Abstract
In laying hens, a diet supplemented with tryptophan (Trp) has been shown to affect their pecking behavior. However, unlike this positive effect, Trp is also involved in negative effects on behavior and stress through indolic pathways. Indole production can be reduced by probiotics (Pro), thus we hypothesized that Pro may prevent negative effects of Trp and increase beneficial effects on behavior in birds. Combined effects of Pro and Trp were also expected. To investigate the effects on behavior in birds of supplementing with a high level of Trp with or without Pro, Japanese quail were used because their behavior can be influenced by Pediococcus acidilactici, and they can be highly aggressive. Quails (n = 120) were assigned to 4 groups in a 2 × 2 factorial design for 55 d: C-C (control diet with usual Trp level, 0.3%; without Pro; n = 30), Trp-C (Trp: 2%; without Pro; n = 30), C-Pro (control diet; with Pro: 1 x 109 CFU/L P. acidilactici in drinking water; n = 30), and Trp-Pro (Trp 2%; with Pro; n = 30). Body weight was measured every week, and different tests were conducted to investigate behavioral characteristics of each quail. Contrary to our hypothesis, there was almost no interaction between Trp and Pro treatments. Tryptophan supplementation significantly (P < 0.05) reduced live weight up to 27 d, whereas Pro treatment had no effect. There was no significant difference between groups for tonic immobility variables (P > 0.05). The birds fed the high Trp diet spent significantly less time in the periphery of the open field than those fed the control diet and moved less in the arena during the social isolation test. Interindividual distances were significantly lower in males fed with Trp 2% than with the control diet, whereas Trp and Pro supplements interacted in females. The treatments did not affect sexual motivation in males. These results indicate that a high level of Trp reduced growth and appeared to enhance emotional reactivity in quails and that supplementing with Pro did not reduce these effects. In conclusion, feeding high Trp for 55 d cannot be recommended as a strategy to improve social behavior unlike effects observed in laying hens.
Key words: tryptophan, probiotic, behavior, microbiota-gut-brain-axis, quail
Introduction
L-tryptophan is a nutritionally essential amino acid for monogastric animals and preweaning ruminants because it cannot be synthesized in the body. Tryptophan (Trp) plays various roles in nutrition and physiology, particularly in food intake, neurological function, and immunity. Moreover, tryptophan is a precursor of serotonin (5-hydroxytryptamine), melatonin, and niacin. Serotonin is a mediator between the brain and the intestine, and it has been reported that tryptophan affects the behavior of birds through the synthesis of important neurotransmitters such as serotonin (Corzo et al., 2005a), and the role of the Trp-kynurenine pathway is under investigation regarding feather pecking behavior in hens (Birkl et al., 2019). Consequently, increased dietary Trp may affect an animal's response to stress through its ability to increase serotonin levels (Liu et al., 2015). Previous studies have indicated positive effects of supplementing diets with Trp on the behavior of farm animals by reducing aggressiveness and stress responses (Savory et al., 1999; Koopmans et al., 2005; Poletto et al., 2010; Shen et al., 2012) or feather pecking in laying hens (van Hierden et al., 2004). Intake of Trp can increase or decrease the emotional state of mammals (Young, 2013). Tryptophan may also affect behavior through indolic pathways. Indole and indolic compounds (indole propionate and indole acetate) are metabolic products of aromatic amino acids (tyrosine, phenylalanine, Trp) originating from dietary proteins, and indole could be a metabolite involved in mechanisms of the gut–brain axis (Crumeyrolle-Arias et al., 2014). We can thus postulate that probiotics (Pro) may modulate the effects of Trp on behavior.
Probiotics are defined as microbial cell preparations or components of microbial cells that have a beneficial effect on health. Probiotics, especially lactic acid bacteria, have been used for many centuries for different purposes including in food production (Song et al., 2012). They maintain a healthy balance of bacteria in the gastrointestinal tract by competitive exclusion, the process by which beneficial bacteria exclude potentially pathogenic bacteria through competing for attachment sites and nutrients in the intestine (Harimurti and Hadisaputro, 2015). Moreover, Pro reduce the intestinal absorption of toxic substances, such as indole, amine, and ammonia (Pathmakanthan et al., 2000). Commensal bacteria affect a variety of complex behaviors, including social, emotional, and anxiety-like behaviors, and contribute to brain development and function in mice and humans (Sampson and Mazmanian, 2015). These experiments have led to the concept of the microbiota–gut–brain axis, and this concept is now in use with farm animals because of the increasing evidence that commensal microbes and Pro influence their behavior (Kraimi et al., 2019a).
Only a few studies have investigated the gut–brain axis in birds. Following on from results of Pro and Trp use in poultry, we decided to investigate their effects on bird behavior and test whether their effects interacted. We chose the quail to test this hypothesis because its social and fear behaviors are well known under experimental conditions, and the microbiota–gut–brain axis has been investigated in this species (Kraimi et al., 2018, 2019b); moreover, aggressiveness can be high in species under rearing conditions. The objective of this study was to investigate whether supplementing with Trp and/or Pro influenced behavioral characteristics of quail.
Materials and methods
Ethics Statement
The experimental animal protocol describing the management, behavior procedures, and animal care and also the study protocol were evaluated and approved by the Animal Welfare and Ethics Committee of INRA Val-de-Loire Center, Nouzilly, France (ref. 2015-19). All animal behavior and care procedures throughout this study followed French Experimental Animal Care and Use Guidelines.
Animals, Housing, Diets, and Experimental Design
Quail eggs originating from a nonselected genetic line were provided by the experimental unit 1295 (UE PEAT) and UMR 85, Physiologie de la Reproduction et des Comportements, INRA Val de Loire Center, Nouzilly, France.
They were incubated for 17 d in a collective incubator in our laboratory (38.5°C, 45% humidity for 15 d, and 65% humidity for the last 2 d). After hatching, a total of one hundred twenty 1-day-old wing-tagged quail chicks of both sexes were randomly assigned to 4 groups: C-C (control diet with Trp; 0.295%; without Pro), Trp-C (Trp: 2%; without Pro), C-Pro (control diet; with Pro: 1 x 109 CFU/L Pediococcus acidilactici in drinking water, Bactocell ME, Lallemand), Trp-Pro (Trp 2%; with Pro: P. acidilactici supplementation). A dose of 2%Trp was chosen based on previous findings indicating that this level attenuates stress in chicks and laying hens (Savory et al., 1999; van Hierden et al., 2004). Water with Pro was prepared ex temporane with water at room temperature. Drinking water with and without Pro was changed every day to make sure the provision of Pro remained available and unaltered. Three cages of 10 birds for each group were raised until 21 d of age (30 birds in each group). At 3 wk of age, the birds' sex was determined according to feather dimorphism (plumage color), and thereafter, males and females were reared separately in battery cages until the end of the trial (2 cages of 7 to 8 quails for each sex in each group, 30 birds in each group) to avoid aggressiveness of males toward females. Lighting was continuous for the first 3 d and then gradually decreased to 14 hr per day on day 15 (22L:2D at day 4; 20L:4D at day 5; 18L:6D day 6; 16L:8D at day 10; 15L:9D at day 14 and 14L:8D, thereafter). The temperature was progressively reduced from 38°C to 20°C. Water and food were provided ad libitum. All quail were weighed every 7 d from hatching to day 55. Male quail were withdrawn from the experiment on day 42 because their aggressive behavior prevented them from being kept in collective cages.
The control feed was formulated according to standard recommendations. Both the control and high Trp feeds were isoenergetic and isoproteic and based on wheat, maize, and soybean meal (Table 1). The control feed contained 0.295% Trp, whereas the experimental feed contained 2%Trp. Regarding Pro treatment, the control drinking water contained no Pro, whereas the experimental drinking water contained 1 x 109 CFU/L Pro (P. acidilactici CNCM MA 18/5M, Bactocell ME, Lallemand).
Table 1.
Composition and nutrient level of dietary raw materials (g/kg, air-dry basis).
| Ingredients | Normal trytophan | High tryptophan |
|---|---|---|
| Maize | 24.80 | 24.00 |
| Wheat | 500.00 | 486.80 |
| Soybean meal | 247.00 | 244.00 |
| Soybean oil | 55.90 | 55.90 |
| Wheat distillery by-product | 30.00 | 30.00 |
| Rapeseed meal | 50.00 | 50.00 |
| Extruded soybean | 50.00 | 50.00 |
| DL-methionine | 3.25 | 3.25 |
| Calcium carbonate | 7.25 | 7.25 |
| Dicalcium phosphate | 19.00 | 19.00 |
| Vitamin-mineral premix1 | 5.00 | 5.00 |
| Salt | 4.00 | 4.00 |
| Lysine HCl | 2.80 | 2.80 |
| Threonine | 1.00 | 1.00 |
| Tryptophan | - | 17.00 |
| Calculated nutrient composition | ||
| ME (kcal/kg) | 3,000 | 3,000 |
| Crude protein (g/kg) | 220.00 | 220.00 |
| Lysine(g/kg) | 13.020 | 13.020 |
| Methionine (g/kg) | 6.400 | 6.400 |
| Methionine + cystine (g/kg) | 10.180 | 10.180 |
| Tryptophan (g/kg) | 2.950 | 20.000 |
| Threonine (g/kg) | 8.910 | 8.910 |
| Calcium (g/kg) | 10.460 | 10.460 |
| Available phosphorus (g/kg) | 4.480 | 4.480 |
Each kg of vitamin–mineral premix contained: 2 mg propyl gallate (E310); 329 mg ethoxyquin (E324); 3,000,000 UI vitamin A; 860,000 vitamin D3; 20,000 UI vitamin E; 1,000 mg vitamin K3; 1,000 mg vitamin B1; 1,600 mg vitamin B2; 20,000 mg vitamin B3; 5,000 mg vitamin B5; 1,400 mg vitamin B6; 5.2 mg vitamin B12; 600 mg folic acid; 60 mg biotin; 110,000 mg choline; trace elements: 4,000 mg Cu; 10,000 mg Fe; 18,000 mg Zn; 16,000 mg Mn; 400 mg I; 40.0 mg Se.
The live weight on day 0, 6, 13, 20, 27, 34, 41, 48, and 55 and size of the cloacal vent on day 34 were measured in both sexes. Cloacal vent width was measured with a digital caliper to the nearest 0.1 mm.
Emotional Reactivity Testing
Tonic Immobility
To evaluate the emotional reactivity of quail, we conducted a tonic immobility test on day 7 or day 8 on each bird (n = 25–29 per group). In poultry, the duration of tonic immobility was considered to be a standard and robust measure of fearfulness (Mills and Faure, 1991). Each quail was placed on its back in a U-shaped wooden cradle and held by the experimenter with one hand over the sternum and one hand gently covering the bird's head. The quail was restrained for 10 s before being released. The duration of tonic immobility was measured as the time the bird remained motionless on its back after the initial 10 s and before it escaped. In cases where tonic immobility did not occur, another induction attempt was conducted, and the number of inductions was recorded. When tonic immobility could not be attained after 5 induction attempts, a score of 0 s was assigned. When the quail did not stand up within 300 s, the test was stopped, and a maximum duration of 300 s was allocated.
Open Field
The open field test was performed on day 28 and day 29. It assessed reactivity to a novel environment, that is an arena (80 × 80 × 50 cm) made of wood, with beige linoleum on the floor. This arena was surrounded by a green curtain to avoid the quail escaping during the test. Each quail (n = 27–30 per group) was then placed in the center of the open field arena and left alone for 6 min. A digital camera (Ethovision tracking system, v. XT7.0, Noldus Technology, Wageningen, the Netherlands) was fixed above the arena to record the location of the birds. The tracking program recorded the entries, latency to first movement, the time spent in the central and peripheral areas, and the total distance traveled to determine differences in levels of locomotor activity between birds. This test evaluates the fearfulness of quails in relation to both a novel environment and separation from conspecifics; fearful quails tend to stay immobile and spent a long time in the central zone where they are placed at the start of the test.
Social Motivation Testing
Social Reinstatement Behavior (Treadmill Test)
The treadmill test was performed on 14-day-old to 16-day-old quails (Mills and Faure, 1991). Each quail (n = 25–29 per group) was placed on the center of the treadmill with the possibility of reaching 2 extremities, either a cage with conspecifics or a dead end. Social motivation was estimated by the distance run on the treadmill to join the conspecifics. This variable recorded as DistSR is higher in more socially motivated birds.
Reaction to Social Isolation:
The test was performed between day 22 and day 24. Groups of 10 quails were first habituated to a wooden cage (64 × 38 × 90 cm) for at least 1 h before testing. Each quail (n = 25–29 per group) was then placed alone in a similar cage. Its movements were analyzed for 5 min using monitoring software (Ethovision tracking system; v. XT7.0, Noldus Technology, Wageningen, the Netherlands). The monitoring system was placed in a separate room to avoid disturbing the animals. After testing, quail were returned to the habituation cage. Apart from the testing periods, birds were always housed in groups. When all of the 10 quails had been tested, they were returned to their rearing cage. The total distance traveled, DistIso was recorded simultaneously. This distance was considered as a measure of social motivation, with greater distances traveled indicating higher motivation.
Interindividual Distances
This test was carried out in 24 to 26 quails per group. Between day 30 and day 31 for males and day 49 to day 50 for females, 3 quails were placed together in the open field, and after 5 min of habituation to allow interindividual distances to stabilize, the position of each quail and its 2 conspecifics was recorded (Ethovision tracking system) for 10 min. The interindividual distances (IID) were measured for each quail, and the maximum IID, the greatest distance between any 3 birds within the group, was used to quantify the social bonding: a high maximum IID would indicate a weaker bonding than a low IID.
Sexual Motivation Testing
The treadmill test was performed when quails were 51-day-old to test sexual motivation in males only (n = 14–16 per group) because this test does not enable sexual motivation to be measured in females. Sexual motivation was estimated by the distance run on the treadmill to join 3 females from the same group. This variable was recorded as DistSex.
Statistical Analysis
The results were processed with the XLSTAT (2015) software for Windows. Each animal was considered to be the fundamental experimental unit, and the cage effect was taken into account by using a cage repetition effect in a nested model designed for ANOVA. The analysis of variance was then performed to investigate the effects of the diet (control diet with 0.295% Trp vs. diet with 2% Trp), the effect of supplementing the drinking water with Pro, the effect of cage repetition, and the diet by Pro interaction and Yijk = Di + Pj + Ck + DPij + Eijl, where Di was the Diet effect, Pj was the Pro effect, Ck was the Cage repetition effect, and DPij the interaction between Diet and Pro effects and Eijkl the residual. An LSD-Fisher test was used for multiple comparisons. The number of birds moving to join conspecifics on the treadmill was analyzed using a chi-square test. Data were expressed as mean of each group ± SEM.
Results
Body Weight and Cloacal Vent Width
There was no interaction between Trp and Pro effects on live weight (ANOVA, P > 0.05). There were no significant differences in the initial live weight between control and treatment groups. Quail females were heavier than males which is a classical feature; however, sex effect had no interaction with Trp and Pro effects. The female and male quail fed the control diet were significantly heavier than those supplemented with Trp 2% on day 6, 13, 20, and 27 (ANOVA, P = 0.035; P = 0.004; P = 0.009 and P = 0.046 respectively; Figure 1), but this effect was no longer significant after day 27 (P > 0.05). The treatment with Pro (P. acidilactici) did not significantly affect quail growth. Neither Trp nor Pro or their interactions significantly influenced body weight of females on day 55 (ANOVA, P > 0.05 for each effect and for the interaction).
Figure 1.
Body weight in groups receiving the control and Trp diets. Bars represent means ± SEM. ANOVA, ∗P < 0.05, ∗∗P < 0.01; Indicates a significant effect of Trp treatment. Only females were kept in the experiment until day 55. Abbreviation: Trp, tryptophan.
Cloacal vent width was not different between male and females (P > 0.05), and there was no interaction between sex effect and Trp or Pro effect. There was a significant interaction between the treatments on cloacal vent width (Figure 2; ANOVA, P = 0.051), whereas there was no general effect of Trp or Pro (ANOVA, P > 0.05). The results showed that high Trp reduced cloacal vent width, but the combination of Trp and Pro brought up the width and reached the same level as the control group.
Figure 2.
Effect of tryptophan and/or probiotic on cloacal vent width of quail of both sexes on day 34. Results are expressed as means ± SEM. (ANOVA, ∗P < 0.05; letters indicate a significant difference between groups). Abbreviations: Pro, probiotic; Trp, tryptophan.
Emotional Reactivity
Tonic Immobility Test
There were no significant differences between groups in the duration of tonic immobility (C-C: 59.80 ± 8.38 s, Trp-C: 44.80 ± 5.44 s, C-Pro: 43.91 ± 6.87 s, Trp-Pro: 56.6 ± 7.53 s; ANOVA, P > 0.05).
Open-Field Test
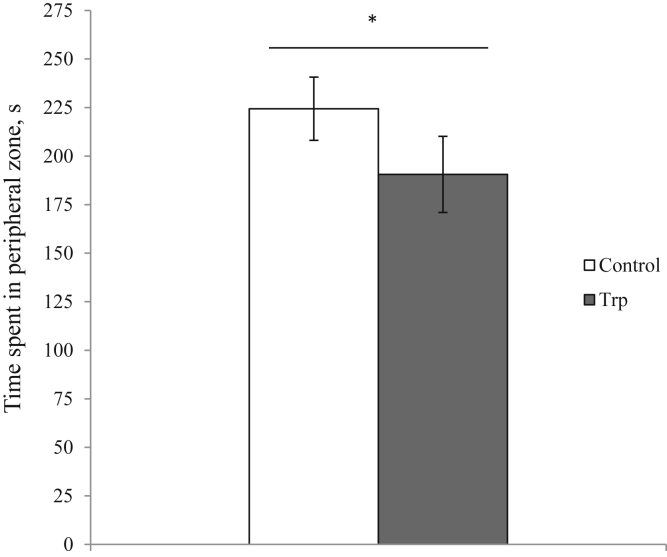
There was no effect of Trp, Pro, or interaction effect between Trp and Pro on the total distance traveled (C-C: 813.1 ± 102.2 cm, Trp-C: 749.3 ± 104.7 cm, C-Pro: 785.5 ± 84.0 cm, Trp-Pro: 671.6 ± 127.9 cm; ANOVA, P > 0.05). High-dietary Trp reduced the time spent in the peripheral zone (Figure 3; ANOVA, P = 0.033), and this duration was not affected by Pro effect or interaction between Trp and Pro effects (ANOVA, P > 0.05). The latency to first move to the peripheral zone was not affected by Trp effect or Trp and Pro interaction, but the birds receiving Pro went into the periphery zone earlier than those without (latency with Pro: 20.6 ± 6.1 s; latency without Pro: 43.3 ± 13.0 s; ANOVA, P = 0.032).
Figure 3.
The time spent in the peripheral zone in the open field. Results are expressed as means ± SEM. (ANOVA, ∗P = 0.033). Abbreviation: Trp, tryptophan.
Social Motivation
Social Reinstatement Behavior
There was no difference in the distance traveled on the treadmill between groups on DistSR in the birds that move on the treadmill (C-C: 879 ± 119, Trp-C: 862 ± 140, C-Pro: 829 ± 139, Trp-Pro: 951 ± 129 arbitrary unit; ANOVA, P > 0.05). The proportion of the birds moving toward conspecifics on the treadmill was 78.3% and tended to be higher in birds fed the control diet than those fed with the high Trp diet (85.5 vs. 70.6%; chi-square, P = 0.06).
Social isolation Test
The interaction between Trp and Pro effects on the total distance traveled in the arena during the social isolation test was not significant (ANOVA, P > 0.05). This distance tended to be smaller in birds fed Trp 2% diet than those fed the control diet (Figure 4; ANOVA, P = 0.062).
Figure 4.
The total distance traveled during social isolation (Distlso) obtained from quail fed the control or the Trp 2% diets. Results are expressed as means ± SEM (ANOVA, #P = 0.062). Abbreviation: Trp, tryptophan.
Interindividual Distances
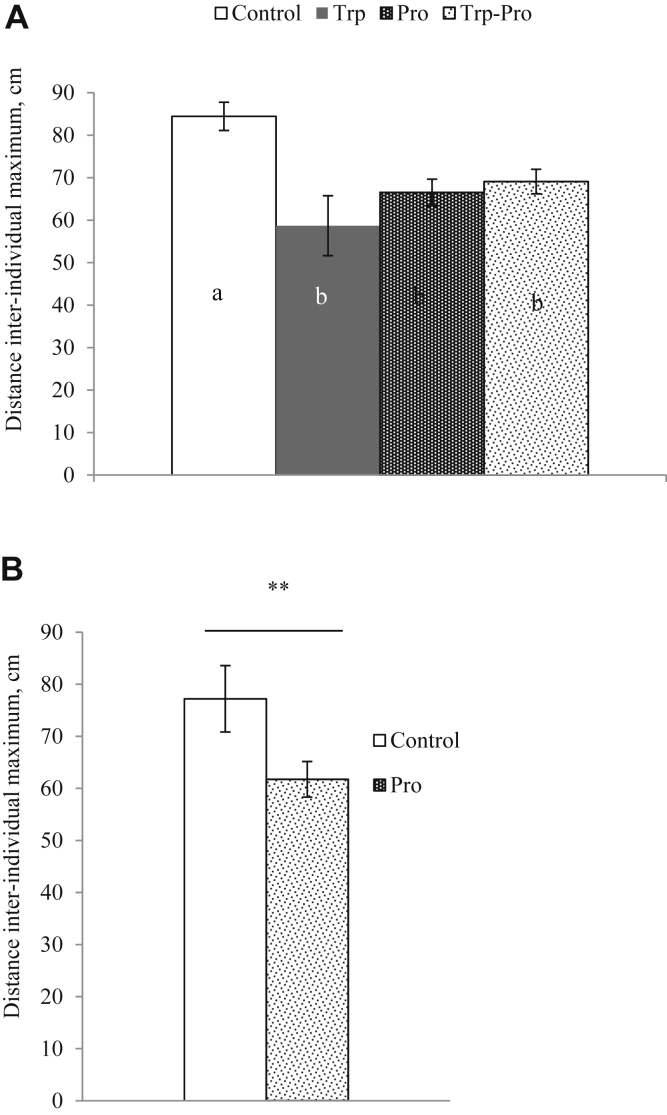
The IID measured in females were influenced by Trp treatment (ANOVA, P = 0.018) and by the interaction between Trp and Pro treatments (ANOVA, P = 0.003; Figure 5A). In males, IID were shorter in males supplemented with Pro (ANOVA, P = 0.01, Figure 5B), and there was no effect of Trp treatment or interaction between the 2 treatments (ANOVA, P > 0.05)
Figure 5.
The interindividual distance (IID) in female (A) and male quail (B). Results are expressed as means ± SEM. In females, there was a significant interaction between Trp and Pro effects (ANOVA, ∗∗P = 0.003; letters indicate a significant difference between groups). In males, there was a significant effect of Pro treatment (ANOVA, ∗∗P = 0.01). Abbreviations: Trp, tryptophan; Pro, probiotic.
Sexual Motivation
There were no significant differences in the sexual motivation between groups measured in males at day 51 (DistSex) (C-C: 885 ± 211, Trp-C:1155 ± 275, C-Pro: 866 ± 203, Trp-Pro 998 ± 238 arbitrary unit; ANOVA, P > 0.05).
Discussion
We investigated the hypothesis that a high dietary level of Trp would modify emotional and social behavior in Japanese quail and that Pro supplementation would interact and help this Trp effect. Our data demonstrated a few effects of high dietary Trp and/or Pro supplementation on emotional and social behavior of Japanese quails but also negative effects of high Trp on body growth.
Body Development
From day 6 to 27, mean live weights were significantly reduced with the dietary Trp supplement (2%) compared with the control diet. High dietary Trp has already been shown to impair growth performance in quail based on previous reports of a negative effect on appetite physiology, that is, decrease in live weight and feed intake in broiler chicks (Corzo et al., 2005b; Duarte et al., 2013). Likewise, Wang et al. (2016) showed that Trp was involved in the regulation of feed intake, and higher levels of Trp inhibited feed intake and thus inhibited weight gain in broiler chicks. In the current study, the additional quantity of Trp consumed by the quail was not determined, because feed intake was not measured. Moreover, an adequate Trp content brings about an optimal amino acid balance, whereas an excess supply of dietary Trp can dramatically disrupt this balance, thus resulting in poor growth (Castro et al., 2000; Wei et al., 2011). Some studies have reported that dietary Trp supplementation effectively improves growth performance under stress conditions (Shen et al., 2012; Liu et al., 2015). Changes in body weight of quail fed with a high Trp diet could lead to slower development and sexual maturation which could explain the smaller cloacal vent width in Trp birds at day 34.
Probiotics can improve growth performance as they increase the population of beneficial microorganisms and inhibit the proliferation of pathogens in the intestinal microbiota. The Pro P. acidilactici is known to improve growth performance of broiler chickens (Murshed and Abudabos, 2015; Gheisar et al., 2016). However in the present study, Pro alone had no additional effect on quail growth. This is in line with some studies which found no influence of Pro supplementation on Japanese quail growth reared under unstressed conditions (Parois et al., 2017). The lack of a significant influence of the dietary treatment on quail growth during our experiment could be attributed to the single strain Pro bacteria, its activity, the composition of the control diet, and/or the environmental conditions.
Sociosexual Behavior and Trp Supplementation
The quail that traveled on the treadmill to reach the conspecifics tended to be less numerous when fed the high Trp compared with the control diet. Since social reinstatement behavior can be quantified in runway or treadmill tests when young birds are separated from their conspecifics (Mills and Faure, 1991), this observation reflects a lower motivation of the treated quails. This is in line with results obtained for the isolation test for which quail fed the high Trp diet traveled a shorter total distance (Distlso). In fact, these quail decreased their locomotor activity and reduced exploratory behavior suggesting an overall reduction in locomotor activity. This could also explain the effects of Trp on IID of females placed in groups of 3, because female quails fed the high Trp diet showed lower IID than control group females. However, the same effect was not seen in males. Another explanation could thus be that Trp induced a reduction in social motivation in both sexes (treadmill and isolation tests) when quails were young, but this would be later masked by changes in hormonal status. In males, IID were equal between treatments, and this may be accounted for by similar/the same social motivation. On the contrary, social motivation would be enhanced in Trp females because the test took place during hormonal changes that would interact with Trp provision at this stage. This would be in line with a study showing that excessive Trp influences behavioral responses during the laying period in fish (Raghuveer et al., 2011).
Sociosexual Behavior and Supplementing With Pro
Interestingly, Pro males had lower IID than those without Pro in their water, and this effect was not present in females. This sex difference might be explained by the difference in test days (day 30–31 for males and day 49–50 for females) and because the effect in each sex may result from different mechanisms. This difference in the Pro effect between males and females is in line with recent data from microbiota sequencing that have shown differences between sexes in most sections of the intestinal tract in Japanese quail (Wilkinson et al., 2016). In males, quail that received Pro tended to remain closer together than those without Pro. These results may be explained in the light of studies which have found that manipulating bacteria found in the gut can affect emotional behavior in quail because of an interaction between gut microbes and the brain (Parois et al., 2017; Kraimi et al., 2018, 2019a) but also in other farm animals (Kraimi et al., 2019b). The reason why we did not observe the effect of Pro on IID in the female quails on day 30–31 could be because of hormonal changes previously mentioned and the physiological stress response to the development of yellow follicles in the ovary during the prelaying period.
Emotional Reactivity
Our results revealed that neither high dietary Trp nor Pro modulated the fear levels measured by tonic immobility reaction. Tonic immobility is a reversible spontaneous response induced by a constraint and is considered to be positively correlated with underlying fearfulness in poultry (Jones, 1996). It is widely accepted that brain serotonin is implicated in the control of tonic immobility duration (Henning et al., 1986), and the level of individual Trp uptake could change brain serotonin concentrations (Gudev et al., 2011). Our results are in agreement with those reported by Gudev et al. (2011), who found no effect of a 1% Trp level for broiler chickens from day 36 to 51 of age. On the other hand, Newberry and Blair (1993) claimed a shorter duration of tonic immobility in broiler chickens supplemented with Trp (2%). In contrast, Gallup et al. (1977) reported a dose-dependent increase in tonic immobility following systemic injections of Trp. These inconsistent results could be because of the different routes, level, and duration of Trp supplementation. Nevertheless, our measurement of emotional reactivity through the open-field would be in line with that of Gallup. The open-field test has been widely used to assess fear (Jones, 1996). During open-field testing, supplementing with high Trp levels reduced the time spent in the peripheral zone indicating an increase in emotional reactivity (Jones, 1996).
In our study, Pro supplementation had no effect on tonic immobility, and the effect in the open-field test was weak because only latency to move to the periphery was reduced, indicating lower stress at the beginning of the test. This lack of difference was unexpected because the anxiolytic effects of Pro are now well documented as demonstrated in broiler chickens and also by Ghareeb et al. (2008) who found effects on tonic induction and duration in birds fed diets supplemented with Pro (Lactobacillus sp., 1 x 108 CFU/kg) for 5 wk. However, Parois et al. (2017) showed that host genetics can influence the effect of P. acidilactici in quail, which is probably the explanation for the differences between results in experiments using Pro. Overall, our data indicate a slight increase in emotional reactivity in the Trp group particularly.
Interaction Between Trp and Pro Effects
Tryptophan is converted by bacterial action to indole, indican, and indolic acid derivates such as indolyl-3-acetic acid, indolyl-acetyl-glutamine, indolyl-propionic acid, indolyl-lactic acid, indolyl-acrylic acid, and indolyl-acryloyl-glycine (Peters, 1991). Indole and its derivatives are the main degradation products of microbial metabolism of L-Trp. The possible Trp overload in the intestine could lead to increased production of bacterial degradation products whose effects could not be counteracted by the Pro. In the present study, only cloacal vent showed a positive effect of Pro that counteracted the reduction of the vent length induced by high Trp in the diet. The low impact of combined Trp and Pro on body weight and behavior could be because P. acidilactici was not effective in reducing bacterial degradation products of excessive Trp and/or the poor absorption of these products by the gut wall. However, it should be underlined that the effects of supplementing diets with Pro in poultry can be extremely variable, depending on age and sanitary conditions, composition of diet, and quantity and type of Pro added to the diet.
Conclusion
In conclusion, feeding a high Trp diet is not recommended as a strategy to improve social behavior because its action appears to be different from that observed in laying hens. Supplementing diets with Trp and/or Pro did not provide beneficial effects on growth and behavior. Future studies are necessary to explore further the usefulness of this dietary supplement for poultry to optimize their efficacy and to elucidate the mechanisms by which different levels of high dietary Trp and/or Pro can induce positive effects on behavior in quail.
Acknowledgments
This work was funded by INRAE, UMR85 Physiologie de la Reproduction et des Comportements and an International Postdoctoral Research Fellowship Program of the Scientific and Technological Research Council of Turkey (TUBITAK, Grant No. BIDEB-2219). The authors are grateful to Sue Edrich for her revision of the English manuscript.
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Birkl P., Chow J., Forsythe P., Gostner J.M., B Kjaer J., A Kunze W., McBride P., Fuchs D., Harlander-Matauschek A. The role of tryptophan-kynurenine in feather pecking in domestic chicken lines. Front. Vet. Sci. 2019;6:209. doi: 10.3389/fvets.2019.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A.J., Gomes P.C., Pupa J.M.R., Rostagno H.S., Albino L.F.T., Nascimento A.H. Exigência de triptofano para frangos de corte de 1 a 21 dias de idade. R. Bras. Zootec. 2000;29:1743–1749. [Google Scholar]
- Corzo A., Moran E.T., Hoehler D., Lemmell A. Dietary tryptophan need of broiler males from forty-two to fifty-six days of age. Poult. Sci. 2005;84:226–231. doi: 10.1093/ps/84.2.226. [DOI] [PubMed] [Google Scholar]
- Corzo A., Kidd M.T., Thaxton J.P., Kerr B.J. Dietary tryptophan effects on growth and stress responses of male broiler chicks. Br. Poult. Sci. 2005;46:478–484. doi: 10.1080/00071660500157974. [DOI] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M., Jaglin M., Bruneau A., Vancassel S., Cardona A., Daugé V., Naudon L., Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Duarte K.F., Junqueira O.M., Filardi R.D.S., Siqueira J.C.D., Puzotti M.M., Garcia E.A., Molino A.D.B., Laurentiz A.C.D. Digestible tryptophan requirements for broilers from 22 to 42 days old. R. Bras. Zootec. 2013;42:728–733. [Google Scholar]
- Gallup G.G., Wallnau L.B., Boren J.L., Gagliardi G.J., Maser J.D., Edson P.H. Tryptophan and tonic immobility in chickens: effect of dietary and systemic manipulations. J. Comp. Physiol. Psychol. 1977;1:642–648. doi: 10.1037/h0077353. [DOI] [PubMed] [Google Scholar]
- Ghareeb K., Awad W.A., Nitsch S., Abdel-Raheem S., Böhm J. Effects of transportation on stress and fear responses of growing broilers supplemented with prebiotic or probiotic. Int. J. Poult. Sci. 2008;7:678–685. [Google Scholar]
- Gheisar M.M., Hosseindoust A., Kim I.H. Effects of dietary Enterococcus faecium on growth performance, carcass characteristics, faecal microbiota, and blood profile in broilers. Vet. Med. 2016;61:28–34. [Google Scholar]
- Gudev D., Moneva P., Sredkova V. Tonic immobility and adrenal response in chickens fed supplemental tryptophan. Bulg. J. Agric. Sci. 2011;17:560–566. [Google Scholar]
- Harimurti S., Hadisaputro W. Beneficial Microorganisms in Agriculture, Aquaculture and Other Areas. Springer International Publishing; New York, NY: 2015. Probiotics in poultry; pp. 1–19. [Google Scholar]
- Henning C.W., Steinhoff W.C., Booth J.V. Central and peripheral effects of serotonin on the immobility response in chickens. Pharmacol. Biochem. Behav. 1986;24:1623–1627. doi: 10.1016/0091-3057(86)90496-x. [DOI] [PubMed] [Google Scholar]
- Jones R.B. Fear and adaptability in poultry: insights. Worlds Poult. Sci. J. 1996;52:131–170. [Google Scholar]
- Koopmans S., Ruis M., Dekker R., Vandiepen H., Korte M., Mroz Z. Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs. Physiol. Behav. 2005;85:469–478. doi: 10.1016/j.physbeh.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Kraimi N., Calandreau L., Biesse M., Rabot S., Guitton E., Velge P., Leterrier C. Absence of gut microbiota reduces emotional reactivity in Japanese quails (Coturnix japonica) Front. Physiol. 2018;9:603. doi: 10.3389/fphys.2018.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraimi N., Dawkins M., Gebhardt-Henrich S.G., Velge P., Rychlik I., Volf J., Creach P., Smith A., Colles F., Leterrier C. Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: a review. Physiol. Behav. 2019;210:112658. doi: 10.1016/j.physbeh.2019.112658. [DOI] [PubMed] [Google Scholar]
- Kraimi N., Calandreau L., Zemb O., Germain K., Dupont C., Velge P., Guitton E., Lavillatte S., Parias C., Leterrier C. Effects of a gut microbiota transfer on emotional reactivity in Japanese quails (Coturnix japonica) J. Exp. Biol. 2019;222(Pt 10) doi: 10.1242/jeb.202879. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yuan J.M., Zhang L.S., Zhang Y.R., Cai S.M., Yu J.H., Xia Z.F. Effects of tryptophan supplementation on growth performance, antioxidative activity, and meat quality of ducks under high stocking density. Poult. Sci. 2015;94:1894–1901. doi: 10.3382/ps/pev155. [DOI] [PubMed] [Google Scholar]
- Mills A.D., Faure J.M. Divergent selection for duration of tonic immobility and social reinstatement behavior in Japanese quail (Coturnix coturnix japonica) chicks. J. Comp. Psychol. 1991;105:25–38. doi: 10.1037/0735-7036.105.1.25. [DOI] [PubMed] [Google Scholar]
- Murshed M.A., Abudabos A.M. Effects of the dietary inclusion of a probiotic, a prebiotic or their combinations on the growth performance of broiler chickens. Rev. Bras. Cienc. Avic. 2015;17(SPE):99–103. [Google Scholar]
- Newberry R.C., Blair R. Behavioral responses of broiler chickens to handling: effects of dietary tryptophan and two lighting regimens. Poult. Sci. 1993;71:1237–1244. doi: 10.3382/ps.0721237. [DOI] [PubMed] [Google Scholar]
- Parois S., Calandreau L., Kraimi N., Gabriel I., Leterrier C. The influence of a probiotic supplementation on memory in quail suggests a role of gut microbiota on cognitive abilities in birds. Behav. Brain Res. 2017;331:47–53. doi: 10.1016/j.bbr.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Pathmakanthan S., Meance C.A., Edwards S. Probiotics: a review of human studies to date and methodological approaches. Microb. Ecol. Health Dis. 2000;12:10–30. [Google Scholar]
- Peters J.C. Tryptophan nutrition and metabolism: an overview. Adv. Exp. Med. Biol. 1991;294:345–358. doi: 10.1007/978-1-4684-5952-4_32. [DOI] [PubMed] [Google Scholar]
- Poletto R., Meisel R.L., Richert B.T., Cheng H.W., Marchant-Forde J.N. Aggression in replacement grower and finisher gilts fed a short-term high tryptophan diet and the effect of long-term human–animal interaction. Appl. Anim. Behav. Sci. 2010;122:98–110. [Google Scholar]
- Raghuveer K., Sudhakumari C.C., Senthilkumaran B., Kagawa H., Dutta-Gupta A., Nagahama Y. Gender differences in tryptophan hydroxylase-2 mRNA, serotonin, and 5-hydroxytryptophan levels in the brain of catfish, Clarias gariepinus, during sex differentiation. Gen. Comp. Endocrinol. 2011;171:94–104. doi: 10.1016/j.ygcen.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savory C.J., Mann J.S., Macleod M.G. Incidents of pecking damage in growing bantams in relation to food form, group size, stocking density, dietary tryptophan concentration and dietary protein source. Br. Poult. Sci. 1999;40:579–584. doi: 10.1080/00071669986936. [DOI] [PubMed] [Google Scholar]
- Shen Y.B., Voilque G., Kim J.D., Odle J., Kim S.W. Effects of increasing tryptophan intake on growth and physiological changes in nursery pigs. J. Anim. Sci. 2012;90:2264–2275. doi: 10.2527/jas.2011-4203. [DOI] [PubMed] [Google Scholar]
- Song D., Ibrahim S., Hayek S. Recent application of probiotics in food and agricultural science. Probiotics. 2012;10:1–34. [Google Scholar]
- van Hierden Y.M., Koolhaas J.M., Korte S.M. Chronic increase of dietary L-tryptophan decreases gentle feather pecking behavior. Appl. Anim. Behav. Sci. 2004;89:71–84. [Google Scholar]
- Wang B., Min Z., Yuan J. Apparent ileal digestible tryptophan requirements of 22-to 42-day-old broiler chicks. J. Appl. Poult. Res. 2016;25:54–61. [Google Scholar]
- Wei Z.Y., Wang L., Ji Y., Yu L.H., Pan X.H., Wang M.Z., Wang H.R. Effects of dietary tryptophan supplementation and feed restriction on growth performance and carcass characteristics of goslings. J. Anim. Vet. Adv. 2011;10:2079–2083. [Google Scholar]
- Wilkinson N., Hughes R.J., Aspden W.J., Chapman J., Moore R.J., Stanley D. The gastrointestinal tract microbiota of the Japanese quail, Coturnix japonica. Appl. Microbiol. Biotechnol. 2016;100:4201–4209. doi: 10.1007/s00253-015-7280-z. [DOI] [PubMed] [Google Scholar]
- Young S.N. Acute tryptophan depletion in humans: a review of theoretical, practical and ethical aspects. J. Psychiatry Neurosci. 2013;38:294–305. doi: 10.1503/jpn.120209. [DOI] [PMC free article] [PubMed] [Google Scholar]