Abstract
The prevalence of crossed beaks ranging from 0.2 to 7.4% was documented in at least 12 chicken strains. Previous studies focused largely on candidate molecules, whereas the morphological observation was missing. This study reported a detailed phenotype and prevalence of crossed beaks based on morphological observation in nine thousand nine hundred 1-day-old female Beijing-You chicks. Affected chicks were classified into 2 categories based on the direction of the mandibular deformation: left and right. Each category was selected to sacrifice for the measurement of length, width, and thickness of the bilateral mandibular ramus (MR). The normal chicks were used as controls. Paraffin section was made for the bilateral MR of a crossed beak and a normal control for histology analysis. A total of 97 out of 9,900 chickens showed beak deformity including 71 crossed beaks (0.72%) and 26 side beaks (0.26%) for which the upper and lower beak were both bent in the same direction. There was no difference in the direction of the bend of the lower beak in crossed beaks (P > 0.05). The incidence of crossed beaks increased quickly from 0 to 56 d and no new incidence after 56 d. The angle of the crossed beaks was below 5° in the first week and had grown more severe with age until 56 d. The mandible structure showed that condyle served as a growth center for the MR extension. The short-side MR of crossed beaks was thicker than normal ones (P < 0.05) and caused the mandible deviated to the same direction. Meanwhile, the short-side MR prevented the occlusion, leading the jugal arch deformity, which in turn resulted in a bent maxillary horizontally. Similarly, chicks with side beaks also had asymmetry in MR length and the deformities of the jugal arch after dissection. In summary, asymmetric growth of bilateral MR induced crossed beaks and side beaks; the mandibular condyle could be an ideal sample for the related molecular mechanism studies underlying this trait.
Key words: beak deformity, chicken, mandibular ramus, mandibular condyle, jugal arch
Introduction
The beak of birds consists of the maxillary and mandible and is the dominant facial feature (Gottschaldt and Lausmann, 1974). The crossed beak is a deformity defined earlier as misalignment of the upper and lower beak (Pomeroy, 1962). Based on the relevant literatures, crossed beaks have been reported in at least 12 chicken strains around the world, including commercial strains like White Leghorn (Landauer, 1938), Rhode Island Red (Landauer, 1956), and native chickens like Appenzeller Barthuhn, Schweizerhuhn (Joller et al., 2018), Silkies (Bai et al., 2014, 2016), Beijing-You (Bai et al., 2018a), and Huiyang Bearded (Hong et al., 2019). The frequency in the literatures ranged from 0.2 to 7.4%. In addition, crossed beaks exist in about 30% of 114 Chinese native chicken strains, according to our survey of one–third of the breeds. Further, 0.05 to 2.0% of the incidence in our incomplete statistical result.
Beak functions are similar to the lips and teeth of mammals, being used for the intake of food and water (Benkman and Lindholm, 1991), grooming, and parasite removal (Chen et al., 2011; Giuseppe et al., 2015). However, birds with crossed beaks have reduced feed intake, inhibited growth, poor production performance, and shorter survival (Bai et al., 2018a, 2018b; Joller et al., 2018; Hong et al., 2019). Therefore, crossed beaks represent an economic as well as an animal welfare problem in the poultry industry.
Many studies suggested that the crossed beak was caused by genetic factors (Landauer, 1938; Bai et al., 2016, 2018a; Joller et al., 2018), although, it also could be a result of physical injury like the beak-trimming (Yamauchi et al., 2017; Struthers et al., 2019). Landauer (1938) developed a classification of crossed beaks based on descriptive observations. The first 2 categories included crossed beaks accompanied by abnormalities of the eyes and the skull during unfavorable incubation condition and have survived the early lethal period with lethal alleles. Moreover, the after 2 categories have indicated hereditable that showed asymmetry in nasals and orbits. The third category comprised of crossed beaks that does not become apparent until the chicks are between 1 and 2 mo old, whereas the fourth category consisted of crossed beaks present at hatching but later grow into a normally developed beak. However, no true-breeding individuals were obtained in spite of considerable inbreeding (Landauer, 1938) and crossing between parents with crossed beaks gave exclusively normal offspring (Bai, 2017; Joller et al., 2018). Meanwhile, the heritability was estimated at 0.10 in Beijing-You chickens, and the incidence of crossed beaks did not differ between male and female progeny (Bai et al., 2018b). These studies provide accumulative evidence that crossed beak is a complex mode of inheritance.
To understand the genetic determinants of beak deformity, Bai et al. (2014) employed digital gene expression to compare the difference of mRNA profile in the mandible of chickens of 56 d of age and highlighted several genes as potential candidates. Moreover, Hong et al. (2019) performed qRT-PCR to compare bone morphogenetic protein 4 expression in different parts of the affected chickens' skull of 105 d of age and observed that it was the highest in mandible, premaxilla, lacrimal, frontal, and parietal bones, but not different in nasal, turbinate, or occipital bones. Sun et al. (2019) employed a proteomics method to explore the differentially expressed proteins of the whole beak from the affected and normal birds of 12 d of age and suggested parvalbumin and lipoprotein lipase as key proteins. However, the genetic determinants of crossed beaks remain incompletely understood. The fact that the phenotype of beak deformity was not well-defined and therefore the sampling was not evidence-based could be the crucial factors accounting for the nonconsensus among these different studies.
The research of crossed beaks has been increasing in recent years, and the morphological information of this trait needs further study urgently. The objective of this study was to identify the detailed phenotype of beak deformity based on Beijing-You chickens and provide the guidance for sampling and new insights underlying crossed beaks.
Materials and methods
Ethics Statement
The study was performed in accordance with local ethical guidelines and met the requirement of the institutional animal care and use committee (No. IAS2020-8). The osteological terminology used here follows Baumel et al. (1993), but terms are translated into English.
Animals and Prevalence of Beak Deformity
As the incidence of crossed beaks did not differ between male and female progeny based on our previous study (Bai, 2017), 1-day-old female Beijing-You chicks (n = 9,900) from Beijing Bainianliyuan Ecological Agriculture Co., Ltd., located in Beijing, China, were used in this study. The chicks were not beak-trimmed and housed in a light-controlled brooding facility. Feed and water were provided according to the chicken feeding standards (Ministry of Agriculture, 2004). All chicks were under observation for the occurrences of beak deformity until 70 d of age. Moreover, a portion of 2,140 chicks were randomly selected and examined weekly for the beak morphology and prevalence of crossed beak. Based on the beak morphology in this study, all chicks were classified into 3 groups (Figure 1): crossed beak (G1), which similar to the third category in Landauer (1938) that crossed beaks does not become apparent until the chicks grow for a while; side beak (G2), that is the upper and lower beak were both bent in the same direction; and normal beak (G3).
Figure 1.
The morphological observation of 3 beak types in Beijing-You chickens. Note: side beak indicated the upper and lower beaks were both bent in the same direction. a, mandibular condyle; b, mandibular ramus.
Skull Anatomy
Each chicken of G1, G2, and G3 of 28 d of age and 2 chickens with crossed beak of 120 d of age (die of limited feed intake because of the beak deformity) were selected for the skull anatomy analysis. The head of these chickens was isolated after the direct cervical dislocation and boiled in 100°C water for 10 min. After removal of the muscle tissue, the skulls were further soaked in 1% sodium hydroxide for 1 d and in an organic solvent for 7 d to remove the lipids substantially and dried by exposure to the sun.
Change in BW and Angle
Each chick from G1 and G2 and 30 chicks from G3 were weighed weekly from 0 to 70 d of age.
The average angle in the crossed beak of G1 was measured weekly using a protractor according to Bai (2017) by the same technician until 70 d of age.
Mandibular Ramus Characteristics
Chicks of G1 were further classified into 2 categories based on the direction of the mandibular deformation: 1) the mandible was bent to left (LEFT) and 2) the mandible was bent to right (RIGHT). The measurement of mandibular ramus (MR) characteristics was conducted on 14, 28, and 70 d, because of hardly obtain large samples at 1 time and affected birds had a higher mortality rate than normal. Three or 4 chicks of each category were randomly selected to sacrifice for the characteristic of the MR. The weight of bilateral mandible was recorded, and the MR length was measured by Digimizer 5.3.4 MedCalc software (Ostend, Belgium). The thickness and width of bilateral MR were averaged at 3 parts (near, middle, and far) by a Vernier caliper, respectively. Moreover, normal chicks of the same age were used as controls for the parameters estimated here.
The following formula proposed by Habets et al. (1988) was used to evaluate the symmetry of the bilateral MR length:
Symmetry (%) = (left MR length − right MR length)/(left MR length + right MR length) × 100, for which, 0% is complete symmetry and 100% is complete asymmetry. The ratio of higher than 3% is considered as anatomical asymmetry based on standard of Habets et al. (1988).
Histology of the Mandibular Condyle and Ramus
The bilateral mandible of each chick in LEFT and NORMAL were removed at 7 d of age, sectioned sagittal, and fixed in buffered 4% formalin. After decalcification in 7% EDTA, they were embedded in paraffin and sectioned at 4 μm thick. These sections were then stained with Harris hematoxylin and eosin for histomorphology analysis the structure of mandibular condyle and ramus using A Zeiss Axioskop (Carl Zeiss, Thornwood, NY) equipped with a QICam digital camera operated with NIS Elements Software (Nikon Instruments, Melville, NY).
Statistical Analysis
The significance of occurrence in each category was analyzed by Chi-square test. The MR characteristics and symmetry ratio were analyzed using the GLM procedure of SAS 9.1 (SAS Institute Inc., Cary, NC). The main effect in the model was the direction of the mandibular deformation. Means were compared by Student-Newman-Keuls multiple-range tests when a significant difference was detected. The length, thickness, and width of bilateral MR were analyzed using a 2-sample paired t test. Significance was designated as P < 0.05.
Results
Prevalence of Beak Deformity
A total of 97 out of 9,900 (0.98%) showed beak deformity including 71 crossed beaks (0.72%) and 26 side beaks (0.26%) in Beijing-You chickens within 70 d of age (Table 1). There was no difference in the direction of the bend of the lower beak in crossed beaks group (chi-square = 0.03; P > 0.05). However, the frequency of left deviation was higher than the right in side beaks group (chi-square = 4.04; P < 0.05).
Table1.
The occurrence of beak deformity in Beijing-You chickens at 70 d of age.
| Population | Number | Subtype | Occurrence | Chi-square | P-value |
|---|---|---|---|---|---|
| Total observations | 9,900 | - | - | - | - |
| Normal beaks | 9,803 (99.02%) | - | - | - | - |
| Crossed beaks | 71 (0.72%) | Left1 | 36 (0.36%) | 0.03 | >0.05 |
| Right2 | 35 (0.35%) | ||||
| Side beaks | 26 (0.26%) | Left3 | 18 (0.18%) | 4.04 | <0.05 |
| Right4 | 8 (0.08%) |
The lower beak was bent to left.
The lower beak was bent to right.
The upper and lower beaks were both bent to left.
The upper and lower beaks were both bent to right.
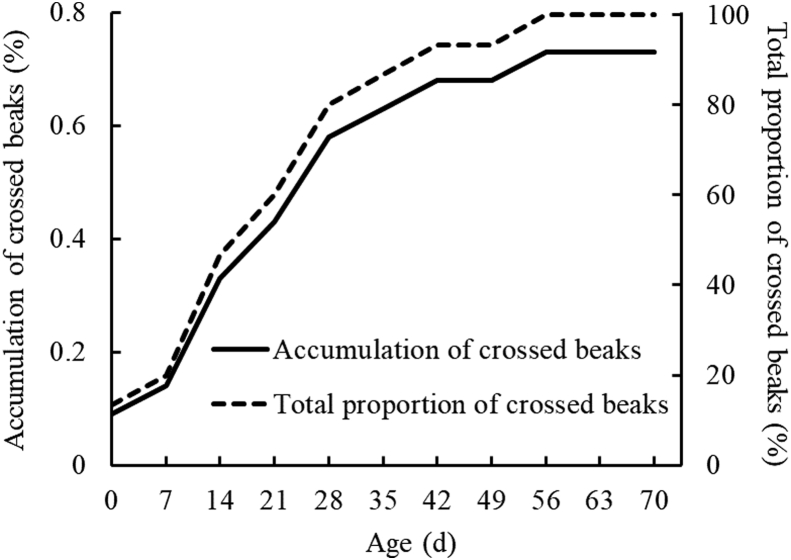
The crossed beaks could be observed as early as hatched (0.09%; Figure 2), and the incidence increased quickly at a rate of nearly 20% per week from 7 to 28 d of age. Moreover, the 93.33% chicks with crossed beaks could be identified before 42 d of age, and no new incidence after 56 d of age.
Figure 2.
The prevalence of crossed beaks in Beijing-You chickens from 0 to 70 d of age.
Skull Anatomy
The crossed beak is characterized by one or both beaks deviating laterally from the longitudinal axis of the head. Meanwhile, the crossed beaks and side beaks showed asymmetric length of bilateral MR (Figure 1).
The view from the ventral of the affected chicks indicated that the short-side MR caused the mandible bent in the same direction, and the bilateral synovial joint in the quadrate bone was asymmetry (Figure 3A). The jugal arch was bent by the short MR, resulting in the deformity of the jugal arch, which in turn caused the maxillary curved horizontally at the base along and bend to the direction of the mandibular deformation (Figures 3B, 3C).
Figure 3.
The morphological observation of crossed beaks in Beijing-You chickens. A, View from ventral; B, View from dorsal; C, View from front. a, Synovial joint in the quadrate bone; b, Curved jugal arch.
Change in BW and Angle
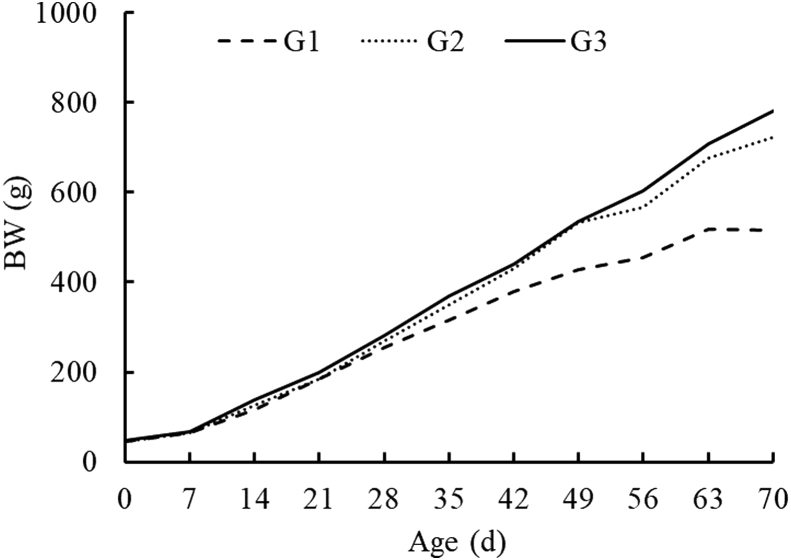
The BW of G1 increased slowly after 28 d of age as compared with G2 and G3 (Figure 4).
Figure 4.
Change in BW of Beijing-You chickens with crossed beak (G1), side beak (G2), and normal beak (G3) with age until 70 d of age.
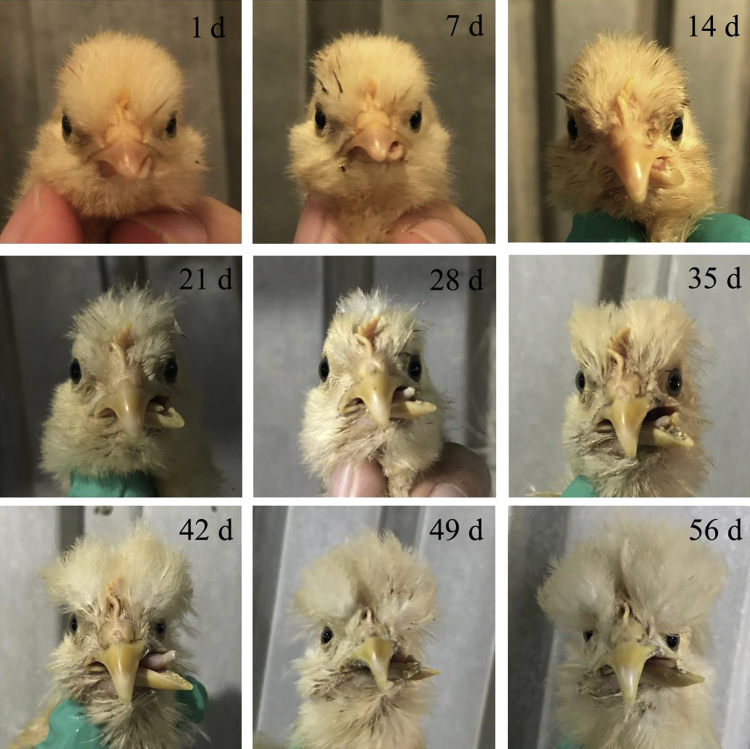
The average angle of the crossed beaks was below 5° in the first week and had grown more severe with age until 56 d of age (Figure 5), as an example in Figure 6.
Figure 5.
Change in the average angle of Beijing-You chickens with crossed beaks from 0 to 70 d of age.
Figure 6.
Morphology change of a crossed beak in an affected Beijing-You chicken with age until 56 d of age.
Mandibular Ramus Characteristics
Mandibular ramus characteristics at 14 d, 28 d, and 70 d of age were shown in 2, 3, 4, respectively. The MR length difference (left-right) in LEFT and RIGHT were lower and higher than NORMAL, respectively (P < 0.05), and the absolute value of symmetry ratio of LEFT and RIGHT were higher than 3%. The LEFT had shorter and thicker left MR than those of NORMAL and RIGHT (P < 0.05), but there was no difference between NORMAL and RIGHT (P > 0.05). The RIGHT had shorter and thicker right MR than those of NORMAL and LEFT (P < 0.05).
Table 2.
The mandibular ramus characteristics of crossed beaks (n = 8) and normal beaks (n = 4) in Beijing-You chickens at 14 d of age.
| Group | Crossed beaks |
Normal beaks |
SEM | P-value | ||
|---|---|---|---|---|---|---|
| LEFT | RIGHT | NORMAL | ||||
| Mandible weight, g | Left | 0.15 | 0.16 | 0.17 | 0.01 | 0.52 |
| Right | 0.16 | 0.17 | 0.18 | 0.01 | 0.62 | |
| SEM | 0.01 | 0.01 | 0.01 | |||
| P-value | 0.32 | 0.41 | 0.38 | |||
| MR1 thickness, mm | Left | 1.03 | 0.87B | 0.85 | 0.04 | 0.13 |
| Right | 0.77c | 1.06A,a | 0.93b | 0.05 | 0.01 | |
| SEM | 0.07 | 0.04 | 0.03 | |||
| P-value | 0.07 | 0.01 | 0.12 | |||
| MR width, mm | Left | 2.64 | 2.96A | 2.98 | 0.10 | 0.30 |
| Right | 2.88 | 2.69B | 2.98 | 0.07 | 0.24 | |
| SEM | 0.13 | 0.10 | 0.05 | |||
| P-value | 0.23 | <0.01 | 1.00 | |||
| MR length, cm | Left | 1.40B,b | 2.09A,a | 2.05a | 0.10 | <0.01 |
| Right | 2.02A,a | 1.52B,b | 2.07a | 0.08 | <0.01 | |
| SEM | 0.12 | 0.11 | 0.01 | |||
| P-value | <0.01 | <0.01 | 0.96 | |||
| MR length difference (left-right), cm | −0.61c | 0.57a | −0.02b | 0.12 | <0.01 | |
| Symmetry2, % | −18.05c | 15.82a | −0.53b | 2.78 | <0.01 | |
a–cValues within a row with different superscripts differ significantly at P < 0.05.
A, BValues within a column of each trait with different superscripts differ significantly at P < 0.05.
MR, Mandibular ramus.
Symmetry (%) = (left MR length − right MR length)/(left MR length + right MR length) × 100.
Table 3.
The mandibular ramus characteristics of crossed beaks (n = 6) and normal beaks (n = 3) in Beijing-You chickens at 28 d of age.
| Group | Crossed beaks |
Normal beaks |
SEM | P-value | ||
|---|---|---|---|---|---|---|
| LEFT | RIGHT | NORMAL | ||||
| Mandible weight, g | Left | 0.25 | 0.22B | 0.23 | 0.01 | 0.37 |
| Right | 0.27 | 0.25A | 0.25 | 0.01 | 0.57 | |
| SEM | 0.01 | 0.01 | 0.01 | |||
| P-value | 0.60 | 0.03 | 0.06 | |||
| MR1 thickness, mm | Left | 1.11A,a | 0.83b | 0.85b | 0.06 | 0.04 |
| Right | 0.84B,b | 1.11a | 0.84b | 0.06 | 0.04 | |
| SEM | 0.07 | 0.09 | 0.01 | |||
| P-value | <0.01 | 0.07 | 0.69 | |||
| MR width, mm | Left | 3.78A | 3.51B | 3.47 | 0.06 | 0.05 |
| Right | 3.51B,b | 4.00A,a | 3.46b | 0.1 | <0.01 | |
| SEM | 0.08 | 0.11 | 0.07 | |||
| P-value | <0.01 | 0.01 | 0.87 | |||
| MR length, cm | Left | 1.91B,b | 2.59A,a | 2.65a | 0.12 | <0.01 |
| Right | 2.69A,a | 1.91B,b | 2.66a | 0.13 | <0.01 | |
| SEM | 0.18 | 0.16 | 0.03 | |||
| P-value | <0.01 | <0.01 | 0.63 | |||
| MR length difference (left-right), cm | −0.78c | 0.68a | −0.01b | 0.21 | <0.01 | |
| Symmetry2, % | −16.91c | 15.18a | −0.15b | 4.64 | <0.01 | |
a–cValues within a row with different superscripts differ significantly at P < 0.05.
A, BValues within a column of each trait with different superscripts differ significantly at P < 0.05.
MR, Mandibular ramus.
Symmetry (%) = (left MR length − right MR length)/(left MR length + right MR length) × 100.
Table 4.
The mandibular ramus characteristics of crossed beaks (n = 8) and normal beaks (n = 4) in Beijing-You chickens at 70 d of age.
| Group | Crossed beaks |
Normal beaks |
SEM | P-value | ||
|---|---|---|---|---|---|---|
| LEFT | RIGHT | NORMAL | ||||
| Mandible weight, g | Left | 0.38 | 0.4 | 0.44 | 0.01 | 0.17 |
| Right | 0.36 | 0.42 | 0.44 | 0.02 | 0.28 | |
| SEM | 0.01 | 0.02 | 0.02 | |||
| P-value | 0.32 | 0.41 | 0.38 | |||
| MR1 thickness, mm | Left | 1.51A,a | 1.11B,b | 1.10b | 0.07 | <0.01 |
| Right | 1.12B,b | 1.59A,a | 1.14b | 0.08 | <0.01 | |
| SEM | 0.08 | 0.11 | 0.03 | |||
| P-value | <0.01 | 0.04 | 0.14 | |||
| MR width, mm | Left | 4.58a,b | 4.24b | 4.94a | 0.11 | 0.03 |
| Right | 4.30 | 4.52 | 4.89 | 0.14 | 0.28 | |
| SEM | 0.08 | 0.19 | 0.07 | |||
| P-value | 0.13 | 0.23 | 0.49 | |||
| MR length, cm | Left | 2.27B,b | 3.41A,a | 3.43a | 0.18 | <0.01 |
| Right | 3.39A,a | 2.42B,b | 3.48a | 0.16 | <0.01 | |
| SEM | 0.22 | 0.19 | 0.05 | |||
| P-value | <0.01 | <0.01 | 0.10 | |||
| MR length difference (left-right), cm | −1.12c | 0.99a | −0.05b | 0.29 | <0.01 | |
| Symmetry2, % | −19.83c | 17.05a | −0.78b | 5.01 | <0.01 | |
a–cValues within a row with different superscripts differ significantly at P < 0.05.
A, BValues within a column of each trait with different superscripts differ significantly at P < 0.05.
MR, Mandibular ramus.
Symmetry (%) = (left MR length − right MR length)/(left MR length + right MR length) × 100.
Furthermore, the left MR was shorter and thicker than that of the right-side in LEFT (P < 0.05), and the right MR was shorter and thicker than that of the left-side in RIGHT (P < 0.05). However, there was no difference in mandible weight among LEFT, RIGHT, and NORMAL (P > 0.05).
Histology of the Mandibular Condyle and Ramus
The mandibular condyle and ramus structure of both affected and normal chicks showed 3 distinctive principal zones: the resting zones, proliferative zones, and hypertrophic zones (Figure 7). The mandibular condyle serves as an important growth center for the MR extension. However, no obvious difference in the histology with bilateral mandibular condyle was observed between affected and normal chicks.
Figure 7.
Histology analysis of hematoxylin and eosin stained sections of the mandibular condyle and ramus in Beijing-You chickens at 7 d of age. A and B: left mandibular condyle and ramus of a crossed beak (the lower beak was bent to left); C and D: right mandibular condyle and ramus of a crossed beak (the lower mandible was bent to left); E and F: right mandibular condyle and ramus of a normal beak. B, D, and F detail of mandibular condyle marked by the rectangle in A, C, and E, respectively. Bar = 500 μm in A, C, and E. Bar = 50 μm in B, D, and F. a, cartilage cell; b, marrow cavity; c, bone trabecula; d, proliferative zones; e, hypertrophic zones.
Discussion
Prevalence of Beak Deformity
In Beijing-You chickens as observed here, the morphology and angle of the crossed beaks were more and more severe with age until 56 d of age. Meanwhile, the occurrence of crossed beaks increased 20% per week from 7 to 28 d of age, and no new incidence after 56 d of age. This is similar to Type III as classified by Landauer (1938), for which the chicks are normal at hatch, and the crossed beaks does not become apparent until 1 to 2 mo old. The occurrence of the beak deformity in Beijing-You chickens was 0.98%, and the crossed beaks reached 0.72%. This result was similar to the 0.8% in Schweizerhuhn, an indigenous Swiss chicken strain (Joller et al., 2018), and lower than 2.5 to 3.3%, 7.4, 1.5, and 2.4% in Silver Spangled Hamburgh (Landauer, 1938), Appenzeller Barthuhn, Appenzeller Spitzhaubenhuhn (Joller et al., 2018), and Huiyang Bearded chickens which an indigenous Chinese chicken strain (Hong et al., 2019), respectively.
Mandibular Ramus Characteristics
Chicks with crossed beaks had asymmetry MR length, whereas the shorter one is shorter than normal, and the longer one is similar to the normal at 14 d, 28 d, and 70 d of age. The mandible consists of dental bone, ramus, and condyle. Many researches indicated that the mandibular growth is mainly through endochondral bone formation at the condyle and intramembranous bone formation at the periosteum (Meikle, 1973; Enlow and Hans, 1996; Feng et al., 2013). Meanwhile, the condyle is essential for normal mandibular growth, in particular for the enlargement of the ramus (Meikle, 1973). In the present study, the structure of mandible further proved that the mandibular condyle served as a growth center for the developing of ramus at early stages. An endochondral growth mechanism is required for this part of the mandible. Our results are similar to the etiology of abnormal mandibular growth that condylar hyperactivity on unilaterally in humans (Pirttiniemi et al., 2009). Fukaya et al. (2017) reported that local unilateral insulin-like growth factors 1 injection to the condyle induced unilateral growth of the mandible and consequently caused facial asymmetry in mice. The mandibular length was significantly longer in the insulin-like growth factors 1 injection side than that on the control side. Therefore, the short MR of crossed beaks could be associated with the functional damage of the mandibular condyle.
Besides length, the short-side MR of the crossed beak was thicker than that of the normal ones, whereas the other side was normal in this study. This phenotype could be a protective measure to prevent the short-side MR from crushing and breaking. The force exerted from the other side of the MR on the short side may lead to an increase in bone mass and thickness according to mechanostat, a mechanism proposed by Frost (1987): the adaptive structure of bone tissue is a negative feedback system, the strain is the input signal, and the output is the bone mineral content and structure. Frost (1987) indicated that bone strains increase cortical bone mass, and many following studies also supported this mechanism (Gross et al., 1997; Kotha et al., 2004; Lambers et al., 2011; Meakin et al., 2014).
Skull Anatomy of Chickens With Beak Deformity
The upper beak of the crossed beak often bends horizontally at the base along, besides the affected mandible, and the skull (mainly nasals and orbits) is also asymmetric. This phenotype was also reported in native Swiss chicken strain (Joller et al., 2018). Meanwhile, Huiyang beard chickens with crossed beaks showed the same phenotype that the periorbital area is affected occasionally (Hong et al., 2019). Further dissection in this study showed that these chicks had deformed the jugal arch on the short-side MR. The jugal arch is a slender, generally straight, bar that connects the maxillary with the quadrate bone. Normally, the mandible is located medial to the jugal arch. When crossed beak occurs, however, the short-side MR crossed with the jugal arch and prevented the normal occlusion. The jugal arch is bent gradually by the mandible to ensure the drinking and feeding. The curved jugal arch and the muscle attached may have tension on the maxillary, resulting in the upper beak bent horizontally and the skull asymmetry.
Moreover, the small proportion (0.26%) of chicks with side beaks in this study that their upper and lower beak were both bent in the same direction. These chicks could not be identified as affected until their asymmetry in MR length, and the deformities of the jugal arch on the same side was observed after dissection. This nonobvious deformity without impaired growth may be hard eliminated from breeding and caused the persistent inheritance of teratogenic genes of crossed beaks.
Conclusion
The asymmetric growth of bilateral MR caused crossed beaks and side beaks; the mandibular condyle could be an ideal sample for the related molecular mechanism studies.
Acknowledgments
The financial support of this study was provided by the Beijing Municipal Science and Technology Project (No. D171100007817005), the Chinese Agricultural Research System (No. CARS-40), Key Laboratory of Animal (Poultry) Genetics Breeding and Reproduction, Ministry of Agriculture and Rural Affairs (No. Poultrylab2019-1), and the Agricultural Science and Technology Innovation Program (No. ASTIPIAS04).
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
Contributor Information
Yanyan Sun, Email: yanyansun2014@163.com.
Jilan Chen, Email: chen.jilan@163.com.
References
- Bai H. CAAS; China: 2017. Genome-wide Association Studies and Genome-wide Detection of Copy Number Variations for Beak Deformity in Chickens. PhD Diss. [Google Scholar]
- Bai H., Sun Y., Liu N., Liu Y., Xue F., Li Y., Xu S., Ni A., Ye J., Chen Y., Chen J. Genome-wide detection of CNVs associated with beak deformity in chickens using high-density 600K SNP arrays. Anim. Genet. 2018;49:226–236. doi: 10.1111/age.12652. [DOI] [PubMed] [Google Scholar]
- Bai H., Sun Y., Liu N., Xue F., Li Y., Xu S., Ye J., Zhang L., Chen Y., Chen J. Single SNP- and pathway-based genome-wide association studies for beak deformity in chickens using high-density 600K SNP arrays. BMC Genomics. 2018;19:501. doi: 10.1186/s12864-018-4882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H., Sun Y., Zhu J., Liu N., Li D., Xue F., Li Y., Chen J. Study on LOC426217 as a candidate gene for beak deformity in chicken. BMC Genet. 2016;17:44. doi: 10.1186/s12863-016-0353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H., Zhu J., Sun Y.Y., Liu R.R., Liu N., Li D.L., Wen J., Chen J.L. Identification of genes related to beak deformity of chickens using digital gene expression profiling. PLoS One. 2014;9:e107050. doi: 10.1371/journal.pone.0107050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumel J.J., King A.S., Breazile J.E., Evans H.E., Van den Berge J.C. Publications of the Nuttall Ornithological Club; Cambridge: 1993. Handbook of Avian Anatomy: Nomina Anatomica Avium. [Google Scholar]
- Benkman C.W., Lindholm A.K. The advantages and evolution of a morphological novelty. Nature. 1991;349:519–520. [Google Scholar]
- Chen B.L., Haith K.L., Mullens B.A. Beak condition drives abundance and grooming-mediated competitive asymmetry in a poultry ectoparasite community. Parasitology. 2011;138:748–757. doi: 10.1017/S0031182011000229. [DOI] [PubMed] [Google Scholar]
- Enlow D.H., Hans M.G. WB Saunders Company; Philadelphia, PA: 1996. Essentials of Facial Growth. [Google Scholar]
- Feng Z., Li L., Zhou Z., He D., Lai R., Yang C. The role of retaining condylar cartilage in open surgery of intracapsular condylar fracture in growing goats: histological analysis. CJOMS. 2013;11:459–467. [Google Scholar]
- Frost H.M. Bone “mass” and the “mechanostat”: a proposal. Anat. Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- Fukaya S., Kanzaki H., Miyamoto Y., Yamaguchi Y., Nakamura Y. Possible alternative treatment for mandibular asymmetry by local unilateral IGF-1 injection into the mandibular condylar cavity: experimental study in mice. Am. J. Orthod. Dentofacial. Orthop. 2017;152:820–829. doi: 10.1016/j.ajodo.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Giuseppe V., Mullens B.A., Mench J.A. Relationships between beak condition, preening behavior and ectoparasite infestation levels in laying hens. Poult. Sci. 2015;94:1997–2007. doi: 10.3382/ps/pev171. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K., Lausmann S. The peripheral morphological basis of tactile sensibility in the beak of geese. Cell Tissue Res. 1974;153:477–496. doi: 10.1007/BF00231542. [DOI] [PubMed] [Google Scholar]
- Gross T.S., Edwards J.L., Mcleod K.J., Rubin C.T. Strain gradients correlate with sites of periosteal bone formation. J. Bone Miner. Res. 1997;12:982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- Habets L.L., Bezuur J.N., Naeiji M., Hansson T.L. The Orthopantomogram, an aid in diagnosis of temporomandibular joint problems. II. The vertical symmetry. J. Oral Rehabil. 1988;15:465–471. doi: 10.1111/j.1365-2842.1988.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Hong Y., Pang Y., Zhao H., Chen S., Tan S., Xiang H., Yu H., Li H. The morphology of cross-beaks and BMP4 gene expression in Huiyang bearded chickens. Animals. 2019;9:1143. doi: 10.3390/ani9121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller S., Bertschinger F., Kump E., Spiri A., von Rotz A., Schweizer-Gorgas D., Drögemüller C., Flury C. Crossed beaks in a local Swiss chicken breed. BMC Vet. Res. 2018;14:68. doi: 10.1186/s12917-018-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha S.P., Hsieh Y.F., Strigel R.M., Müller R., Silva M.J. Experimental and finite element analysis of the rat ulnar loading model—correlations between strain and bone formation following fatigue loading. J. Biomech. 2004;37:541–548. doi: 10.1016/j.jbiomech.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Lambers F.M., Schulte F.A., Kuhn G., Webster D.J., Müller R. Mouse tail vertebrae adapt to cyclic mechanical loading by increasing bone formation rate and decreasing bone resorption rate as shown by time-lapsed in vivo imaging of dynamic bone morphometry. Bone. 2011;49:1340–1350. doi: 10.1016/j.bone.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Landauer W. Notes on cross-beak in fowl. J. Genet. 1938;37:51–68. [Google Scholar]
- Landauer W. Hereditary and induced cross-beak of fowl. J. Exp. Zool. 1956;132:25–38. [Google Scholar]
- Meakin L.B., Price J.S., Lanyon L.E. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front. Endocrinol. 2014;5:154. doi: 10.3389/fendo.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle M.C. The role of the condyle in the pstinatal growth of the mandible. Am. J. Orthod. 1973;64:50–62. doi: 10.1016/0002-9416(73)90280-7. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture . China Agriculture Press; Beijing, China: 2004. Feeding Standard of Chicken (NY/T33-2004) [Google Scholar]
- Pirttiniemi P., Peltomaki T., Muller L., Luder H.U. Abnormal mandibular growth and the condylar cartilage. Eur. J. Orthod. 2009;31:1–11. doi: 10.1093/ejo/cjn117. [DOI] [PubMed] [Google Scholar]
- Pomeroy D.E. Birds with abnormal bills. Br. Birds. 1962;55:233–238. [Google Scholar]
- Struthers S., Classen H.L., Gomis S., Crowe T.G., Schwean-Lardner K. The impact of beak tissue sloughing and beak shape variation on the behavior and welfare of infrared beak-treated layer pullets and hens. Poult. Sci. 2019;98:4269–4281. doi: 10.3382/ps/pez274. [DOI] [PubMed] [Google Scholar]
- Sun Y., Liu N., Bai H., Li Y., Xue F., Ye J., Ma H., En H., Chen J. Differential proteomic analysis to identify proteins associated with beak deformity in chickens. Poult. Sci. 2019;98:1833–1841. doi: 10.3382/ps/pey519. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y., Yoshida S., Matsuyama H., Obi T., Takase K. Morphologically abnormal beaks observed in chickens that were beak-trimmed at young ages. J. Vet. Med. Sci. 2017;79:1466–1471. doi: 10.1292/jvms.17-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]