Abstract
Studies demonstrated that chicken ghrelin mRNA was expressed in immune organs of chicken. However, it was not known for its functions in chicken immune system. This study aimed to investigate the effects of ghrelin on infectious bursal disease virus (IBDV)-induced acute inflammatory and bursal injury. Chickens were divided into 4 groups. One group was used as control (“C”). The other three groups incubated with IBDV on the 19th d, of which 2 were injected intraperitoneally with 0.5 nmol (“LG”) or 1.0 nmol (“HG”) ghrelin/100g body weight from 18th to 22nd d, respectively, and one was injected intraperitoneally with PBS (“I”). Results showed that cytokines including interleukin (IL)-6, IL-1β, and IL-8 mRNA expression in I group were upregulated significantly after chickens infected with IBDV from 1 d post-infection (dpi) to 3 dpi (P < 0.05). However, the expression level of IL-6, IL-1β, and IL-8 mRNA in LG and HG groups was 7.3, ∼43.3% as much as that of the I group at 2 dpi and 3 dpi (P < 0.05). Moreover, ghrelin administration attenuated significantly the bursal injury from 1 dpi to 7 dpi and prevents the reduction of bird weight gain at 5 dpi and 7 dpi, which were induced by IBDV (P < 0.05). The results indicated that ghrelin could play an important role in the immune system of chicken.
Key words: ghrelin, inflammatory response, bursal injury, IBDV, chicken
Introduction
Infectious bursal disease virus (IBDV) is an immunosuppressive chicken Avibirnavirus of the family Birnaviridae. It mainly infects and destructs dividing B-lymphocytes and causes pronounced necrosis or apoptosis of B lymphocytes within the follicles of the bursa of Fabricius (BF) during 3–5 d post-infection (dpi) of IBDV (Jungmann et al., 2001). The loss of B lymphocytes leads to a suppressed primary antibody response, which decreases the susceptibility of chickens to other vaccines and resistibility to other disease and further results in the increase of chicken morbidity and mortality. Therefore, IBDV remains a threat to the commercial poultry industry. In addition, viral replication in the bursa is associated with the infiltration of inflammatory cells such as T lymphocytes, macrophages, and heterophils in the BF (Rautenschlein et al., 2002; Khatri et al., 2005; Palmquist et al., 2006; Li et al., 2018). These inflammatory cells produce cytokines such as interleukin 6 (IL-6), interleukin-1β (IL-1β), and interleukin 8 (IL-8), which are important in initiating the local inflammatory response at the site of infection. However, overproduction of proinflammatory cytokines may destruct tissue, retard recovery, and harm the host. Many studies suggest that excessive production of inflammatory cytokines that results from viral infection may lead to “cytokine storm” and then autologous cell and tissue destruction and further death of animals (O'Shea et al., 2002; Xie et al., 2018). Therefore, controlling the overproduction of proinflammatory cytokines and tissue injury from virus infection is important.
Ghrelin is a peptide hormone with many physiological functions. In the last 20 yr, studies have demonstrated that ghrelin plays a vital role in the immune system of humans and animals. For example, ghrelin promotes the age-associated changes in thymic architecture and thymocyte numbers and enhances the proliferation of murine CD4+ primary T cells and a CD4+ T-cell line through activating the ERK1/2 and Akt signaling pathways via upstream activation of phosphatidylinositol-3-kinase and protein kinase C (Dixit et al., 2007; Lee et al., 2014). Reports have also demonstrated that ghrelin inhibits proinflammatory cytokines IL-6, IL-1β, and tumor necrosis factor alpha expression and promote anti-inflammatory cytokines interleukin-10 (IL-10) expression (Dixit et al., 2004; Waseem et al., 2008; Ziko et al., 2018; de Paula Silva et al., 2019). In addition, ghrelin has been considered as a potent anti-inflammatory mediator in mammals (Baatar et al., 2011). In some animal models of diseases such as colitis and sepsis, the administration of ghrelin attenuates the inflammatory and autoimmune response (Gonzalez-Rey et al., 2006; Zeng et al., 2015). Ghrelin effectively suppresses expression of tumor necrosis factor alpha–induced inflammatory factors including intercellular adhesion molecular-1, vascular cell adhesion molecule 1, monocyte chemoattractant protein-1, and IL-1β (Zhang et al., 2019). Moreover, many reports suggest that ghrelin protects organs from damage induced by burn, medicine, and diseases in different animal models (Dembinski et al., 2003; Kiang et al., 2014; Zeng et al., 2015). Recently, Martínez et al. (2016) found that a novel synthetic growth hormone secretagogues A233 (ghrelin-like) could promote the expression of antiviral protein complexes such as mitochondrial antiviral-signaling protein, enhance superoxide anion production and the interferon-γ level in murine macrophage J774A.2 cells, and reduce viral load in a dengue virus mouse model of infection, which suggested that ghrelin also had the antiviral activities.
Chicken ghrelin consists of 26 amino acids and has high homology with mammals (Kaiya et al., 2002), and its mRNA is also expressed in different organs including the bursa and spleen (Ma et al., 2015). However, whether chicken ghrelin has the same function as mammals in immune system is not known. In the previous study, we demonstrated that plasma ghrelin levels increased sharply after chickens were infected with IBDV with robustly increasing cytokines level (Yu et al., 2019). Moreover, this increase positively correlated with the inflammatory cell infiltration in bursa of chicken (Yu et al., 2019). This observation prompted us to speculate that ghrelin may modulate chicken acute inflammatory response induced by IBDV. Thus, in the present study, we performed an investigation to determine the effect of ghrelin administration on the early inflammatory responses and whether ghrelin attenuates the bursal injury of chicken infected with IBDV.
Materials and methods
Experimental Design and Sample Collection
One hundred and eighty specific pathogen-free Hyline brown laying chickens (presented by HULAN BIO COMPANY, Xinxiang, Henan) were raised in negative-pressure isolators (GJ-1; Suzhou Fengshi Laboratory animal equipment Co., Ltd.) at a specific pathogen-free animal laboratory on the hatch day. The fodder was purchased from Merial Vital Laboratory Animal Technology Co., Ltd. (Beijing, China), and sterile water was given ad libitum. At the 18th d of raising (D18), the chickens were weighed, and those weighing approximately 270 g were selected and randomly divided into 4 groups (n = 21 + 21). From day 18 (D18) to D22, one group of chickens (LG group) was injected intraperitoneally with 0.5 nmol of ghrelin/100 g body weight (BW) (Abcam, ab120231) according to previous studies (Furuse et al., 2001; Geelissen et al., 2006). The second group of chickens (HG group) was injected intraperitoneally with 1.0 nmol of ghrelin/100 g BW. At D19, the third group of birds (I group), LG group, and HG group were inoculated with the virulent IBDV strain BC6/85 (purchased from China Institute of Veterinary Drug Control, Beijing, China) at a dose containing 106.23 EID50/0.1 mL each through ocular-nasal. The fourth group (C group) was mock treated with sterile PBS (pH = 7.38). Chickens of each pen in I group, LG group, and HG group were raised in different negative-pressure isolators in one room, and chickens of 2 pens of the C group were raised in different negative-pressure isolators in another room. From 1 dpi to 3 dpi, six chickens were randomly collected from each group (3 chickens per pen), weighed, and then euthanized with cervical dislocation under deep nembutal anesthesia (45 μg/g of BW, intraperitoneal injection; Shanghai Chemical Factory, Shanghai, China). The BF was quickly collected from each chicken. One part of each BF was fixed in 4% paraformaldehyde (Solarbio, P1110, Beijing, China) for hematoxylin and eosin (HE) staining, and the other part of BF was frozen in liquid nitrogen and stored at −80°C for total RNA isolation. The remaining chickens of different groups were weighed at 4, 5, and 7 dpi and then euthanized to collect BF. The BF was fixed in 4% paraformaldehyde for HE staining. All animal experiments in the study were approved by the scientific ethical committee of the Henan Institute of Science and Technology.
Histopathological Examination and Analysis
The deparaffinized tissue sections were stained with HE for histopathological examination. A semi-quantitative histopathological assessment was based on the following scoring system (Table 1) according to a previous study (Shaw and Davision, 2000). Three histological features of the BF were scored from 0 to 3, including bursal follicle, fibro in bursa interstitium, and infiltrated inflammatory cells, and the total score was the last score. All the samples in each group were assessed, and the data were shown as means ± SD.
Table 1.
The scoring system showing the bursal injury after chickens were infection with IBDV.
| Score | Bursal follicle | Fibro in bursa interstitium | Infiltrated inflammatory cells |
|---|---|---|---|
| 0 | No bursal damage in any follicle, clear demarcation of medulla and cortex | Size of bursal interstitium is normal | A few of inflammatory cells in bursa interstitium |
| 1 | <50% of follicles with severe lymphocyte depletion | Size of bursal interstitium become bigger, connective tissue emerge | Many inflammatory cells infiltrated in bursa interstitium |
| 2 | >50% of follicles with severe lymphocyte depletion | Connective tissue take up < 50% area of bursal interstitium | A large number of inflammatory cells infiltrated in bursa interstitium |
| 3 | Follicular outlines only remaining | Connective tissue take up > 50% area of bursal interstitium | A large number of inflammatory cells infiltrated both in bursa interstitium and follicle |
Quantification of IL-6, IL-1β, and IL-8 in BF
For quantitative real-time PCR, total RNA was extracted from BF with TRIzol reagent (Life Technologies, NY), and cDNA was synthesized by using a PrimeScriptRT reagent kits with gDNA eraser (TakaRa, Dalian, China) as previously described (Ma et al., 2015). Three pairs of primers screened were used to detect the expression of IL-6 (forward: AAATCCCTCCTCGCCAATCTG, reverse: CCTCACGGTCTTCTCCATAAACG, HM179640), IL-1β (forward: ATGTCGTGTGTGATGAGCGGC, reverse: AGGCGGTAGAAGATGAAGCGG, NM_204524), IL-8 (forward: GCTGCTCTGTCGCAAGGTAGG, reverse: ACATCTTGAATGGATTTAGGGTGG, HQ008779), respectively, and β-actin (forward: TTGTGATGGACTGGTGATGGTG, reverse: TTCTCTCTCGGCTGTGGTGGTG, NM_205518) was applied as an internal standard. The primer pairs for IL-6, IL-1β, and IL-8 and β-actin were synthesized. The real-time PCR procedure was described briefly as follows: an initial incubation for 30 s at 95°C, 40 cycles of 5 s at 95°C, 20 s at 58°C, and 20 s at 72°C. The quantification was based on the fluorescence detection by real-time PCR with SYBR Premix Ex Taq II (Takara, RR820 A, China). The specificities of the PCR products were assessed by dissociation curve analyses. Each sample was repeated in triplicate. The data were analyzed with the QuantStudio 5 (Applied Biosystems, Thermo Fisher Scientific, Marsiling, Singapore) using the 2−ΔΔCT method. The relative expression of cytokines was that target gene relative to the housekeeping gene.
Statistical Analysis
All the data in the present experiments were analyzed with multiple comparison (LSD based on proc anova) by using SAS software (version 8.01; SAS Institute, Cary, NC) to measure the differences. A P value of less than 0.05 was considered as statistically significant.
Results
Ghrelin Reduces the Expression of Chicken Pro-inflammatory Cytokines Induced by IBDV
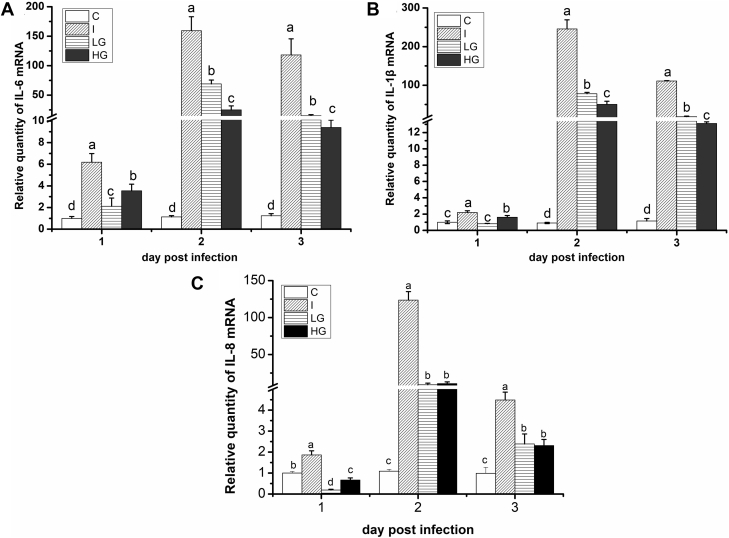
In the present study, cytokines in the BF were first detected to explore whether exogenous ghrelin could modulate chicken pro-inflammatory cytokines induced by IBDV (Figures 1A–C). At 1 dpi, the IL-6, IL-1β, and IL-8 mRNA expression levels increased in the I group, which are 6.19-, 2.19-, and 1.86-fold higher than those of the C group, respectively (P < 0.05). However, in the LG and HG groups, the IL-6, IL-1β, and IL-8 mRNA expression levels were significantly lower than those of the I group (P < 0.05) but significantly higher than those of the C group except for IL-8 mRNA expression, which was significantly lower than that of the C group. At 2 dpi, the IL-6, IL-1β, and IL-8 mRNA expression level in the BF increased abruptly in the I group, which were 140.04-, 268.97-, and 112.51-fold higher than that of the C group. However, the IL-6, IL-1β, and IL-8 mRNA expression level in the LG and HG groups decreased significantly compared with that of the I group. They were 7.30 to 43.34% lower than that of the I group (P < 0.05), although they were all significantly higher than that of the control. At 3 dpi, the IL-6, IL-1β, and IL-8 mRNA expression level in the BF in different treatment groups decreased compared with that of 2 dpi. However, the pattern of IL-6, IL-1β, and IL-8 mRNA expression at 3 dpi in different treatment groups was similar to that of 2 dpi, of which was the IL-6, IL-1β, and IL-8 mRNA expression levels in the I group which were significantly higher than those of the C, LG, and HG groups (P < 0.05) and significantly lower in the C group than those of the LG and HG groups (P < 0.05). Therefore, IBDV induced high expression of pro-inflammatory cytokines in chicken, and ghrelin administration downregulated their high expression evoked by IBDV.
Figure 1.
Effect of ghrelin on proinflammatory cytokines of chickens infected with IBDV. The image shows that ghrelin deduces the proinflammatory cytokine increase induced by IBDV infection. (A) Relative quantity of IL-6 mRNA; (B) relative quantity of IL-1β mRNA; (C) relative quantity of IL-8 mRNA. The same letter represented no significance (P > 0.05), and different letters represented significant difference (P < 0.05).
Ghrelin Attenuates the Bursal Injury of Chicken Induced by IBDV
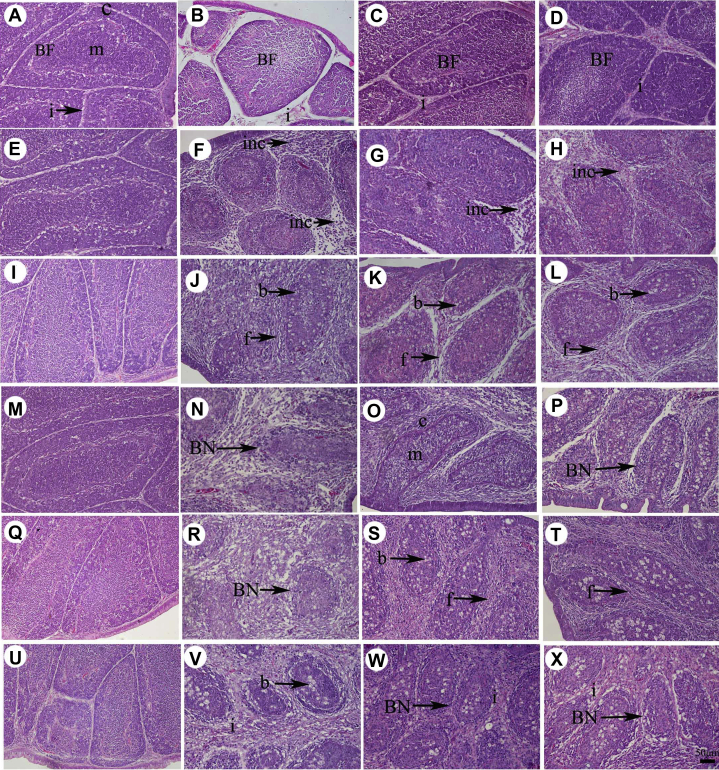
Reports suggested that excessive production of inflammatory cytokines, which resulted from viral infection, might lead to tissue destruction. Hence, we investigated whether ghrelin attenuated the injury of the chicken bursa induced by IBDV. Histological examination of bursal tissues revealed that bursal injury induced by IBDV varied over time and depending on administration of ghrelin (Table 2). The bursal follicle of chicken in the C group was intact, the demarcation of the cortex and medulla was clear, and size of interstitium was normal (Figures 2A, E, I, M, Q, U), and therefore, the scores were 0 during the entire experiment (Table 2). However, when chickens were inoculated with IBDV, the bursal follicle of the chicken bursa became smaller, the demarcation of cortex and medulla disappeared, and interstitial size became bigger in the I group at 1 dpi (Figure 2B), and according to the scoring system, the score was 2.00 ± 0.00. While in the LG and HG groups, only connective tissue emerged in bursal interstitium (Figures 2C, 2D), and the score was 1.00 ± 0.00 (Table 2). At 2 dpi, the number of lymphocytes in bursal follicular depleted, but <50%. Connective tissue emerged in the bursa interstitium. And, a large number of inflammatory cells infiltrated into both bursa interstitium and bursal follicle except in the LG group (Figures 2F–2H). According to the scoring system, the score of LG was lower significantly than that of the I and HG groups (P < 0.05; Table 2). At 3 dpi, still <50% of lymphocytes in bursa follicle depleted; infiltrated inflammatory cells distributed mainly in bursal interstitium in the I, LG, and HG groups. However, connective tissue took up >50% of bursal interstitium in I and HG groups (Figures 2J–2L). Therefore, the pathological scores were 5.67 ± 1.53, 4.00 ± 1.00, and 4.33 ± 0.58 in the I, LG, and HG groups, respectively, and bursa injury was less severe in the LG and HG groups than in the I group (P < 0.05; Table 2). At 4 dpi, >50% of lymphocytes in bursa follicle depleted, a large number of infiltrated inflammatory cells distributed in bursal interstitium and connective tissue took up >50% of bursal interstitium in the I group (Figure 2N), and the pathological score was 7.67 ± 0.58. While in the LG and HG groups, many lymphocytes came back to the bursal follicle and demarcation of cortex and medulla emerged; many infiltrated inflammatory cells were distributed in the bursal interstitium. Connective tissue also took up >50% of bursal interstitium (Figures 2O, 2P). Therefore, the total scores were 3.67 ± 0.58 and 3.33 ± 0.58 in the LG and HG groups, respectively (Table 2). At 5 dpi, in the I group, depletion of lymphocytes in bursa follicle was very severe, and only the follicular outlines remained; connective tissue nearly took up all the bursal interstitium, and a large number infiltrated inflammatory cells were found distributed in the bursal interstitium (Figure 2R) and the total score was 8.00 ± 1.00 (Table 2). However, in LG and HG groups, the injury became severe again compared with the LG and HG groups at 4 dpi. Demarcation of cortex and medulla disappeared, and more but <50% of lymphocytes in bursal follicle depleted. Connective tissue still took up nearly all the bursal interstitium, and many of the infiltrated inflammatory cells were found distributed mainly in the bursa interstitium (Figures 2S, 2T). Therefore, the pathological scores were 5.33 ± 1.15 and 5.00 ± 1.00 in LG and HG groups, respectively (Table 2). At 7 dpi, in the I group, a few of the lymphocytes came back into the bursa follicle and the follicular outlines were more clear, connective tissue continued to take up all the bursal interstitium and a large number infiltrated inflammatory cells were found distributed in bursal interstitium (Figure 2V), and the total score was 6.00 ± 1.00 (Table 2). In the LG and HG groups, <50% of lymphocytes in bursa follicle depleted, connective tissue took up nearly all the bursal interstitium, and the infiltrated inflammatory cells were found distributed mainly in the bursa interstitium (Figures 2W, 2X). Therefore, the pathological scores were 5.00 ± 1.00 and 5.33 ± 1.15 in the LG and HG groups, respectively (Table 2). From these results, ghrelin administration could attenuate the bursa injury of chicken induced by IBDV infection significantly.
Table 2.
Effects of ghrelin on the bursal injury in induced by IBDV.
| Day post infection | Total score of bursal injury, means ± SD |
|||
|---|---|---|---|---|
| C Group | I Group | LG group | HG group | |
| 1 | 0.00 ± 0.00c | 2.00 ± 0.00a | 1.00 ± 0.00b | 1.00 ± 0.00b |
| 2 | 0.00 ± 0.00c | 4.00 ± 1.00a | 2.33 ± 0.57b | 4.00 ± 1.00a |
| 3 | 0.00 ± 0.00c | 5.67 ± 1.53a | 4.00 ± 1.00b | 4.33 ± 0.58b |
| 4 | 0.00 ± 0.00c | 7.67 ± 0.58a | 3.67 ± 0.58b | 3.33 ± 0.58b |
| 5 | 0.00 ± 0.00c | 8.00 ± 1.00a | 5.33 ± 1.15b | 5.00 ± 1.00b |
| 7 | 0.00 ± 0.00c | 6.00 ± 1.00a | 5.00 ± 1.00b | 5.33 ± 1.15a,b |
The same letter represented no significance (P > 0.05), and different letters represented significant difference (P < 0.05) among different groups at the same day.
Figure 2.
Bursal histopathology of chickens in different treatment groups. A, E, I, M, Q, and U represent the bursal follicle in the C group at 1, 2, 3, 4, 5, and 7 dpi; B, F, J, N, R, and V represent bursal follicle in the I group at 1, 2, 3, 4, 5, and 7 dpi; C, G, K, O, S, and W represent the bursal follicle in the LG group at 1, 2, 3, 4, 5, and 7 dpi; D, H, L, P, T, and X represent the bursa nodules in the HG group at 1, 2, 3, 4, 5, and 7 dpi. Abbreviations: b, bubble; BF, bursal follicle; c, cortex; f, fibro; inc, inflammatory cells; m, medulla.
Ghrelin Prevents the Reduction of Bird Weight Gain Induced by IBDV
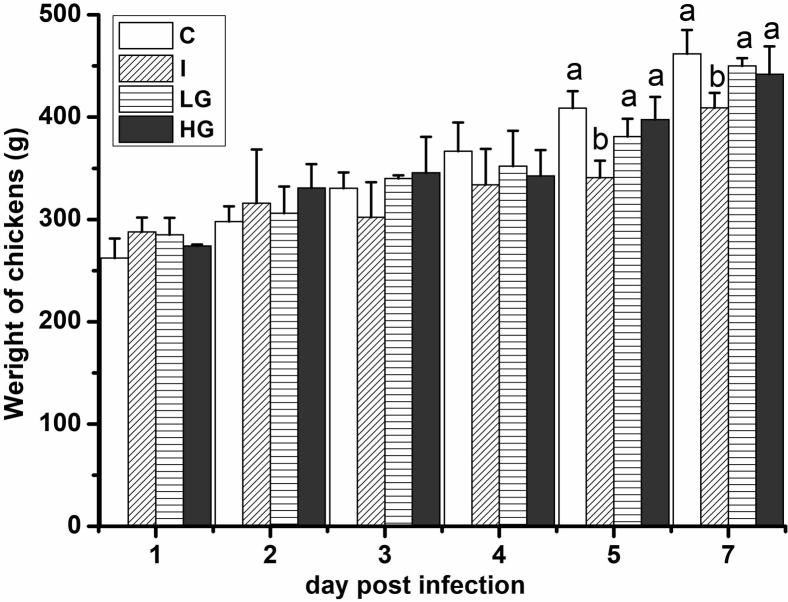
Weight increase is an important index to measure the health of animals. Therefore, the weight increase of chicken was demonstrated in the next study. The results showed that the average weight of chickens in different treatment groups had no significant difference compared with that of the C group from 1 dpi to 4 dpi. However, at 5 and 7 dpi, the weight of chickens in the I group was 19.6 and 12.7% lower than that of the C group, respectively, and reached a significant difference (P < 0.05; Figure 3) resulting from slow weight gain from 1 dpi to 4 dpi. The average weight of chickens in the LG and HG groups had no significant difference from that of the C group at 5 dpi and 7 dpi (P > 0.05), although they were slightly lighter than that of the C group, indicating that ghrelin prevented the reduction of bird weight gain (BWG) of chickens induced by IBDV.
Figure 3.
Effect of ghrelin on weights of chickens in different treatment groups. The image shows that ghrelin prevents reduction of BWG induced by IBDV infection. The same letter represented no significance (P > 0.05), and different letters represented significant difference (P < 0.05).
Discussion
Studies demonstrated that proinflammatory cytokines increased quickly after chickens were infected with IBDV, and as a result, acute inflammation occurred (Liu et al., 2010; Rasoli et al., 2015). In the present study, mRNA expression of cytokines such as IL-6, IL-8, and IL-1β was upregulated by significantly from 1 to 3 dpi in the I group compared with that in the C group. Considerable evidences showed that exogenous ghrelin could modulate the release of inflammatory cytokines in mammals, as reviewed by Baatar et al. (2011). In addition, our previous study has shown that the plasma ghrelin level and ghrelin mRNA expression levels in the BF increased sharply after chickens were infected with IBDV, which was accompanied with high level of cytokine expression and correlated positively with the inflammatory cell infiltration in the BF of chicken (Yu et al., 2019). Therefore, we hypothesized that ghrelin might modulate chicken acute inflammation induced by IBDV. The results showed that mRNA expression of cytokines IL-6, IL-1β, and IL-8 in the BF in the LG and HG groups was significantly lower than that of the I group during the early stage of chicken infection with IBDV. Thus, for the first time, our study has proven ghrelin plays a role in regulating cytokine expression in chicken similar to that of mammals. Further investigation should be performed using different avian disease models and different methods in vivo and in vitro.
In general, the overproduction of proinflammatory cytokines that resulted from viral infection might lead to tissue damage and harm of the host. Therefore, we tested whether downregulating cytokines expression induced by ghrelin would alleviate bursal injury compared with that of the I group. In mammals, a number of studies demonstrated that ghrelin alleviated organ injury in different disease models. For example, ghrelin protected the heart against ischemia/reperfusion injury (Wang et al., 2017), ameliorated acute lung injury after hemorrhagic shock (Zhang et al., 2019), and regulated sepsis-induced rat acute gastric injury (Li et al., 2019). Our results indicated that ghrelin could protect the chicken bursa from damage similar to that of mammals through decreasing the production of cytokines in the bursa evoked by IBDV.
In the present study, chickens infected by IBDV had diarrhea, depression, feather ruffling, and loss of appetite, resulting in the slow increase of weight. However, in the LG and HG groups, these clinical signs of chickens became lighter and weight increased faster than in the I group, which suggested that ghrelin administration prevented the reduction of BWG of chickens induced by IBDV. This seemed paradoxical with previous studies' reports that ghrelin inhibited chickens' feeding (Furuse et al., 2001; Geelissen et al., 2006; Ocłoń and Pietras, 2011; Kaiya et al., 2013). One explanation was that IL-1β and IL-6 could control food intake and energy homeostasis through acting on the central nervous system (Dantzer, 2001). Loss of appetite in IBDV-infected chickens might be mediated via proinflammatory cytokines. Therefore, a reduction of the IL-1β and IL-6 levels in ghrelin-treated chickens might be the cause of a comparatively maintained BW and feeding in acute inflammation.
In conclusion, our study investigated the effect of ghrelin administration on the proinflammatory cytokine increase and bursa injury evoked by IBDV. The results showed that ghrelin attenuated the early inflammatory response and bursal injury of chickens induced by the virus. This study was the first direct evidence that ghrelin could regulate cytokine expression and protect organs from damage in chicken, which suggested that ghrelin played a vital role in the innate immune system of chicken. The primary data would lead us to perform further investigation on other functions of ghrelin in the chicken immune system.
Acknowledgments
The authors are grateful to Jiabin Zhang and Xingchen Guo, undergraduate students of College of Animal Science and Veterinary Medicine, Henan Institute of Science and Technology, for their assistance in the process of experiment. The research was supported by National Natural Science Foundation of China-Henan Joint Fund (no. U1904117), Program for Innovative Research Team (in Science and Technology) in University of Henan Province (no. 18IRTSTHN019), and National Natural Science Foundation of China (no. 31702199).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Baatar D., Patel K., Taub D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell. Endocrinol. 2011;340:44–58. doi: 10.1016/j.mce.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. N. Y. Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dembinski A., Warzecha Z., Ceranowicz P., Tomaszewska R., Stachura J., Konturek S.J., Konturek P.C. Ghrelin attenuates the development of acute pancreatitis in rat. J. Physiol. Pharmacol. 2003;54:561–573. [PubMed] [Google Scholar]
- de Paula Silva F., da Costa C.M.B., Pereira L.M., Lessa D.F.S., Pitol D.L., Issa J.P.M., do Prado Júnior J.C., Abrahão A.A.C. Effects of ghrelin supplementation on the acute phase of Chagas disease in rats. Parasites Vectors. 2019;12:532. doi: 10.1186/s13071-019-3787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V.D., Schaffer E.M., Pyle R.S., Collins G.D., Sakthivel S.K., Palaniappan R., Lillard J.W., Taub D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V.D., Yang H., Sun Y., Weeraratna A.T., Youm Y.H., Smith R.G., Taub D.D. Ghrelin promotes thymopoiesis during aging. J. Clin. Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Tachibana T., Ohgushi A., Ando R., Yoshimatsu T., Denbow D.M. Intracerebroventricular injection of ghrelin and growth hormone releasing factor inhibits food intake in neonatal chicks. Neurosci. Lett. 2001;301:123–126. doi: 10.1016/s0304-3940(01)01621-4. [DOI] [PubMed] [Google Scholar]
- Geelissen S.M.E., Swennen Q., van der Geyten S., Kühn E.R., Kaiya H., Kangawa K., Decuypere E., Buyse J., Darras V.M. Peripheral ghrelin reduces food intake and respiratory quotient in chicken. Domest. Anim. Endocrinol. 2006;30:108–116. doi: 10.1016/j.domaniend.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E., Chorny A., Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Jungmann A., Nieper H., Müller H. Apoptosis is induced by infectious bursal disease virus replication in productively infected cells as well as in antigen-negative cells in their vicinity. J. Gen. Virol. 2001;82:1107–1115. doi: 10.1099/0022-1317-82-5-1107. [DOI] [PubMed] [Google Scholar]
- Kaiya H., Kangawa K., Miyazato M. Update on ghrelin biology in birds. Gen. Comp. Endocrinol. 2013;190:170–175. doi: 10.1016/j.ygcen.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Kaiya H., van der Geyten S., Kojima M., Hosoda H., Kitajima Y., Matsumoto M., Geelissen S., Darras V.M., Kangawa K. Chicken ghrelin: purification, cDNA cloning, and biological activity. Endocrinology. 2002;143:3454–3463. doi: 10.1210/en.2002-220255. [DOI] [PubMed] [Google Scholar]
- Kiang J.G., Zhai M., Liao P.J., Elliott T.B., Gorbunov N.V. Ghrelin therapy improves survival after whole-body ionizing irradiation or combined with burn or wound: amelioration of leukocytopenia, thrombocytopenia, splenomegaly, and bone marrow injury. Oxid. Med. Cell. Longev. 2014;2014:215858. doi: 10.1155/2014/215858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri M., Palmquist J.M., Cha R.M., Sharma J.M. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res. 2005;113:44–50. doi: 10.1016/j.virusres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Patel K., Tae H.J., Lustig A., Kim J.W., Mattson M.P., Taub D.D. Ghrelin augments murine T-cell proliferation by activation of the phosphatidylinositol-3-kinase, extracellular signal-regulated kinase and protein kinase C signaling pathways. FEBS Lett. 2014;588:4708–4719. doi: 10.1016/j.febslet.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Lin Q., Guo H., Liu L., Li Y. Ghrelin regulates sepsis-induced rat acute gastric injury. Mol. Med. Rep. 2019;19:5424–5432. doi: 10.3892/mmr.2019.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Kubasová T., Rychlik I., Hoerr F.J., Rautenschlein S. Infectious bursal disease virus infection leads to changes in the gut associated-lymphoid tissue and the microbiota composition. PLoS One. 2018;13:e0192066. doi: 10.1371/journal.pone.0192066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang M., Han H., Yuan J., Li Z. Comparison of the expression of cytokine genes in the bursal tissues of the chickens following challenge with infectious bursal disease viruses of varying virulence. Virol. J. 2010;7:364. doi: 10.1186/1743-422X-7-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Yu Y., Xue J., Ou C., Mo H., Liu X. Tissue distribution and developmental changes of ghrelin and GOAT expression in broiler chickens during embryogenesis. Gen. Comp. Endocrinol. 2015;213:130–135. doi: 10.1016/j.ygcen.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Martínez R., de Villavicencio-Díaz T.N., Sánchez A., Ramos Y., Ferro J.N., González L.G., Méndez E.M., Rodríguez E., Marcos E., Sánchez B., Masforrol Y., Garay H., Albericio F., Hermida L., González L.J., Vonasek E., Estrada M.P., Besada V. Comparative proteomic analysis of growth hormone secretagogue A233 treatment of murine macrophage cells J774A.2 indicates it has a role in antiviral innate response. Biochem. Biophys. Rep. 2016;5:379–387. doi: 10.1016/j.bbrep.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocłoń E., Pietras M. Peripheral ghrelin inhibits feed intake through hypothalamo-pituitary-adrenal axis-dependent mechanism in chicken. J. Anim. Feed Sci. 2011;20:118–130. [Google Scholar]
- O'Shea J.J., Ma A., Lipsky P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- Palmquist J.M., Khatri M., Cha R.M., Goddeeris B.M., Walcheck B., Sharma J.M. In vivo activation of chicken macrophages by infectious bursal disease virus. Viral Immunol. 2006;19:305–315. doi: 10.1089/vim.2006.19.305. [DOI] [PubMed] [Google Scholar]
- Rasoli M., Yeap S.K., Tan S.W., Roohani K., Kristeen-Teo Y.W., Alitheen N.B., Rahaman Y.A., Aini I., Bejo M.H., Kaiser P., Omar A.R. Differential modulation of immune response and cytokine profiles in the bursae and spleen of chickens infected with very virulent infectious bursal disease virus. BMC Vet. Res. 2015;11:75. doi: 10.1186/s12917-015-0377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenschlein S., Yeh H.Y., Njenga M.K., Sharma J.M. Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Arch. Virol. 2002;147:285–304. doi: 10.1007/s705-002-8320-2. [DOI] [PubMed] [Google Scholar]
- Shaw I., Davison T.F. Protection from IBDV-induced bursal damage by a recombinant fowlpox vaccine, fpIBD1, is dependent on the titre of challenge virus and chicken genotype. Vaccine. 2000;18:3230–3241. doi: 10.1016/s0264-410x(00)00133-x. [DOI] [PubMed] [Google Scholar]
- Waseem T., Duxbury M., Ito H., Ashley S.W., Robinson M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macro-phages through distinct signaling pathways. Surgery. 2008;143:334–342. doi: 10.1016/j.surg.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Lin P., Li P., Feng L., Ren Q., Xie X., Xu J. Ghrelin protects the heart against ischemia/reperfusion injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci. 2017;186:50–58. doi: 10.1016/j.lfs.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Xie J., Wang M., Cheng A., Zhao X., Liu M., Zhu D., Chen S., Jia R., Yang Q., Wu Y., Zhang S., Liu Y., Yu Y., Zhang L., Sun K., Chen X. Cytokine storms are primarily responsible for the rapid death of ducklings infected with duck hepatitis A virus type 1. Sci. Rep. 2018;8:6596. doi: 10.1038/s41598-018-24729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhang Y.H., Xu Z.Y., Liu T.Y., Wang Q.X., Ou C.B., Ma J.Y. Effects of IBDV infection on expression of ghrelin and ghrelin-related genes in chicken. Poult. Sci. 2019;98:119–127. doi: 10.3382/ps/pey328. [DOI] [PubMed] [Google Scholar]
- Zeng M., He W., Li L., Li B., Luo L., Huang X., Guan K., Chen W. Ghrelin attenuates sepsis-associated acute lung injury oxidative stress in rats. Inflammation. 2015;38:683–690. doi: 10.1007/s10753-014-9977-z. [DOI] [PubMed] [Google Scholar]
- Zhang L.N., Gong W.D., Luo J., Yu Y.J., Qi S.H., Yue Z.Y. Exogenous ghrelin ameliorates acute lung injury by modulating the nuclear factor κB inhibitor kinase/nuclear factor κB inhibitor/nuclear factor κB pathway after hemorrhagic shock. Int. Immunopharmacol. 2019;69:95–102. doi: 10.1016/j.intimp.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Ziko I., Sominsky L., De Luca S.N., Lelngei F., Spencer S.J. Acylated ghrelin suppresses the cytokine response to lipopolysaccharide and does so independently of the hypothalamic-pituitary-adrenal axis. Brain Behav. Immun. 2018;74:86–95. doi: 10.1016/j.bbi.2018.07.011. [DOI] [PubMed] [Google Scholar]