Abstract
Vitamin D deficiency has become a global pandemic affecting approximately one billion people worldwide. Much attention has been paid to the association of low serum 25-hydroxyvitamin D (25(OH)D) levels and various chronic diseases, especially heart failure (HF). A clear role of vitamin D deficiency has been established, with increased mortality and morbidity in heart failures. However, previous randomized control trials have failed to show improvement in clinical outcomes with calciferol supplementation in these patients. Therefore, it is still unclear whether calciferol therapy can be added to the standard care in congestive heart failure (CHF) patients with deficiency. Hence, to evaluate the role of vitamin D supplementation in CHF patients with low serum 25(OH)D, we conducted an extensive search in the PubMed and Google Scholar databases using various combinations of keywords. All potentially eligible studies that evaluated the effects of vitamin D supplementation on clinical outcomes in HF patients were retrieved and extensively studied. We also checked the references of all eligible studies to identify additional relevant publications. In this study, we reviewed various mechanisms of vitamin D affecting the cardiovascular system and examined the impact of deficiency on heart failures in terms of mortality and hospitalizations. In conclusion, vitamin D supplementation has failed to improve the clinical outcomes in HF patients. The possible long-term benefits of supplementation cannot be excluded. Therefore, for future clinical trials, we recommend considering large sample sizes, longer follow-up durations, along with optimal dosage and appropriate dosing frequency.
Keywords: vitamin d deficiency, heart failure, chf, congestive heart failure, vitamin d supplementation, vitamin d, long-term clinical outcomes, cholecalciferol, 25 hydroxyvitamin d, 25(oh)d
Introduction and background
Over the last two decades, vitamin D deficiency is a major topic of debate even though a remarkable amount of research has been done in the past. Indeed, according to recent research, 90% of physicians believe that they overprescribe calciferol supplements, considering the lack of side effects [1]. This overprescribing is due to the lack of specific guidelines for screening and treating patients with low serum cholecalciferol levels. Nearly one billion people worldwide are affected by either a vitamin D deficiency (<20 ng/ml) or insufficiency (21-29 ng/ml) [2]. A 2018 study that took data from the National Health and Nutrition Examination Survey (NHANES) estimated that the prevalence of vitamin D deficiency was 28·9% [3]. Researchers are aware that the role of vitamin D is not just limited to bone health, however, its role outside bone health is poorly understood. Research has shown that vitamin D deficiency has a negative impact on morbidity and mortality outcomes in patients who have chronic illnesses such as heart disease, diabetes mellitus, cancer, pulmonary hypertension, and auto-immune disorders [4-5]. Unfortunately, none of the current evidence was able to explain the association between vitamin D deficiency and these chronic diseases. A study by the Agency for Health Research and Quality reviewed nearly 250 studies and concluded that the results of these studies are inconsistent, making it difficult to establish a possible link between vitamin D and health outcomes [6]. Although a few studies have found a statistically significant reduction in all-cause mortality with supplementation of 25-hydroxyvitamin D (25(OH)D), further evaluation of these results did not show such reduction [7-8].
Among these chronic diseases, cardiovascular disease is the most common cause of death in the world and accounts for 30% of deaths, leading to a huge burden on health care systems [9]. Although there are a variety of cardiovascular diseases affected by vitamin D deficiency, congestive heart failure is the major one, accounting for the healthcare burden in terms of hospitalizations, healthcare usage, morbidity, and mortality primarily affecting middle and old age people [10-11]. As of 2017, almost 6.5 million adults have heart failure and contribute to one in every eight deaths [12]. To be more specific, outcomes in these patients can be changed and, in some cases, can prevent life-threatening events with simple lifestyle changes such as the intake of foods rich in vitamin D, dietary supplements, and exposure to sunlight.
A recent study reported that vitamin D deficiency increases the risk of hospitalization and mortality in these patients, but it is still unclear whether supplementation improves the outcome or not [13]. Results from the current literature have shown a wide range of variations and inconsistencies [14-19]. Thus, the effectiveness of supplementation is still unclear. Therefore, no specific guidelines were made for the inclusion of vitamin D therapy in the standard care of HF patients.
From this review, we are trying to assess the benefits of giving vitamin D supplements to CHF patients and its effects on clinical outcomes of CHF patients with low vitamin D. This study aims to summarize the results from previous studies, compare the conclusions, and finally draw inferences on whether vitamin D supplements improve the outcomes. We are also trying to summarize the underlying pathogenesis and mechanism of cardiac dysfunction in these patients. This summary of evidence will provide an update for current clinical practice; it will also help to counsel HF patients regarding misconceptions of vitamin D use and provide recommendations for future researchers.
Review
Effects of vitamin D on the cardiovascular system and its role in heart failure patients
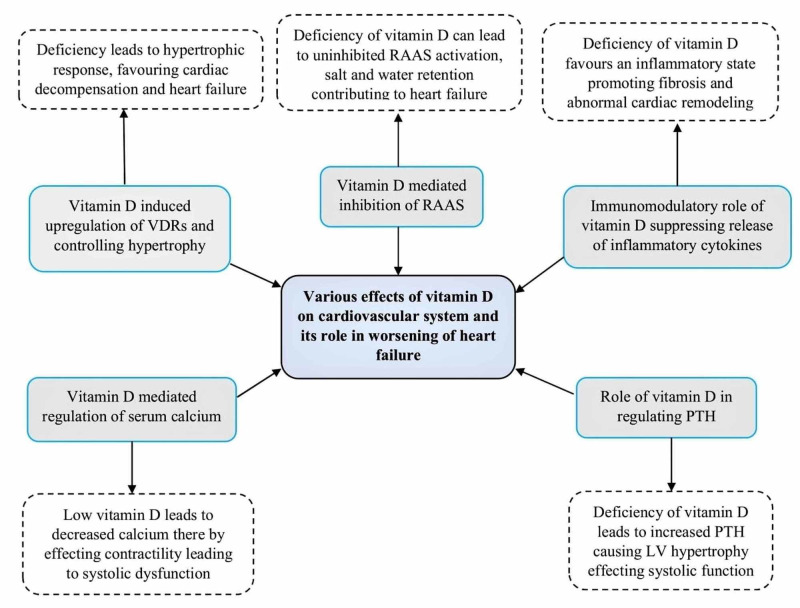
Extensive research has established a clear role of 25-hydroxycholecalciferol in promoting the health of the cardiovascular system. Of the various functions of vitamin D, regulation of the Renin-Angiotensin activation system (RAAS) is the major cardiac protective action. Therefore, the deficiency can lead to uninhibited RAAS activation and contribute to the worsening of heart failure by the retention of salt and water. Data from a study conducted by Resnick et al. have shown an inverse relationship between vitamin D and serum renin levels. This relationship is mainly due to the suppression of the renin transcription with the help of vitamin D receptors (VDRs) [20]. Coming to the defensive role of cholecalciferol in controlling cardiac hypertrophy, in-vitro evidence has shown that VDRs in cardiac myocytes and fibroblasts are upregulated as a counter-regulatory mechanism to hypertrophy [21]. The absence of this role favors hypertrophic response leading to cardiac decompensation and heart failure. Another important role of vitamin D is its immunomodulatory mechanism, which decreases inflammatory effects by promoting anti-inflammatory T-helper cells like Th2 cells and inhibiting Th1 and Th17 cells that are responsible for inflammatory cytokines like interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-a). Few studies also reported that vitamin D suppresses the release of pro-inflammatory cytokines [22]. Furthermore, a negative correlation was found between serum 25(OH)D levels and these cytokines, resulting in an inflammatory state in deficient patients [23]. Vitamin D also regulates the metabolism of calcium and parathyroid hormone (PTH) and, in fact, these two also play a major role in cardiac contractility and remodeling, respectively. Low 25(OH)D levels lead to decreased calcium thereby effecting contractility leading to systolic dysfunction. In addition, deficiency may cause an increase in PTH, which, in turn, causes myocardial fibrosis and hypertrophy affecting LV systolic function. So, fluctuations in vitamin D can lead to alterations in serum levels of calcium and PTH, thus affecting the function of cardiac muscle indirectly. Taking into consideration all these different actions of 25-hydroxycholecalciferol on cardiovascular health, researchers believe that improving the vitamin D status in deficient patients may improve the clinical outcomes and overall mortality in heart failures. However, few recent clinical trials have found that there was no added advantage even with supplementation of vitamin D in these patients [14-17]. Therefore, the role of vitamin D as standard therapy in CHF patients is still unclear. But there are few studies that have shown some positive impacts on these patients appealing to researchers in need of further studies in the future to have a clear picture. Figure 1 below gives an overview of different mechanisms that are affecting cardiac muscle either directly or indirectly.
Figure 1. Various mechanisms involved in the pathophysiology of vitamin D deficiency-associated effects in heart failures.
RAAS=Renin Angiotensin Activation System, VDRs=Vitamin D Receptors, PTH=Parathyroid Hormone
Role of vitamin D supplementation in CHF patients
Several investigators have attempted to examine the benefits of vitamin D supplementation in CHF patients and their results have suggested few recommendations for the current standard of care. A variety of regimens (such as high dose and low dose) for different durations (long-term and short-term) and different frequencies (daily, weekly, and monthly) were used in several clinical trials.
A recent study evaluated the effects of short-term vitamin D supplementation in heart failure patients for eight weeks [24]. They studied its relation to blood pressure (BP) and physical activity and concluded that supplementation has failed to improve BP and the Six-Minute Walk Test (6MWT) [24]. However, these results should be taken with caution, as the sample of this study was very small, and therapy was given for a short period without maintenance [24]. In contrast to this, a long-term clinical trial was conducted for three years on 400 HF patients who received daily 4000 IU with all-cause mortality as the primary endpoint [15]. No difference in mortality was observed between the treatment and control groups, with 19.6% of deaths in the treatment group and 17.9% of deaths in the placebo group, concluding its importance in advanced HF [15]. Their data also revealed no difference in hospitalizations, the need for cardiac resuscitation, and transplantation [15]. Furthermore, they noticed a greater need for mechanical circulatory support implants in the intervention group indicating caution with respect to long-term vitamin D supplementation [15]. In fact, this was the only RCT that provided evidence for the detrimental effects of moderately high-dose cholecalciferol in HF patients. They cited high plasma calcium concentrations as a reason for the adverse effects in these patients [25]. Coming to the limitations, this study has restricted their analysis to male patients only, and further, it could not prove the causal association [25]. A clinical trial was conducted by Scragg and his colleagues to examine whether a monthly high-dose vitamin D supplementation can prevent cardiovascular disease in the general population regardless of their serum 25(OH)D status [26]. A total of 5108 participants were selected, and half of them were randomly given oral D3 in an initial dose of 200,000 IU followed by a monthly 100,000 IU for a median of 3.3 years. Their primary outcome revealed no purpose of giving supplements, and it did not prevent any cardiovascular disease (CVD) events [26]. They stated that this non-significance may be due to their monthly regimen, however, a daily or weekly regimen can be more effective [26]. This suggests the importance of dosing frequency. To understand its effects better, Witte et al. conducted a randomized controlled trial with 229 patients with CHF and vitamin D deficiency [27]. Participants received either 4,000 IU of oral D3 or placebo for one year, the results showed clinically significant improvements in ejection fraction, LV dimensions, and volumes highlighting the role in the reversal of cardiac remodeling [27]. But supplementation had no effect on their primary endpoint of 6MWT [27]. Similar results were also found in another RCT conducted by Dalbeni et al., in addition to an increase in ejection fraction, the study also reported a beneficial effect on systolic blood pressure [28]. But when compared to Witte et al., the major drawback of this study was its small sample of 23 CHF patients and supplements given for only a short duration of six months [28].
Coming to the effects of vitamin D supplementation on serum renin levels, a study by Schroten et al. reported that there was a drastic decrease in renin levels in the treatment group [29]. They concluded that this reduction in renin cannot be translated as improved outcomes in these patients, as the absolute reduction was small [29]. But from the fact that sustained elevation in plasma renin activity (PRA) is an independent factor that predicts adverse outcomes in HF patients [30], we can consider reduced PRA as a positive interaction. They found no significant effect on natriuretic peptides that correlate with the severity of CHF [29]. The short duration of six weeks could be a limitation of this study. Another study showed a dramatic increase in serum 25(OH)D levels within six months of weekly high-dose therapy with a proportional decrease in PTH [31]. However, this increase could not be correlated clinically, as they found no difference when compared to the placebo [31]. The relatively small sample size of 30 subjects and short duration of six months were the drawbacks of this study. A unique study from 2011 tried to evaluate optimal treatment options for the adverse effects of vitamin D deficiency in heart failure patients [32]. In this study, weekly high-dose cholecalciferol of 50,000 IU along with daily calcium was supplemented. Their major finding was a decrease in plasma 8-isoprostane, which is a marker of oxidative stress and lipid peroxidation [32], and this reduced oxidative stress was clinically correlated with improved LVEF (Left Ventricular Ejection Fraction). Nevertheless, this study had its own limitations, first, the subjects recruited were restricted to African Americans with heart failure and vitamin D deficiency and followed up for 14 weeks only. Second, as the treatment regimen includes both cholecalciferol and calcium, their results cannot be applied to cholecalciferol alone, and, importantly, they did not have a control group to compare the findings [32].
A compelling amount of evidence has shown that inflammation plays a major role in the pathogenesis of HF. Few studies tried to evaluate the effects of supplementation on inflammatory markers [17,33]. A study published by Witham et al. found no significant difference in TNF-a levels in the treatment group when compared to placebo [17]. No changes were noticed in renin or aldosterone levels, but they found a clinically significant drop in brain natriuretic peptide (BNP) levels when compared to placebo, demonstrating the effects of vitamin D on the cardiovascular system [17]. Although BNP levels were reduced, they found no improvement in ejection fraction [17]. Another randomized control trial by Schleithoff et al. assessed the role of 25-hydroxycholecalciferol in reducing pro-inflammatory markers [33]. A higher level of IL-10 (anti-inflammatory) and lower levels of TNF-a (pro-inflammatory) were noticed, thereby improving the background inflammatory state in CHF patients [33]. Table 1 summarizes several clinical trials that evaluated the role of vitamin D supplementation in CHF patients.
Table 1. List of clinical trials that evaluated the role of vitamin D supplementation in CHF patients.
CHF=Congestive Heart Failure, 6MWT=6 Minute Walk Test, MCS=Mechanical Circulatory Support, LVEF=Left Ventricular Ejection Fraction, HR=Hazard Ratio, PTH: Parathyroid Hormone, TNF-a=Tumour Necrosis Factor-alpha, IL=Interleukin, BNP=Brain Natriuretic Peptide, ANP=Atrial Natriuretic Peptide, f/b=Followed By, 25-OHD=25-Hydroxyvitamin D, IU=International Units
| Study details (year) | Follow-up | Vit D dose | Results |
| 1. Hosseinzadeh et al. 2020 [24] | 8 weeks | 50,000 IU weekly | No improvements were found in BP and 6MWT when compared to the placebo. Levels of vitamin D were increased significantly in the intervention group. With short-term no improvement in HF patients’ physical activity consistent with previous studies. |
| 2. Zittermann et al. 2017 [15] | 3 years | 4000 IU daily | No significant effect was found with supplements in advanced HF patients. The study also concluded that group on vitamin D had a greater need for MCS implants in the intervention group with HR 1.96 (1.04–3.66). No difference was found between the intervention and the placebo group in terms of mortality with HR 1.09 (0.69-1.71). Data also suggests caution with prolonged supplementation of high doses. |
| 3. Scragg et al. 2017 [26] | 3.3 years | 200 000 IU f/b 100 000 IU monthly | Monthly high dose vitamin D did not prevent cardiovascular disease with HR 1.02 (0.87-1.21). Results also suggest that there was no purpose of supplementation in relation to heart failure with HR of 1.19 (0.84-1.68). This study also stated that high dose Vit D can be less effective than weekly or daily supplements in preventing a cardiovascular event. |
| 4. Turrini et al. 2017 [16] | 6 months | 300,000 IU f/b 50,000 monthly | Treatment of deficiency did not influence the final outcome when compared to the placebo group. Supplements improved functional capacity and PTH levels at 3 months but this was not observed at 6 months. Concludes baseline vitamin D levels did not affect the functional capacity. |
| 5. Witte et al. 2016 [27] | 1 year | 4000 IU daily | Results showed a significant improvement of cardiac function showing a mean change of +6.7 % LVEF (3.20-8.95) on echocardiogram. 1-year supplementation of daily Vit D did not improve 6-minute walk distance. It also has beneficial effects on LV structure and function in patients on current standard medical therapy. |
| 6. Dalbeni et al. 2014 [28] | 6 months | 4000 IU daily | Results showed that there was a significant improvement in EF of 6.71% in elderly patients with HF and vitamin D deficiency. Therapy also improved SBP after 6 months from 129.6 to 122.7 mm Hg, but no significant variations were found on other parameters. |
| 7. Schroten et al. 2013 [29] | 6 weeks | 2000 IU daily | Short term supplementation improved the Vit D levels from 48 nmol/L to 80 nmol/L. Results showed Plasma renin activity decrease from 6.5 ng/mL per hour (3.8-11.2) to 5.2 ng/mL per hour (2.9-9.5) in 6 weeks. No significant changes were seen in natriuretic peptides (BNP, ANP) and other markers of fibrosis. |
| 8. Boxer et al. 2013 [30] | 6 months | 50,000 IU Weekly | High dose Vit D improved serum 25-OH D levels from baseline 19.1 ± 9.3 ng/ml to 61.7 ± 20.3 ng/ml. Vitamin D supplements did not improve physical performance for patients with HF despite a good increase in serum 25-OHD |
| 9. Zia et al. 2011 [31] | 14 weeks | 50,000 IU weekly for 8 weeks f/b 1400 IU daily | Although a small patient population, results suggest improvement in secondary hyperparathyroidism, oxidative stress, and ventricular function (LVEF). Serum PTH was reduced at 14 weeks from 104.8 to 73.8 pg/mL, Plasma 8-isoprostane a marker of oxidative stress was reduced at 14 weeks to 117.8 from 136.1 pg/ml. With baseline EF of 24.3 ± 1.7% at entry was improved to 31.3 ± 4.3%. |
| 10. Witham et al. 2010 [17] | 20 weeks | 100 000 IU and in the 10th week | Supplementation did not improve functional capacity or quality of life in heart failure patients with vitamin D insufficiency. B-type natriuretic peptide levels decreased in the treatment group when compared with placebo |
| 11. Schleithoff et al. 2006 [32] | 9 months | 1000 IU daily | Significant treatment effects were observed, parathyroid hormone levels were significantly decreased when compared to the baseline. Anti-inflammatory cytokine IL-10 was significantly higher in the intervention group at 9 months and pro-inflammatory cytokine TNF-a was increased in controls but remain constant in the therapy group. Vitamin D reduced the inflammatory state in CHF patients and might be a new anti-inflammatory agent in the future. But during follow-up at 15 months, the survival rate did not differ significantly. |
Role of Vitamin-D as a predictor for risk of hospitalization and poor clinical outcomes
A considerable amount of literature has established the association of vitamin D deficiency and poor clinical outcomes in patients with HF. The majority of these studied the outcomes in terms of hospitalizations, risk of mortality, impact on LVEF, and effect on physical activity. A recent study reported vitamin D deficiency as an independent risk factor for hospitalization in patients with CHF [13]. Furthermore, this risk was more consistent in frail veterans when compared to non-frail veterans. An important aspect of this study was that its results failed to show any relationship between mortality and deficiency [13]. The main reasons for this lack of effect on mortality may be due to the small sample size and short follow-up period [13]. Another interesting study tried to determine the role of 25(OH)D levels on the clinical outcomes of HF patients undergoing cardiac resynchronization therapy (CRT) [34]. They concluded that serum calciferol levels less than 24.12 ng/ml has a significant impact on heart failure patients [34]. Deficient patients were more likely to show a lack of response to CRT and deficiency also predicts long-term mortality in these patients. This evidence convinced that the monitoring of vitamin D levels in HF patients could be of greater clinical significance by identifying high-risk groups. Similarly, another clinical trial also tried to demonstrate a response to CRT in HF patients with vitamin D deficiency [35]. Although the sample size was small, their results indicate that adequate levels of cholecalciferol can significantly improve the functional capacity of HF patients after undergoing CRT [35]. To understand its effects better, a key study from 2019 studied the pattern of mortality rates and the risk of hospitalizations in HF patients with respect to vitamin D status [36]. Findings from this study demonstrated a significant increase in rates of cardiovascular hospitalizations, but no association was found in terms of mortality, ejection fraction, and diastolic dysfunction [36]. Another longitudinal study examined the reason for increased hospitalizations and mortality rates in vitamin D-deficient HF patients [37]. Data from this study revealed no association with the risk of hospitalizations, however, they found significantly higher mortality in deficient patients when compared to non-deficient subjects [37]. This study also stated that a 2.72-fold increase in serum 25(OH)D levels can lower mortality by 14% [37]. A more interesting study attempted to assess the risk of readmission and infection rates after left ventricular assist device (LVAD) in HF patients with vitamin D deficiency [38]. They concluded that low vitamin D levels were independently associated with a higher risk of readmission and driveline infection risk [38]. Similarly, a cohort study investigated the relationship between serum vitamin D and the incidence of hospitalization in heart failure patients [39]. A greater risk of hospitalization was observed in deficient patients with a hazard ratio of 1.61 when compared to those with normal levels [39].
Due to the high prevalence of vitamin D deficiency in elderly patients, Porto et al. conducted a unique randomized clinical trial that evaluated the risk of heart failure in the elderly population [40]. A significant association was found between low levels of 25(OH)D and incidence of heart failure, this was significant especially in male patients and obese subjects. The higher incidence of heart failure in male subjects was strongly supported by the fact that vitamin D is positively associated with testosterone levels [41]. Testosterone, in turn, also has a protective effect on the myocardium, decreasing the risk of heart failure [42]. Thus, the combined effect of decreased testosterone levels and increased prevalence of vitamin D deficiency in elderly males led to a higher risk of HF [43]. In the same way, obese people are more prone to vitamin D deficiency, both obesity and vitamin D deficiency were independent risk factors for heart failure [44]. Another well-organized RCT from 2016 examined the role of vitamin D in predicting the rate of hospitalizations and mortality in HF patients [45]. This study finally concluded vitamin D as an independent predictor of hospitalizations and the risk of mortality [45]. The limitations of this study were the small sample size and small control group. Similar results were found by a study conducted by Liu et al. in 2012 and concluded that inadequate serum calciferol levels have a higher risk of mortality not only in HF patients but also in all cardiovascular diseases [46]. In another major study, vitamin D was found to be an independent factor in predicting survival rates in HF patients [19]. In this study, they evaluated the role of oral D3 supplementation and its effects on mortality. The treatment group was followed for 518 days and stated that vitamin D supplements improved the survival rates in these patients [19], thus highlighting the importance of including vitamin D therapy in the standard care of heart failures. Coming to the prognostic role of vitamin D in HF patients, a study by Liu et al. suggested that a low vitamin D concentration is associated with poor prognosis [47]. They also found high C-reactive protein (CRP) levels and increased renin activity in patients with low serum 25(OH)D [47]. Therefore, we can consider high levels of CRP and increased plasma renin activity as poor prognostic factors in HF patients. Table 2 summarizes studies that have evaluated the clinical outcomes in HF patients with vitamin D deficiency.
Table 2. List studies that evaluated the clinical outcomes in HF patients with vitamin D deficiency.
HF=Heart Failure, CRT=Cardiac Resynchronization Therapy, LVAD=Left Ventricular Assist Device
| Study details | Measured outcomes | Risk estimates |
| 1. Ugarriza et al. 2019 [13] | Hospitalization | 1.8 (1.3-2.5) |
| Mortality | 0.83 (0.56-1.2) | |
| Hospitalization in frail subjects | 1.7 (1.2-2.7) | |
| Mortality in frail subjects | 0.84 (0.50-1.4) | |
| 2. Perge et al. 2019 [33] | 5-year all-cause mortality | 1.92 (1.02-1.45) |
| Poor response to CRT | 2.62 (1.01-6.25) | |
| 3. Nolte et al. 2019 [35] | Hospitalizations | 1.74 (1.08-2.80) |
| 5-year mortality | 1.55 (1.00-2.42) | |
| 4. Cubbon et al. 2019 [36] | Mortality | 1.24 (1.05-1.46) |
| 5. Obeid et al. 2018 [37] | Risk of readmission after LVAD | 2.46 (1.07-5.77) |
| Risk of driveline infection within 1 year after LVAD | 6.18 (0.80-49.2) | |
| 6. Costanzo et al. 2018 [38] | Incidence of hospitalization | 1.61 (1.06-2.43) |
| 7. Porto et al. 2017 [39] | Incidence of heart failure | 12.19 (4.23-35.2) |
| Incidence of HF in males | 15.32 (3.39-69.2) | |
| Incidence of HF in obese subjects | 4.17 (1.36-12.81) | |
| 8. Belen et al. 2016 [44] | Hospitalizations (lower vs higher) | 23.4% vs 7.3% |
| Mortality (lower vs higher) | 16.1% vs 1.2% | |
| Hospitalizations in higher levels | 0.89 (0.84-0.95) | |
| Mortality in higher levels | 0.83 (0.75-0.92) | |
| 9. Liu et al. 2012 [45] | Mortality | 2.06 (1.01-4.25) |
| 10. Gotsman et al. 2012 [19] | Mortality | 1.52 (1.21-1.92) |
| Mortality after supplementation | 0.68 (0.54-0.85) |
Conclusions
In conclusion, our current review evaluated the role of vitamin D supplementation in CHF patients and explained various consequences of low serum 25-hydroxycholecalciferol in these patients. No significant difference was noticed with daily supplementation of vitamin D and similar results were observed with high-dose monthly or weekly supplements. We also found that deficiency of vitamin D is associated with increased risk of hospitalizations, mortality, and poor clinical outcomes. However, residual confounding lifestyle factors could also explain this inverse association. Possible long-term benefits from vitamin D supplementation cannot be excluded. To demonstrate effects on mortality and hospitalization more efficiently we require clinical trials with larger sample sizes along with longer follow-up durations. Therefore, further studies are essential in the future to comment on optimal dosing, frequency, and ideal serum target levels to improve outcomes in HF patients.
Acknowledgments
We thank Dr. Hassan Tohid and Dr. Syeda Sidra Hasnain for their guidance and editorial assistance throughout this paper.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.SUN-P295: vitamin D testing and supplementation in clinical practice: a cross-sectional study among Lebanese physicians in a middle eastern urban setting. Aoun A, Karimeh T, Gerges N El, Obeid C, Wehbe A, Hlais S. Clin Nutr. 2017;36:162–163. [Google Scholar]
- 2.Medical progress: vitamin D deficiency. Holick MF. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Liu X, Baylin A, Levy PD. Br J Nutr. 2018;119:928–936. doi: 10.1017/S0007114518000491. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine Committee. Dietary Reference Intakes for Calcium and Vitamin D. Washington: The National Academies Press; [Jul;2020 ]. 2011. Dietary Reference Intakes for Adequacy: Calcium and Vitamin D. [PubMed] [Google Scholar]
- 5.Vitamin D deficiency among patients with pulmonary hypertension. Atamañuk AN, Litewka DF, Baratta SJ, et al. BMC Pulm Med. 2019;19:258. doi: 10.1186/s12890-019-1011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitamin D and calcium: a systematic review of health outcomes (update) Newberry SJ, Chung M, Shekelle PG, et al. https://archive.ahrq.gov/research/findings/evidence-based-reports/er217-abstract.html. Evid Rep Technol Assess (Full Rep) 2014;217:1–929. doi: 10.23970/AHRQEPCERTA217. [DOI] [PubMed] [Google Scholar]
- 7.Vitamin D supplementation and total mortality. A meta-analysis of randomized controlled trials. Autier P, Gandini S. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 8.Can vitamin D reduce total mortality? Giovannucci E. Arch Intern Med. 2007;167:1709–1710. doi: 10.1001/archinte.167.16.1709. [DOI] [PubMed] [Google Scholar]
- 9.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Naghavi M, Wang H, Lozano R, et al. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitamin D intake and risk of CVD and all-cause mortality: evidence from the Caerphilly prospective cohort study. Guo J, Cockcroft JR, Elwood PC, Pickering JE, Lovegrove JA, Givens DI. Public Health Nutr. 2017;20:2744–2753. doi: 10.1017/S1368980017001732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitamin D status and cardiovascular outcome. Saponaro F, Marcocci C, Zucchi R. J Endocrinol Invest. 2019;42:1285–1290. doi: 10.1007/s40618-019-01057-y. [DOI] [PubMed] [Google Scholar]
- 12.Heart disease and stroke statistics—2019 update: a report from the American heart association. Benjamin EJ, Muntner P, Alonso A, et al. Circulation. 2019;139:56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 13.Is vitamin D deficiency related to a higher risk of hospitalization and mortality in veterans with heart failure? Aparicio-Ugarriza R, Salguero D, Mohammed YN, et al. Maturitas. 2020;132:30–34. doi: 10.1016/j.maturitas.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. Manson JAE, Cook NR, Lee IM, et al. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Zittermann A, Ernst JB, Prokop S, et al. Eur Heart J. 2017;38:2279–2286. doi: 10.1093/eurheartj/ehx235. [DOI] [PubMed] [Google Scholar]
- 16.Effects of cholecalciferol supplementation in patients with stable heart failure and low vitamin D levels (ECSPLOIT-D): a double-blind, randomized, placebo-controlled pilot study. Turrini F, Scarlini S, Giovanardi P, et al. Minerva Cardioangiol. 2017;65:553–562. doi: 10.23736/S0026-4725.17.04340-7. [DOI] [PubMed] [Google Scholar]
- 17.The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure. A randomized controlled trial. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo MET. Circ Heart Fail. 2010;3:195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 18.Systematic vitamin D supplementation and monitoring: improving outcomes in heart failure? Gruson D, Pouleur AC, Makris K, Chrysohoou C. Eur J Heart Fail. 2017;19:686–687. doi: 10.1002/ejhf.717. [DOI] [PubMed] [Google Scholar]
- 19.Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Gotsman I, Shauer A, Zwas DR, Hellman Y, Keren A, Lotan C, Admon D. Eur J Heart Fail. 2012;14:357–366. doi: 10.1093/eurjhf/hfr175. [DOI] [PubMed] [Google Scholar]
- 20.1,25-dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. Li YC, Kong J, Wei M, Chen Z-F, Liu SQ, Cao L-P. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Expression of the vitamin D receptor is increased in the hypertrophic heart. Chen S, Glenn DJ, Ni W, et al. Hypertension. 2008;52:1106–1112. doi: 10.1161/HYPERTENSIONAHA.108.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plasma cytokine parameters and mortality in patients with chronic heart failure. Rauchhaus M, Doehner W, Francis DP, et al. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 23.Correlation of vitamin D with inflammatory cytokines, atherosclerotic parameters, and lifestyle factors in the setting of heart failure: a 12-month follow-up study. Roffe-Vazquez DN, Huerta-Delgado AS, Castillo EC, et al. Int J Mol Sci. 2019;20:5811. doi: 10.3390/ijms20225811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An empirical study on the effect of short-term regular vitamin D3 supplement therapy on blood pressure and exercise tolerance in heart failure patients. Hosseinzadeh F, Oskouei NJ, Ghavamzadeh S. Clin Nutr Res. 2020;9:20–31. doi: 10.7762/cnr.2020.9.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the atherosclerosis risk in communities (ARIC) study. Lutsey PL, Alonso A, Michos ED, Loehr LR, Astor BC, Coresh J, Folsom AR. Am J Clin Nutr. 2014;100:756–764. doi: 10.3945/ajcn.114.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study. A randomized clinical trial. Scragg R, Stewart AW, Waayer D, et al. JAMA Cardiol. 2017;2:608–616. doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. Witte KK, Byrom R, Gierula J, et al. J Am Coll Cardiol. 2016;67:2593–2603. doi: 10.1016/j.jacc.2016.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double-blind controlled trial. Dalbeni A, Scaturro G, Degan M, Minuz P, Delva P. Nutr Metab Cardiovasc Dis. 2014;24:861–868. doi: 10.1016/j.numecd.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open-label, blinded end point, randomized prospective trial (VitD-CHF trial) Schroten NF, Ruifrok WPT, Kleijn L, et al. Am Heart J. 2013;166:357–364. doi: 10.1016/j.ahj.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Prognostic value of renin and prorenin in heart failure patients with decreased kidney function. Szymanski MK, Damman K, van Veldhuisen DJ, van Gilst WH, Hillege HL, de Boer RA. Am Heart J. 2011;162:487–493. doi: 10.1016/j.ahj.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 31.A randomized controlled trial of high-dose vitamin D3 in patients with heart failure. Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Piña IL. JACC Heart Fail. 2013;1:84–90. doi: 10.1016/j.jchf.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supplemental vitamin D and calcium in the management of African Americans with heart failure having hypovitaminosis D. Zia AA, Komolafe BO, Moten M, et al. Am J Med Sci. 2011;341:113–118. doi: 10.1097/MAJ.0b013e3182058864. [DOI] [PubMed] [Google Scholar]
- 33.Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 34.Vitamin D deficiency predicts poor clinical outcomes in heart failure patients undergoing cardiac resynchronization therapy. Perge P, Boros AM, Gellér L, et al. Dis Markers. 2019;2019:4145821. doi: 10.1155/2019/4145821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitamin D deficiency and functional response to CRT in heart failure patients. Separham A, Pourafkari L, Kazemi B, et al. Herz. 2019;44:147–154. doi: 10.1007/s00059-017-4630-x. [DOI] [PubMed] [Google Scholar]
- 36.Vitamin D deficiency in patients with diastolic dysfunction or heart failure with preserved ejection fraction. Nolte K, Herrmann-Lingen C, Platschek L, et al. ESC Heart Fail. 2019;6:262–270. doi: 10.1002/ehf2.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vitamin D deficiency is an independent predictor of mortality in patients with chronic heart failure. Cubbon RM, Lowry JE, Drozd M, et al. Eur J Nutr. 2019;58:2535–2543. doi: 10.1007/s00394-018-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Effect of vitamin D level on clinical outcomes in patients undergoing left ventricular assist device implantation. Obeid FA, Yost G, Bhat G, Drever E, Tatooles A. Nutr Clin Pract. 2018;33:825–830. doi: 10.1002/ncp.10078. [DOI] [PubMed] [Google Scholar]
- 39.Serum vitamin D deficiency and risk of hospitalization for heart failure: prospective results from the Moli-sani study. Costanzo S, de Curtis A, di Castelnuovo A, et al. Nutr Metab Cardiovasc Dis. 2018;28:298–307. doi: 10.1016/j.numecd.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Association between vitamin D deficiency and heart failure risk in the elderly. Porto CM, Silva VDL, da Luz JSB, Filho BM, da Silveira VM. ESC Heart Fail. 2018;5:63–74. doi: 10.1002/ehf2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Association between plasma 25-OH vitamin D and testosterone levels in men. Nimptsch K, Platz EA, Willett WC, Giovannucci E. Clin Endocrinol. 2012;77:106–112. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Testosterone and heart failure. Volterrani M, Rosano G, Lellamo F. Endocrine. 2012;42:272–277. doi: 10.1007/s12020-012-9725-9. [DOI] [PubMed] [Google Scholar]
- 43.Vitamin D deficiency in older men. Orwoll E, Nielson CM, Marshall LM, et al. J Clin Endocrinol Metab. 2009;94:1214–1222. doi: 10.1210/jc.2008-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obesity and vitamin D deficiency: a systematic review and meta-analysis. Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obes Rev. 2015;16:341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 45.Vitamin D levels predict hospitalization and mortality in patients with heart failure. Belen E, Sungur A, Sungur MA. Scand Cardiovasc J. 2016;50:17–22. doi: 10.3109/14017431.2015.1098725. [DOI] [PubMed] [Google Scholar]
- 46.Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Liu L, Chen M, Hankins SR, et al. Am J Cardiol. 2012;110:834–839. doi: 10.1016/j.amjcard.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Vitamin D status and outcomes in heart failure patients. Liu LC, Voors AA, van Veldhuisen DJ, et al. Eur J Heart Fail. 2011;13:619–625. doi: 10.1093/eurjhf/hfr032. [DOI] [PubMed] [Google Scholar]