin, Email: qinshunyi@163.com.
References
- Aristides L.G.A., Venancio E.J., Alfieri A.A., Otonel R.A.A., Frank W.J., Oba A. Carcass characteristics and meat quality of broilers fed with different levels of saccharomyces cerevisiae fermentation product. Poult. Sci. 2018;97:3337–3342. doi: 10.3382/ps/pey174. [DOI] [PubMed] [Google Scholar]
- Baowei W., Guoqing H., Qiaoli W., Bin Y. Effects of yeast selenium supplementation on the growth performance, meat quality, immunity, and antioxidant capacity of goose. J. Anim. Physiol. Anim. Nutr. 2011;95:440–448. doi: 10.1111/j.1439-0396.2010.01070.x. [DOI] [PubMed] [Google Scholar]
- Calvo L., Segura J., Toldrá F., Flores M., Rodríguez A.I., López-Bote C.J., Rey A.I. Meat quality, free fatty acid concentration, and oxidative stability of pork from animals fed diets containing different sources of selenium. Food Sci. Technol. Int. 2017;23:716–728. doi: 10.1177/1082013217718964. [DOI] [PubMed] [Google Scholar]
- Calvo L., Toldrá F., Rodríguez A.I., López-Bote C., Rey A.I. Effect of dietary selenium source (organic vs. Mineral) and muscle ph on meat quality characteristics of pigs. Food Sci. Nutr. 2017;5:94–102. doi: 10.1002/fsn3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu J., Li C. Effect of different selenium sources on production performance and biochemical parameters of broilers. J. Anim. Physiol. Anim. Nutr. 2014;98:747–754. doi: 10.1111/jpn.12136. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhu L., Qiu H., Qin S. Selenium-enriched saccharomyces cerevisiae improves growth, antioxidant status and selenoprotein gene expression in arbor acres broilers. J. Anim. Physiol. Anim. Nutr. 2017;101:259–266. doi: 10.1111/jpn.12571. [DOI] [PubMed] [Google Scholar]
- Couloigner F., Jlali M., Briens M., Rouffineau F., Geraert P.-A., Mercier Y. Selenium deposition kinetics of different selenium sources in muscle and feathers of broilers. Poult. Sci. 2015;94:2708–2714. doi: 10.3382/ps/pev282. [DOI] [PubMed] [Google Scholar]
- Delesalle C., de Bruijn M., Wilmink S., Vandendriessche H., Mol G., Boshuizen B., Plancke L., Grinwis G. White muscle disease in foals: Focus on selenium soil content. A case series. BMC Vet. Res. 2017;13:121. doi: 10.1186/s12917-017-1040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng C.-Y., Ren L.-P., Zhou Z.-M., Chang Y., Meng Q.-X. Comparison of active dry yeast (saccharomyces cerevisiae) and yeast culture for growth performance, carcass traits, meat quality and blood indexes in finishing bulls. Anim. Sci. J. 2016;87:982–988. doi: 10.1111/asj.12522. [DOI] [PubMed] [Google Scholar]
- Habibian M., Ghazi S., Moeini M.M. Effects of dietary selenium and vitamin e on growth performance, meat yield, and selenium content and lipid oxidation of breast meat of broilers reared under heat stress. Biol. Trace Elem. Res. 2016;169:142–152. doi: 10.1007/s12011-015-0404-6. [DOI] [PubMed] [Google Scholar]
- Han X.J., Qin P., Li W.X., Ma Q.G., Ji C., Zhang J.Y., Zhao L.H. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult. Sci. 2017;96:3973–3980. doi: 10.3382/ps/pex216. [DOI] [PubMed] [Google Scholar]
- Hosnedlova B., Kepinska M., Skalickova S., Fernandez C., Ruttkay-Nedecky B., Malevu T.D., Sochor J., Baron M., Melcova M., Zidkova J., Kizek R. A summary of new findings on the biological effects of selenium in selected animal species-a critical review. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Lin Y., Zhou G., Luo L., Jiang S., Chen F. Effects of dietary selenomethionine supplementation on growth performance, meat quality and antioxidant property in yellow broilers. J. Agric. Food Chem. 2009;57:9769–9772. doi: 10.1021/jf902411c. [DOI] [PubMed] [Google Scholar]
- Jose C.G., Jacob R.H., Gardner G.E., Pethick D.W., Liu S.M. Selenium supplementation and increased muscle glutathione concentration do not improve the color stability of lamb meat. J. Agric. Food Chem. 2010;58:7389–7393. doi: 10.1021/jf100191k. [DOI] [PubMed] [Google Scholar]
- Juniper D.T., Phipps R.H., Ramos-Morales E., Bertin G. Effect of high dose selenium enriched yeast diets on the distribution of total selenium and selenium species within lamb tissues. Livestock Sci. 2009;122:63–67. [Google Scholar]
- Khan A.Z., Kumbhar S., Liu Y., Hamid M., Pan C., Nido S.A., Parveen F., Huang K. Dietary supplementation of selenium-enriched probiotics enhances meat quality of broiler chickens (gallus gallus domesticus) raised under high ambient temperature. Biol. Trace Elem. Res. 2018;182:328–338. doi: 10.1007/s12011-017-1094-z. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Park W.Y., Choi I.H. Effects of dietary alpha-tocopherol, selenium, and their different combinations on growth performance and meat quality of broiler chickens. Poult. Sci. 2010;89:603–608. doi: 10.3382/ps.2009-00280. [DOI] [PubMed] [Google Scholar]
- Leeson S., Namkung H., Caston L., Durosoy S., Schlegel P. Comparison of selenium levels and sources and dietary fat quality in diets for broiler breeders and layer hens. Poult. Sci. 2008;87:2605–2612. doi: 10.3382/ps.2008-00174. [DOI] [PubMed] [Google Scholar]
- Li J.L., Zhang L., Yang Z.Y., Zhang Z.Y., Jiang Y., Gao F., Zhou G.H. Effects of different selenium sources on growth performance, antioxidant capacity and meat quality of local Chinese subei chickens. Biol. Trace Elem. Res. 2018;181:340–346. doi: 10.1007/s12011-017-1049-4. [DOI] [PubMed] [Google Scholar]
- Markovic R., Ciric J., Drljacic A., Šefer D., Jovanovic I., Jovanovic D., Milanovic S., Trbovic D., Radulovic S., Baltic M.Ž., Starcevic M. The effects of dietary selenium-yeast level on glutathione peroxidase activity, tissue selenium content, growth performance, and carcass and meat quality of broilers. Poult. Sci. 2018;97:2861–2870. doi: 10.3382/ps/pey117. [DOI] [PubMed] [Google Scholar]
- Mechlaoui M., Dominguez D., Robaina L., Geraert P.-A., Kaushik S., Saleh R., Briens M., Montero D., Izquierdo M. Effects of different dietary selenium sources on growth performance, liver and muscle composition, antioxidant status, stress response and expression of related genes in gilthead seabream (sparus aurata) Aquaculture. 2019;507:251–259. [Google Scholar]
- Papadomichelakis G., Zoidis E., Pappas A.C., Danezis G., Georgiou C.A., Fegeros K. Dietary organic selenium addition and accumulation of toxic and essential trace elements in liver and meat of growing rabbits. Meat Sci. 2018;145:383–388. doi: 10.1016/j.meatsci.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Pappas A.C., Zoidis E., Papadomichelakis G., Fegeros K. Supranutritional selenium level affects fatty acid composition and oxidative stability of chicken breast muscle tissue. J. Anim. Physiol. Anim. Nutr. 2012;96:385–394. doi: 10.1111/j.1439-0396.2011.01152.x. [DOI] [PubMed] [Google Scholar]
- Payne R.L., Southern L.L. Comparison of inorganic and organic selenium sources for broilers. Poult. Sci. 2005;84:898–902. doi: 10.1093/ps/84.6.898. [DOI] [PubMed] [Google Scholar]
- Qin S., Huang B., Ma J., Wang X., Zhang J., Li L., Chen F. Effects of selenium-Chitosan on blood selenium concentration, Antioxidation status, and cellular and Humoral immunity in Mice. Biol. Trace. Elem. Res. 2015;165:145–152. doi: 10.1007/s12011-015-0243-5. [DOI] [PubMed] [Google Scholar]
- Rayman M. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Shi L., Xun W., Yue W., Zhang C., Ren Y., Shi L., Wang Q., Yang R., Lei F. Effect of sodium selenite, se-yeast and nano-elemental selenium on growth performance, se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011;96:49–52. [Google Scholar]
- Silva J.S., Rodriguez F.D., Trettel M., Abal R.T., Lima C.G., Yoshikawa C.Y.C., Zanetti M.A. Performance, carcass characteristics and meat quality of nellore cattle supplemented with supranutritional doses of sodium selenite or selenium-enriched yeast. Animal. 2020;14:215–222. doi: 10.1017/S1751731119001265. [DOI] [PubMed] [Google Scholar]
- Sun B., Wang R., Li J., Jiang Z., Xu S. Dietary selenium affects selenoprotein w gene expression in the liver of chicken. Biol. Trace Elem. Res. 2011;143:1516–1523. doi: 10.1007/s12011-011-8995-z. [DOI] [PubMed] [Google Scholar]
- Wang R., Sun B., Zhang Z., Li S., Xu S. Dietary selenium influences pancreatic tissue levels of selenoprotein w in chickens. J. Inorg. Biochem. 2011;105:1156–1160. doi: 10.1016/j.jinorgbio.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhan X., Zhang X., Wu R., Yuan D. Comparison of different forms of dietary selenium supplementation on growth performance, meat quality, selenium deposition, and antioxidant property in broilers. Biol. Trace Elem. Res. 2011;143:261–273. doi: 10.1007/s12011-010-8839-2. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang X., Wu L., Liu Q., Zhang D., Yin J. Expression of selenoprotein genes in muscle is crucial for the growth of rainbow trout (oncorhynchus mykiss) fed diets supplemented with selenium yeast. Aquaculture. 2018;492:82–90. [Google Scholar]
- Wu X., Huang K., Wei C., Chen F., Pan C. Regulation of cellular glutathione peroxidase by different forms and concentrations of selenium in primary cultured bovine hepatocytes. J. Nutr. Biochem. 2010;21:153–161. doi: 10.1016/j.jnutbio.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Xiao X., Yuan D., Wang Y.-X., Zhan X.-A. The protective effects of different sources of maternal selenium on oxidative stressed chick embryo liver. Biol. Trace Elem. Res. 2016;172:201–208. doi: 10.1007/s12011-015-0541-y. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang M., Zhou Y. Effects of selenium-enriched bacillus sp. Compounds on growth performance, antioxidant status, and lipid parameters breast meat quality of Chinese huainan partridge chicks in winter cold stress. Lipids Health Dis. 2019;18:63. doi: 10.1186/s12944-019-1015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H., Zhang Y. Systems biology of selenium and complex disease. Biol. Trace Elem. Res. 2019;192:38–50. doi: 10.1007/s12011-019-01781-9. [DOI] [PubMed] [Google Scholar]
- Yuan D., Zhan X.A., Wang Y.X. Effect of selenium sources on the expression of cellular glutathione peroxidase and cytoplasmic thioredoxin reductase in the liver and kidney of broiler breeders and their offspring. Poult. Sci. 2012;91:936–942. doi: 10.3382/ps.2011-01921. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang Y.X., Zhou Y., Zheng L., Zhan X.A., Pu Q.H. Different sources of maternal selenium affect selenium retention, antioxidant status, and meat quality of 56-day-old offspring of broiler breeders. Poult. Sci. 2014;93:2210–2219. doi: 10.3382/ps.2013-03605. [DOI] [PubMed] [Google Scholar]
- Zhou X., Wang Y. Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in guangxi yellow chicken. Poult. Sci. 2011;90:680–686. doi: 10.3382/ps.2010-00977. [DOI] [PubMed] [Google Scholar]
- Zoidis E., Seremelis I., Kontopoulos N., Danezis G.P. Selenium-dependent antioxidant enzymes: Actions and properties of selenoproteins. Antioxidants (Basel) 2018;7:66. doi: 10.3390/antiox7050066. [DOI] [PMC free article] [PubMed] [Google Scholar]