Abstract
Sperm mobility is a major determinant of sperm quality in the domesticated chicken (Gallus domesticus) and is therefore an area of interest for improving fertility. Sperm-associated antigen 6 (SPAG6) is an important flagellar protein implicated to be necessary for flagellar function but negatively associated with rooster fertility. This study was aimed to characterize the expression of SPAG6 and investigate its utility as a protein biomarker of sperm mobility. By western analysis, relative SPAG6 abundances were compared between the testicular, epididymal, and vasal tissues and in sequentially maturing sperm. Immunocytochemistry techniques were used to detect localization of SPAG6 in chicken sperm. Last, western analysis was used to compare relative SPAG6 abundances in sperm of differing mobility. SPAG6 was found in higher abundance in epididymal tissues and in highest abundance in vasal tissues, relative to that of the testis. SPAG6 was also found to sequentially increase in abundance in maturing sperm. SPAG6 localizes between the axonemal central pair of microtubules in the sperm flagella, but it is also found in lower concentration in the acrosomal region. SPAG6 was not a significant predictor of sperm mobility. SPAG6 abundance, alone, is not a strong predictor of sperm mobility. Its impact on rooster fertility is likely unrelated to its impact on sperm mobility.
Key words: sperm-associated antigen 6, sperm mobility, chicken, flagellar protein, protein abundance
Introduction
Successful internal fertilization of an egg is dependent on the ability of sperm to migrate to the egg at its site of fertilization (Froman et al., 1997, 1999; Mortimer, 1997; Birkhead et al., 1999). Sperm mobility, or the directional, progressive movement of a sperm population, is a quantifiable and heritable trait which may be measured to determine the success of this sperm migration in chickens (Gallus domesticus) (Froman and Feltmann, 1998; Froman et al., 2001). Not only is a minimum level of sperm mobility necessary for delivery of sperm to the egg but the hen's oviduct also selects for sperm with adequate mobility (Bakst et al., 1994). Only 1–2% of sperm reach the sperm storage tubules of the uterovaginal junction, and this selection results from barriers exhibited in the distal end of the hen's oviduct, with adequate sperm mobility being necessary for entrance into the sperm storage tubules (Steele, 1992; Bakst et al., 1994; Froman et al., 1999). In the domestic chicken, sperm mobility is considered a primary determinant of overall rooster fertility, and selection of semen donors by measures of sperm mobility leads to an increase in fertilization success (Froman et al., 1997, 1999; Birkhead et al., 1999). Owing to the demonstrated selection in the hen's oviduct for highly mobile sperm, an understanding of flagellar proteins and their influence on this mobility is important for informing any proteomic efforts toward improving rooster fertility.
Sperm-associated antigen 6 (SPAG6) is the vertebrate ortholog of Chlamydomonas PF16, the axonemal central apparatus protein (Sapiro et al., 2000; Teves et al., 2016). PF16 is shown in Chlamydomonas and Plasmodium studies to be essential for flagellar motility and stabilization of correct structure of the central apparatus in the axoneme (Smith and Lefebvre, 1996; Straschil et al., 2010). Knockout of the Spag6 gene in mice resulted in decreased male fertility and sperm motility, and epididymal sperm exhibited abnormal twitching and abnormal flagella (Sapiro et al., 2002). Other, nonsperm tissues where Spag6 is expressed exhibit a decrease in cilia beat in their epithelia in mouse Spag6 knockouts (Teves et al., 2014). SPAG6/PF16 stabilizes the central apparatus by binding to the C1 central microtubule of the axoneme through a series of armadillo repeats, which facilitate protein-protein interactions (Smith and Lefebvre, 1996, 1997; Sapiro et al., 2002). The stability of the central apparatus contributed by SPAG6 is essential for proper development of the sperm flagellum (Sapiro et al., 2002).
Recently, the expression of SPAG6 was significantly increased in a line of pigs with increased reproductive efficiency and was positively related to higher litter size and improved motility after preservation (Xinhong et al. 2018). SPAG6 was also identified to be differentially expressed in fertile and subfertile roosters. Unlike the expression of SPAG6 in porcine, subfertile roosters, defined as roosters with fertilizing efficiency below 40%, were found to have sperm exhibiting a 1.1-fold increase in SPAG6 relative to sperm from fertile roosters, defined as those with a fertilizing efficiency above 70% (Soler et al., 2016). Given the previously described relationship between SPAG6 expression and fertility as well as the fact that chicken SPAG6 shares an 86% homology with human SPAG6, an antigen for which polyclonal antibodies are commercially available, SPAG6 made an ideal candidate for investigation as a biomarker of rooster sperm mobility (Hamada et al., 2010). The objectives of this study were to (1) characterize the expression of SPAG6 in rooster sperm and (2) investigate SPAG6 as a biomarker of rooster sperm mobility.
Materials and methods
Animals
Athens-Canadian random bred (ACRB) roosters were reared in individual cages under photostimulation of 14 h of light per day. ACRB roosters were 55 weeks of age (WOA) and provided free access to a commercial layer diet (Southern States, Richmond, VA) with 2,700 kcal/kg metabolizing energy and 16% CP. Aviagen Yield Plus (AYP) broiler breeder roosters were kept in individual cages and photostimulated at 21 WOA with 15 h light per day. The AYP were fed a standard breeder layer diet (Spradley et al. 2008) with amount allocated based on Avigen Yield Plus Weight and feed standards (Avigen, 2016). AYP roosters were 42 WOA at the time of individual sample collection and 44 WOA at collection of pooled samples. Although both ACRB and AYP roosters were at an advanced age, there are few commonly assessed sperm quality measures, such a mobility, that have been shown to be impacted at this age (Gumułka and Kapkowska, 2005; Shanmugam et al., 2014). All animals were reared with water ad libitum and given a standard broiler breeder ration. Roosters were maintained in accordance with the rules set forth by the University of Georgia's Institutional Animal Care and Use Committee (07-018-Y2-A1).
Collection and Preparation of Tissues and Sequentially Maturing Sperm
Ejaculated semen samples were collected from 30 ACRB roosters by dorsoabdominal massage method (Burrows and Quinn, 1937) and washed twice by dilution 1:2 in phosphate buffered saline (PBS; pH 7.4) and centrifugation at 1,000 × g for 10 min. Samples were reconstituted 1:5 in lysis buffer (LB: 50 mM NaCl, 10 mM Tris base, 1 mM egtazic acid, 1 mM EDTA, and 1% (v/v) Triton X) and sonicated using an Artek Model 150 sonic dismembrator (Thermo Fisher Scientific, Waltham, MA) at medium power for 5 repetitions of 15 s with a 1-min remission period on ice. Sonicated samples were centrifuged at 10,000 × g for 30 min at 4°C, and lysate was collected. In groups of 10, sperm lysates were pooled, resulting in 3 pooled samples of ejaculated-sperm lysates.
Three days later, the same ACRB roosters were euthanized by CO2 gas asphyxiation. Roosters were dissected, and their reproductive tracts were collected and transported in PBS on ice. Vasal sperm were expressed from the vas deferens with tweezers, and vasal tissues were then separated from the epididymis. Epididymal sperm were expressed from the epididymis with tweezers, and then the epididymal tissues were separated from the testes. Testis samples were sliced open longitudinally at the site of epididymal attachment, and testicular sperm were expressed from the testes with manual pressure. Sperm samples were diluted 1:2 in PBS and washed by centrifugation at 1,000 × g for 10 min. Ten immature testicular, epididymal, and vasal sperm samples were pooled in the same fashion as the ejaculated sperm lysates, resulting in 3 pooled samples of each stage of maturation. Pooled sperm samples were diluted 1:5 in LB. Testicular, epididymal, and vasal tissue samples were flushed of any residual sperm with LB, placed in 5 times tissue volume of LB, and homogenized on ice using a 10 mm × 115 mm saw-tooth generator probe (VWR International, Radnor, PA). Sperm and homogenized tissue samples in LB were sonicated and lysates collected as described previously. Tissue lysates were pooled in the same fashion as the sperm samples, resulting in 3 pooled samples of 10 lysates from each tissue type. Protein quantitation was performed on all samples using the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Inc, Hercules, CA) with bovine serum albumin (BSA) as the standard. The measurements were carried out according to the manufacturer's instructions. Lysates were aliquoted and stored at −80°C.
Collection, Characterization, and Preparation of Mature Sperm
Uncontaminated semen, devoid of transparent fluid, was collected from 24 randomly selected AYP roosters using the dorsoabdominal massage method (Burrows and Quinn, 1937). Immediately after collection, fresh semen samples were assessed for progressive mobility by the Accudenz assay, as described by Froman and McLean (1996), with the following modifications. One-milliliter aliquots of Accudenz solution (6% Accudenz [w/v], 0.6 mM KCl, 41.2 mM TES, 88.9 mM NaCl, 20.0 mM glucose, 3.20 mM CaCl2; pH 7.4) were heated to 41°C in polystyrene cuvettes and overlaid with 100-μL semen. Cuvettes containing semen overlays were incubated at 41°C for an additional 10 min, and absorbance read at 550 nm using a DU 530 spectrophotometer (Beckman Coulter, Inc, Brea, CA) for relative measures of sperm progressive mobility. Two hundred fifty microliters of individual semen samples were diluted 1:2 with PBS and washed two times by centrifugation at 1,000 × g for 10 min to discard seminal plasma. After washing, sperm pellets were reconstituted to original volume in mobility buffer (111 mM NaCl, 25.0 mM glucose, 4.00 mM CaCl2, 50.2 mM TES; pH 7.4). Sperm were centrifuged again at 1,000 × g for 10 min, and sperm pellets were resuspended 1:5 in LB.
Semen samples were collected from an additional 24 randomly selected AYP roosters by dorsoabdominal massage, and 4 pooled samples were generated by combining 6 individual samples. Three milliliters of each pooled sample were diluted 1:2 in PBS and subjected to Percoll density gradient centrifugation (PDGC) as described by Ahammad et al. (2018) in triplicate. After PDGC, low-quality sperm from the top of the gradient and high-quality sperm from the bottom of the gradient were collected, washed as described previously in PBS and resuspended 1:5 in LB. Individual sperm samples, low-quality and high-quality sperm samples, were sonicated, and lysates quantitated in the same fashion as described previously for tissue and sequentially maturing sperm lysates.
SDS-PAGE and Western Blot Analysis
Protein samples were thawed and diluted to 1.2 mg/mL in LB. Samples were diluted further 1:1 with 2x sample buffer (4% SDS, 20% glycerol, 120 mM Tris-HCl, 200 mM dithiothreitol, 0.02% bromophenol blue, pH 6.8) and denatured at 95°C for 5 min. Thirty micrograms of protein were loaded into each lane of Mini-PROTEAN TGX Stain-Free 10% precast gels (Bio-Rad) alongside Precision Plus Protein All Blue Prestained Protein Standards (Bio-Rad). Gels were subjected to SDS-PAGE in 1X Tris-glycine-SDS (25 mM Tris, 192 mM glycine, and 0.1% (w/v) SDS, pH 8.3) running buffer under 70 V for 10 min followed by 120 V for 60 min in a Mini-PROTEAN Tetra Cell system (Bio-Rad). After SDS-PAGE, gels were UV-activated using a ChemiDoc MP Imaging System (Bio-Rad). Proteins were then transferred from the activated gels to Immun-Blot polyvinylidene difluoride membranes (Bio-Rad) in Towbin transfer buffer (25 mM Tris base, 192 mM glycine, and 20% (v/v) methanol) using a wet-blot transfer system (Bio-Rad) at 100V for 1 h. The polyvinylidene difluoride membranes were washed momentarily in Tris-buffered saline (0.02 M Tris base and 0.15 M NaCl, pH 7.4) containing 0.1% Tween 20 (TBST) followed by blocking in 5% (w/v) skim milk in TBST for 30 min. The membranes were then imaged using the ChemiDoc MP Imaging System for total protein normalization followed by probing with a polyclonal human anti-SPAG6 antibody (1:200 dilution) produced in rabbit (Sigma-Aldrich, St. Louis, MO) in TBST overnight at 4°C under constant slow rocking. After washing in TBST, the membrane was probed with 1:10,000 horseradish peroxidase (HRP)-conjugated goat antirabbit IgG secondary antibody (Sigma-Aldrich) in TBST at room temperature (RT) for 1 h and then washed 3 times in TBST for 10 min each. Membrane probed with secondary antibody alone served as a negative control, whereas membrane probed with the SPAG6 antibody preincubated with its corresponding blocking peptide, the SPAG6 antigen (Sigma-Aldrich), for 2 h at RT under constant slow rocking followed by probing with secondary antibody to verify the binding affinity for the primary antibody for its antigen. All membranes were finally subjected to visualization by Clarity Western enhanced chemiluminescent blotting substrate (Bio-Rad), and the images were acquired on the ChemiDoc MP Imaging System. Abundances of SPAG6 protein expression were quantitated by normalizing the densities of the protein bands to that of the total loaded protein using Image Lab Software (version 5.2.1; Bio-Rad).
Immunocytochemistry
Immunocytochemistry was used to localize the presence of SPAG6 antigen in the sperm, according to the method described previously (Bi et al., 2012). Briefly, a 20-μl aliquot of sperm suspension containing 1 × 105 sperm cells from ACRB roosters at room temperature was placed on a clean microscope slide; a smear was prepared and allowed to air dry for 40 s. The air-dried slides were fixed with 4% formaldehyde in PBS for 10 min. The sperm cells were subjected to permeabilization with 0.2% Triton-X 100 in PBS for 20 min at room temperature and blocked with 1% skim milk for 30 min. Blocked cells were probed with anti-SPAG6 antibody overnight at 4°C. Probed samples were washed with PBS 5 times for 3 min each and probed with HRP-conjugated goat antirabbit Alexa Fluor 488 secondary antibody (Thermo Fisher Scientific) for 1 h at RT. Samples probed with only HRP-conjugated goat antirabbit Alexa Fluor 488 secondary antibody served as a negative control. After incubation, the sections were washed five times with PBS for 3 min each and overlaid with a coverslip. Images were visualized and captured using an TH4 100 fluorescence microscope (Olympus Corp., Tokyo, Japan).
Immunogold Transmission Electron Microscopy
A post embedding immunogold transmission electron microscopy protocol was followed to confirm the localization of SPAG6 antigen in the sperm flagella. Fixation of sperm was carried out with electron microscopy grade 4% formaldehyde, 1% glutaraldehyde (v/v) (Electron Microscopy Sciences, Hatfield, PA) in 1x Ca2+- and Mg2+-free PBS (pH 7.4) overnight at 4°C. After fixation, the samples were dehydrated in graded ethanol solutions (50% ethanol for 15 min, 75% ethanol 2 times for 30 min each at RT). The samples were then infiltrated with 50% and 75% (v/v) LR White hydrophilic acrylic resin (Polysciences, Inc, Warrington, PA) in 95% ethanol for 1 h and overnight, respectively. The final infiltrations were performed with 100% LR White resin two times, each for 1 h. The specimens were then embedded in gelatin capsules with fresh LR White resin and heated to 60°C for 24 h for polymerization. The samples were sliced into ultrathin 70-nm sections on an RMC MT-X ultramicrotome (Boeckeler Instruments, Inc, Tuscon, AZ) and mounted on Formvar-carbon coated nickel grids (Electron Microscopy Sciences). Blocking of sections was performed with 1% (w/v) bovine serum albumin (Sigma-Aldrich) in PBS (BSA-PBS) for 30 min, and grids were then jet washed with 1% BSA-PBS for 1 min. Then, grids were incubated with rabbit antihuman SPAG6 primary antibody (Sigma-Aldrich) diluted 1:50 in 1% BSA-PBS overnight at 4°C in a moist chamber and then jet washed with 1% BSA-PBS for 1 min. Grids were incubated with 10-nm gold-conjugated goat antiserum against rabbit IgG secondary antibody (BBI Solutions, Crumlin, UK) diluted 1:20 in 1% BSA-PBS for 1 h at RT and then jet washed for 1 min with 1% BSA-PBS followed by 1 min with deionized water. The sections were then poststained with 0.5% uranyl acetate for 5 min followed by staining with lead citrate (Sigma-Aldrich) for 5 min at RT; grids were jet washed with deionized water for 1 min after each staining step. The stained sections were observed and analyzed using a JEOL JEM1011 transmission electron microscope (JEOL, Inc, Peabody, MA). Imaging were captured using a charge-coupled device camera (Advanced Microscopy Techniques, Corp., Woburn, MA).
Statistical Analysis
Statistical analyses were performed with R 3.5.1 software (The R Foundation for Statistical Computing, Vienna, Austria). Differences in relative expression of SPAG6 between tissues of the reproductive tract, stages of sperm maturation, and high- and low-quality sperm were compared by two-way ANOVA, with experimental replicates considered for blocking variables. The relationship between SPAG6 abundance and sperm progressive mobility was tested by the Spearman rank correlation coefficient test. Differences were considered significant at P < 0.05, and significant differences were compared by Tukey's honest significant difference test post hoc.
Results
Sequential Expression of SPAG6
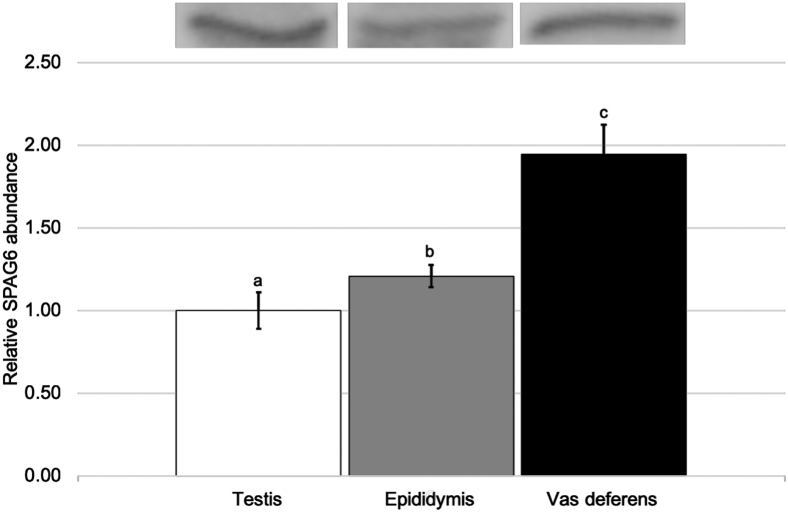
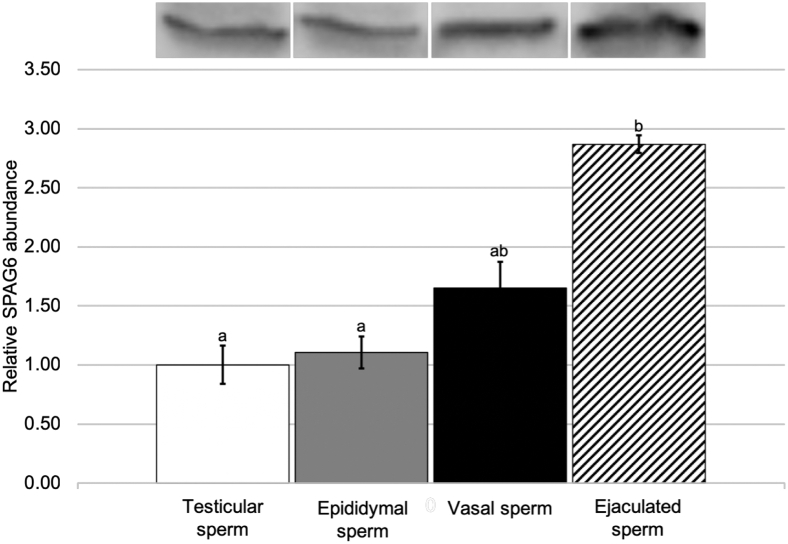
Abundance of SPAG6 was evaluated by Western blotting in tissues of the male reproductive tract and in maturing sperm isolated from each area of the reproductive tract. Western analysis of testicular, epididymal, and vasal tissues reveals a sequential increase in SPAG6 in the tissues (Figure 1). Testicular tissues exhibited the lowest concentration of SPAG6, vasal tissues exhibited the highest, at nearly twofold the concentration of that found in testicular tissues, and epididymal tissues had 21% higher SPAG6 abundance than testicular tissues (P < 0.05). Maturing sperm exhibited a similar pattern, with SPAG6 increasing in abundance as sperm progressed from the testis, epididymis, and vas deferens to mature, ejaculated sperm (Figure 2). Ejaculated sperm had almost threefold the SPAG6 of sperm found in the testis (P < 0.05).
Figure 1.
The relative abundance of SPAG6 found in the testis, epididymis, and vas deferens tissues. Values represent mean ± SEM relative to the mean testis tissue SPAG6 abundance. a–cIndicates significant difference in means, P < 0.05 (n = 3).
Figure 2.
The relative abundance of SPAG6 found in sperm collected from the testis, epididymis, vas deferens, and in ejaculate. Values represent mean ± SEM relative to the mean testicular sperm SPAG6 abundance. a–bIndicates significant difference in means, P < 0.05 (n = 3).
Localization of SPAG6
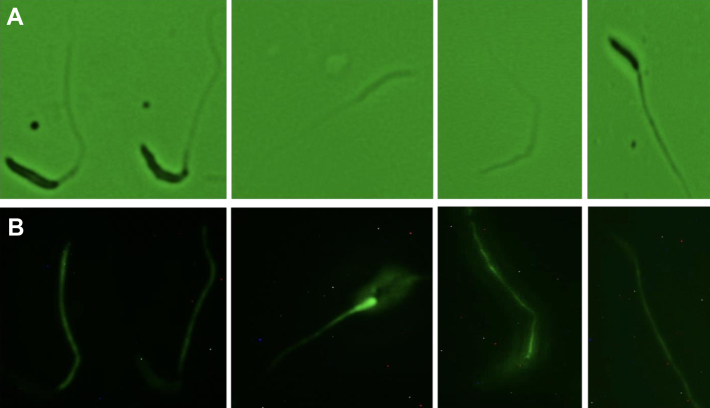
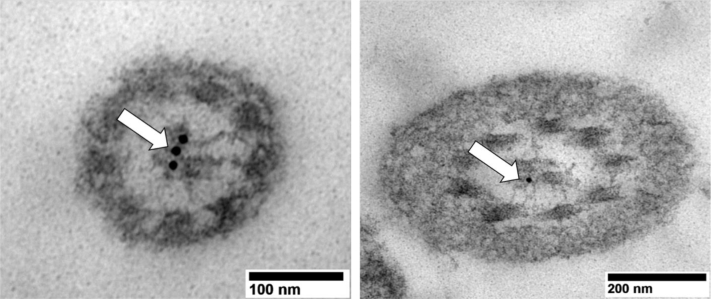
Fluorescence immunocytochemistry and immunogold transmission electron microscopy were used to verify localization of SPAG6 in the sperm flagella of rooster sperm. Fluorescence immunocytochemistry against SPAG6 resulted in strong, consistent fluorescence of the tail regions of spermatozoa, with some strong fluorescence in the midpiece and mild fluorescence in the head region (Figure 3). Transmission electron microscopy displayed distinct gold-nanoparticle labeling in the space between the central pair of microtubules of the flagellar axoneme but no clear pattern of labeling elsewhere on the sperm cell (Figure 4).
Figure 3.
Location of sperm SPAG6 by fluorescence microscopy. (A) Phase contrast microscopic images of sperm treated for fluorescence microscopy. (B) Fluorescence imagery corresponding to each phase contrast image, displaying clear localization of SPAG6 in the flagellum, as well as strong fluorescence in the sperm midpiece and mild fluorescence in the head region.
Figure 4.
Immunogold transmission electron microscopy of the localization of SPAG6 in the rooster sperm axoneme. White arrows emphasize presence of gold nanoparticle, indicating localization of SPAG6 between the central pair of microtubules.
Relationship Between SPAG6 Abundance and Sperm Mobility
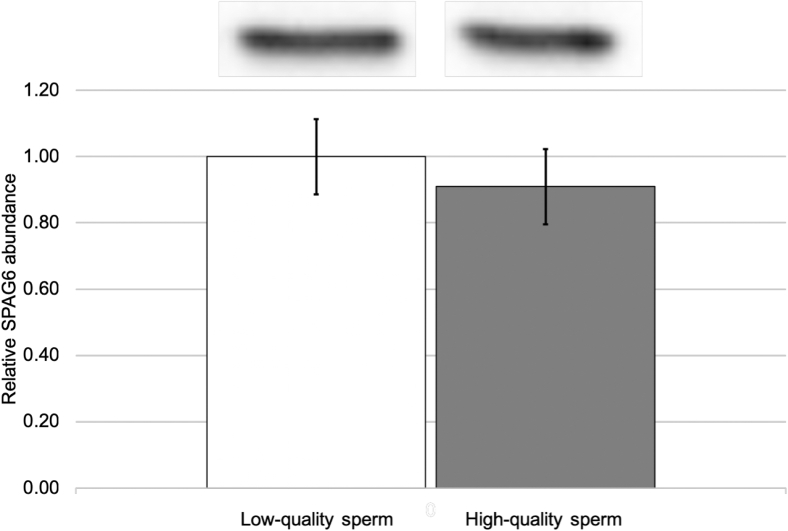
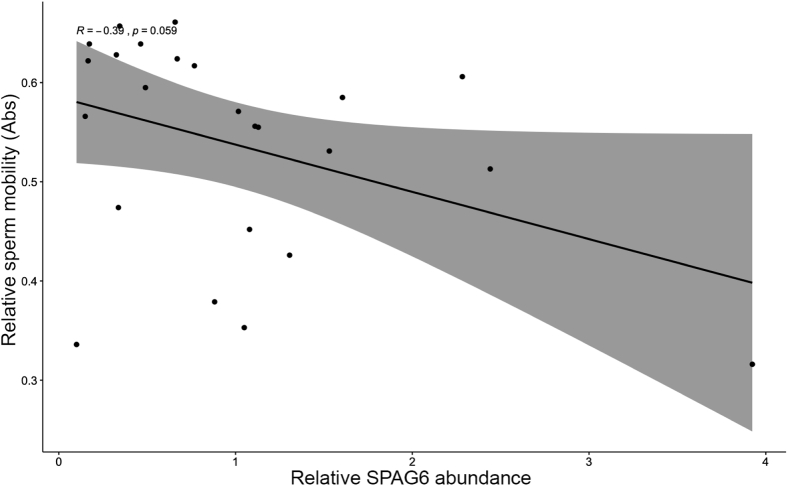
The relative differences in SPAG6 abundance by mobility were tested by Western analysis of low- and high-quality sperm as well as individual sperm samples characterized by their mobility. Sperm separated into low- and high-quality groups by PDGC exhibited no significant differences in SPAG6 abundance (Figure 5). When sperm mobilities of individual sperm samples were compared by their SPAG6 abundance, a correlation approaching significance was found (Figure 6). Sperm mobility of a sample tended to decrease with increasing SPAG6 abundance (P = 0.059).
Figure 5.
The relative abundance of SPAG6 found in low- and high-quality sperm separated by Percoll density gradient centrifugation. Values represent mean ± SEM, P > 0.05 (n = 4).
Figure 6.
The correlation between SPAG6 abundance and sperm mobility. The shaded area represents the confidence interval of the regression line shown in black. P > 0.05, R = −0.39 (n = 24).
Discussion
Sperm quality can be assessed by a variety of sperm characteristics in the rooster. The most commonly assessed parameters in broiler breeder roosters are sperm count, viability, IPVL-binding ability, mobility, and/or motility and metabolism (Jones and Wilson, 1967; Donoghue et al., 1996; Barbato et al., 1998; McDaniel et al., 1998; Froman et al., 1999; Korn et al., 2002; Ahammad et al., 2011). These qualities may be assessed with less resolution by a general measure of sperm fertility, artificial insemination of a hen, followed by assessing eggs for percent fertility (Soler et al., 2016; Wolc et al., 2019). Generation of a broiler breeder line with sustained and improved fertility will require correction of fertility-related traits as part of the breeding goals, without impacting current meat-producing capabilities of broiler lines (Decuypere et al., 2010; Thiruvenkadan et al., 2011). An understanding of the genetic, transcriptomic, proteomic, and metabolomic factors positively and negatively influencing the fertility-related traits of broiler breeders will support targeting of these factors as biomarkers in marker-assisted selection (MAS) breeding programs to improve reproductive fitness of broiler breeders with minimal or no impact on broiler production (Dekkers, 2004; Kovac et al., 2013). As these technologies become more widely available and used, they will allow for rapid improvement of these traits in broiler breeder lines (Hocking, 2014).
A new trend in reproductive biotechnology is the elucidation of infertility etiology through proteomics. This methodology is warranted since mature sperm are transcriptionally and translationally quiescent, so the functionality of sperm is based on early gene expression during spermatogenesis and in proteomic and posttranscriptional modification that occur during maturation. Mass spectrometry (MS) techniques have been used in poultry to search for biomarkers associated with rooster fertility (Labas et al., 2015; Borziak et al., 2016; Soler et al., 2016; Atikuzzaman et al., 2017; Gurjot et al., 2019). These studies have been important for identifying potential fertility biomarkers in rooster that can be targeted for further study. For example, Labas et al., 2015 identified SPINK2 by MS, a serine protease inhibitor associated with higher rooster fertility, and followed this up with characterizing its role in improving fertility and identifying it as a good candidate biomarker to predict fertility in roosters (Thélie et al., 2019).
As MS analysis previously identified SPAG6 to be associated with fertility in both the rooster (Soler et al., 2016) and boar (Xinhong et al. 2018), the objectives of this work were to characterize the expression of SPAG6 in roosters and to investigate SPAG6 as a biomarker of rooster sperm mobility. Our results show that SPAG6 is expressed throughout the male reproductive tract, that it increases in abundance as sperm mature, localizes primarily into the flagellar axoneme, and that SPAG6 abundance is not a strong predictor of sperm mobility.
The sequential expression of SPAG6 observed indicates that SPAG6 continues to accumulate in sperm after development in the testis. This is despite SPAG6 typically being found in the flagellar axoneme, which completes development within the testis (Sapiro et al., 2000; Lehti and Sironen, 2017). The increased abundance of SPAG6 in the epididymis is likely confounded by the fact that the epididymis contains stereocilia, nonmotile modifications to the cell closely related to microvilli (Tingari, 1971). The stereocilia in the epididymis have not been shown to contain SPAG6, but stereocilia found in other tissues have been demonstrated to express the protein (Wang et al., 2015). The possibility of SPAG6 presence in stereocilia of the epididymis does not account for the increased abundance of SPAG6 in the vas deferens or in matured sperm relative to sperm from the testes. Despite being an important protein in the sperm flagellum, SPAG6 has been found in the acrosomal region of mammalian sperm in addition to being in the tail and midpiece, which is consistent with our findings from fluorescence microscopy (Phillips and Verstegen, 2009; Li et al., 2020). While an important protein for flagellar structure, SPAG6 may also be secreted onto the surface of the acrosomal region as one of the many sperm maturation proteins secreted from the reproductive tract during extragonadal maturation (Asano and Tajima, 2017).
SPAG6 is reported to be found in sperm from subfertile roosters at 1.1-fold the abundance of that found in sperm from fertile roosters (Soler et al., 2016). Upon investigating the impact of SPAG6 abundance on sperm mobility, given its function in the sperm flagellum, no significant relationship was observed. Degree of abundance of SPAG6 is likely to impact sperm mobility, as knockout studies have shown that SPAG6 is necessary for adequate sperm motility and that mice possessing only one functional copy of the Spag6 gene had higher sperm motility than those with two functional copies (Sapiro et al., 2002). This considered, SPAG6 abundance alone was not a significant predictor of sperm mobility.
SPAG6, alone, would not function well as a biomarker of sperm mobility; however, it may serve as a biomarker of male fertility when used in conjunction with others. SPAG6 has been demonstrated to be a protein biomarker of subfertility in roosters, and SPAG6 appears to localize in areas other than the flagellar axoneme of sperm (Phillips and Verstegen, 2009; Li et al., 2020). This indicates that the impact of SPAG6 abundance on rooster fertility may not be entirely due to an impact on sperm mobility. For instance, SPINK2, which is secreted by epididymal epithelium during passage through the avian male tract, was shown to be an important actor of fertility in rooster seminal plasma through its inhibitory action on acrosin (Thélie et al., 2019). SPAG6 was shown to be a binding partner with SPINK2, and SPAG6 knockouts have a significantly reduced level of SPINK2 (Li et al., 2020). Recently, Li et al. (2020) found SPINK2 was more prominent in the seminal plasma of roosters with low sperm motility and low fertility, which contradicts the findings of Thélie et al. (2019). Li et al. (2020) suggested their contradictory finding was due to failure of epididymis secreted SPINK2 from binding to the membrane and acrosome of the damaged and dysfunctional spermatozoa. As there is a linked expression pattern and an established binding relationship of SPAG6 and SPINK2, it would be interesting to see if seminal protein expression of SPAG6, which was not tested in the current research, would be associated with improved fertility and provide clarity concerning these contradictory results. If so, this finding would be similar to that of SPINK2, which showed increased expression in seminal plasma of highly fertile roosters, but no relationship was found in SPINK2 protein differences between spermatozoa of differing fertility and mobility (Thélie et al., 2019).
More work is needed to elucidate the purpose of SPAG6 localization outside of the sperm tail region and its relationship with other biomarkers, such as SPINK2. This will help to determine the overall effect of the degree of SPAG6 abundance on rooster fertility and to evaluate the amount of information which may be inferred from the abundance of SPAG6 in a semen sample for MAS breeding programs. In order to find good candidates for MAS breeding programs, further research is needed to characterize the proteins that previous MS studies have identified to be associated with rooster fertility. The current lack of commercially available antibodies that target avian species and the absence of a SILAC-MS chicken model for quantitative proteomics makes this a challenging undertaking.
Acknowledgments
Conflict of interest statement: The authors did not provide a conflict of interest statement.
References
- Ahammad M.U., Nishino C., Tatemoto H., Okura N., Kawamoto Y., Okamoto S., Nakada T. Maturational changes in motility, acrosomal proteolytic activity, and penetrability of the inner perivitelline layer of fowl sperm, during passage through the male genital tract. Theirogenology. 2011;76:1100–1109. doi: 10.1016/j.theriogenology.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Ahammad M.U., Jarrell Z.R., Benson A.P. Sperm collection of differential quality using density gradient centrifugation. J. Vis. Exp. 2018;141:e58833. doi: 10.3791/58833. [DOI] [PubMed] [Google Scholar]
- Asano A., Tajima A. Development and preservation of avian sperm. In: Sasanami T., editor. Avian Reproduction: From Behavior to Molecules. Springer Nature; Singapore, Singapore: 2017. pp. 59–74. [Google Scholar]
- Atikuzzaman M., Sanz L., Pla D., Alvarez-Rodriguez M., Rubér M., Wright D., Calvete J., Rodriguez-Martinez J. Selection for higher fertility reflects in the seminal fluid proteome of modern domestic chicken. CBPD: Genomics and Proteomics. 2017;21:27–40. doi: 10.1016/j.cbd.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Avigen . Avigen Inc.; Huntsville, Alabama: 2016. Yield Plus Weight and Feed Standards. [Google Scholar]
- Bakst M.R., Wishart G., Brillard J.P. Oviducal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 1994;5:117–143. [Google Scholar]
- Barbato G., Cramer P.G., Hammerstedt R.H. A pracical in-vitro sperm-egg binding assay that detects subfertile males. Biol. Reprod. 1998;58:686–699. doi: 10.1095/biolreprod58.3.686. [DOI] [PubMed] [Google Scholar]
- Bi Y., Liu M., Tu W., Wu Y., Guo X., Zhou Z., Sha J. The expression and localization of a novel protein phosphatase inhibitor 2810408A11Rik in mouse testis and sperm. J. Biomed. Res. 2012;26:110–116. doi: 10.1016/S1674-8301(12)60020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead T.R., Martínez J.G., Burke T., Froman D.P. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. Lond. 1999;266:1759–1764. doi: 10.1098/rspb.1999.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borziak K.A., Álvarez-Fernández, Karr T.L., Pizzari T., Dorus S. The seminal fluid proteome of the polyandrous Red junglefowl offers insights into the molecular basis of fertility, reproductive ageing and domestication. Sci. Rep. 2016;6:35854. doi: 10.1038/srep35864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows W.H., Quinn J.P. The collection of spermatozoa from the domestic fowl and Turkey. Poult. Sci. 1937;16:19–24. [Google Scholar]
- Decuypere E., Bruggeman V., Everaert N., Li Y., Boonen R., De Tavernier J., Janssens S., Buys N. The broiler breeder paradox: ethical, genetic and physiological perspectives, and suggestions for solutions. Br. Poult. Sci. 2010;51:569–579. doi: 10.1080/00071668.2010.519121. [DOI] [PubMed] [Google Scholar]
- Dekkers J.C.M. Commercial application of marker- and gene-assisted selection in livestock: strategies and lessons. J. Anim. Sci. 2004;82:313–328. doi: 10.2527/2004.8213_supplE313x. [DOI] [PubMed] [Google Scholar]
- Donoghue A.M., Thistlethwaite D., Donoghue D.J., Kirby J.D. A new rapid determination of sperm concentration in Turkey semen. Poult. Sci. 1996;75:785–789. doi: 10.3382/ps.0750785. [DOI] [PubMed] [Google Scholar]
- Froman D.P., McLean D.J. Objective measurement of sperm motility based upon sperm penetration of Accudenz. Poult. Sci. 1996;75:776–784. doi: 10.3382/ps.0750776. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Feltmann A.J., McLean D.J. Increased fecundity resulting from semen donor selection based upon in vitro sperm motility. Poult. Sci. 1997;76:73–77. doi: 10.1093/ps/76.1.73. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Feltmann A.J. Sperm mobility: a quantitative trait of the domestic fowl (Gallus domesticus) Biol. Reprod. 1998;58:379–384. doi: 10.1095/biolreprod58.2.379. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Feltmann A.J., Rhoads M.L., Kirby J.D. Sperm mobility: a primary determinant of fertility in the domestic fowl. Biol. Reprod. 1999;61:400–405. doi: 10.1095/biolreprod61.2.400. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Pizzari T., Feltmann A.J., Castillo-Juarez H., Birkhead T.R. Sperm mobility: Mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc. R. Soc. Lond. 2001;269:607–612. doi: 10.1098/rspb.2001.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumułka G., Kapkowska E. Age effect of broiler breeders on fertility and sperm penetration of the perivitelline layer of the ovum. Anim. Reprod. Sci. 2005;90:135–148. doi: 10.1016/j.anireprosci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Gurjot K.M., Dubey P.P., Chema R.S., Bansal B.K. Characterization of fertility associated sperm proteins in Aseel and Rhode Island Red chicken breeds. Anim. Reprod. Sci. 2019;203:94–104. doi: 10.1016/j.anireprosci.2019.02.012. [DOI] [PubMed] [Google Scholar]
- Hamada T., Teraoka M., Imaki J., Ui-Tei K., Ladher R.K., Asahara T. Gene expression of Spag6 in chick central nervous system. Anatomia Histologia Embryologia. 2010;39:227–232. doi: 10.1111/j.1439-0264.2010.01000.x. [DOI] [PubMed] [Google Scholar]
- Hocking P.M. Unexpected consequences of genetic selection in broilers and turkeys: Problems and solutions. Br. Poult. Sci. 2014;55:1–12. doi: 10.1080/00071668.2014.877692. [DOI] [PubMed] [Google Scholar]
- Jones J.E., Wilson H.R. Use of an electronic counter for sperm concentration determination in chicken semen. Poult. Sci. 1967;46:532–533. [Google Scholar]
- Korn N., Scott T.R., Pooser B.P., Thurston R.J. Production and characterization of a Turkey sperm mitochondrial monoclonal antibody and its usefulness for assessment of sperm integrity. Poult. Sci. 2002;81:1077–1085. doi: 10.1093/ps/81.7.1077. [DOI] [PubMed] [Google Scholar]
- Kovac J.R., Pastuszak A.W., Lamb D.J. The use of genomic, proteomics and metabolomics in identifying biomarkers of male infertility. Fertil. Steril. 2013;99:998–1007. doi: 10.1016/j.fertnstert.2013.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labas V., Grasseau I., Cahier K., Gargaros A., Harichaux G., Teixeira-Gomes A.P., Alves S., Bourin M., Gérard N., Blesbois E. Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteomics. 2015;112:313–335. doi: 10.1016/j.jprot.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Lehti M.S., Sironen A. Formation and function of sperm tail structures in association with sperm motility defects. Biol. Reprod. 2017;97:522–536. doi: 10.1093/biolre/iox096. [DOI] [PubMed] [Google Scholar]
- Li Y., Sun Y., Ni A., Shi L., Wang P., Isa A.M., Ge P., Jiang L., Fan J., Ma H., Yang G., Chen J. Seminal plasma proteome as an indicator of sperm dysfunction and low sperm motility. Mol. Cell. Proteomics. 2020;19:1035–1046. doi: 10.1074/mcp.RA120.002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel C.D., Hannah J.L., Parker H.M., Smith T.W., Schultz C.D., Zumwalt C.D. Use of a sperm analyzer for evaluating broiler breeder males 1. Effects of altering sperm quality and quantity on the sperm motility index. Poult. Sci. 1998;77:888–893. doi: 10.1093/ps/77.6.888. [DOI] [PubMed] [Google Scholar]
- Mortimer S.T. A critical review of the physiological importance and analysis of sperm movement in mammals. Hum. Reprod. Update. 1997;3:403–439. doi: 10.1093/humupd/3.5.403. [DOI] [PubMed] [Google Scholar]
- Phillips T.C., Verstegen J.P. Identification of sperm associated antigen 6 (SPAG6) on canine sperm. Biol. Reprod. 2009;81:457. [Google Scholar]
- Sapiro R., Tarantino L.M., Velazquez F., Kiriakidou M., Hecht N.B., Bucan M., Strauss J.F. Sperm antigen 6 is the murine homologue of the Chlamydomonas reinhardtii central apparatus protein encoded by the PF16 locus. Biol. Reprod. 2000;62:511–518. doi: 10.1095/biolreprod62.3.511. [DOI] [PubMed] [Google Scholar]
- Sapiro R., Kostetskii I., Olds-Clarke P., Gerton G.L., Radice G.L., Strauss J.F. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol. Cell Biol. 2002;22:6298–6305. doi: 10.1128/MCB.22.17.6298-6305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam A., Vinoth A., Rajaravindra K.S., Rajkumar U. Evaluation of semen quality in roosters of different age during hot climatic condition. Anim. Reprod. Sci. 2014;145:81–85. doi: 10.1016/j.anireprosci.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Smith E.F., Lefebvre P.A. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J. Cell Biol. 1996;132:359–370. doi: 10.1083/jcb.132.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.F., Lefebvre P.A. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil. Cytoskeleton. 1997;38:1–8. doi: 10.1002/(SICI)1097-0169(1997)38:1<1::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Steele M.G. Dundee Institute of Technology; Dundee, UK: 1992. A Study of the Influence of Sperm Surface Proteins on the Activity of Avian Spermatozoa in Vitro and in Vivo. PhD Diss. [Google Scholar]
- Soler L., Labas V., Thélie A., Grasseau I., TeixeiraGomes A., Blesbois E. Intact cell MALDI-TOF MS on sperm: a molecular test for male fertility diagnosis. Mol. Cell Proteomics. 2016;15:1998–2010. doi: 10.1074/mcp.M116.058289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spardley J.M., Freeman M.E., Wilson J.L., Davis A.J. The influence of twice-a-day feeding regimen after photostimulation on the reproductive performance of broiler breeder hens. Poult. Sci. 2008;87:561–568. doi: 10.3382/ps.2007-00327. [DOI] [PubMed] [Google Scholar]
- Straschil U., Talman A.M., Ferguson D.J.P., Bunting K.A., Xu Z., Bailes E., Sinden R.E., Holder A.A., Smith E.F., Coates J.C., Tewari R. The armadillo repeat protein PF16 is essential for flagellar structure and function in Plasmodium male gametes. PLoS One. 2010;5:e12901. doi: 10.1371/journal.pone.0012901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves M.E., Sears P.R., Li W., Zhang Z., Tang W., van Reesema L., Costanzo R.M., Davis C.W., Knowles M.R., Strauss J.F., Zhang Z. Sperm-associated antigen 6 (SPAG6) deficiency and defects in ciliogenesis and cilia function: Polarity, density, and beat. PLoS One. 2014;9:e107271. doi: 10.1371/journal.pone.0107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves M.E., Nagarkatti-Gude D.R., Zhang Z., Strauss J.F. Mammalian axoneme central pair complex proteins: Broader roles revealed by gene knockout phenotypes. Cytoskeleton. 2016;73:3–22. doi: 10.1002/cm.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thélie A., Rehault-Godbert S., Poirier J.C., Govoroun M., Fouchécourt S., Blebois E. The seminal acrosin-inhibitor CITI1/SPINK2 is a fertility-associated marker in the chicken. Mol. Reprod. Dev. 2019;86:762–775. doi: 10.1002/mrd.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruvenkadan A.K., Prabakaran R., Panneerselvam S. Broiler breeding strategies over the decades: an overview. Worlds Poult. Sci. J. 2011;67:309–336. [Google Scholar]
- Tingari M.D. On the structure of the epididymal region and ductus deferens of the domestic fowl (Gallus domesticus) J. Anat. 1971;109:423–435. [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li X., Zhang Z., Wang H., Li J. Expression of prestin in OHCs is reduced in Spag6 gene knockout mice. Neurosci. Lett. 2015;592:42–47. doi: 10.1016/j.neulet.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolc A., Arango J., Settar P., Fulton J.E., O’Sullivan N.P., Dekkers J.C.M. Genetics of male reproductive performance in White Leghorns. Poult. Sci. 2019;98:2729–2733. doi: 10.3382/ps/pez077. [DOI] [PubMed] [Google Scholar]
- Xinhong L., Zhen L., Fu J., Wang L., Yang Q., Li P., Li Y. Quantitative proteomic profiling indicates the difference in reproductive efficiency between Meishan and Duroc boar spermatozoa. Theirogenology. 2018;116:71–82. doi: 10.1016/j.theriogenology.2018.04.025. [DOI] [PubMed] [Google Scholar]