Abstract
Heat stress (HS) is a major problem in poultry business which affects chickens' performance and may trigger large economic losses. This study intends to analyze the impact of HS on broiler chickens' performance compared with those under normal condition. A literature search was performed on PubMed, Web of Science, and Cochrane Library for studies published in English up to January 17, 2020. Outcomes of body weight gain (BWG), feed intake (FI), feed conversion ratio (FCR), and mortality were calculated by weighted difference (WMD) or odds ratio (OR) with 95% confidence interval (CI). A total of 12 studies with 470 broiler chickens were included. HS significantly decreased FI (11 trials: WMD = −97.95, 95% CI: −141.70, −54.20) and BWG (7 trials: WMD = −151.40, 95% CI: −198.59, −104.21) and significantly increased FCR (9 trials: WMD = 0.17, 95% CI: 0.04, 0.29) and mortality (8 trials: OR = 3.74, 95% CI: 1.39, 10.12) compared with the control. In conclusion, HS significantly affected broiler chickens' BWG, FI, FCR, and mortality, indicating the importance to control housing temperature to avoid unnecessary costs.
Key words: heat stress, thermal condition, broiler chicken, feed intake, meta-analysis
Introduction
The poultry industry is important around the world that about 103.5 million tons of annual global chicken meat production accounted for 34.3% of the global meat production in 2012 (Pawar et al., 2016). Besides, chicken meat and eggs are regarded as the most efficient protein sources as well as a healthy alternative to red meat or other protein production systems (Williams et al., 2006). However, owing to the advance of global warming, heat stress (HS) has become a challenge for the poultry industry especially in tropical and subtropical regions (Gregory, 2010). Chicken is most vulnerable to HS for its inability to dissipate body heat production resulting from feather covering and limited sweat glands (Zhang et al., 2017). HS always contributes to a series of physiological disturbances, including systemic immune dysregulation, endocrine disorders, respiratory alkalosis, and electrolyte imbalance, which affect the health and performance of the chickens (Teeter et al., 1985; Sohail et al., 2010; Lara and Rostagno, 2013). Numerous researches have reported the negative influence of HS on poultry production, such as decreased body antioxidant capacity and intestinal immunity as well as impaired intestinal morphology (Sahin et al., 2017; He et al., 2018; Song et al., 2018).
A previous meta-analysis (da Fonseca De Oliveira et al., 2018) has revealed the impact of HS on swine performance in terms of average daily gain, average daily feed intake (FI), and feed gain ratio; another one (Grasteau et al., 2014) demonstrated the effect of HS on laying hens regarding genotype, age, group size, and amplitude of temperature variation. Broiler chicken is an important source of meat production; however, there is no meta-analysis study focused on the performance of broiler chickens exposed to HS. Therefore, the present study was conducted to analyze the impact of HS on broiler chicken by making a systematic review and meta-analysis on the published researches.
Materials and methods
This study was conducted following the PRISMA guidelines for reporting systematic review and meta-analysis.
Search Strategy and Study Selection
Keyword search was performed in PubMed, Web of Science, and Cochrane Library for studies published in English up to January 17, 2020. The following keywords with mapping of term to subject headings and abstracts were used in the database search: HS, thermal condition, hot environment, high temperature, chicken, broiler, poultry, and performance. Titles or abstracts of the studies identified through the keyword search were screened against the study selection criteria. If the title and abstract failed to present adequate information to include or exclude the study for analysis, the study was reviewed in full text. Discrepancies were resolved by discussion meeting each week. Potentially relevant studies were retrieved for evaluation of the full text. Reference lists of retrieved articles were manually examined to further identify potentially relevant publications.
Inclusion criteria are as follows: (1) chicks either exposed to thermal conditions or thermoneutral conditions; (2) experiments were performed under controlled temperature conditions; (3) reported at least one of the endpoints including body weight gain (BWG), FI, feed conversion ratio (FCR), and mortality. Nonoriginal studies such as review, letter, and comment were excluded. For relevant studies that did not provide necessary data for analysis, we contacted the corresponding author of the articles for information. If we did not receive author's response in a reasonable amount of time, the study was excluded.
Data Abstraction
After the initial review, data of potentially relevant articles were extracted by reviewers. A standardized data extraction form was applied to collect information on publication year, first author name, chick strain, number and age of chick for experiment, temperature for the HS group and normal control, period for experiment, and data related to BWG, FI, FCR, and mortality at the end of the experiment.
Statistical Analysis
STATA V12.0 (Stata Corporation, College Station, TX) was used to perform statistical analysis. Size effect of the continuous outcomes was calculated by weighted difference (WMD) with 95% confidence interval (CI), while that of the dichotomous outcomes were calculated by odds ratio (OR). Heterogeneity among studies were examined by Cochran's Q statistic and I2 test. Substantial heterogeneity occurred if P value < 0.05 (Q statistic) and/or I2 > 50, and then the random-effect model was applied, otherwise, the fixed-effect model was used. Sensitivity analysis was performed to confirm the robustness of the results and avoid arbitrary and unclear ones by omitting at least one study at a time. According to the Cochrane Handbook, risk of publication bias was assessed using Begg's test if the included trials up to 10. A P value > 0.05 was considered as no publication bias.
Results
Study Selection Process
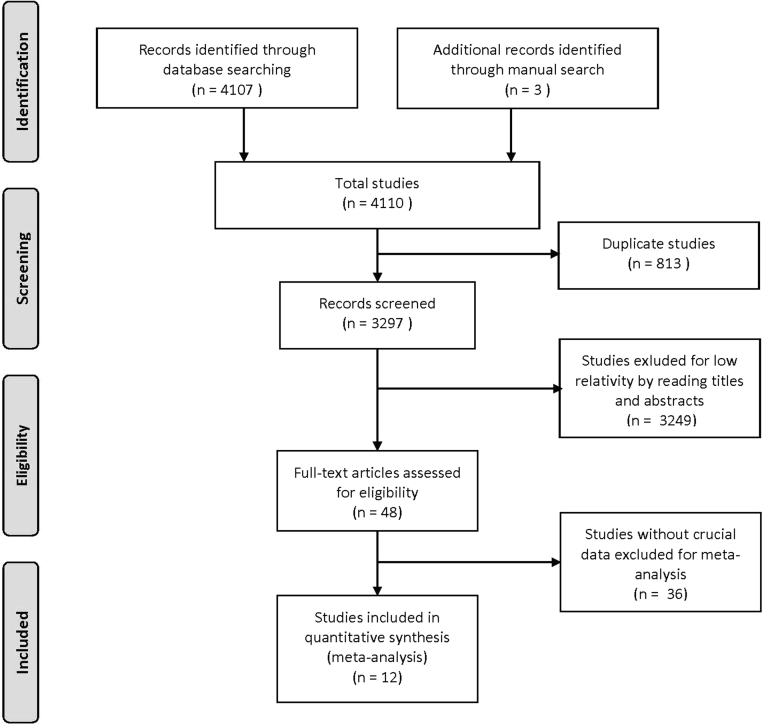
A total of 4,107 studies were retrieved from the electronic databases, and 3 studies were identified through manual search. A total of 813 studies were excluded for duplicate, and the title and abstract of 3,297 studies were screened, among which a total of 3,249 studies were excluded for low relativity, inappropriate article type such as review, comment, and letter, without comparison, and involved other variables. A total of 48 studies were fully reviewed, and 36 were excluded for lack of data of interest. Finally, 12 studies were included in the analysis (Quinteiro-Filho et al., 2010; Sohail et al., 2010; Imik et al., 2012; Quinteiro-Filho et al., 2012a; Alhenaky et al., 2017; Del Vesco et al., 2017; Khatlab et al., 2018; Ma et al., 2018; Olfati et al., 2018; Aswathi et al., 2019; Awad et al., 2020; Goo et al., 2019). Details are presented in Figure 1.
Figure 1.
Study selection process. A total of 4,107 studies were retrieved from the electronic databases (PubMed, Web of Science, and Cochrane Library), and 3 studies were identified through manual search. Finally, a total of 12 studies were included in the study.
Study Characteristics
There were 2 trials in the studies by Quinteiro (Quinteiro-Filho et al., 2010) and Alhenaky (Alhenaky et al., 2017), which were considered as independent studies for analysis. As shown in Table 1, Cobb 500 broiler chickens were used in 4 studies; Hubbard classic, Ross 308, Ross 708, CARIBRO-Vishal, and Arbor Acres were used in 5 studies; the rest 3 studies did not specify the strain of the broiler chicken. Temperature for the thermoneutral control group ranged from 18°C to 25.9°C, except in 2 studies with ladder-type control temperature decreasing from 34°C to 27°C to 18°C (Del Vesco et al., 2017) and from 37°C to 24°C (Sohail et al., 2012). Chronic HS was used in 9 studies, ranging from 28°C to 38°C for 8–22 d, while acute HS was used in 3 studies, ranging from 30°C to 38°C for 4–24 h. As only FI and FCR contained 10 trials, Begg's test was performed on these endpoints, and the results showed that no publication bias was observed (P = 0.17; P = 0.94).
Table 1.
Characteristics of included studies.
| Study | Strain | No. of chick/group | Age for experiment (d) | Thermoneutral conditions | Heat stress conditions |
|---|---|---|---|---|---|
| Alhenaky et al., 2017 | Hubbard classic broiler chicks | 24 | 26 | 20°C ± 2°C | Chronic: 30°C ± 2°C, 24 h/d for 10 d Acute: 35°C ± 2°C for 4 h and then returned back to the thermoneutral conditions |
| Aswathi et al., 2019 | CARIBRO-Vishal broiler breeder hens | 45 | Not provided | Normal temperature | 37°C ± 1°C for 6 h/d up to 10 d |
| Awad et al., 2020 | Cobb 500 and Ross 308 male broiler chicks | 60 | 22 | 23°C | 34°C, 6 h/d for 13 d |
| Del Vesco et al., 2017 | Cobb 500 male broilers | 50 | 20 | 34°C for the first 7 d, decreased gradually to 27°C until day 20, and then decreased gradually to 18°C until day 42 | 38°C for 21 d |
| Goo et al., 2019 | Cobb 500 mixed | 285 | 21 | 20°C for 14 d | 27.8°C for 14 d |
| Imik et al., 2012 | Ross 308 mixed | 45 | 15 | 24°C for 20 d | 34°C, 6 h/D for 20 d |
| Khatlab et al., 2018 | Cobb 500 mixed | 60 | 22 | 19°C for 19 d | 38°C for 19 d |
| Ma et al., 2018 | Arbor Acres male broilers | 48 | 28 | 22°C for 14 d | 32°C for 14 d |
| Olfati et al., 2018 | Broiler chickens | 60 | 22 | 23.9°C ± 2°C for 22 d | 33°C ± 3°C for 22 d |
| Quinteiro-Filho et al., 2010 | Broiler chickens | 80 | 34 | 21°C ± 1°C for 8 d | 31°C ± 1/36°C ± 1°C, 10 h/d for 8 d |
| Quinteiro-Filho et al., 2012b | Male broiler chickens | 60 | 35 | 21°C ± 1°C for the entire day | 31°C ± 1°C for 10 h in the 35th d |
| Sohail et al., 2012 | Ross-708 broilers mixed sex | 90 | 1 | 35°C ± 2°C at day 1 and decreased 3°C per week until reached 26°C ± 2°C | 35°C ± 2°C for 42 d |
Feed Intake
A total of 11 trials from 10 studies (Quinteiro-Filho et al., 2010; Imik et al., 2012; Quinteiro-Filho et al., 2012a; Sohail et al., 2012; Del Vesco et al., 2017; Khatlab et al., 2018; Olfati et al., 2018; Aswathi et al., 2019; Awad et al., 2020; Goo et al., 2019) reported FI between HS and the control group. Substantial heterogeneity was observed, thereby the random-effect model was applied (I2 = 99.4%, P = 0.00). As shown in Figure 2, FI was significantly decreased in the chickens exposed to HS compared with the normal control (WMD = −97.95, 95% CI: −141.70, −54.20).
Figure 2.
The forest plot of feed intake between the broiler chickens exposed to heat stress and thermoneutral condition. Feed intake was significantly decreased in the chickens exposed to heat stress compared with the normal control (WMD = −97.95, 95% CI: −141.70, −54.20).
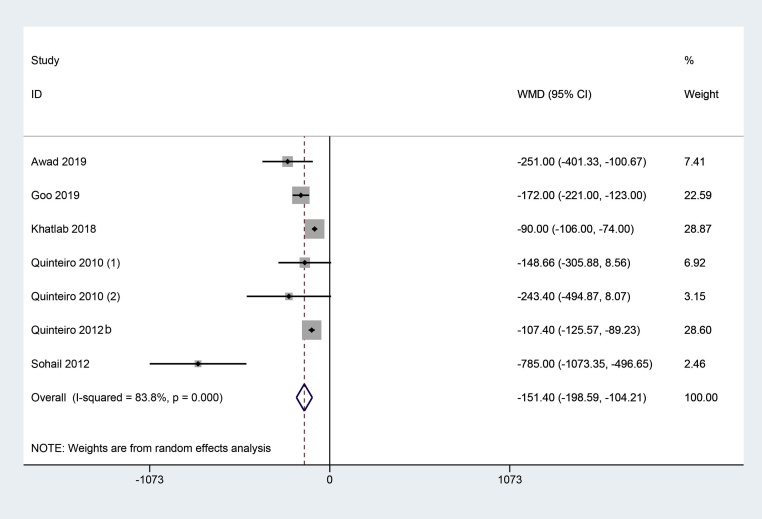
Body Weight Gain
A total of 6 studies (Quinteiro-Filho et al., 2010; Quinteiro-Filho et al., 2012a; Sohail et al., 2012; Khatlab et al., 2018; Awad et al., 2020; Goo et al., 2019) with 7 trials reported BWG between HS and the control group. The random-effect model was used because of substantial heterogeneity (I2 = 83.80%, P = 0.00). Figure 3 showed that HS significantly decreased the chickens' BWG compared with the control (WMD = −151.40, 95% CI: −198.59, −104.21).
Figure 3.
The forest plot of body weight gain between the broiler chickens exposed to heat stress and thermoneutral condition. Heat stress significantly decreased chickens' birth weight gain compared with the control (WMD = −151.40, 95% CI: −198.59, −104.21).
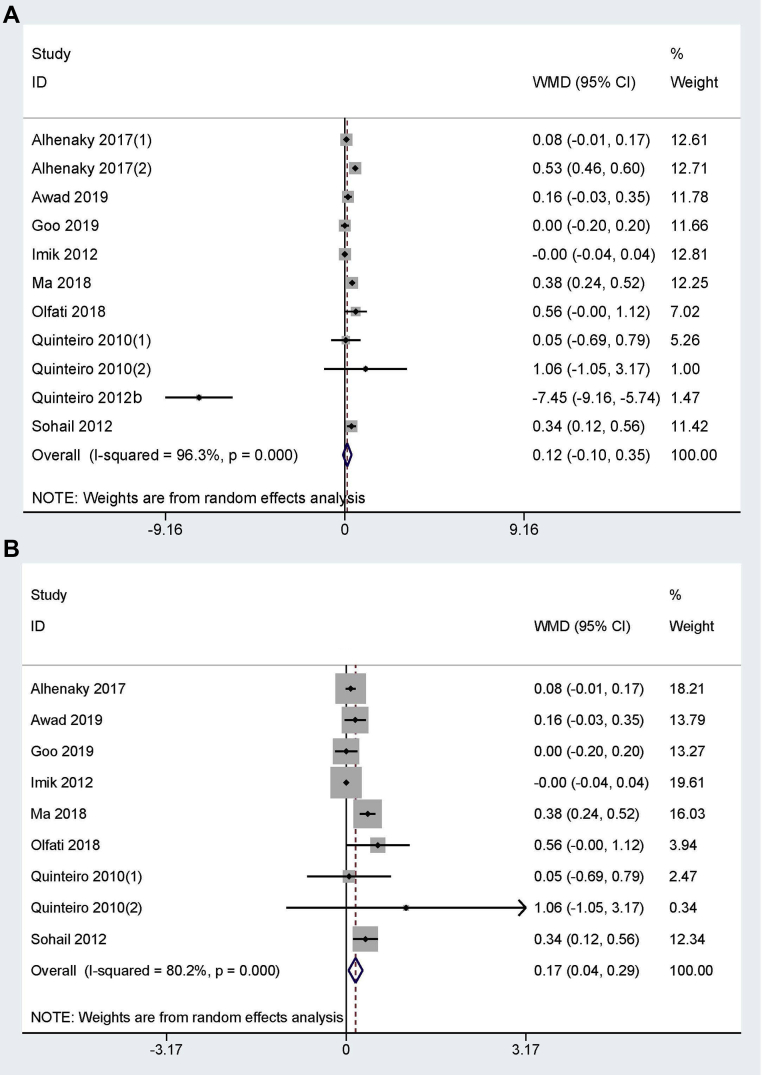
Feed Conversion Ratio
A total of 11 trials from 9 studies (Quinteiro-Filho et al., 2010; Imik et al., 2012; Quinteiro-Filho et al., 2012a; Sohail et al., 2012; Alhenaky et al., 2017; Ma et al., 2018; Olfati et al., 2018; Awad et al., 2020; Goo et al., 2019) reported FCR between HS and the control group. Substantial heterogeneity was observed and the random-effect model was used (I2 = 96.3%, P = 0.00). As presented in Figure 4A, there was no significant difference between HS and the control group (WMD = 0.12, 95% CI: −0.10, 0.35). However, the pooled result would become significantly different with decreased heterogeneity when omitted (Alhenaky et al., 2017 and Quinteiro-Filho et al., 2012a) (WMD = 0.17, 95% CI: 0.04, 0.29; I2 = 80.2%, P = 0.00), indicating that HS significantly increased FCR (Figure 4B).
Figure 4.
The forest plot of feed conversion ratio between the broiler chickens exposed to heat stress and thermoneutral condition. No significantly difference was observed between groups (A), while feed conversion ratio was significantly increased in heat stress group if omitted Alhenaky 2017(2) and Quinteiro 2012 (B).
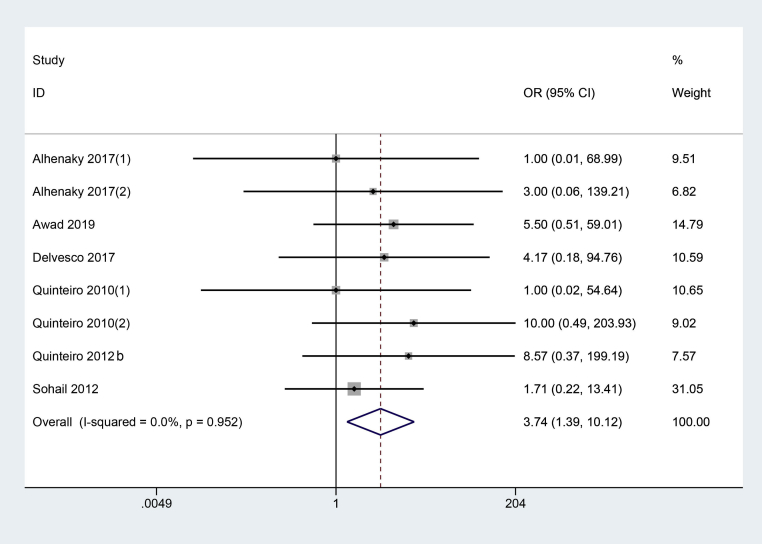
Mortality
A total of 6 studies (Quinteiro-Filho et al., 2010; Quinteiro-Filho et al., 2012a; Sohail et al., 2012; Alhenaky et al., 2017; Del Vesco et al., 2017; Awad et al., 2020) with 8 trials reported chicken mortality between HS and control group. The fixed-effect model was used because of less heterogeneity (I2 = 0%, P = 0.95). As shown in Figure 5, HS significantly increased mortality compared with the control (OR = 3.74, 95% CI: 1.39, 10.12).
Figure 5.
The forest plot of mortality between the broiler chickens exposed to heat stress and thermoneutral condition. Heat stress significantly increased mortality compared with the control.
Discussion
In the current meta-analysis with 12 included studies, we found that HS significantly decreased BWG and FI and significantly increased FCR and mortality in broiler chickens.
It is reported that HS induces the secretion of stress hormones, which alter the chickens' neuroendocrine system by activating the hypothalamic-pituitaty-adrenal axis and thereby increasing the plasma corticosterone levels (Quinteiro-Filho et al., 2012b). Corticosterone is associated with a higher degree of body protein breakdown (Yunianto et al., 1997), which affects the digestive system, nutrient utilization, and digestibility (Olfati et al., 2018). Furthermore, HS has been reported to disturb intestinal barrier function, lead to inflammatory responses, and compromise performance. A study by Alhenaky et al. (2017) showed that HS, whether chronic or acute, impaired intestinal integrity and increased intestinal permeability to endotoxins and Salmonella spp, which may explain the higher mortality in the HS group than the control in the present study.
In addition, modern broiler chickens are genetically selected strains with better growth rate, which is associated with higher FI. Under HS conditions, however, chickens may spend more energy for maintenance and acclimation, which thereby reduce the energy for growth and lead to a decrease in BWG (Mujahid et al., 2007), which is consistent to our findings as well as the previous meta-analysis on layer hens (Grasteau et al., 2014).
FCR did not show significant differences between HS and the control group. However, HS group would presented significantly higher FCR with less heterogeneity than the control if excluded (Quinteiro-Filho et al., 2012a and Alhenaky et al., 2017). After reviewing these 2 studies, we found that the HS strategies used in both studies were acute stress that lasted less than 24 h, while other studies used chronic stress that lasted for 8 to 42 d, which may explain the changes identified from sensitivity analysis and reveal that HS may lead to more costs on animal feed. Nevertheless, exclusion of these 2 studies did not lead to significant changes in other endpoints.
There are some limitations within the present study. First, owing to the small number of included studies, we were unable to perform subgroup analysis based on broiler strain, HS temperature, and age for experiment. Second, significant heterogeneity was common in the endpoints which may lead by the difference of temperature strategy in each study.
In conclusion, this study revealed the negative impacts of high temperature on broiler chickens regarding BWG, FI, FCR, and mortality, indicating the importance and emergency for the poultry industry to seek a way to mitigate the temperature influence and prevent economic losses.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (no.: 31602025, 31540058); the Natural Science Foundation of Hunan Province (no.: 2016jj3059); and the Scientific Research Foundation of Hunan Provincial Education Department (no.: 17C0652).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Alhenaky A., Abdelqader A., Abuajamieh M., Al Fataftah A.R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017;70:9–14. doi: 10.1016/j.jtherbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Aswathi P.B., Bhanja S.K., Kumar P., Shyamkumar T.S., Mehra M., Bhaisare D., Rath P. Effect of acute heat stress on the physiological and reproductive parameters of broiler breeder hens – a study under controlled thermal stress. Indian J. Anim. Res. 2019;53:1150–1155. [Google Scholar]
- Awad E.A., Najaa M., Zulaikha Z.A., Zulkifli I., Soleimani A.F. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Asian-Australas J. Anim. Sci. 2020;33:778–787. doi: 10.5713/ajas.19.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca De Oliveira A., Vanelli K., Sotomaior C., Weber S., Costa L. Impacts on performance of growing-finishing pigs under heat stress conditions: a meta-analysis. Vet. Res. Commun. 2018;43:37–43. doi: 10.1007/s11259-018-9741-1. [DOI] [PubMed] [Google Scholar]
- Del Vesco A.P., Khatlab A.S., Goes E.S.R., Utsunomiya K.S., Vieira J.S., Oliveira Neto A.R., Gasparino E. Age-related oxidative stress and antioxidant capacity in heat-stressed broilers. Animal. 2017;11:1783–1790. doi: 10.1017/S1751731117000386. [DOI] [PubMed] [Google Scholar]
- Goo D., Kim J.H., Park G., Reyes J., Kil D. Effect of heat stress and Stocking Density on growth performance, Breast meat quality, and intestinal barrier function in broiler chickens. Animals (Basel) 2019;9:107. doi: 10.3390/ani9030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasteau S., Moreri U., Narcy A., Rousseau X., Rodenburg B., Tixier-Boichard M., Zerjal T. Robustness to chronic heat stress in laying hens: a meta-analysis. Poult. Sci. 2014;94:586–600. doi: 10.3382/ps/pev028. [DOI] [PubMed] [Google Scholar]
- Gregory N.G. How climatic changes could affect meat quality. Food Res. Int. 2010;43:1866–1873. [Google Scholar]
- He X., Lu Z., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018;98:4471–4478. doi: 10.1002/jsfa.8971. [DOI] [PubMed] [Google Scholar]
- Imik H., Atasever M.A., Urcar S., Ozlu H., Gumus R., Atasever M. Meat quality of heat stress exposed broilers and effect of protein and vitamin E. Br. Poult. Sci. 2012;53:689–698. doi: 10.1080/00071668.2012.736609. [DOI] [PubMed] [Google Scholar]
- Khatlab A., Vesco A., Goes E., Neto A., Menck-Soares M., Gasparino E. Short communication: Gender and heat stress effects on hypothalamic gene expression and feed intake in broilers. Span J. Agric. Res. 2018;16:e04SC02. [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals (Basel) 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., He X., Lu Z., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress affects muscle hypertrophy, muscle protein synthesis and uptake of amino acid in broilers via insulin like growth factor-mammalian target of rapamycin signal pathway. Poult. Sci. 2018;97:4150–4158. doi: 10.3382/ps/pey291. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Akiba Y., Toyomizu M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult. Sci. 2007;86:364–371. doi: 10.1093/ps/86.2.364. [DOI] [PubMed] [Google Scholar]
- Olfati A., Mojtahedin A., Sadeghi T., Akbari M., Martínez-Pastor F. Comparison of growth performance and immune responses of broiler chicks Reared under heat stress, Cold stress and thermoneutral conditions. Span J. Agric.Res. 2018;16 [Google Scholar]
- Pawar S.S., Sajjanar B., Lonkar V.D., Nitin K.P., Kadam A.S., Nirmale A.V., Brahmane M.P., Bal S.K. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016;4:332–341. [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sá L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Rodrigues M.V., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sa L.R., Ferreira A.J., Palermo-Neto J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012;90:1986–1994. doi: 10.2527/jas.2011-3949. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Gomes A.V.S., Pinheiro M.L., Ribeiro A., Ferraz-de-Paula V., Astolfi-Ferreira C.S., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012;41:421–427. doi: 10.1080/03079457.2012.709315. [DOI] [PubMed] [Google Scholar]
- Sahin N., Hayirli A., Orhan C., Tuzcu M., Akdemir F., Komorowski J.R., Sahin K. Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult. Sci. 2017;96:4317–4324. doi: 10.3382/ps/pex249. [DOI] [PubMed] [Google Scholar]
- Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Ijaz A., Sohail A., Shabbir M.Z., Rehman H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Science. 2012;91:2235–2240. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- Sohail M.U., Ijaz A., Yousaf M.S., Ashraf K., Zaneb H., Aleem M., Rehman H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010;89:1934–1938. doi: 10.3382/ps.2010-00751. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Teeter R.G., Smith M.O., Owens F.N., Arp S.C., Sangiah S., Breazile J.E. Chronic heat stress and respiratory alkalosis: occurrence and treatment in broiler chicks. Poult. Sci. 1985;64:1060–1064. doi: 10.3382/ps.0641060. [DOI] [PubMed] [Google Scholar]
- Williams A., Audsley E., Sandars D. Cranfield University and Defra; Bedford: 2006. Determining the Environmental Burdens and Resource Use in the Production of Agricultural and Horticultural Commodities. Main Report. Defra Research Project IS0205. [Google Scholar]
- Yunianto V.D., Hayashi K., Kaneda S., Ohtsuka A., Tomita Y. Effect of environmental temperature on muscle protein turnover and heat production in tube-fed broiler chickens. Br. J. Nutr. 1997;77:897–909. doi: 10.1079/bjn19970088. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]