Abstract
This study investigated the effects of dietary corn-resistant starch on lipid metabolism of broilers and its potential relationship with cecal microbiota modulation. A total of three hundred twenty 1-day-old male broilers were randomly assigned into 5 dietary treatments: 1 normal corn–soybean (NC) diet, 1 corn–soybean–based diet supplementation with 20% corn starch (CS), and 3 corn–soybean–based diets supplementation with 4, 8, and 12% corn resistant starch (RS) (identified as 4%RS, 8%RS, and 12%RS, respectively). Each group had 8 replicates with 8 broilers per replicate. The experiment lasted 21 d. The results showed that the abdominal fat percentage were lower in birds from 8%RS and 12%RS groups (0.75 and 0.58%, respectively) than those from NC and CS groups (1.20 and 1.28%, respectively; P < 0.05). The birds from 8%RS and 12%RS groups exhibited lower concentrations of blood triglyceride and nonestesterified fatty acid than those in the NC and CS groups (P < 0.05). Moreover, birds fed diets supplementation with 12% RS decreased the relative mRNA expressions of peroxisome proliferator-activated receptor gamma, ATP citrate-lyase, fatty acid synthase, and acetyl-CoA carboxylase in liver, and glycerol-3-phosphate acyltransferase in abdominal adipose tissue (P < 0.05). Microbiota analysis revealed that birds fed diets supplementation with 8 and 12% RS decreased the abundance of cecal Firmicutes by 23.08 and 20.47% and increased the proportion of Bacteroidetes by 24.33 and 21.92%, respectively, compared with the NC group (P < 0.05). In addition, correlation analysis revealed that many Firmicutes members had highly positive relationship with blood lipid levels and fat storage capacity, which might contribute to the lower abdominal fat phenotype. Overall, broilers receiving diets containing a higher concentration of RS harbor less Firmicutes, which decreased liver fatty acid synthesis and suppress abdominal fat deposition of birds during the starter phase. These findings provide a profound understanding about the relationship between gut microbial composition and lipid metabolism in broilers.
Key words: broiler, corn resistant starch, fat deposition, cecum, microbiota
Introduction
Dietary starch structure plays an important role in lipid metabolism of animals. Starch presents as the major source of carbohydrate in broiler diet, and it generally classified into rapidly digestible starch, slowly digestible starch, and resistant starch (RS) based on its digestion and absorption rate in small intestine (Englyst et al., 1992). Diets rich in highly digestible carbohydrates can lead to high levels of triglycerides (TG) production and remnant accumulation of body fat (Jeffcoat, 2007). Excessive fat deposition decreases feed efficiency of live birds, and abdominal fat is often discarded during meat processing, causing considerable economic losses to poultry industry (Han et al., 2016). Diets rich in slowly digestible carbohydrates are thought to reduce blood lipid levels (Liu et al., 2010). Therefore, the rate of starch digestion and absorption may have a profound impact on lipid metabolism of chickens.
Resistant starch is resistant to digestion in small intestine but fermented by the gut microbiota in large intestine, which has similar physiological functions as dietary fiber (Nugent, 2005). A recent study revealed that broilers receiving RS-treated diets exhibited poor growth performance and undesired carcase traits (Liu et al., 2020). Increasing evidence suggests that RS has beneficial effects in preventing fat deposition (Keenan et al., 2006; So et al., 2007). One of the reasons is that hormones concentrations are severely influenced by dietary RS. The supplementation of RS in diets can effectively improve insulin sensitivity and increase the concentrations of plasma peptide YY and glucagon-like peptide-1 (Robertson et al., 2003; Zhou et al., 2008), whereas these hormones are usually resistant to fat storage. In addition, RS also exerts a positive effect in lowering blood lipid levels, especially for TG and cholesterol (Han et al., 2003; Kim et al., 2003). Unlike mammal, adipose tissue of birds is served only as a fat storage site, and fat synthesis mainly occurs in the liver, and thus the development of adipose tissue is depended on the utilization of plasma TG (O'Hea and Leveille, 1968; Hermier, 1997). Therefore, we hypothesized that RS may effectively inhibit abdominal fat deposition of broilers by regulating plasma TG concentration.
Firmicutes and Bacteroidetes are dominant phylum in gut microbiota. Many members of Firmicutes and Bacteroidetes can encode carbohydrate-active enzymes, which would contribute to the hydrolysis and the utilization of carbohydrates (El Kaoutari et al., 2013). Meanwhile, Firmicutes can generate more harvestable energy than Bacteroidetes, and obese individuals have more Firmicutes (Turnbaugh et al., 2006). Therefore, microbial composition contributes a lot to lipid metabolism by regulating carbohydrate metabolism. It has been shown that Bacteroidetes is more likely to be enriched by dietary RS (Upadhyaya et al., 2016), which implies that RS may have a profound effect on lipid metabolism through regulating gastrointestinal microbial composition. Large numbers of bacteria are colonized in cecum of broilers and participate in the digestion and metabolism of nondigestible carbohydrates (Pan and Yu, 2013). Therefore, this study aimed to investigate the effects corn RS on broiler lipid metabolism and its potential relationship with cecal microbiota populations.
Materials and methods
Experimental Design, Animals, and Management
All experimental design and procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of three hundred twenty 1-day-old male Arbor Acres broiler chicks were purchased from a commercial hatchery (Hewei Agricultural Development Co. Ltd., Xuancheng, China). Chicks were weighed and randomly assigned to 5 dietary treatments: 1 normal corn–soybean (NC) diet, 1 corn–soybean–based diet supplementation with 20% corn starch (CS) (maize starch, 99% purity, YuXing Inc., Hebei, China), and 3 corn–soybean–based diets supplementation with 4, 8 and 12% corn RS (identified as 4%RS, 8%RS and 12%RS, respectively) by replacing CS with 6.67, 13.33, and 20% of Hi-Maize 260 (type II RS, 60% purity; Ingredion Inc., Westchester, IL), respectively. The composition and nutrition levels of all diets are given in the Table 1. Each group had 8 replicates with 8 broilers per replicate. The size of the replicate cage was 100 × 60 × 40 cm. Birds were allowed free access to feed and water in a temperature-controlled room at Nanjing Kangxin Poultry Industry (Nanjing, China) throughout a 21-d experiment. At the first wk, the environment temperature in the chicken house was kept at 33°C and then gradually reduced by 3°C per week to a final temperature of around 26°C. Birds were exposed to light for 23 h/d throughout the whole experimental period.
Table 1.
Ingredients and nutrient composition of experimental diets.
| Items | Treatments1 |
||||
|---|---|---|---|---|---|
| NC | CS | 4%RS | 8%RS | 12%RS | |
| Ingredients (%) | |||||
| Corn | 57.00 | 36.50 | 36.50 | 36.50 | 36.50 |
| Soybean meal (44% crude protein) | 31.50 | 28.15 | 28.15 | 28.15 | 28.15 |
| Corn gluten meal (63.5% crude protein) | 3.40 | 8.15 | 8.15 | 8.15 | 8.15 |
| Corn starch | - | 20.00 | 13.33 | 6.67 | - |
| Hi-Maize 260 (60% resistant starch) | - | - | 6.67 | 13.33 | 20.00 |
| Soybean oil | 3.10 | 2.20 | 2.20 | 2.20 | 2.20 |
| Limestone | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Dicalcium phosphate | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| L-Lysine | 0.34 | 0.34 | 0.34 | 0.34 | 0.34 |
| DL-Methionine | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Zeolite powder | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Premix2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Calculated nutrient levels | |||||
| Metabolizable energy (MJ/kg) | 12.52 | 12.50 | - | - | - |
| Crude protein (%) | 21.33 | 21.00 | 21.00 | 21.00 | 21.00 |
| Calcium (%) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Available phosphorus (%) | 0.46 | 0.45 | 0.45 | 0.45 | 0.45 |
| Lysine (%) | 1.21 | 1.12 | 1.12 | 1.12 | 1.12 |
| Methionine (%) | 0.50 | 0.51 | 0.51 | 0.51 | 0.51 |
| Methionine + cysteine (%)) | 0.86 | 0.85 | 0.85 | 0.85 | 0.85 |
| Arginine (%) | 1.27 | 1.18 | 1.18 | 1.18 | 1.18 |
| Threonine (%) | 0.83 | 0.81 | 0.81 | 0.81 | 0.81 |
| Analysed nutrient levels | |||||
| Crude protein (%) | 20.91 | 20.40 | 20.61 | 20.39 | 20.64 |
| Starch (%) | 51.25 | 51.23 | 51.34 | 52.30 | 52.01 |
| RS (%) | 3.03 | 4.18 | 7.33 | 11.02 | 14.16 |
NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS and 12%RS, the corn–soybean–based diets supplementation with 4, 8 and 12% corn resistant starch (RS), respectively.
Premix provided per kilogram of diet: trans-retinyl acetate, 30 mg; cholecalciferol, 0.075 mg; DL-α-tocopherol acetate, 30 mg; menadione, 1.3 mg; thiamine, 2.2 mg; riboflavin, 8.0 mg; nicotinamide, 40 mg; choline, 400 mg; pantothenic acid, 15 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; cobalamin, 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8.0 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc sulfate), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
Sample Collection and Preparation
At 21 d of age, 1 bird per replicate with a BW close to the average weight of the replicate was selected and weighed for the sample collection. Blood samples from jugular vein were centrifuged at 4,000 × g for 10 min at 4°C to separate plasma and then stored at −20°C for further analysis. The birds were stunned electrically and then slaughtered and eviscerated. The fat from the abdomen, gizzard, bursa of fabricius, cloaca, and adjacent muscles were removed and weighed. Meanwhile, several fragments were cut from the middle of the abdominal fat pad and the left lobe of the liver, immediately frozen in liquid nitrogen, and then stored at −80°C. Two ceca of each bird were removed, and the digesta were squeezed into the RNA-free tubes in super clean bench and then stored in liquid nitrogen for microbiota analysis.
Abdominal Fat Percentage Measurements
The trachea, esophagus, craw, gut, spleen, pancreas, gallbladder, reproductive organs, heart, liver, lung, abdominal fat, head, and feet were removed from the carcasses to obtain the eviscerated weight. The abdominal fat percentage (%) was calculated according to the Performance Ferms and Measurement for Poultry (2004) using the following equation:
Percentage of abdominal fat (%) = Abdominal fat weight/(Eviscerated weight + Abdominal fat weight) × 100%
Histochemical Analysis of the Abdominal Adipose Tissue
Approximately 1 cm2 of the abdominal adipose tissue segments were excised and fixed in 4% paraformaldehyde for at least 24 h. The paraformaldehyde-fixed tissues were cut into 7 μm cross-sections and then stained with hematoxylin-eosin for morphology observation. Using an Olympus DP12 CCD digital camera attached to a Olympus light microscope, fields that were occupied mainly by fat cells and photographed at 400 × magnification. The average diameters of adipocytes in photographs were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD).
Plasma Biochemical Analysis
The concentrations of plasma TG (catalog no. A110-1), nonesterified free fatty acid (NEFA, catalog no. A042-2), and glucose (catalog no. F006-1) were determined with corresponding commercial diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Activity Analysis of lipoprotein lipase and hormone sensitive lipase in Abdominal Adipose Tissue
The activities of lipoprotein lipase (LPL, catalog no. H238) and hormone sensitive lipase (HSL, catalog no. A067-1) in abdominal adipose tissue were determined with corresponding commercial diagnostic kits.
RNA Extraction and Real-Time PCR Analysis
Total RNA was isolated from the liver and abdominal adipose tissue samples using Trizol reagent (Takara Biotechnology Co. Ltd., Dalian, China). The purity and quantity of the RNA were measured using an ultramicro spectrophotometer (Thermo Scientific, Wilmington, DE). Reverse transcription of total RNA was completed using a PrimeScript RT Master Mix kit (Takara Biotechnology Co. Ltd.). The reverse-transcription (RT) reactions were incubated for 15 min at 37°C, followed by 5 s at 85°C. The RT products (cDNA) were stored at −20°C.
Real-time quantitative PCR was performed using the ABI PRISM 7500 Detection System (Applied Biosystems, Foster City, CA) using SYBR Premix Ex Taq kits (Takara Biotechnology Co. Ltd.). The total PCR volume was 20 μL, including 10 μL of SYBR Premix Ex Taq, 7.8 μL of double distilled water, 1 μL of cDNA, 0.4 μL of ROX Reference Dye II, and 0.4 μL of each primer. The PCR cycling conditions included initial denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s and anneal at 60°C for 30 s, and the collection of the fluorescence signal at 60°C. All of the specific primers used are listed in Supplementary Table 1. The expression of target gene relative to 18S rRNA was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Bacterial Genomic DNA Extraction, 16S rRNA Gene Sequencing, and Analysis
Total bacterial genomic DNA of cecal digesta was extracted from all 40 samples of 5 treatments according to a bead-beating method (Zoetendal et al., 1998). Then, the DNA concentration of each sample was determined using a Nano-Drop 1000 spectrophotometer (Thermo Fisher Scientific) and diluted to 1 ng/μL with sterile water. The V3-V4 regions of 16S rRNA gene were amplified using the universal primers (341F: CCTAYGGGRBGCASCAG, 806R: GGACTACNNGGGTATCTAAT). The PCR volume was 30 μL, including 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs, Beverly, MA), 0.2 μM of forward and reverse primers, 10 ng of template DNA, and double-distilled water filled to 30 μL. The thermal cycling condition was as follows: 95°C for 2 min; 25 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by 72°C for 5 min. Then, the Illumina MiSeq platform was used to sequence the library.
MOTHUR (http://www.mothur.org) software was used to remove impurities, filter, align, and classify the original sequence. Raw data were processed to remove sequences less than 200 bp. Sequences with greater than 97% similarity are assigned to the same operational taxonomic units. For each operational taxonomic unit, use the Ribosomal Database Project classifier to annotate classification information. Alpha diversity (Chao1, Shannon, and Simpson) were analyzed with MOTHUR. Beta diversity was analyzed based on unweighted UniFrac distance matrices for principal coordinate analysis.
Statistical Analysis
Data of abdominal fat percentage, adipocyte diameter, lipid metabolism parameters, gene mRNA expressions, phylum, and genus abundance of cecal microbiota were tested for significance using one-way analysis of variance using SPSS statistical software (version 16.0, SPSS Inc., Chicago, IL). Each result represents the mean value ± SE, data are means of 8 replicates (n = 8). Significant differences were declared when P < 0.05. Differential genera were evaluated using linear discriminant analysis. The box plot of differential genera was drawn with OriginPro 2019 software (Version 9.6.0.37, OriginLab Inc, Northampton, MA). Correlations between differential genera and plasma parameters were assessed by Pearson's correlation test.
Results
Abdominal Fat Deposition
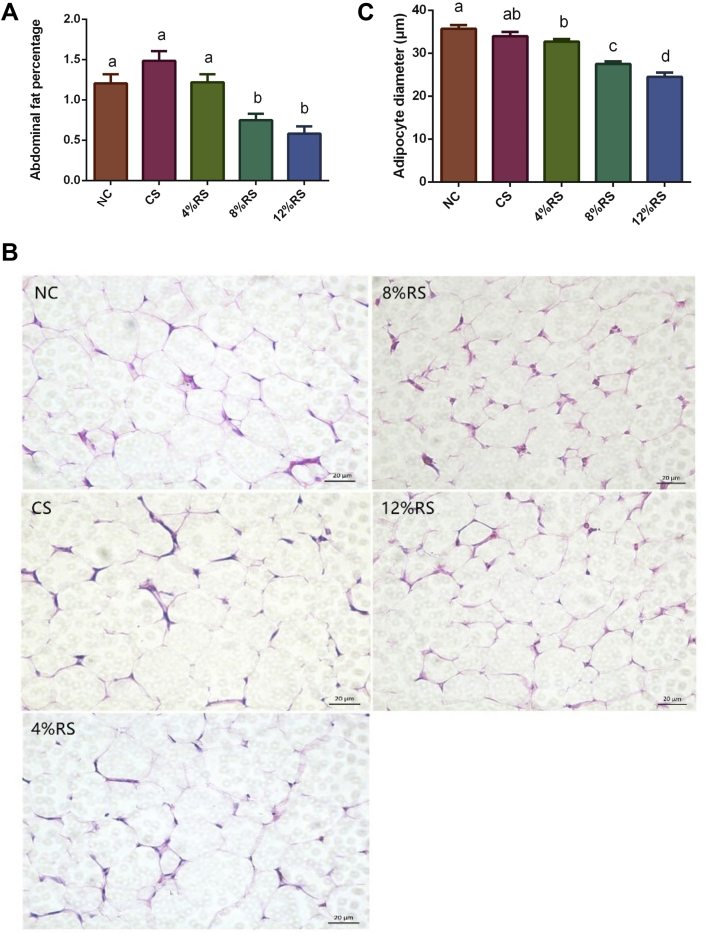
As shown in Figure 1A, the abdominal fat percentage of birds were lower in the 8%RS and 12%RS groups (0.75 and 0.58%, respectively) than those of the NC and CS groups (1.20 and 1.28%, respectively; P < 0.05). The adipose cell morphological observation were presented in Figure 1B, and the average adipocytes diameters of birds from 8%RS and 12%RS (27.27 μm and 24.69 μm) group were lower than those of the NC and CS groups (36.73 μm and 34.04 μm, respectively; P < 0.05; Figure 1C).
Figure 1.
(A) Effect of dietary corn resistant starch (RS) on abdominal fat percentage of 21-day-old broilers. (B) Morphological observation of abdominal adipose tissue of 21-day-old broilers based on HE staining. (C) Adipocyte diameter (μm) of abdominal adipose tissue. Values without a common letter (a-d) significantly differ (P < 0.05; n = 8). Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS, and 12%RS, the corn-soybean based diets supplementation with 4, 8, and 12% corn resistant starch (RS), respectively.
Plasma Variables and Activities of LPL and HSL in Abdominal Adipose Tissue
In contrast to the NC group, broilers from CS group exhibited lower concentrations of plasma TG and NEFA and a higher plasma glucose concentration (P < 0.05; Table 2). However, plasma glucose concentrations in 8%RS and 12%RS groups were increased in comparison to those of the CS group (P < 0.05). There was no significant difference on the activities of LPL and HSL in abdominal adipose tissue between NC and CS groups, whereas birds of 12%RS group showed lower LPL activity and higher HSL activity than those of the NC and CS group (P < 0.05; Table 2).
Table 2.
Effects of corn resistant starch on parameters of lipid metabolism in broilers.
| Items2 | Treatments1 |
||||||
|---|---|---|---|---|---|---|---|
| NC | CS | 4%RS | 8%RS | 12%RS | SEM | P value | |
| Plasma | |||||||
| TG (mmol/L) | 0.41a | 0.29b | 0.34a,b | 0.27b | 0.28b | 0.01 | 0.002 |
| NEFA (mmol/L) | 0.40a | 0.18b | 0.22b | 0.14b | 0.19b | 0.02 | <0.001 |
| Glucose (mmol/L) | 6.98c | 8.85b | 8.01b,c | 10.22a | 10.48a | 0.28 | <0.001 |
| Abdominal adipose tissue | |||||||
| LPL (U/mg of protein) | 0.87a | 0.83a,b | 0.80a,b | 0.73b,c | 0.65c | 0.02 | 0.015 |
| HSL (U/g of protein) | 40.46c | 47.03b,c | 39.89c | 59.07a,b | 71.67a | 2.81 | 0.001 |
a-cMeans in a row without a common superscript letter significantly differ (P < 0.05; n = 8).
NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS and 12%RS, the corn–soybean–based diets supplementation with 4%, 8% and 12% corn resistant starch (RS), respectively.
TG, triglycerides; NEFA, nonesterified free fatty acid; LPL, lipoprotein lipase; HSL, hormone sensitive lipase.
Gene mRNA Expression
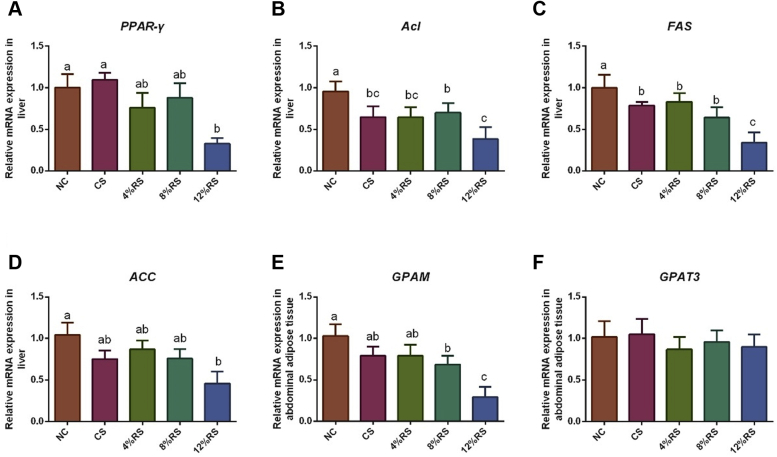
Compared with the NC group, birds in CS group showed lower relative mRNA expressions of ATP citrate-lyase (Acl) and fatty acid synthase (FAS) in abdominal adipose tissue (P < 0.05; Figure 2). The mRNA expressions of peroxisome proliferator-activated receptor gamma (PPAR-γ) and FAS in liver and glycerol-3-phosphate acyltransferase (GPAM) in abdominal adipose tissue of the birds from 12%RS group were lower than those of the CS group (P < 0.05).
Figure 2.
The relative mRNA expressions of PPAR-γ (A), Acl (B), FAS (C), and ACC (D) in liver and GPAM (E) and GPAT3 (F) in abdominal adipose tissue of 21-day-old broilers. Values without a common letter (a-c) significantly differ (P < 0.05; n = 8). Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch; 4%RS, 8%RS, and 12%RS, the corn–soybean–based diets supplementation with 4, 8, and 12% corn resistant starch (RS), respectively; ACC, Acetyl-CoA carboxylase; Acl, ATP citrate-lyase; FAS, fatty acid synthase; GPAM, glycerol-3-phosphate acyltransferase; GPAT3, glycerol-3-phosphate acyltransferase 3; PPAR-γ, peroxisome proliferator-activated receptor gamma.
Characterizations of Cecal Microbiota
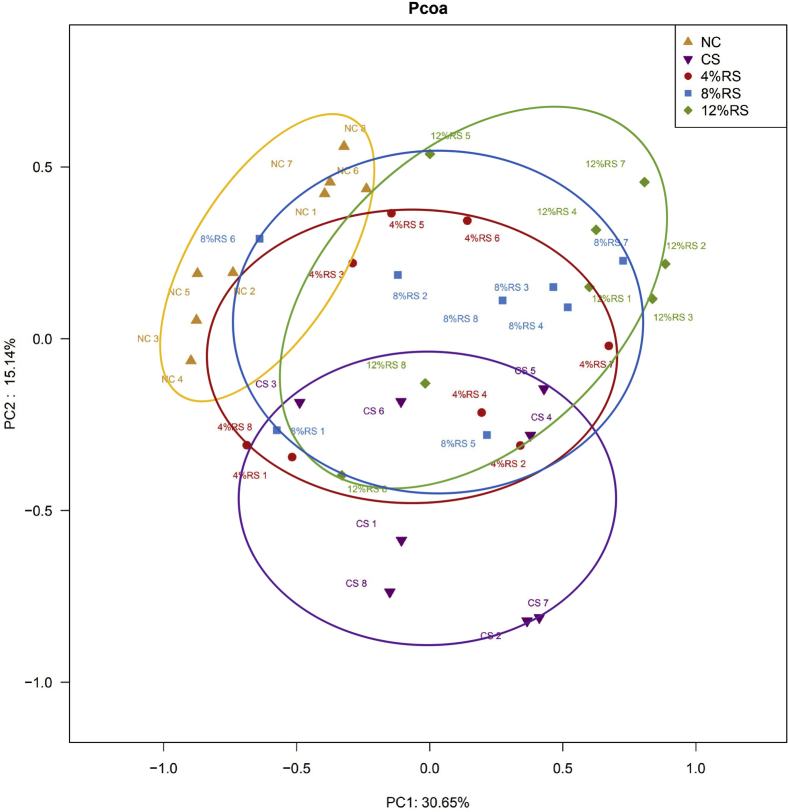
The alpha diversity metrics of cecal microbiota among groups are presented in Figure 3. No significant differences in Chao1, Shannon, and Simpson indices were observed among the groups (P > 0.05; Figure 3). Principal coordinate analysis revealed that bacterial communities in cecal samples from the CS and all RS supplementation diet group clustered separately from the bacteria of the NC group, whereas the sample from CS and 4%RS groups clustered more closely (Figure 4).
Figure 3.
The (A) Chao 1, (B) Shannon, and (C) Simpson indices were used to show the alpha diversity among groups. Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS, and 12%RS, the corn–soybean–based diets supplementation with 4, 8, and 12% corn resistant starch (RS), respectively.
Figure 4.
Principal coordinate analysis (PCoA) of bacterial communities in cecal digesta of broilers based on unweighted UniFrac distance metrics. Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS, and 12%RS, the corn–soybean–based diets supplementation with 4, 8, and 12% corn resistant starch (RS), respectively.
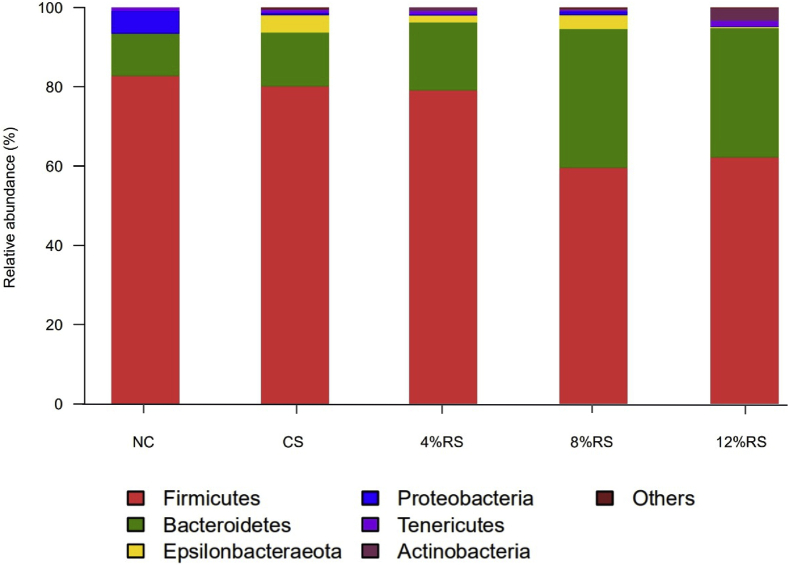
As shown in Figure 5, phylum level microbiota analysis revealed that Firmicutes, Bacteroidetes, Epsilonbacteraeota, Proteobacteria, Tenericutes, and Actinobacteria were the most 6 cecal dominants in all groups, together accounting for over 99.80% of the total sequences. Birds fed diets supplementation with 8 and 12% RS increased the abundance of cecal Bacteroidetes by 24.33 and 21.92% and decreased the abundance of Firmicutes by 23.08 and 20.47%, respectively, compared with the NC group (P < 0.05). Moreover, the abundance of Actinobacteria was increased in the 12%RS group compared with other groups.
Figure 5.
Relative abundance of cecal microbial communities of 21-day-old broilers on the phylum level. Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS, and 12%RS, the corn–soybean–based diets supplementation with 4%, 8%, and 12% corn resistant starch (RS), respectively.
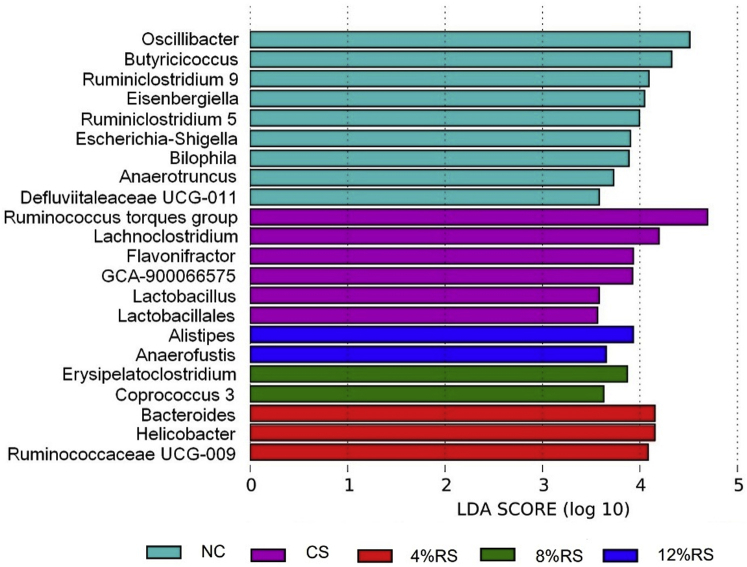
Figure 6 shows the differential genera in cecal digesta of 21-day-old broilers at the genus level. Compared with the NC group, CS group was characterized by higher relative abundances of Alistipes, Bacteroides, Campylobacter, GCA-900066575, Lactobacillus, and Ruminococcus torques group (P < 0.05; Figure 7A), as well as lower proportions of Anaerotruncus, Bilophila, Butyricicoccus, Defluviitaleaceae UCG-011, Escherichia-Shigella, Oscillibacter, Ruminiclostridium 5, and Ruminiclostridium 9 (P < 0.05; Figure 7B). When comparing CS and all RS groups directly, there was a higher abundance of Ruminococcaceae UCG-009 in 4%RS group, and a higher proportion of Coprococcus 3 in 8%RS group (P < 0.05; Figure 7C). Conversely, genera including Eisenbergiella were decreased in 8%RS and 12%RS groups, as well as Lachnoclostridium were decreased in all RS groups (P < 0.05; Figure 7D).
Figure 6.
Histogram of the LDA scores of the significantly altered genera among groups. The default parameter is LDA Score >2 and P < 0.05. Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS, and 12%RS, the corn–soybean–based diets supplementation with 4, 8, and 12% corn resistant starch (RS), respectively.
Figure 7.
Relative abundances of all altered genera of 21-day-old broilers. (A) Corn starch enriched genera, (B) Corn starch depleted genera, (C) Corn resistant starch enriched genera when compared with corn starch and (D) Corn resistant starch depleted genera when compared with corn starch. Values without a common letter (a-c) significantly differ (P < 0.05; n = 8). Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch; 4%RS, 8%RS, and 12%RS, the corn–soybean–based diets supplementation with 4, 8, and 12% corn resistant starch (RS), respectively.
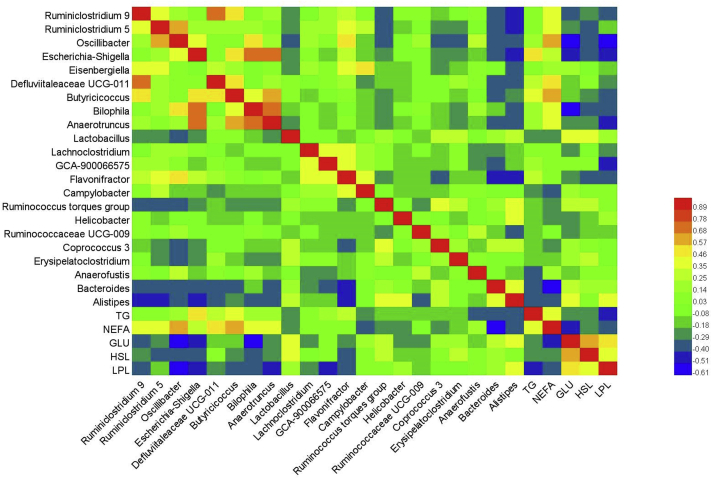
Correlation Between Cecal Altered Genera and Lipid Metabolism Parameters
Figure 8 revealed a range of correlation coefficients for the altered cecal genera and lipid metabolism parameters in plasma and abdominal adipose tissue, ranging from 1.0 (maximum positive correlation) to −1.0 (maximum anti-correlation), and 0 indicating no correlation. Obviously, genera including Ruminiclostridium 9, Ruminiclostridium 5, Oscillibacter, Escherichia-Shigella, Defluviitaleaceae UCG-011, Butyricicoccus, Bilophila, and Anaerotruncus were positively correlated with plasma NEFA concentration, while Lactobacillus, Campylobacter, Bacteroides, and Alstipes were negatively correlated with plasma NEFA concentration. Plasma TG concentration was positively correlated with cecal Escherichia-Shigella and Butyricicoccus, but negatively correlated with Anaerofuslis, Bacteroides, and Alstipes. Moreover, Oscillibacter, Escherichia-Shigella, Bilophila, and Flavonifractor were negatively correlated with plasma glucose concentration and activities of LPL and HSL.
Figure 8.
Pearson's correlations between altered cecal genera and parameters of lipid metabolism. Abbreviations: NC, a basic normal corn–soybean diet; CS, a corn–soybean–based diet supplementation with 20% corn starch (CS); 4%RS, 8%RS, and 12%RS, the corn–soybean–based diets supplementation with 4, 8, and 12% corn resistant starch (RS), respectively; HSL, hormone sensitive lipase; LPL, lipoprotein lipase; NEFA, nonesterified free fatty acid.
Discussion
Excessive abdominal fat is associated with the many chronic diseases of human, especially diabetes and cardiovascular (Lehmann et al., 1995; Miyazaki et al., 2002). For poultry industry, abdominal fat is the main source of waste which reduces feed efficiency and carcass yield (Moreira et al., 2018). It has been reported that RS from the high amylose maize reduced abdominal fat deposition in rats (Vidrine et al., 2014), which is consistent with our present findings that diets supplementation with 8 and 12% RS decreased abdominal fat percentage of broilers. Moreover, birds receiving RS treated diets exhibited smaller adipocyte diameters of abdominal adipose tissue than those receiving the control diet. These results demonstrated that feeding birds with diets containing higher concentrations of RS may alter lipid metabolism and then reduce fat deposition. It has been shown that dietary nondigestible carbohydrates exerts beneficial effects on blood lipid profiles (Roberfroid, 1993). In the present study, we observed that dietary supplementation with RS decreased the concentrations of plasma NEFA and TG. It is well known that dietary fat enters into the portal blood system and blood lipids mainly enter liver to be metabolized (Hermier, 1997). Therefore, the hepatic mRNA expressions of PPAR-γ, Acl, FAS, and acetyl-CoA carboxylase were determined for evaluating the ability of fatty acids synthesis of the liver. Maybe in response to the decreased lipid in blood, the mRNA expressions of PPAR-γ, Acl, FAS, and acetyl-CoA carboxylase were decreased in 12%RS treatment, indicating the inhibition of fatty acids synthesis in liver. In avian species, most fatty acids are synthesized in liver, then transported and stored as TG in adipose tissues (Griffin et al., 1992). To investigate whether the decreased liver fatty acids synthesis would further affect fat storage in the adipose tissue, the activities of LPL and HSL, as well as the mRNA expressions of GPAM and glycerol-3-phosphate acyltransferase 3 in the abdominal adipose tissue were determined. Lipoprotein lipase is responsible for the hydrolysis of plasma lipoprotein TG, and it plays a major role in TG deposition in adipose tissue (Cryer, 1981). In the present study, LPL activities were significantly decreased in bird from 8%RS and 12%RS groups, suggesting that RS could inhibit the conversion of TG to fat. Glycerol-3-phosphate acyltransferase is a rate-limiting enzyme catalyzing the initial step in TG biosynthesis (Wendel et al., 2009). The ability of TG resynthesize was also inhibited inferred from lower mRNA expressions of hepatic GPAM of birds both from 8%RS and 12%RS groups. In addition, hepatic HSL activity was higher both in birds from both 8%RS and 12%RS groups, indicating that body fat mobilization was strengthened, which further exacerbated the reduction of fat storage.
The thinner individuals often have a distinct gut microbiota composition compared with the obesity ones (Turnbaugh et al., 2006). In this study, birds fed with higher RS had less abdominal fat accompanied by more Bacteroidetes and less Firmicutes in their cecum. There is growing evidence that obesity has been indicated to correlate with abundances of Bacteroidetes and Firmicutes (Ley et al., 2006). Individuals with more Bacteroidetes and less Firmicutes may be thinner (Ley et al., 2005), which is in line with our current findings. We therefore speculated that the dietary RS-induced change in cecal microbiota may be part of the underlying reason for the reduction of abdominal fat deposition in broilers. Moreover, Proteobacteria was abundant in the NC group, whereas Epsilonbacteraeota was abundant in the CS group. However, there was no difference in fat deposition between the 2 groups, which demonstrated that Proteobacteria and Epsilonbacteraeota may contribute less to the fat deposition.
Pearson correlation analysis revealed that most genera depleted by dietary RS and CS were positively correlated with plasma NEFA and TG concentrations (e.g., Escherichia-Shigella and Butyricicoccus), whereas the genera enriched by RS and CS were negatively correlated with plasma NEFA concentration (e.g., Lactobacillus, Campylobacter, Bacteroides, and Alstipes), indicating that individuals who harbor more Lactobacillus, Campylobacter, Bacteroides, and Alstipes accompanied by less Escherichia-Shigella and Butyricicoccus can lower blood lipids. This finding was in agreement with Kalavathy et al. (2003) who reported that supplementation of Lactobacillus strains in broiler diets decreases serum TG concentration. Lactobacillus and Bacteroides have long been considered to be associated with lipid metabolism (Neyrinck et al., 2011; Xie et al., 2011). Furthermore, a negative correlation between activities of liver LPL, HSL and cecal Oscillibacter, Escherichia-Shigella, and Bilophila suggested that individuals who have less of these bacteria may have a higher fat storage capacity.
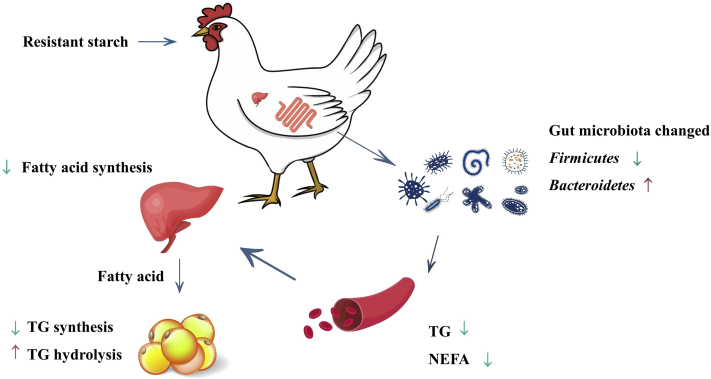
Overall, this study explored a relationship between lipid metabolism and cecal microbial composition in broilers fed diets supplementation with RS (Figure 9). Our data revealed that broilers receiving diets containing a higher concentration of RS harbor less Firmicute, which decreased liver fatty acid synthesis and suppress abdominal fat deposition of birds during the starter phase.
Figure 9.
Effects of corn-resistant starch on cecal microbial composition and lipid metabolism of broilers. In this study, we discovered that dietary corn-resistant starch could alter cecal microbiota, decrease blood lipid levels and fat storage capacity of abdominal adipose tissue as well as fatty acids synthesis of liver, which eventually led to the reduction in the abdominal fat deposition. Abbreviations: NEFA, nonesterified free fatty acid; TG, triglycerides.
Acknowledgments
This study was financially supported by the National Key Research and Development Program of China (2017YFD0500505), the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2019]425), and the Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents (2018).
Conflicts of Interest Statement: All authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.07.042.
Contributor Information
Lin Zhang, Email: zhanglin2012@njau.edu.cn.
Feng Gao, Email: gaofeng0629@sina.com.
Supplementary data
References
- Cryer A. Tissue lipoprotein lipase activity and its action in lipoprotein metabolism. Int. J. Biochem. 1981;13:525–541. doi: 10.1016/0020-711x(81)90177-4. [DOI] [PubMed] [Google Scholar]
- El Kaoutari A., Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- Englyst H.N., Kingman S.M., Cummings J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992;2:S33–S50. [PubMed] [Google Scholar]
- Griffin H.D., Guo K., Windsor D., Butterwith S.C. Adipose tissue lipogenesis and fat deposition in leaner broiler chickens. J. Nutr. 1992;122:363–368. doi: 10.1093/jn/122.2.363. [DOI] [PubMed] [Google Scholar]
- Han K., Fukushima M., Kato T., Kojima M., Ohba K., Shimada K., Sekikawa M., Nakano M. Enzyme-resistant fractions of beans lowered serum cholesterol and increased sterol excretions and hepatic mRNA levels in rats. Lipids. 2003;38:919–924. doi: 10.1007/s11745-003-1145-2. [DOI] [PubMed] [Google Scholar]
- Han J., Li L., Wang D., Ma H. (−)-Hydroxycitric acid reduced fat deposition via regulating lipid metabolism-related gene expression in broiler chickens. Lipids Health Dis. 2016;15:37. doi: 10.1186/s12944-016-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermier D. Lipoprotein metabolism and Fattening in poultry. J. Nutr. 1997;127:805S–808S. doi: 10.1093/jn/127.5.805S. [DOI] [PubMed] [Google Scholar]
- Jeffcoat R. Obesity-A perspective based on the biochemical interrelationship of lipids and carbohydrates. Med. Hypotheses. 2007;68:1159–1171. doi: 10.1016/j.mehy.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kalavathy R., Abdullah N., Jalaludin S., Ho Y.W. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br. Poult. Sci. 2003;44:139–144. doi: 10.1080/0007166031000085445. [DOI] [PubMed] [Google Scholar]
- Keenan M.J., Zhou J., McCutcheon K.L., Raggio A.M., Bateman H.G., Todd E., Jones C.K., Tulley R.T., Melton S., Martin R.J., Hegsted M. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity. 2006;14:1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- Kim W.K., Chung M.K., Kang N.E., Kim M.H., Park O.J. Effect of resistant starch from corn or rice on glucose control, colonic events, and blood lipid concentrations in streptozotocin-induced diabetic rats. J. Nutr. Bilchem. 2003;14:166–172. doi: 10.1016/s0955-2863(02)00281-4. [DOI] [PubMed] [Google Scholar]
- Lehmann R., Vokac A., Niedermann K., Agosti K., Spinas G.A. Loss of abdominal fat and improvement of the cardiovascular risk profile by regular moderate exercise training in patients with NIDDM. Diabetologia. 1995;38:1313–1319. doi: 10.1007/BF00401764. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Liu X., Ogawa H., Kishida T., Ebihara K. The effect of high-amylose cornstarch on lipid metabolism in OVX rats is affected by fructose feeding. J. Nutr. Biochem. 2010;21:89–97. doi: 10.1016/j.jnutbio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Liu Y.S., Zhang Y.Y., Li J.L., Wang X.F., Xing T., Zhu X.D., Zhang L., Gao F. Growth performance, carcass traits and digestive function of broiler chickens fed diets with graded levels of corn resistant starch. Bri. Poult. Sci. 2020;61:146–155. doi: 10.1080/00071668.2019.1694137. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Glass L., Triplitt C., Wajcberg E., Mandarino L.J., Defronzo R.A. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- Moreira G.C.M., Boschiero C., Cesar A.S.M., Reecy J.M., Godoy T.F., Pértille F., Ledur M.C., Moura A.S.A.M.T., Garrick D.J., Coutinho L.L. Integration of genome wide association studies and whole genome sequencing provides novel insights into fat deposition in chicken. Sci. Rep. 2018;8:16222. doi: 10.1038/s41598-018-34364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrinck A.M., Possemiers S., Druart C., Van de Wiele T., De Backer F., Cani P.D., Larondelle Y., Delzenne N.M. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One. 2011;6:e20944. doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A.P. Health properties of resistant starch. Food Nutr. Bull. 2005;30:27–54. [Google Scholar]
- O'Hea E.K., Leveille G.A. Lipogenesis in isolated adipose tissue of the domestic chick (Gallus domesticus) Comp. Biochem. Physiol. 1968;26:111–120. doi: 10.1016/0010-406x(68)90317-4. [DOI] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2013;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Performance Ferms and Measurement for Poultry . China Agricultural Press; Beijing, China: 2004. Ministry of Agricultural of the People’s Republic of China. NY/T823–2004. [Google Scholar]
- Roberfroid M. Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. Crit. Rev. Food Sci. Nutr. 1993;33:103–148. doi: 10.1080/10408399309527616. [DOI] [PubMed] [Google Scholar]
- Robertson M.D., Currie J.M., Morgan L.M., Jewell D.P., Frayn K.N. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. 2003;46:659–665. doi: 10.1007/s00125-003-1081-0. [DOI] [PubMed] [Google Scholar]
- So P., Yu W., Kuo Y., Wasserfall C., Goldstone A.P., Bell J.D., Frost G. Impact of resistant starch on body fat patterning and central appetite regulation. PLoS One. 2007;2:e1309. doi: 10.1371/journal.pone.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Upadhyaya B., Mccormack L., Fardinkia A.R., Juenemann R., Nichenametla S., Clapper J., Specker B., Dey M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016;6:28797. doi: 10.1038/srep28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine K., Ye J., Martin R.J., McCutcheon K.L., Raggio A.M., Pelkman C., Durham H.A., Zhou J., Senevirathne R.N., Williams C., Greenway F., Finley J., Gao Z., Goldsmith F., Keenan M.J. Resistant starch from high amylose maize (HAM-RS2) and dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity. 2014;22:344–348. doi: 10.1002/oby.20501. [DOI] [PubMed] [Google Scholar]
- Wendel A.A., Lewin T.M., Coleman R.A. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. Acta. 2009;1791:501–506. doi: 10.1016/j.bbalip.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie N., Cui Y., Yin Y., Zhao X., Yang J., Wang Z., Fu N., Tang Y., Wang X., Liu X., Wang C., Lu F. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement. Altern. Med. 2011;11:53. doi: 10.1186/1472-6882-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Martin R.J., Tulley R.T., Raggio A.M., McCutcheon K.L., Shen L., Danna S.C., Tripathy S., Hegsted M., Keenan M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal E.G., Akkermans A.D., De Vos W.M. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 1998;64:3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.