Abstract
In recent years, several studies emphasize the deleterious effects of Campylobacter jejuni on the chicken intestine. In this context, it was shown that C. jejuni, contrary to the general belief, has a negative influence on the gut barrier in chickens. More precisely, we demonstrated that C. jejuni affects gut physiology characterized by changes in ion transport and transepithelial ion conductance, but the underlying mechanism is yet to be investigated. In the actual study, to determine epithelial paracellular permeability, the mucosal to serosal flux of 14C-mannitol in the small and large intestine was measured applying Ussing chamber. A total of seventy-five 1-day-old Ross 308 broiler chickens were housed in floor pens on wood shavings with feed and water provided ad libitum. Birds were randomly allocated to 3 different groups (n = 25 with 5 replicates/group) and infected at 14 d of age with a high (108 colony forming units [CFU]) or a low (104 CFU) dose of C. jejuni and a third group kept as noninfected control. Infection with the low dose of C. jejuni resulted in delayed cecal colonization but equalized at 21 d postinfection, independent of the dose. Invasion of liver and spleen with C. jejuni was only noticed in birds infected with 108 (CFU). Body weight (BW) and body weight gain of all birds infected with C. jejuni were lower than in the control group and varied with the dose of infection, confirming a negative correlation between the infection dose and birds BW. Mannitol flux in jejunum and cecum was significantly (P < 0.05) higher in all C. jejuni infected birds compared with control birds. Likewise, significant differences in mannitol flux of both jejunum and cecum were detected depending on the infection dose of C. jejuni. The correlation analyses revealed a positive relationship between Campylobacter dose and mannitol flux of both jejunum and cecum. Altogether, the actual results emphasize that the adverse effect of C. jejuni on gut permeability arises in a dose-dependent manner.
Key words: Campylobacter jejuni, bacterial dose, colonization, permeability, broiler chickens
Introduction
Producing safe and high-quality poultry products and decreasing the incidence of zoonotic diseases are common goals of animal production worldwide (EFSA, 2011). To achieve these goals, poultry health and nutritional aspects are of outstanding importance. A high number of broiler chickens are colonized with Campylobacter, and poultry products represent the main source of Campylobacter jejuni infections in humans (EFSA, 2011; Wassenaar, 2011; Skarp et al., 2016). Infections of humans with Campylobacter are continuously rising, with severe consequences on human health and the economy. Despite the global health impact and economic burden of C. jejuni, data on the interaction with the chicken host are very limited, and it is a general opinion that these bacteria are rather commensals in chickens than pathogens, neglecting most recent findings (Awad et al. 2018). In some of those studies, it was reported that Campylobacter is able to invade the chicken's intestinal mucosa and spread to internal organs (Lamb-Rosteski et al., 2008; Van Deun et al., 2008; Awad et al., 2015a). This might contribute to the translocation of bacteria which seems more widespread than believed, based on a recently published comprehensive epidemiological study in which 12.6% of Campylobacter-positive broilers harbored the bacteria at extraintestinal sites (Weber et al., 2014).
Experimentally, an increased intestinal permeability or “leaky gut” following Campylobacter infection has been demonstrated in different studies with consequences on the translocation of luminal bacteria to the underlying tissues (Lamb-Rosteski et al., 2008; Kalischuk et al., 2009, 2010, Awad et al., 2015a, b, 2017). In this context, studies in mammals and chickens showed that C. jejuni promotes not only the translocation of C. jejuni itself but also the spread of Escherichia coli to internal organs (Kalischuk et al., 2009, 2010; Awad et al., 2016a, b).
Ussing chamber technique allows the quantitative analyses of flux rates or the uptake of defined molecules (e.g., electrolytes or sugars) across the intestinal epithelium, as indicators for intestinal permeability at given sites. In such studies, we previously found that C. jejuni induced an increased intestinal permeability in chickens which might have contributed to the translocation of the bacteria itself, either paracellular or transcellular (Awad et al., 2015a). In humans, it was reported that C. jejuni can cross the epithelial barrier by the paracellular and/or transcellular route (Konkel et al., 1992; Van Deun et al., 2008). However, Boehm et al. (2012) demonstrated that the paracellular route is mainly used to reach basolateral surfaces, exemplarily demonstrated for C. jejuni strains 81–176 and NCTC 11168. Although both pathways can be involved in bacteria translocation, the paracellular pathway appears to be of particular importance for bacteria to disseminate toward inner organs as this way of transfer is unmediated (no transporters) and passive (no energy expenditure) (Schwartz et al., 1995).
To date, nothing is known about the mechanisms C. jejuni applies to breach the gut epithelial barrier and transmigrate across epithelial cells of chickens. The aim of the actual study was to determine whether (i) C. jejuni uses the paracellular route to cross the gut barrier, (ii) this phenomenon is linked with the translocation of C. jejuni to inner organs (liver and spleen), and (iii) a dose-dependence of this effect is existing. For this, birds were experimentally infected with C. jejuni, and the flux of 14C mannitol as an indicator for paracellular permeability was determined by Ussing chamber technique.
Materials and methods
Ethics Statement
The animal trial was approved by the institutional ethics committee of the University of Veterinary Medicine and the Ministry of Research and Science under the license number GZ 68.205/0159-WF/V/3b/2017.
Bacterial Strains, Media, and Growth Conditions
C. jejuni reference strain NCTC 12744 was cultivated at 41.5°C for 48 h under microaerophilic conditions (Genbox microaer, BioMerieux, Vienna, Austria) on Campylobacter Selective Agar (CASA, BioMerieux), which was also used to determine bacterial load in different organs. Modified charcoal–cefaprazone–deoxycholate agar (CM0739, OXOID, Hampshire, UK) was used to determine fecal shedding based on cloacal swabs.
Experimental Design
A total of seventy-five 1-day-old broiler chickens (Ross-308 males and females) were obtained from a commercial hatchery (Gefluegelhof Schulz, Graz, Austria). The birds were housed on wood shavings and were given ad libitum access to water and diet. The diet was based on corn, soybean meal, soybean oil, sunflower oil, and a premix with vitamins, minerals, amino acids, salt, and monocalcium phosphate. At 1 and 14 d of age, cloacal swabs were taken and investigated to confirm the Campylobacter-free status. For this, swabs were streaked onto modified charcoal–cefaprazone–deoxycholate agar (CM0739, OXOID) and incubated for 48 h under microaerophilic condition at 41.5°C.
Infection of Birds
The birds were randomly divided into 3 groups (25 birds/5 replicates/group): 1 control group without infection (inoculated with phosphate-buffered saline, PBS) and 2 groups in which birds were orally inoculated via feeding tube (gavage) at 14 d of age with 1-mL of a PBS suspension containing different doses of Campylobacter reference strain NCTC 12744, either 1 × 104 or 1 × 108 CFU/bird. At least twice per day, all animals were controlled for adverse clinical signs to ensure their health and welfare. At 7, 14, and 21 d postinfection (dpi), cloacal swabs were taken to monitor C. jejuni excretion and shedding, and 5 birds from each group were euthanized by injection of thiopental (20 mg/kg) into the wing vein and by bleeding of the jugular vein. During postmortem examination, samples from liver, spleen, jejunum, and ceca were taken aseptically and processed for Campylobacter enumeration. The trial was terminated at 21 dpi when birds were 35 d of age.
Assessment of Birds' Performance
Body weight (BW) was determined as an individual measurement at weekly intervals, on days 1, 7, 14, 21, 28, and 35 and the body weight gain (BWG) was calculated as the difference between the final and initial bird weight during each of the intervals. Furthermore, feed intake over the course of the experiment was measured for control and infected birds and consequently feed conversion ratio (FCR) was calculated.
Intestinal Load and Extraintestinal Spread of C. jejuni
To determine the level of gut colonization, tissue from jejunum and cecum including content (1 g) were collected from each individual bird necropsied at 7, 14 and 21 dpi. Similarly, liver and spleen tissues (1 g) were collected aseptically from individual birds to determine the presence of C. jejuni in these tissues. Samples were diluted 1:10 (wt:vol) in PBS (BR0014G, OXOID), and the mixture was homogenized using an Ultra-Turrax (IKA, Staufen, Germany). Afterwards, 1:10 serial dilutions (up to 1010) were made from the stock suspension, and each dilution was direct-plated in duplicate on CASA Agar. The plates were incubated microaerobically at 41.5°C for 48 h. After incubation, typical Campylobacter spp. colonies were counted as colony-forming units per gram, and final bacterial counts were expressed as the average from both plates.
Jejunal and Cecal Barrier Function
Following the end of sequential killing at 21 dpi, 10 birds per treatment were kept for up to 5 d at the same housing and feeding conditions for the Ussing chamber in vitro experiments. Following such experiments, the whole trial was terminated. Immediately after exsanguination, segments of jejunum and cecum (4 samples/bird) were harvested and prepared for Ussing chamber studies to measure the paracellular mannitol fluxes. Manipulation and experimental procedures were performed in accordance with Awad et al. (2019). Briefly, the intestinal segments were placed into ice-cooled buffer solution (contained in mmol/L): NaCl, 115; KCl, 5; CaCl2, 1.5; MgCl2, 1.2; NaH2PO4, 0.6; Na2HPO4, 2.4; L-glutamine, 1; Na-D/L-lactate, 5; HEPES-free acid, 10; NaHCO3, 25; and mannitol, 10; pH 7.4 oxygenated with carbogen (95% O2/5% CO2). The intestinal segments were opened along the mesenteric border, and the intestinal content was flushed out with buffer solution at 4°C. The underlying serosal layer was stripped off, and the epithelial sheets were mounted in Ussing chambers. Epithelial sheets had an exposed serosal area of 1.1 cm2 and were incubated with 12 mL of buffer solution on their mucosal and serosal sides under short-circuit conditions. Bathing solutions were oxygenated (95% O2/5% CO2) and circulated in water-jacketed reservoirs maintained at 38°C. Flux rates of mannitol (Jman) were measured at a bilateral concentration of 10 mmol/L. The radioactive tracer, 14C-mannitol (0.1 mCi/mL; Hartmann Analytic), was added to the mucosal solution. After a 30-min equilibration period, standards were taken from the mucosal side of each chamber, and a 30 min flux period was established by taking 0.6-mL from the serosal compartment. Hot samples (100 μL) were collected at the beginning and end of the entire sampling period, whereas cold samples (600 μL) were collected at the start of each flux period. Epithelia were incubated for 3 h divided into 3 flux periods (baseline measurement period) and subsequent exposure to mucosal hyperosmolarity (challenge period, increasing the luminal 14C mannitol concentration from 10 mmol/L to 20 mmol/L) and persistent effects of the hyperosmolarity challenge (persistent period). The presence of 14C mannitol was established by measuring β emission in a liquid scintillation counter after addition of a liquid scintillation fluid (Aquasafe 300 Plus, Zinsser Analytic, Maidenhead, UK). Unidirectional 14C mannitol fluxes from mucosa to serosa (Jms) were evaluated by calculating the net appearance of 14C overtime in the serosal bathing solution as a ratio of flux/concentration as described previously.
Statistical Analysis
Data are presented as means with SEM. Following tests for normality (Kolmogorov-Smirnov test), statistical analysis of performance and mannitol fluxes data were evaluated for significant differences between the 3 groups using one-way ANOVA and Duncan's multiple range test. Differences were considered significant at a level of P ≤ 0.05. The Pearson's correlation was performed to measure the strength of association between the dose of infection and performance data as well as to the mannitol fluxes in jejunum and cecum. Statistical analyses were performed using IBM SPSS (version 24, SPSS Inc., Chicago, IL).
Results
C. jejuni-Associated Changes in Birds' Performance
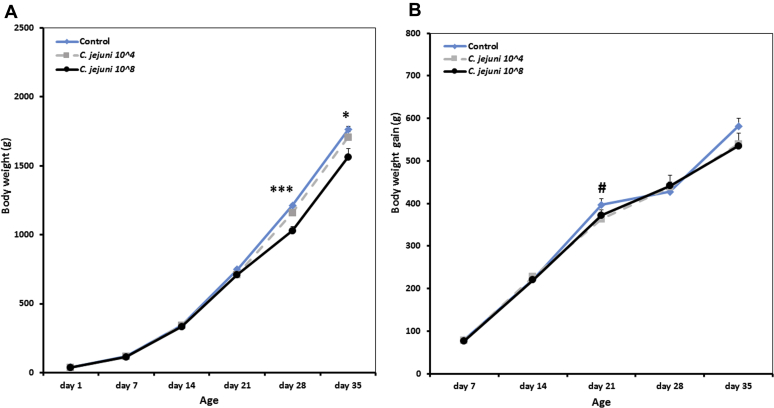
No adverse clinical signs were observed in any of the groups independent whether birds were infected or not with Campylobacter. Data for BW and BWG of the broilers from 1 to 35 d of age are shown in Figure 1. Growth performance of infected birds (either104 or 108 CFU), in terms of the average BW, started to decrease numerically at 7 dpi (709 ± 16, 710 ± 22 g) compared with the controls (747 ± 15). Later on, at 14 and 21 dpi, the BW of birds infected with a higher dose of C. jejuni (1,028 ± 32 and 1,563 ± 63 g) was significantly (P < 0.001) reduced compared with birds infected with the lower dose (1,160 ± 22, 1,706 ± 39) and the noninfected controls (1,212 ± 14, 1,763 ± 109 g). Furthermore, there was a reduction in the overall BWG by 11% in the group of birds infected with the higher dose of Campylobacter compared with the control birds. A negative Pearson's rank correlation coefficient was found between the dose of Campylobacter and both BW and overall BWG (Pearson's rank correlation coefficient: −0.398 and −0.385, respectively) at a significance level of P < 0.05.
Figure 1.
Effect of different doses of C. jejuni on body weight (A) and body weight gain (B) of broiler chickens. Results are presented as mean values and SEM. Asterisks mark differences with P ≤ 0.1 (#), P ≤ 0.05 (∗), or P ≤ 0.001 (∗∗∗).
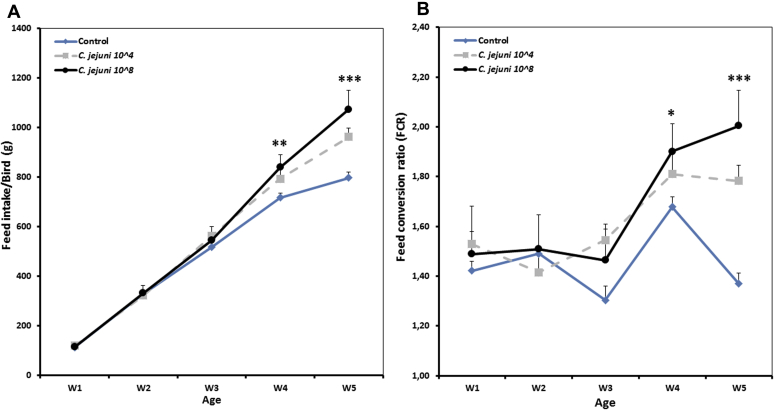
Moreover, at 14 and 21 dpi, the average feed intake was significantly (P < 0.01) increased in C. jejuni infected birds compared with the controls (Figure 2). This effect was most pronounced and significant in birds infected with the higher dose compared with the other groups. In this context, the FCR of infected birds with the higher dose was significantly higher (2.04 ± 0.06) compared with control birds (1.36 ± 0.04). Additionally, a positive correlation between Campylobacter dose and both feed intake and FCR was found (Pearson's rank correlation coefficient: 0.705) at a significance level of P < 0.01.
Figure 2.
Effect of different doses of C. jejuni on feed intake (A) and feed conversion ratio (B) of broiler chickens. Results are presented as mean values and SEM. Asterisks mark differences with P ≤ 0.05 (∗), P ≤ 0.01 (∗∗), or P ≤ 0.001 (∗∗∗).
C. jejuni Colonization of the Gut and Extraintestinal Spread
No Campylobacter were detected in swab samples taken from day-old birds and before infection at 14 d of age. Noninfected birds stayed Campylobacter-negative throughout the experiment. Fecal droppings remained normal in both control and infected birds, with no signs of diarrhea over the course of the animal trial.
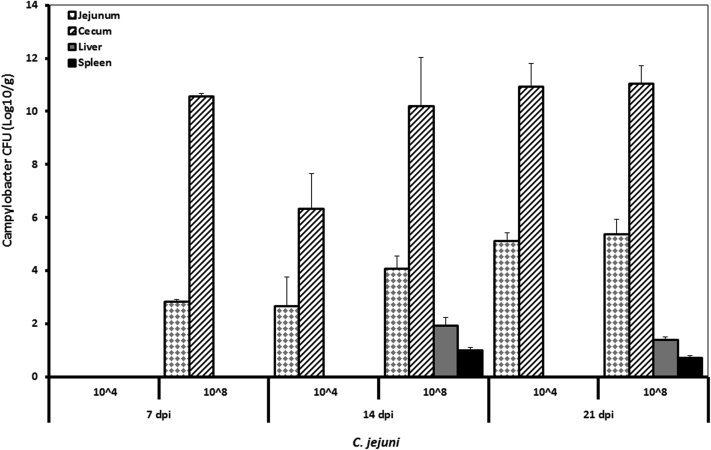
Following oral inoculation of birds with a dose of 108 CFU of C. jejuni, Campylobacter could be reisolated from jejunum and cecum of chickens at 7 dpi. However, no colonization of the gut by C. jejuni was found at 7 dpi of chickens infected with 104 which changed at 14 dpi (Figure 3). Both doses were similarly (P > 0.05) able to colonize the ceca of broilers to a high level at 21 dpi, with up to 0.9 × 1011 (low dose) and 1.16 × 1011 (high dose) CFU per gram, respectively. Furthermore, the bacterial load was higher in the jejunum (2.91 × 105 CFU/g) and cecum (1.16 × 1011 CFU/g) at 21 dpi compared with levels reached at 14 dpi in the jejunum (1.50 × 104 CFU/g) and cecum (1.97 × 1010 CFU/g) of chickens infected with 108, respectively. Organ invasion of birds infected with a high dose of C. jejuni showed a marked difference compared with those birds receiving the low dose as liver and spleen invasion was only found in birds infected with the dose of 108 CFU, with values ranging from 0.5 × 101 to 0.85 × 102.
Figure 3.
C. jejuni counts in the jejunum, cecum, liver, and spleen of infected birds at different times post infection. Results are presented as mean values and SEM (n = 5). Numbers of bacteria are expressed in logarithmic form of colony forming units (log CFU/g).
C. jejuni-Associated Changes in Paracellular Permeability
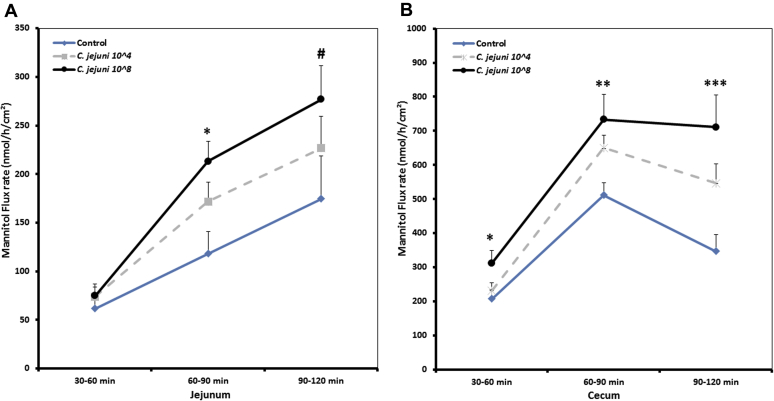
To elucidate whether permeation via the paracellular epithelial pathway is altered by C. jejuni, the fluxes of a known paracellular marker molecule, 14C mannitol, was used. The unidirectional mucosa-to-serosa permeability of 14C mannitol in jejunum and cecum is shown in Figure 4. The results revealed that C. jejuni exposure induces a significant increase in the flux of 14C mannitol in the jejunum in a dose-dependent manner in all flux periods, indicating an increased paracellular leakage. There was a positive Pearson's rank correlation between Campylobacter dose and mannitol flux in the jejunum (Pearson's rank correlation coefficient: 0.407; P < 0.01). During the baseline period, there were significant differences in the flux of the marker molecule in the cecum among the different groups. Furthermore, during the second flux period (from 60 to 90 min), a continuous increase in 14C mannitol flux was found, most probably because of passive diffusion, confirming that the paracellular pathway is much more conductive than the transcellular route. Nevertheless, the impact of the high dose of C. jejuni was much more persistent, either in jejunum or cecum, compared with the lower dose, as there were significant differences throughout the experiment at all flux periods. A positive relationship between Campylobacter dose and mannitol flux could also be observed in the cecum (Pearson's rank correlation coefficient: 0.488; P < 0.01). Moreover, higher (P < 0.001) mannitol fluxes in the cecum in comparison to the jejunum were noticed, independent of the infection status.
Figure 4.
Effect of different doses of C. jejuni on paracellular permeability in jejunum (A) and cecum (B). Mucosal to serosal flux (Jms) of the permeability marker 14C-mannitol were performed in Ussing chambers. Data are presented as the mean values and SEM (n = 10). Asterisks mark differences with P ≤ 0.1 (#), P ≤ 0.05 (∗), P ≤ 0.01 (∗∗), or P ≤ 0.001 (∗∗∗).
Discussion
C. jejuni is the most common reason of bacterial-mediated diarrheal disease in humans worldwide (EFSA, 2014; WHO, 2018). Chickens serve as a major source of human infections, and therefore, infected birds remain a major problem for the poultry industry (Wassenaar, 2011). Meunier et al. (2016) suggested that for reducing the incidence of campylobacteriosis in humans, avian colonization must be combatted, because it has been predicted that decreasing Campylobacter colonization of poultry by 2 to 3 log10 could lead to a substantial decrease in human disease (Romero-Barrios et al., 2013).
Furthermore, despite increased efforts in recent years, there are significant gaps in our knowledge concerning the colonization strategies Campylobacter applies in chickens (Humphrey et al., 2014; Awad et al., 2014, 2018). Although such studies demonstrated a deleterious effect of Campylobacter on the gut barrier. The mechanisms behind are poorly understood especially considering different doses of Campylobacter. The infection doses used in the present study were intended to mimic the greatly varying levels of environmental contamination likely to occur under natural conditions (Kazwala, 1988). The findings revealed that the infective dose had an influence on the colonization of C. jejuni within the gut as the low dose resulted in delayed colonization, although both infected groups reached nearly the same level at 21 dpi. Likewise, Young et al. (1999) determined the dose–response curves for cecal colonization of chicks for 3 different isolates of C jejuni, and they found that cecal colonization occurs when as little as 102 CFU of Campylobacter is orally inoculated into chicks.
Commonly, C. jejuni infection does not cause clinical symptoms in poultry, although behavioral changes were noticed in infected vs. noninfected flocks (Colles et al., 2016). Different studies also report a negative influence on BW following experimental infections, altogether reviewed by Awad et al. (2018). Again, an influence of C. jejuni on the BWG of chickens could be demonstrated in the actual study confirming results of earlier experiments. Nonetheless, the effect was dose-dependent and only noticed in chickens inoculated with a dose of 108 CFU/mL, whereas no significant effect during the 3-wk postinfection period was recorded in birds infected with the lower dose. Pearson's rank correlation analysis between the dose of Campylobacter and BW demonstrated an overall negative relationship.
The negative impact on BW might be directly correlated with a damage of epithelial cells or the induction of an inflammatory response, altogether disturbing the absorptive capacity of chicken's intestine (Awad et al., 2018). In agreement with this, Young et al. (2007) suggested that transmigration across and invasion into intestinal epithelial cells during infection is a major reason of C. jejuni-triggered tissue damage.
In addition to absorbance, translocation of bacteria might also be influenced by disturbance of the gut barrier. Intestinal bacteria can gain access to the lamina propria via the paracellular route, in which bacteria translocate between disrupted epithelial tight junctions (“leaky gut”) (Clayburgh et al., 2004). Organ invasion study with high dose of C. jejuni inocula showed a marked difference between Campylobacter isolates, indicating that different C. jejuni isolates do not only vary with regard to the colonization pattern of the gastrointestinal tract but also in the ability of extraintestinal spread (Young et al., 1999). A correlation between increased intestinal paracellular permeability and bacterial translocation was demonstrated in mice (Ferrier et al., 2003). Furthermore, bacteria have been observed within the paracellular space of polarized enterocyte monolayers (Nazli et al., 2004). Bacteria may also translocate transcellular across the intestinal epithelium, involving endocytic uptake followed by intracellular trafficking. Similarly, Swidsinski et al. (2002) observed the presence of commensal intestinal bacteria within the cytoplasm of enterocytes in human patients with inflammatory bowel disease, although the mechanism remains to be elucidated.
In agreement with a previous study (Awad et al., 2016b), translocation of Campylobacter could again be demonstrated, and the present results indicate that C. jejuni could transmigrate across epithelial cells via the paracellular pathway, as evidenced by increasing fluxes of mannitol in the jejunum and cecum. Furthermore, this effect became more prominent in the cecum, as the cecum is the intestinal segment in which the paracellular permeability in general is quite high which was also confirmed in study. Additionally, in the present study, a positive association was found between Campylobacter dose and mannitol flux in both jejunum and cecum.
In conclusion, the results of the current study support the hypothesis that C. jejuni exacerbates the intestinal paracellular permeability with consequences on the translocation of bacteria across the mucosa and towards internal organs. Furthermore, all parameters investigated in the actual study indicated that the negative effect of C. jejuni on gut permeability is dose-dependent which is of importance for future studies.
Acknowledgments
This work was financed by the Austrian Research Promotion Agency (FFG; 858543), Biomin Holding GmbH (Technopark 1, 3430 Tulln, Austria), and Austrian Science Fund (FWF; P30140). The authors would like to thank D. Jandreski-Cvetkovic for her technical contribution in the laboratory work and V. Stanisavljevic and A. Sandor for their assistance during the animal trial.
Conflict of Interest Statement: The authors did not provide any conflict of interest statement.
References
- Awad W.A., Aschenbach J.R., Ghareeb K., Khayal B., Hess C., Hess M. Campylobacter jejuni influences the expression of nutrient transporter genes in the intestine of chickens. Vet. Microbiol. 2014;172:195–201. doi: 10.1016/j.vetmic.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Dublecz F., Hess C., Dublecz K., Khayal B., Aschenbach J.R., Hess M. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 2016;95:2259–2265. doi: 10.3382/ps/pew151. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Hess C., Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9:60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.,A., Hess C., Hess M. Re-thinking the chicken–Campylobacter jejuni interaction: a review. Avian Pathol. 2018;47:352–363. doi: 10.1080/03079457.2018.1475724. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 2016;6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Molnár A., Aschenbach J.R., Ghareeb K., Khayal B., Hess C., Liebhart D., Dublecz K., Hess M. Campylobacter infection in chickens modulates the intestinal epithelial barrier function. Innate Immun. 2015;21:151–160. doi: 10.1177/1753425914521648. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ruhnau D., Hess C., Doupovec B., Schatzmayr D., Hess M. Feeding of deoxynivalenol increases the intestinal paracellular permeability of broiler chickens. Arch. Toxicol. 2019;93:2057–2064. doi: 10.1007/s00204-019-02460-3. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Smorodchenko A., Hess C., Aschenbach J.R., Molnár A., Dublecz K., Khayal B., Pohl E.E., Hess M. Increased intracellular calcium level and impaired nutrient absorption are important pathogenicity traits in the chicken intestinal epithelium during Campylobacter jejuni colonization. Appl. Microbiol. Biotechnol. 2015;99:6431–6441. doi: 10.1007/s00253-015-6543-z. [DOI] [PubMed] [Google Scholar]
- Boehm M., Hoy B., Rohde M., Tegtmeyer N., Bæk K.T., Oyarzabal O.A., Brøndsted L., Wessler S., Backert S. Rapid paracellular transmigration of Campylobacter jejuni across polarized epithelial cells without affecting TER: role of proteolytic-active HtrA cleaving E-cadherin but not fibronectin. Gut Pathog. 2012;4:3. doi: 10.1186/1757-4749-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayburgh D.R., Shen L., Turner J.R. A porous defense: the leaky epithelial barrier in intestinal disease. Lab. Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- Colles F.M., Cain R.J., Nickson T., Smith A.L., Roberts S.J., Maiden M.C.J., Lunn D., Dawkins M.S. Monitoring chicken flock behaviour provides early warning of infection by human pathogen Campylobacter. Proc. Biol. Sci. 2016;283:20152323. doi: 10.1098/rspb.2015.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011;9:2105. [Google Scholar]
- European Food Safety Authority (EFSA). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2012. EFSA J. 2014;12:312. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier L., Mazelin L., Cenac N., Desreumaux P., Janin A., Emilie D., Colombel J.F., Garcia-Villar R., Fioramonti J., Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., Wigley P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio. 2014;5 doi: 10.1128/mBio.01364-14. e01364–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalischuk L.D., Inglis G.D., Buret A.G. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009;1:2. doi: 10.1186/1757-4749-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalischuk L.D., Leggett F., Inglis G.D. Campylobacter jejuni induces transcytosis of commensal bacteria across the intestinal epithelium through M-like cells. Gut Pathog. 2010;2:14. doi: 10.1186/1757-4749-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazwala R.R. National University of Ireland; Dublin: 1988. Studies on the Origin and Quantitative Distribution of Thermophilic Campylobacters at Various Stages of Poultry Production and Poultry Processing; p. 240. M.V.M. Thesis. [Google Scholar]
- Konkel M.E., Mead D.J., Hayes S.F., Cieplak W., Jr. Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. J. Infect. Dis. 1992;166:308–315. doi: 10.1093/infdis/166.2.308. [DOI] [PubMed] [Google Scholar]
- Lamb-Rosteski J., Kalischuk L., Douglas Inglis G., Buret G. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect. Immun. 2008;76:3390–3398. doi: 10.1128/IAI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M., Guyard-Nicodème M., Hirchaud E., Parra A., Chemaly M., Dory D. Identification of novel vaccine candidates against Campylobacter through reverse vaccinology. J. Immunol. Res. 2016;2016:5715790. doi: 10.1155/2016/5715790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazli A., Yang P.C., Jury J., Howe K., Watson J.L., Soderholm J.D., Sherman P.M., Perdue M.H., McKay D.M. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 2004;164:947–957. doi: 10.1016/S0002-9440(10)63182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Barrios P., Hempen M., Messens W., Stella P., Hugas M. Quantitative microbiological risk assessment(QMRA) of food-borne zoonoses at the European level. Food Control. 2013;29:343–349. [Google Scholar]
- Schwartz R.M., Furne J.K., Levitt M.D. Paracellular intestinal transport of six-carbon sugars is negligible in the rat. Gastroenterology. 1995;109:1206–1213. doi: 10.1016/0016-5085(95)90580-4. [DOI] [PubMed] [Google Scholar]
- Skarp C.P.A., Hänninen M.L., Rautelin H.I.K. Campylobacteriosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Swidsinski A., Ladhoff A., Pernthaler A., Swidsinski S., Loening-Baucke V., Ortner M., Weber J., Hoffmann U., Schreiber S., Dietel M., Lochs H. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- Van Deun K., Pasmans F., Ducatelle R., Flahou B., Vissenberg K., Martel A., Van den Broeck W., Van Immerseel F., Haesebrouck F. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet. Microbiol. 2008;130:285–297. doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Wassenaar T.M. Following an imaginary Campylobacter population from farm to fork and beyond: a bacterial perspective. Lett. Appl. Microbiol. 2011;53:253–263. doi: 10.1111/j.1472-765X.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- Weber R., Auerbach M., Jung A., Glünder G. Campylobacter infections in four poultry species in respect of frequency, onset of infection and seasonality. Berl. Münch. Tierärztl. Wochenschr. 2014;127:257–266. [PubMed] [Google Scholar]
- World Health Organization (WHO). Campylobacter [cited 2018 Jan 24] 2018. https://www.who.int/news-room/fact-sheets/detail/campylobacter Accessed Sep. 2020.
- Young K.T., Davis L.M., Dirita V.J. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- Young C.R., Ziprin R.L., Hume M.E., Stanker L.H. Dose response and organ invasion of day-of-hatch Leghorn chicks by different isolates of Campylobacter jejuni. Avian Dis. 1999;43:763–767. [PubMed] [Google Scholar]