Abstract
Ammonia (NH3), a toxic gas, has deleterious effects on chicken health in intensive poultry houses. MicroRNA can mediate inflammation. The complex molecular mechanisms underlying NH3 inhalation–caused inflammation in animal kidneys are still unknown. To explore the mechanisms, a broiler model of NH3 exposure was established. Kidney samples were collected on day 14, 28, and 42, and meat yield was evaluated on day 42. We performed histopathological examination, detected miR-6615-5p and mothers against decapentaplegic homolog 7 (Smad7), and determined inflammatory factors and cytokines in kidneys. The results showed that excess NH3 reduced breast weight and thigh weight, which indicated that excess NH3 impaired meat yield of broilers. Besides, kidney tissues displayed histopathological changes after NH3 exposure. Meanwhile, the increases of inducible nitric oxide synthase (iNOS) activity and nitric oxide content were obtained. The mRNA and protein expression of inflammatory factors, including nuclear factor-κB (NF-κB), cyclooxygenase-2, prostaglandin E synthases, and iNOS increased, indicating that NF-κB pathway was activated. T-helper (Th) 1 and regulatory T (Treg) cytokines were downregulated, whereas Th2 and Th17 cytokines were upregulated, suggesting the occurrence of Th1/Th2 and Treg/Th17 imbalances. In addition, we found that Smad7 was a target gene of miR-6615-5p in chickens. After NH3 exposure, miR-6615-5p expression was elevated, and Smad7 mRNA and protein expression were reduced. In summary, our results suggest that NH3 exposure negatively affected meat yield; and miR-6615/Smad7 axis and immune imbalance participated in NH3-induced inflammatory injury via the NF-κB pathway in broiler kidneys. This study is helpful to understand the mechanism of NH3-induced kidney injury and is meaningful to poultry health and breed aquatics.
Key words: ammonia, broiler kidney, miR-6615/Smad7 axis, immune imbalance, inflammatory injury
Introduction
Ammonia (NH3) is the most harmful gas in intensive poultry houses and receives the increasing attention. Birds consumed high-protein feed and produced uric acid that ultimately converted to NH3 under favorable conditions (Naseem and King, 2018). Microbial decomposition of litter and manure was also the important source of NH3 emission (Atapattu et al., 2008). United Egg Producers recommended that the NH3 concentration should ideally be less than 10 ppm (equivalent to 8 mg/m3) and not more than 25 ppm (equivalent to 19 mg/m3) (UEP, 2017). However, in some intensive chicken farms, NH3 concentrations exceeded the recommendation. In spring, NH3 concentration reached 26 ppm (equivalent to 20 mg/m3) during a 6-week fattening period in an intensive broiler-breeding facility in northwest Croatia (Vucemilo et al., 2007). In winter, NH3 concentration even exceeded 63 ppm (equivalent to 48 mg/m3) in a broiler house in Brazil (Osorio Hernandez et al., 2016). Exposure to excess NH3 decreased growth performance and meat yield (Miles et al., 2004) and increased mortality (Do et al., 2005) in broilers. Kidney is an important excretory organ in the body, which can reabsorb nutrients and excrete metabolic waste including uric acid and nitrogen (Rani et al., 2018). Previous studies reported that kidney was a target organ of NH3 toxicity (Han et al., 2020). After NH3 exposure, microscopic lesions and inflammation were observed in broiler kidneys (Witkowska et al., 2006). High concentration of NH3 caused a lower kidney weight and renal coagulative necrosis in broilers (Zarnab et al., 2019). However, little is known about the molecular mechanism of NH3-induced kidney tissue damage.
Inflammatory response can cause tissue damages via breaking tissue homeostasis (Attia et al., 2018). MicroRNA (miRNA), a class of noncoding RNA, can regulate posttranscriptional gene expression by binding to target sequences and participate in multiple physiological and pathological processes including apoptosis (Chi et al., 2019), autophagy (Zhang et al., 2019), and necroptosis (Wang et al., 2020). miRNA has been implicated in various inflammatory diseases through regulating target genes. For instance, miR-140-5p suppressed mothers against decapentaplegic homolog 3 (Smad3) in a model of temporomandibular joint osteoarthritis (Li et al., 2019). miR-21 targeted Smad7 and induced renal fibrosis and inflammation in diabetic nephropathy (McClelland et al., 2015). Previous studies also reported that noncoding RNA took part in NH3-caused inflammatory injury in spleens (An et al., 2019) and thymuses (Chen et al., 2020a) of broilers. In another study on NH3 toxicity, miR-6615-5p and Smad7 were differentially expressed genes using high-throughput sequencing in chicken thymuses (D. Chen, Northeast Agricultural University, Harbin, China, personal communication). Smad7 can blockade nuclear factor-κB (NF-κB) activation through inhibitor of NF-κB (IκBα) and mitigate renal inflammation in a rat remnant kidney model (Ng et al., 2005). NF-κB can drive the transcription of proinflammatory genes, such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Shi et al., 2018). COX-2 and iNOS are dominant sources of nitric oxide (NO) and prostaglandin E synthases (PGEs), respectively. A recent study reported that particulate matter (PM) 2.5 caused inflammatory response and lung injury by triggering NF-κB pathway in rats (Shi et al., 2019). Thus, we assumed that miR-6615-5p and NF-κB pathway might be involved in NH3-induced inflammatory injury in broiler kidneys.
Environmental stressors, such as cold stress (Su et al., 2019), atrazine (Cui et al., 2019), lipopolysaccharide (Dong et al., 2020) and cadmium (Chen et al., 2020c), can affect immunological functions in organisms. Four subsets, including T-helper (Th) 1 cells, Th2 cells, Th17 cells, and regulatory T (Treg) cells, are differentiated from naive CD4+ T cells and are involved in immune response and inflammation by releasing cytokines (Zhang and An, 2007). Emerging researches indicated that hydrogen sulfide (H2S) exposure induced inflammatory injury through Th1/Th2 imbalance in chicken lungs (Wang et al., 2018) and jejunums (Zheng et al., 2019). Our previous study also reported that decreased Th1 cytokines and increased Th2 cytokines were involved in NH3-caused inflammatory injury in broiler spleens (An et al., 2019) and thymuses (Chen et al., 2019). Besides, patients with chronic kidney disease had a decreased percentage of Treg cells and an increased percentage of Th17 cells in peripheral blood cells (Zhu et al., 2018). Mice with PM exposure showed a decrease of Treg/Th17 ratio and inflammation in spleens (Li et al., 2016). However, whether Treg/Th17 imbalance was involved in NH3-induced inflammatory injury in chicken kidneys remained to be studied. Therefore, we designed this study to investigate the molecular mechanism of excess NH3-induced inflammatory injury in broiler kidneys. This study is helpful to understand the nephrotoxic effect of NH3 and prevent NH3 poisoning in intensive poultry production.
Materials and methods
Animals and Treatments
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Northeast Agricultural University (protocol number SRM-06). A total of 108 one-day-old Ross 308 broilers were randomly distributed into 3 groups: the control group, the NH3 group 1, and the NH3 group 2. The broilers were raised in 3 environmentally controlled chambers in the Laboratory Animal Center of the Northeast Agricultural University (Harbin, China). Each group had 3 replicate pens with 12 birds per replicate. Standard commercial diet and drinking water were offered ad libitum. This diet met the nutrient requirements recommended by the National Research Council (NRC, 1994). Chicken manure was cleaned once a day. Temperature was maintained at 33°C during the first 3 d and then gradually reduced by 3°C per week until it reached 24°C. Relative humidity was maintained at 65%. Light programs were 23 h of light and 1 h of dark during the first week, and 20 h of light and 4 h of dark until the end of the experiment.
NH3 concentration was monitored using a Luma Sense Photoacoustic Field Gas-Monitor (Innova-1412; Lumasense Technologies Inc, Santa Clara, CA). For the control group, NH3 concentration was less than or equal to 5 mg/m3 during the entire experimental period. For the NH3 group 1 and the NH3 group 2, exogenous NH3 was supplied using a cylinder of compressed anhydrous NH3 (Dawn Gas Co., Ltd., Harbin, China), and NH3 concentrations were 10 ± 0.5 mg/m3 and 20 ± 0.5 mg/m3 from 0 to 21 d of age and 15 ± 0.5 mg/m3 and 45 ± 0.5 mg/m3 from 22 to 42 d of age, respectively.
Sample Collection and Meat Yield Measurement
On day 14, 28, and 42, 12 chickens were randomly selected from each group and euthanized with sodium pentobarbital. Kidney samples were immediately collected. Each kidney sample was divided into 3 parts. The first part was fixed with 10% formalin for histopathology. The second part was homogenized for assay kits. The third part was frozen in liquid nitrogen and then stored at −80°C for quantitative real-time PCR and Western blot. On day 42, left breast meat and left thigh meat were weighted.
Histopathological Examination
The kidney samples on day 42 were obtained from 10% formalin, were dehydrated with a graded series (50, 70, 90, and 100%) of ethanol for 10 min each grade, were cleared in xylol, and were embedded in paraffin. The paraffin sections (5-μm thick) were sliced and stained with hematoxylin-eosin. Histopathological examination was performed using a light microscope (Eclipse 80i; Nikon, Tokyo, Japan).
Assay Kits
The kidney homogenates were centrifuged at 3,000 × g for 10 min, and the supernatants were collected. iNOS activity and NO content were determined according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Quantitative Real-Time PCR
Primers were listed as described previously (Zhao et al., 2013; Wang et al., 2018; An et al., 2019) and were synthesized by the Invitrogen Biotechnology Co., Ltd. (Shanghai, China). Housekeeping genes U6 and β-actin were selected as internal references. Total RNA was extracted with TRIzol reagent (Invitrogen Inc, Carlsbad, CA) according to the manufacturer's instructions. RNA integrity was verified with 1.1% agarose gel electrophoresis. RNA purity and concentration were calculated through detecting optical density at 230 nm, 260 nm, and 280 nm using a spectrophotometer (Nano-400; Hangzhou Allsheng Instruments Co., Ltd., China). cDNA Was synthesized with the miRcute miRNA First-Strand cDNA synthesis kit to detect miRNA expression and with the Quantscript RT kit to detect mRNA expression according to the manufacturer's instructions (Tiangen, Beijing, China). Quantitative real-time PCR was performed using a QuantStudio 3 real-time PCR system (Applied Biosystems, Foster City, CA) with 20 μL of reaction mixture (Tiangen, Beijing, China) for miRNA and with 20 μL of reaction mixture (Roche, Basel, Switzerland) for mRNA.
Western Blot
Total protein was extracted from kidneys on day 42 with the cell lysis buffer for Western and immunoprecipitation (Biosharp, Beijing, China) containing 1-mmol phenylmethylsulfonyl fluoride. Protein concentration was determined with the enhanced bicinchoninic acid protein assay kit (Beyotime, China). Equal amounts of total protein were loaded onto SDS-PAGE and then were transferred to nitrocellulose membrane. The membranes were blocked in 5% skim milk at 37°C for 2 h and were incubated with primary antibodies: β-actin and NF-κB (1:1000; Santa Cruz Biotechnology, CA); iNOS (1:1000; Abcam, Cambridge, UK); and IκBα, COX-2, and Smad7 (1:500; produced by Dr. Shiwen Xu lab, College of Veterinary Medicine, Northeast Agricultural University, Harbin, China) at 4°C for 12 h. After being washed, the membranes were incubated with a horse-radish peroxidase conjugated secondary antibody against rabbit IgG (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at 37°C for 1 h. Immune complex was treated with the enhanced chemiluminescence reagent (Applygen Technologies, Beijing, China). Imaging was performed using a chemiluminescence instrument (AI600 RGB; General Electric Company, Boston, MA). Band intensities were quantified using Image J version 1.8.0 (Rasband, MD).
Statistical Analysis
Statistical analysis was undertaken using SPSS version 20.0 (SPSS Inc., Chicago, IL). Normality was assessed using Shapiro-Wilk normality test. Variance homogeneity was tested using Levene's test. All data showed a normal distribution and satisfied the requirement of variance homogeneity. Statistical significance was evaluated using one-way and two way ANOVA followed by Tukey's multiple comparison test. Pearson's correlation coefficient (PCC) was performed to evaluate correlation between miRNA and target gene. Data were expressed as mean ± SD. Mean values with different lowercase letters represented significant difference (P < 0.05) among different groups at the same time point. Mean values with different uppercase letters represented significant difference (P < 0.05) in the same group at different time points.
Results
Meat Yield
As shown in Table 1, with the increase of NH3 concentration, breast weight and thigh weight were significantly reduced (P < 0.05) on day 42. After 42 d of NH3 exposure, compared with the control group, breast weight and thigh weight were reduced by 21.94 and 19.47% in the NH3 group 1 and were reduced by 38.87 and 37.44% in the NH3 group 2, respectively.
Table 1.
The effects of NH3 exposure on meat yield of broilers.
| Meat yield | The control group | The NH3 group 1 | The NH3 group 2 |
|---|---|---|---|
| Left breast weight (g) | 271.34a | 211.81b | 165.89c |
| Left thigh weight (g) | 169.00a | 136.10b | 105.72c |
a,bDifferent lowercase letters represent significant differences (P < 0.05) in different groups.
Histopathology
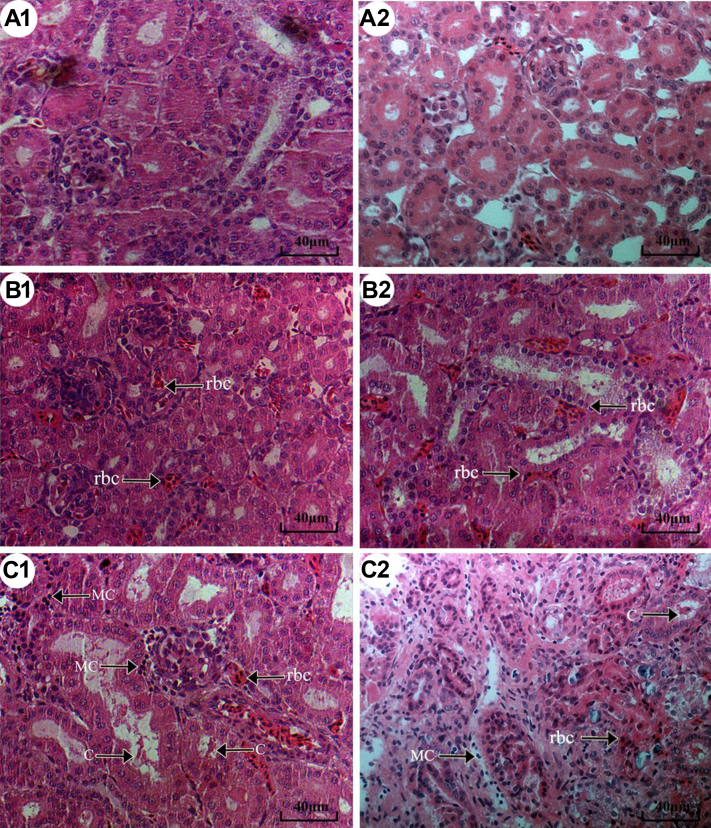
Histopathological observations are displayed in Figure 1. For the control group (Figure 1A1, A2), kidney tissues showed normal glomeruli and tubules. For the NH3 group 1 (Figure 1B1, B2), a large number of red blood cells in renal interstitium and lumen dilatation in renal tubular were observed. For the NH3 group 2 (Figure 1C1, C2), we observed mononuclear cell (MC) infiltrate in glomeruli and renal interstitium; granular cast (C), and lumen dilatation in renal tubular.
Figure 1.
Effects of NH3 on histopathology in chicken kidneys on D 42 (H&E staining, ×400). (A1 and A2) Histopathological observation in the control group. (B1 and B2) Histopathological observation in NH3 group 1. (C1 and C2) Histopathological observation in NH3 group 2. Abbreviations: C, cast; MC, mononuclear cell; rbc, red blood cell.
miR-6615-5p and Smad7
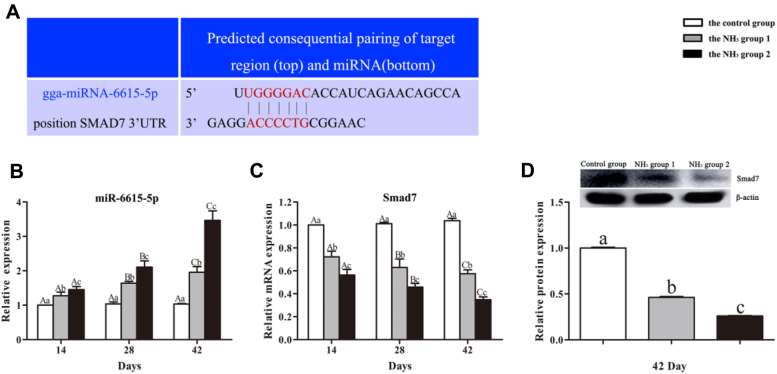
miRanda algorithm (score ≥ 140, score < -15) and RNAhybrid algorithm (score < −20) were used to predict target relationship between miRNAs and mRNAs. We found that Smad7 was one of potential target genes of miR-6615-5p. Moreover, 7 complementary sites between miR-6615-5p and Smad7 were mapped in the 3′ untranslated regions (3′-UTR) using DNAman version 7 (LynnonBiosoft Inc, San Ramon, CA), as shown in Figure 2A, further indicating that Smad7 was a target gene of miR-6615-5p.
Figure 2.
Effects of NH3 on miR-6615-5p and Smad7 in chicken kidneys. (A) Complementary sites between miR-6615-5p and Smad7. (B) Relative expression of miR-6615-5p. (C) Relative mRNA expression of Smad7. (D) Relative protein expression of Smad7.
We also detected miR-6615-5p expression (Figure 2B) and Smad7 mRNA (Figure 2C) and protein expression (Figure 2D). The results showed that miR-6615-5p expression increased significantly (P < 0.05) and Smad7 mRNA expression decreased significantly (P < 0.05) with the increase of NH3 concentration on day 14, 28, and 42. Smad7 protein expression decreased significantly (P < 0.05) at higher NH3 concentrations on day 42. Aforementioned results confirmed that Smad7 was a target gene of miR-6615-5p.
Regarding the control group, there were no significant differences (P > 0.05) in miR-6615-5p expression and Smad7 mRNA expression among different time points. Regarding the NH3 group 1 and the NH3 group 2, miR-6615-5p expression increased significantly (P < 0.05), and Smad7 mRNA expression decreased significantly (P < 0.05) with the increase of NH3 exposure time.
In addition, PCC analysis showed that there was an extremely significant negative relationship (r = −0.832, P < 0.01) between miR-6615-5p expression and Smad7 mRNA expression, further confirming the target relationship between miR-6615-5p and Smad7.
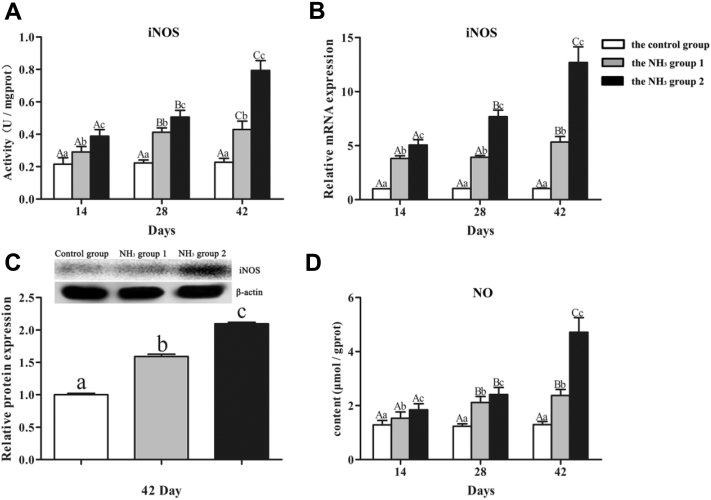
iNOS and NO
In order to investigate if iNOS and NO were involved in NH3-induced inflammatory damage, iNOS activity (Figure 3A), iNOS mRNA (Figure 3B) and protein expression (Figure 3C), and NO content (Figure 3D) were measured. Compared with the control group, iNOS activity, iNOS mRNA expression, and NO content were significantly upregulated (P < 0.05) in the NH3 group 1 at all 3 time points. Compared with the NH3 group 1, iNOS activity, iNOS mRNA expression, and NO content were significantly upregulated (P < 0.05) in the NH3 group 2 at all 3 time points. A similar result was obtained in iNOS protein expression. iNOS protein expression increased significantly (P < 0.05) with the increase of NH3 concentration on day 42.
Figure 3.
Effects of NH3 on iNOS and NO in chicken kidneys. (A) iNOS activity. (B) Relative mRNA expression of iNOS. (C) Relative protein expression of iNOS. (D) NO content.
Regarding the control group, no significant difference (P > 0.05) was found in iNOS activity, iNOS mRNA expression, and NO content among different time points. Regarding the NH3 group 1 and the NH3 group 2, iNOS activity, iNOS mRNA expression, and NO content elevated significantly (P < 0.05) with the increase of NH3 exposure time, except that iNOS mRNA expression on day 42 was significantly (P < 0.05) higher than that on day 28 and 14 in the NH3 group 1; and NO content on day 28 and 42 were significantly (P < 0.05) higher than that on day 14 in the NH3 group 1.
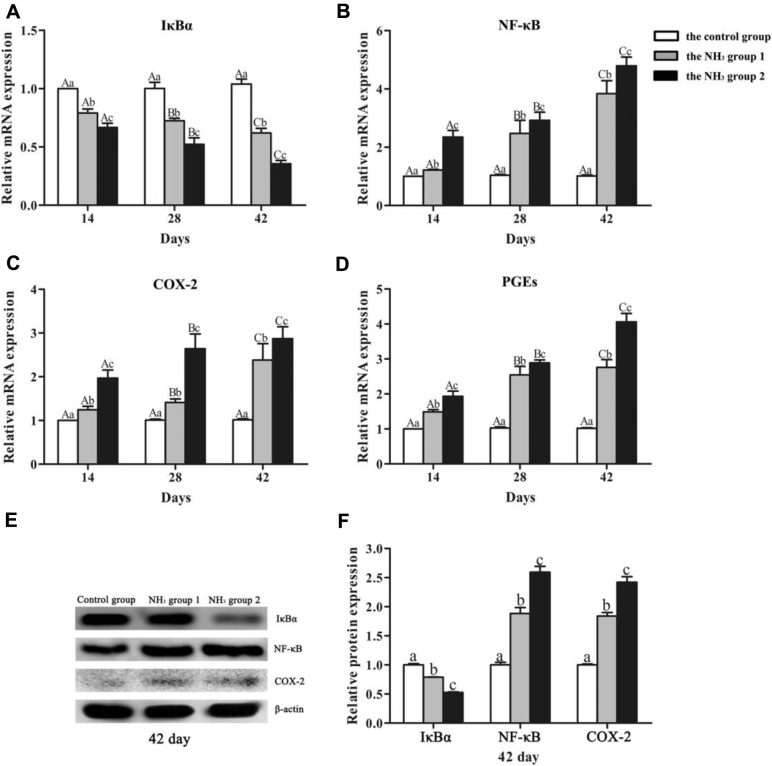
IκBα, NF-κB, COX-2, and PGEs
To explore the mechanism of inflammation caused by NH3, we determined IκBα, NF-κB, COX-2, and PGEs mRNA expression on day 14, 28, and 42 and IκBα, NF-κB, and COX-2 protein expression on day 42 (Figure 4). There were a significant decline (P < 0.05) in IκBα mRNA expression and significant rises (P < 0.05) in mRNA expression of NF-κB, COX-2, and PGEs with the increase of NH3 concentration on day 14, 28, and 42. IκBα protein expression was significantly downregulated (P < 0.05), and NF-κB and COX-2 protein expression were significantly upregulated (P < 0.05) with the increase of NH3 concentration on day 42.
Figure 4.
Effects of NH3 on IκBα, NF-κB, COX-2, and PGEs in chicken kidneys. (A) Relative mRNA expression of IκBα. (B) Relative mRNA expression of NF-κB. (C) Relative mRNA expression of COX-2. (D) Relative mRNA expression of prostaglandin E synthases (PGEs). (E-F) Relative protein expression of IκBα, NF-κB, and COX-2.
Regarding the control group, there was no significant difference (P > 0.05) in mRNA expression of all detected factors among different time points. Regarding the NH3 group 1 and the NH3 group 2, IκBα mRNA expression decreased significantly (P < 0.05), and NF-κB, COX-2, and PGEs mRNA expression increased significantly (P < 0.05) with the increase of NH3 exposure time.
Th1, Th2, Th17, and Treg Cytokines
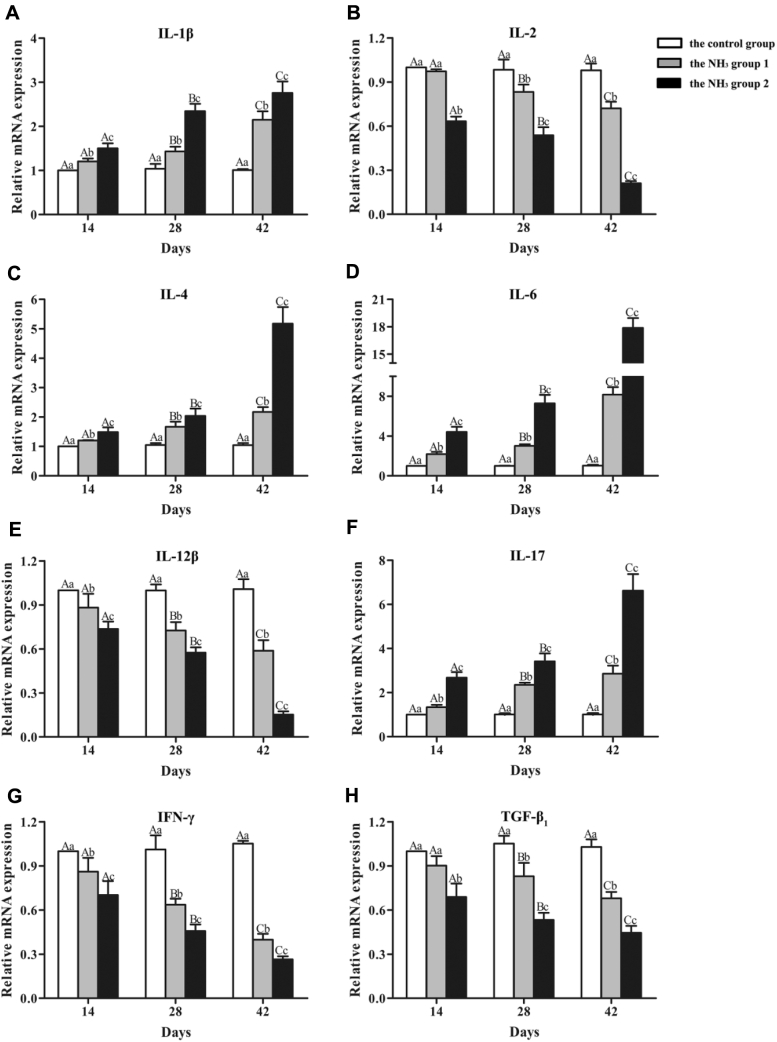
To investigate whether Th1/Th2 and Treg/Th17 imbalances occurred in chicken renal inflammatory injury caused by NH3, Th1 cytokines (IL-2, IL-12, and interferon-γ [IFN-γ]), Th2 cytokines (IL-1β, IL-4, and IL-6), Th17 cytokine (IL-17), and Treg cytokine (transforming growth factor-β1 [TGF-β1]) were detected (Figure 5). The results showed that Th1 cytokines and Treg cytokine decreased significantly (P < 0.05) in a concentration-dependent manner on day 14, 28, and 42 except that IL-2 and TGF-β1 in the NH3 group 2 were higher significantly (P < 0.05) than those in the control group and the NH3 group 1 on day 14. On the contrary, Th2 cytokines and Th17 cytokine increased significantly (P < 0.05) in a concentration-dependent manner at all 3 time points. Strikingly, on day 42, compared with the control group, IL-6 mRNA expression was elevated 7-fold in the NH3 group 1 and 16-fold in the NH3 group 2.
Figure 5.
Effects of NH3 on cytokines in chicken kidneys. (A) Relative mRNA expression of IL-1β. (B) Relative mRNA expression of IL-2. (C) Relative mRNA expression of IL-4. (D) Relative mRNA expression of IL-6. (E) Relative mRNA expression of IL-12β. (F) Relative mRNA expression of IL-17. (G) Relative mRNA expression of IFN-γ. (H) Relative mRNA expression of TGF-β1.
Regarding the control group, all detected cytokines showed no significant differences (P > 0.05) among different time points. Regarding the NH3 group 1 and the NH3 group 2, Th1 and Treg cytokines were significantly downregulated (P < 0.05); Th2 and Th17 cytokines were significantly upregulated (P < 0.05) with the increase of NH3 exposure time.
Discussion
Exposure to high level of NH3 in poultry houses can adversely affect chicken growth and health. Miles et al. (2004) reported that atmospheric NH3 caused a decrease in breast meat yield of broilers. NH3 exposure caused lower breast meat percentage and thigh meat percentage in broilers (Xing et al., 2016). In this study, excess NH3 decreased breast weight and thigh weight. Our results indicated that excess NH3 negatively affected meat yield of broilers. In addition, previous studies found that exposure to NH3 can damage cardiac muscle (Xing et al., 2019), thymuses (Chen et al., 2020b), and spleens (An et al., 2019) in chickens. Other studies have reported the nephrotoxic effect of NH3. Witkowska et al. (2006) observed congestion, glomerulitis, necrotic epithelial cells, and MC infiltration in the kidneys of broilers treated by NH3 (Witkowska et al., 2006). Zarnab et al. (2019) found that high concentration of NH3 caused coagulative necrosis and the detachment of epithelial cells in broilers. In our experiment, histopathological observation revealed MC infiltration, hyperemia, swelling, and granular cast in kidneys of broilers exposed to excess NH3, which indicated that NH3 caused kidney injury in broilers. NH3-induced kidney injury may be due to that inhalation or dermal exposure to NH3 resulted in the elevation of blood NH3 level (hyperammonemia) (Manninen and Savolainen, 1989). Hyperammonemia led to impaired kidney function and sustaining kidney injury (Dasarathy et al., 2017). However, the molecular mechanism of NH3-induced kidney injury remains unclear.
miRNA Can be involved in inflammation through binding to target genes. Transfection with anti-miR-195 led to an increase of Smad7 in Caco-2 cell line, and miR-195 inhibited inflammation by targeting Smad7 in steroid resistance of ulcerative colitis (Chen et al., 2015). miR-21 Contributed to renal inflammation by targeting Smad7 in a model of diabetic nephropathy (McClelland et al., 2015). An et al. (2019) suggested that miR-133a-5p activated NF-κB and promoted inflammatory injury through inhibition of LOC101747543 in broiler spleens. In the present study, Smad7 was identified as a potential target of miR-6615-5p using 2 algorithms. Besides, miRNA binds to target sequence located in the 3′ UTR of their target mRNA through 6- to 8-nt-long complementary sequences (Marques-Rocha et al., 2015). In our experiment, 7-nt complementary sequences were found in the 3′-UTR of Smad7 using bioinformatics software. Moreover, miR-6615-5p expression increased and Smad7 mRNA expression decreased after NH3 exposure, suggesting that Smad7 was the target gene of miR-6615-5p. In addition, PCC analysis presented an extremely significant negative correlation between miR-6615-5p expression and Smad7 mRNA expression. As can be seen, Smad7 was a target gene of miR-6615-5p; and miR-6615/Smad7 axis may take part in inflammatory injury caused by NH3 in broiler kidneys.
Wang et al. (2005) found that Smad7 overexpression upregulated IκBα, inhibited NF-κB activation, and alleviated inflammatory response in a murine model of obstructive kidney disease. Smad7 knockout mice showed a higher level of NF-κB and severe renal inflammation in diabetic kidneys (Chen et al., 2011). NF-κB plays an indispensable role in inflammation. IκBα can inhibit the transcriptional activity of NF-κB (Prigent et al., 2000). The overproduction of NO derived from iNOS can promote inflammatory cell infiltration and contribute to inflammatory injury (Kobayashi, 2010). COX-2–derived PGEs can contribute directly to the classic signs of inflammation—redness, swelling, and fever—because it increases blood flow into the inflamed tissue through augmenting arterial dilatation and increasing microvascular permeability (Ricciotti and FitzGerald Garret, 2011). After NH3 exposure, broilers showed trachea inflammatory injury and increased mRNA and protein expression of NF-κB, iNOS, COX-2, and PGEs (Shi et al., 2018). Wang et al. (2018) found that H2S exposure resulted in chicken pneumonia via elevating NF-κB, COX-2, and iNOS. In our study, excess NH3 decreased IκBα; increased NF-κB, iNOS, COX-2, NO, and PGEs; and caused inflammatory response and tissue damage in broiler kidneys. These results indicated that miR-6615/Smad7 axis mediated inflammatory injury via IκBα/NF-κB pathway in kidneys of broilers exposed to NH3.
Some studies found that immune imbalance participated in inflammation. Th1/Th2 imbalance can drive several inflammatory diseases such as rheumatoid arthritis, allergies, and Crohn’s disease (Boissier et al., 2008). Th1 cells secrete IL-2, IL-12, and IFN-γ (Salem et al., 2004). Th2 cells secrete IL-1β, IL-4, and IL-6 (Romagnani, 2000). Tsuchiya et al. (2015) found that IL-1β stimulation induced IκBα degradation and NF-κB activation and IκBα degradation in canine fibroblasts. Our previous study reported that NH3 enhanced IL-1β, IL-4, and IL-6 mRNA expression, reduced IL-2 and IFN-γ mRNA expression, and caused Th1/Th2 imbalance and spleen inflammatory injury in chickens (An et al., 2019). Increased IL-1β and IL-6 mRNA expression was obtained in NH3-induced thymus damage in chickens (Chen et al., 2019). Hu et al. (2019) found that H2S caused thymus damage through increasing IL-4 mRNA expression and decreasing IL-12 and IFN-γ mRNA expression in chickens. In the present study, NH3 inhalation downregulated IL-2, IL-12β, IFN-γ, and IκBα and upregulated IL-1β, IL-4, IL-6, and NF-κB, meaning that Th1/Th2 imbalance mediated inflammatory injury via IκBα/NF-κB pathway in the kidneys of broilers treated by NH3. It is noteworthy that IL-6 mRNA expression increased 16-fold after 42-day exposure to excess NH3, indicating that IL-6 may be a sensitive gene in NH3-induced chicken kidney inflammatory damage. This result coincided with the result reported by Shi et al. (2018) that IL-6 mRNA expression was upregulated nearly 6-fold in NH3-induced trachea inflammatory damage in chickens.
Disturbed balance of Treg/Th17 plays a role in pathogenesis and progression of IgA nephropathy (Lin et al., 2012). Treg cells produce TGF-β1 that can suppress inflammation (Yoshimura et al., 2010). Th17 cells produce proinflammatory cytokine IL-17 (Turner et al., 2010). TGF-β1–induced IκBα expression inhibited NF-κB activity in human salivary gland cells (Azuma et al., 1999). IL-17 can promote NF-κB activation in human rheumatoid arthritis (Hot and Miossec, 2011). Li et al. (2016) found that PM exposure impaired the function of Treg cells, decreased TGF-β1 secretion, and caused pulmonary inflammation in mice. Zhao et al. (2013) reported that cold stress increased IL-17, decreased TGF-β1, and caused inflammation in broiler small intestines. In our research, NH3 inhalation downregulated TGF-β1 and IκBα and upregulated IL-17 and NF-κB, which indicated that Treg/Th17 imbalance was involved in NH3-caused inflammatory injury through IκBα/NF-κB pathway in broiler kidneys.
In addition, we found that miR-6615-5p expression; iNOS activity; and NF-κB, COX-2, PGEs, IL-1β, IL-4, IL-6, and IL-17 mRNA expression increased in a time- and dose-dependent manner. Smad7, IκBα, IL-12β, and IFN-γ mRNA expression decreased in a time- and dose-dependent manner. IL-2 and TGF-β1 mRNA expression decreased in a time-dependent manner. Smad7 and IκBα protein expression were reduced; NO content and protein expression of NF-κB, iNOS, and COX-2 were elevated in a dose-dependent manner. These results indicated that NH3 caused broiler kidney inflammatory injury in a time- and dose-dependent manner. Our previous study also found similar results. NH3 exposure increased NF-κB, COX-2, and PGEs in a time- and dose-dependent manner and caused spleen inflammatory damage in broilers (An et al., 2019).
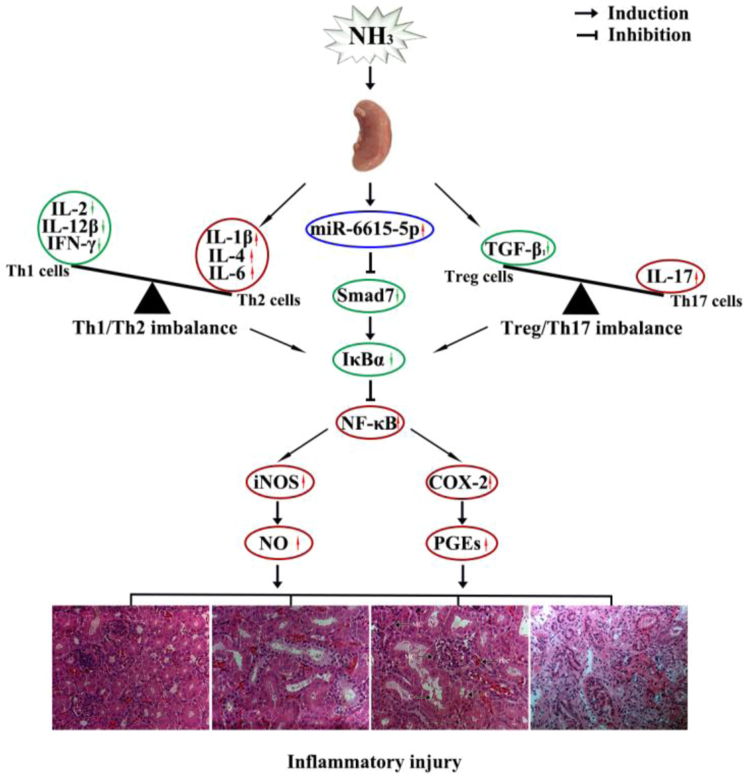
In conclusion, NH3 exposure impaired meat yield, caused inflammatory injury, increased miR-6615-5p and inhibited Smad7, broke Th1/Th2 and Treg/Th17 imbalance, and activated NF-κB pathway in broiler kidneys. For the first time, we discovered the role of Treg/Th17 imbalance in NH3 nephrotoxicity and put forward that IL-6 was a sensitive gene in NH3-induced inflammatory injury in broilers. The relationship between miR-6615-5p and Smad7 needs to be investigated in future vitro study. miR-6615/Smad7 Axis and immune imbalance were involved in NH3-caused inflammatory injury via the NF-κB pathway in chicken kidneys (Figure 6).
Figure 6.
miR-6615/Smad7 and immune imbalance participated in NH3-caused inflammatory injury through NF-κB pathway in broiler kidneys.
Acknowledgments
The study was supported by the National Key Research and Development Program of China (No. 2016YFD0500501), National Natural Science Foundation of China (No. 31972612), Open Project of State Key Laboratory of Animal Nutrition (2004DA125184F1714), and China Agriculture Research System (No. CARS-41).
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Contributor Information
Hongfu Zhang, Email: zhanghongfu@caas.cn.
Xiaohua Teng, Email: tengxiaohua@neau.edu.cn.
References
- An Y., Xing H.J., Zhang Y., Jia P.C., Gu X.H., Teng X.H. The evaluation of potential immunotoxicity induced by environmental pollutant ammonia in broilers. Poult. Sci. 2019;98:3165–3175. doi: 10.3382/ps/pez135. [DOI] [PubMed] [Google Scholar]
- Atapattu N.S.B.M., Senaratna D., Belpagodagamage U.D. Comparison of ammonia emission Rates from three Types of broiler litters. Poult. Sci. 2008;87:2436–2440. doi: 10.3382/ps.2007-00320. [DOI] [PubMed] [Google Scholar]
- Attia H., Nounou H., Shalaby M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat’s brain after oral exposure. Toxics. 2018;6:29–49. doi: 10.3390/toxics6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M., Motegi K., Aota K., Yamashita T., Yoshida H., Sato M. TGF-β1 inhibits NF-κB activity through induction of IκB-α expression in human salivary gland cells: a Possible mechanism of growth suppression by TGF-β1. Exp. Cell Res. 1999;250:213–222. doi: 10.1006/excr.1999.4503. [DOI] [PubMed] [Google Scholar]
- Boissier M.C., Assier E., Falgarone G., Bessis N. Shifting the imbalance from Th1/Th2 to Th17/treg: the changing rheumatoid arthritis paradigm. Joint Bone Spine. 2008;75:373–375. doi: 10.1016/j.jbspin.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Chen G., Cao S., Liu F., Liu Y. miR-195 plays a role in steroid resistance of ulcerative colitis by targeting Smad7. Biochem. J. 2015;471:357–367. doi: 10.1042/BJ20150095. [DOI] [PubMed] [Google Scholar]
- Chen H.Y., Huang X.R., Wang W., Li J.H., Heuchel R.L., Chung A.C., Lan H.Y. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 2011;60:590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.C., Miao Z.Y., Peng M.Q., Xing H.J., Zhang H.F., Teng X.H. The co-expression of circRNA and mRNA in the thymuses of chickens exposed to ammonia. Ecotox. Environ. Safe. 2019;176:146–152. doi: 10.1016/j.ecoenv.2019.03.076. [DOI] [PubMed] [Google Scholar]
- Chen D.C., Ning F.Y., Zhang J.Y., Tang Y., Teng X.H. NF-κB pathway took part in the development of apoptosis mediated by miR-15a and oxidative stress via mitochondrial pathway in ammonia-treated chicken splenic. Sci. Total Environ. 2020;729:139017. doi: 10.1016/j.scitotenv.2020.139017. [DOI] [PubMed] [Google Scholar]
- Chen D.C., Hu G.H., Zhang S., Zhang H.F., Teng X.H. Ammonia-triggere apoptosis via immune function and metabolic. process in the thymuses of chickens by proteomics analysis. Ecotox. Environ. Safe. 2020;198:110619. doi: 10.1016/j.ecoenv.2020.110619. [DOI] [PubMed] [Google Scholar]
- Chen J.Q., Zhang S., Tong J.Y., Teng J.Y., Zhang Z.Y., Li S., Teng X.J. Whole transcriptome-based miRNA-mRNA network analysis revealed the mechanism of inflammation-immunosuppressive damage caused by cadmium in common carp spleens. Sci. Total Environ. 2020;717:137081. doi: 10.1016/j.scitotenv.2020.137081. [DOI] [PubMed] [Google Scholar]
- Chi Q.R., Luan Y.L., Zhang Y.M., Hu X.Y., Li S. The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM. Metallomics. 2019;11:845–857. doi: 10.1039/c9mt00006b. [DOI] [PubMed] [Google Scholar]
- Cui Y., Yin K., Gong Y., Qu Y., Liu H., Lin H. Atrazine induces necroptosis by miR-181-5p targeting inflammation and glycometabolism in carp lymphocytes. Fish Shellfish Immunol. 2019;94:730–738. doi: 10.1016/j.fsi.2019.09.068. [DOI] [PubMed] [Google Scholar]
- Dasarathy S., Mookerjee R.P., Rackayova V., Rangroo Thrane V., Vairappan B., Ott P., Rose C.F. Ammonia toxicity: from head to toe? Metab. Brain Dis. 2017;32:529–538. doi: 10.1007/s11011-016-9938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do J.C., Choi I.H., Nahm K.H. Effects of chemically amended litter on broiler performances, atmospheric ammonia concentration, and phosphorus solubility in litter. Poult. Sci. 2005;84:679–686. doi: 10.1093/ps/84.5.679. [DOI] [PubMed] [Google Scholar]
- Dong N., Li X.R., Xue C.Y., Zhang L., Wang C.S., Xu X.Y., Shan A.S. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J. Cell. Physiol. 2020;235:5525–5540. doi: 10.1002/jcp.29452. [DOI] [PubMed] [Google Scholar]
- Han Q., Zhang J.Y., Sun Q., Xu Y.M., Teng X.H. Oxidative stress and mitochondrial dysfunction involved in ammonia-induced nephrocyte necroptosis in chickens. Ecotox. Environ. Safe. 2020;203:110974. doi: 10.1016/j.ecoenv.2020.110974. [DOI] [PubMed] [Google Scholar]
- Hot A., Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann. Rheum. Dis. 2011;70:727–732. doi: 10.1136/ard.2010.143768. [DOI] [PubMed] [Google Scholar]
- Hu X.Y., Chi Q.R., Liu Q.Q., Wang D.X., Zhang Y.M., Li S. Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-κB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere. 2019;237:124427. doi: 10.1016/j.chemosphere.2019.124427. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010;88:1157–1162. doi: 10.1189/jlb.0310149. [DOI] [PubMed] [Google Scholar]
- Li G., Cao Y., Sun Y., Xu R., Zheng Z., Song H.H. Ultrafine particles in the airway aggravated experimental lung injury through impairment in Treg function. Biochem. Biophys. Res. Commun. 2016;478:494–500. doi: 10.1016/j.bbrc.2016.05.059. [DOI] [PubMed] [Google Scholar]
- Li W., Zhao S.R., Yang H., Zhang C., Kang Q., Deng J., Xu Y., Ding Y., Li S. Potential novel prediction of TMJ-OA: MiR-140-5p regulates inflammation through Smad/TGF-β Signaling. Front. Pharmacol. 2019;10:1–9. doi: 10.3389/fphar.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Jiang G., Shan J., Zhu C., Zou J., Wu X. Imbalance of regulatory T cells to Th17 cells in IgA nephropathy. Scand. J. Clin. Lab. Invest. 2012;72:221–229. doi: 10.3109/00365513.2011.652158. [DOI] [PubMed] [Google Scholar]
- Manninen A.T.A., Savolainen H. Effect of Short-Term ammonia inhalation on selected Amino acids in rat brain. Pharmacol. Toxicol. 1989;64:244–246. doi: 10.1111/j.1600-0773.1989.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Marques-Rocha J.L., Samblas M., Milagro F.I., Bressan J., Martinez J.A., Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015;29:3595–3611. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- McClelland A.D., Herman-Edelstein M., Komers R., Jha J.C., Winbanks C.E., Hagiwara S., Gregorevic P., Kantharidis P., Cooper M.E. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin. Sci. 2015;129:1237–1249. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- Miles D.M., Branton S.L., Lott B.D. Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poult. Sci. 2004;83:1650–1654. doi: 10.1093/ps/83.10.1650. [DOI] [PubMed] [Google Scholar]
- Naseem S., King A.J. Ammonia production in poultry houses can affect health of humans, birds, and the environment-techniques for its reduction during poultry production. Environ. Sci. Pollut. Res. 2018;25:15269–15293. doi: 10.1007/s11356-018-2018-y. [DOI] [PubMed] [Google Scholar]
- Ng Y.-Y., Hou C.-C., Wang W., Huang X.R., Lan H.Y. Blockade of NFκB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int. 2005;67:S83–S91. doi: 10.1111/j.1523-1755.2005.09421.x. [DOI] [PubMed] [Google Scholar]
- National Research Council. 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Osorio Hernandez R., Tinoco I., Osorio Saraz A., De C., Coelho R., Sousa F. Calidad del aire en galpón avícola con ventilación natural durante la fase de pollitos. Rev. Bras. Eng. Agr. Amb. 2016;20:660–665. [Google Scholar]
- Prigent M., Barlat I., Langen H., Dargemont C. IκBα and IκBα/NF-κB complexes are retained in the cytoplasm through interaction with a novel partner, RasGAP SH3-binding Protein 2. J. Biol. Chem. 2000;275:36441–36449. doi: 10.1074/jbc.M004751200. [DOI] [PubMed] [Google Scholar]
- Rani J., Sharma U.K., Sharma D.N. Role of adequate water intake in purification of body. Environ. Conserv. J. 2018;18:183–186. [Google Scholar]
- Ricciotti E., FitzGerald Garret A. Prostaglandins and inflammation. Atertio. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. T-cell subsets (Th1 versus Th2) Ann. Allerg. Asthma. Immunol. 2000;85:9–21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- Salem M.L. Estrogen, A Double-Edged Sword: Modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr. Drug Targets Inflamm. Allergy. 2004;3:97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- Shi Q.X., Wang W., Chen M.H., Zhang H.F., Xu S.W. Ammonia induces Treg/Th1 imbalance with triggered NF-κB pathway leading to chicken respiratory inflammation response. Sci. Total Environ. 2018;659:354–362. doi: 10.1016/j.scitotenv.2018.12.375. [DOI] [PubMed] [Google Scholar]
- Shi Y., Zhao T., Yang X., Sun B., Li Y., Duan J., Sun Z. PM2.5-induced alteration of DNA methylation and RNA-transcription are associated with inflammatory response and lung injury. Sci. Total Environ. 2019;650:908–921. doi: 10.1016/j.scitotenv.2018.09.085. [DOI] [PubMed] [Google Scholar]
- Su Y., Wei H., Bi Y., Wang Y., Zhao P., Zhang R., Li X., Li J., Bao J. Pre-cold acclimation improves the immune function of trachea and resistance to cold stress in broilers. J. Cell. Physiol. 2019;234:7198–7212. doi: 10.1002/jcp.27473. [DOI] [PubMed] [Google Scholar]
- Turner J.-E., Paust H.-J., Steinmetz O.M., Panzer U. The Th17 immune response in renal inflammation. Kidney Int. 2010;77:1070–1075. doi: 10.1038/ki.2010.102. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H., Nakano R., Konno T., Okabayashi K., Narita T., Sugiya H. Activation of MEK/ERK pathways through NF-κB activation is involved in interleukin-1 beta-induced cyclooxygenease-2 expression in canine dermal fibroblasts. Vet. Immunol. Immunopathol. 2015;168:223–232. doi: 10.1016/j.vetimm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- UEP. Animal husbandry guidelines for U.S. egg laying flocks (2017 Edition) 2017. https://uepcertified.com/wp-content/uploads/2015/08/UEP-Animal-Welfare-Guidelines-20141.pdf
- Vucemilo M., Matkovic K., Vinkovic B., Jaksic S., Granic K., Mas N. The effect of animal age on air pollutant concentration in a broiler house. Czech J. Anim. Sci. 2007;52:170–174. [Google Scholar]
- Wang W.S., Huang X.R., Li A.G., Liu F., Li J.H., Truong L.D., Wang X.J., Lan H.Y. Signaling mechanism of TGF-β1 in prevention of renal inflammation: role of Smad7. J. Am. Soc. Nephrol. 2005;16:1371–1383. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- Wang W., Chen M.H., Jin X., Li X.J., Yang Z.J., Lin H.J., Xu S.W. H2S induces Th1/Th2 imbalance with triggered NF-kappaB pathway to exacerbate LPS-induce chicken pneumonia response. Chemosphere. 2018;208:241–246. doi: 10.1016/j.chemosphere.2018.05.152. [DOI] [PubMed] [Google Scholar]
- Wang W., Shi Q., Wang S., Zhang H., Xu S. Ammonia regulates chicken tracheal cell necroptosis via the LncRNA107053293/MiR-148a-3p/FAF1 axis. J. Hazard. Mater. 2020;386:121626. doi: 10.1016/j.jhazmat.2019.121626. [DOI] [PubMed] [Google Scholar]
- Witkowska D., Sowińska J., Mituniewicz T., Wójci J. The effect of a disinfection on the ammonia concentration on the surface of litter, air and the pathomorphological picture of kidneys and livers in broiler chickens. Arch. Tierz. 2006;49:249–256. [Google Scholar]
- Xing H., Luan S.J., Sun Y.B., Sa R.N., Zhang H.F. Effects of ammonia exposure on carcass traits and fatty acid composition of broiler meat. Anim. Nutr. 2016;2:282–287. doi: 10.1016/j.aninu.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H.J., Peng M.Q., Li Z., Chen J.Q., Zhang H.F., Teng X.H. Ammonia inhalation-mediated mir-202-5p leads to cardiac autophagy through PTEN/AKT/mTOR pathway. Chemosphere. 2019;235:858–866. doi: 10.1016/j.chemosphere.2019.06.235. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Wakabayashi Y., Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J. Biochem. 2010;147:781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnab S., Chaudhary M.S., Javed M.T., Khatoon A., Saleemi M.K., Ahmed T., Tariq N., Manzoor F., Javed I., Zhang H., Hua X.Z., Peng Y. Effects of induced high ammonia concentration in air on Gross and histopathology of different body organs in experimental broiler birds and its Amelioration by different Modifiers. Pak. Vet. J. 2019;39:371–376. [Google Scholar]
- Zhang J., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zheng S., Wang S., Wang W., Xing H., Xu S. Chlorpyrifos induced oxidative stress to promote apoptosis and autophagy through the regulation of miR-19a-AMPK axis in common carp. Fish Shellfish Immunol. 2019;93:1093–1099. doi: 10.1016/j.fsi.2019.07.022. [DOI] [PubMed] [Google Scholar]
- Zhao F., Zhang Z., Yao H., Wang L., Liu T., Yu X., Li S., Xu S. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013;95:146–155. doi: 10.1016/j.rvsc.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Zheng S., Jin X., Chen M., Shi Q., Zhang H., Xu S. Hydrogen sulfide exposure induces jejunum injury via CYP450s/ROS pathway in broilers. Chemosphere. 2019;214:25–34. doi: 10.1016/j.chemosphere.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Zhu X., Li S., Zhang Q., Zhu D., Xu Y., Zhang P., Han J., Duan Z., Gao J., Ou Y. Correlation of increased Th17/Treg cell ratio with endoplasmic reticulum stress in chronic kidney disease. Med. 2018;97:e10748. doi: 10.1097/MD.0000000000010748. [DOI] [PMC free article] [PubMed] [Google Scholar]