Abstract
Newcastle disease (ND) is perceived to be the major constraint in village chickens of Ethiopia causing huge economic loss. Village chickens are mobile and pass through markets, and live chicken markets are a highly productive source of ND virus replication, maintenance, and spread. However, in northwest of Ethiopia, there is a dearth of information on the role of live chicken markets in the maintenance and spread of ND in the village chickens. Therefore, a total of 480 apparently healthy chickens in the 4 live chicken markets were sampled with the aim to detect and estimate ND virus infection. Tracheal and cloacal swabs were collected from each bird and processed for virus isolation in 9- to 11-day-old embryonated chicken eggs, and hemagglutination inhibition (HI) assay was performed on all sera samples. The overall infection rate of ND virus was reported to be 39.2% (95% CI: 34.8–43.5). Of all chickens, 34.6% (95% CI: 30.3–38.9) had mean HI titer ≥4 log2, which was considered as protective. The mean hemagglutination titer for the ND virus was reported to be 6.0 log2, and mean antibody titer was reported to be 6.2 log2, with no statistically significant variation among the markets (P > 0.05). Newcastle disease occurrence was detected in all seasons of the year in the live bird markets, with the highest prevalence (55.8%) during the prerainy dry season (April and May), showing evidence for climatic and socioeconomic aspects as a risk factor in the occurrence of ND in indigenous chicken. In vivo virulence tests, mean death time of the embryo, and the intracerebral pathogenicity index revealed the presence of all pathotypes of ND virus strains: velogenic, mesogenic, and lentogenic. Apparently, healthy appearing birds were reported to be reservoirs of velogenic ND virus strains that could initiate endemicity of ND cycles in the village setting. Hence, it is strongly recommended to implement appropriate prevention and control measures to mitigate the economic loss caused by the disease.
Key words: chicken, infection rate, Newcastle disease virus, live chicken market, northwest Ethiopia
Introduction
Ethiopian poultry production is a low-cost investment that plays key role in the local economy, which has the potential to improve food security and assist in poverty alleviation. Village indigenous chickens account for more than 98% of the national poultry population and are more important than the exotic breed kept under the intensive management system with regard to total numbers of egg and poultry meat production (Zeleke et al., 2005). All families at the village level, even the poor and landless, raise chicken for additional income and high quality (Reta, 2009).
Newcastle disease (ND) is among the major poultry diseases incriminated for reduction of total numbers and impairment of productivity. It is an acute and highly contagious viral infection that can affect most species of birds (OIE, 2009). The Newcastle disease virus (NDV) strains are classified as velogenic, mesogenic, and lentogenic based on their pathotypes and virulence (Brown and Bevins, 2017; Schirrmacher, 2017). The pathogenicity of NDV strains is determined using virus isolation followed by in vivo tests such as the intracerebral pathogenicity index (ICPI), intravenous pathogenicity index, and mean death time (MDT) in specified pathogen-free chicken embryo and birds (Alexander et al., 2004; Putri et al., 2017).
The NDV is primarily transmitted via inhalation or ingestion of virus shed in feces and respiratory secretions by infected birds for variable lengths of time (OIE, 2009; Brown and Bevins, 2017). The virus spreads rapidly between premises by the movement of apparently healthy but infected birds, by movement of people and contaminated equipment, food, and water, and by airborne spread from one premise to another (OIE, 2009; Brown and Bevins, 2017; Schirrmacher, 2017).
In most developing countries, the village chicken is an extremely important asset representing a significant source of protein in the form of eggs and meat. However, ND is frequently responsible for devastating losses in village poultry, with flock mortality reaching up to 100%, and the economic impact of trading restrictions (Wambura, 2009).
Similar to other developing countries, ND is one of the major health constraints that cause heavy mortality and morbidity to village chicken and affect the productivity of the system (Tadelle and Jobre, 2004; Mazengia et al., 2010; Chaka et al., 2013). Newcastle disease occurs almost any time of the year, and the velogenic strain is kept in circulation by the wandering unvaccinated rural and commercial poultry sector of Ethiopia (Chaka et al., 2013; Fentie et al., 2014; Damena et al., 2016). Locally, the velogenic strain of NDV is named as “Fengil,” which is translated as sudden dorsal prostration that signifies the severity and fatality of the disease (Chaka et al., 2012).
Village chickens in northwest Ethiopia are mobile and pass through live chicken markets. This condition eases the contact of chickens from different areas at market and facilitate the rapid spread and persistence of ND among village chickens (Serkalem et al., 2005). Live chicken markets are therefore, hypothesized to be a productive source of NDV and an ideal environment for virus amplification. In addition, reports elsewhere showed an important linkage between the live chicken markets and epidemiology of NDV. Despite, there is a dearth of information on the role of chicken markets in maintenance and spread of the disease in village chickens of Ethiopia. Therefore, this study was aimed to identify the role of live chicken market in the perpetuation and dissemination of NDV and to estimate the infection rates of the disease in the study markets and respective districts.
Materials and methods
Study Area and Chicken Population
The study was conducted in selected live chicken markets in 4 districts of North Gondar administrative zone. The study area lies approximately 550 m in western lowland to 4,620 m in Semien Mountain in the north above sea level, and the average annual rainfall varies from 800 mm to 1,772 mm characterized by a monopodial type of distribution. The four selected study market sites are located in districts: Aymba in Dembia, Gondar in Gondar city, Maksegnit in Gondar Zuria, and Ambagiorgis in Wogera district of North Gondar administrative zone. The mean annual minimum and maximum temperature is 10°C in highland and 44.5°C in lowland, respectively. Geographically, the study area lies between 12.3 to 13.80north latitude and 35.350east longitude (CSA, 2009).
In the area, chicken and eggs are sold off from the farm directly to a final consumer or to a local middleman through a local market in both rural and urban areas. Live chicken markets in Gondar city are terminal markets for village chicken collected from producers and primary or secondary markets at district towns. Dealers collect chickens and transport by bus or any truck available with or without crates for retail in the larger towns of the market sheds. Depending on the market sites and market days (higher in holidays), the volume of trade every week is estimated in the range of 500 to 1500 chickens per market.
Study Design
The study was a cross-sectional and seasonal study. Data were collected from 4 potential live chicken markets, one terminal market in Gondar city and 3 primary chicken markets in the district towns. The study districts were selected based on their potential to supply chicken markets every week. One chicken market was randomly selected from each district and studied by visiting live chicken markets once per season (predry season, dry season, prerainy season, and rainy season) to detect the occurrence and dissemination of NDVs.
Sample Size Determination and Sampling Method
The sample size was calculated by assuming a population of an average of 1,000 chickens per market per week. The sample size was calculated using the following equation (Musako and Abolnik, 2012):
where n is the sample size, C is the desired level of confidence, and P is the prevalence of positive samples in the population.
Thirty chickens per market per visit were randomly sampled at 95% level of confidence to detect NDV at 10% expected prevalence. Therefore, a total of 480 samples were collected from the 4 live chicken markets, that is, 120 chicken samples per visit.
Data and Sample Collection
Before sampling birds, general information about the history of vaccination against ND in the last 5 to 6 mo was collected from district animal health workers for they are the only ones to handle ND vaccines and vaccinate birds in the study districts usually to contain outbreaks. Sera samples and swab samples (cloacal and tracheal) were collected directly from study chickens. Cloacal and tracheal swabs were mixed in the sterile bottle containing phosphate-buffered saline (PBS) and antibiotics, transported in cool boxes with ice packs to Veterinary Microbiology Laboratory, University of Gondar, and stored at −20°C until tested. Demographic data were collected by using a pretested and structured questionnaire from local chicken marketing actors (producers, traders, and livestock officers). Interviews, focal group discussion and key informants were also used for generating data. Serological and virological examinations were carried out to detect antibodies and to isolate NDVs.
Virus Isolation
Tracheal and cloacal swabs were clarified by centrifugation, and 0.2 mL of the suspension was inoculated into the allantoic cavity of 9- to 10-day-old embryonated eggs. Two eggs were inoculated per sample. The inoculated eggs were incubated for 5 d at 37°C and chilled at 4°C, and the allantoic fluids were collected. The eggs were candled daily for checking embryonic deaths. Owing to lack of eggs from pathogen-free chicken, we collected fertile eggs from unvaccinated chicken and inoculated the sample suspension into the allantoic cavity after 9 d of incubation. To avoid neutralization of the virus by maternal antibody, we harvested the allantoic fluid after 5 d as the antibody is maintained in the yolk and the virus can reach there after 14 d of incubation (OIE, 2009). The allantoic fluid, about 5 mL in quantity, was aspirated from every embryonated eggs into a pipette, and the harvested allantoic fluids were clarified by centrifugation at 1,000 × g for 10 min and tested for the presence of hemagglutinins through hemagglutination (HA) using 1% chicken red blood cells (RBC). Samples positive by HA were further confirmed using the hemagglutination inhibition (HI) test using positive antiserum against NDV. All virological tests were performed in the microbiology laboratory of College of Veterinary Medicine and Animal Sciences in the University of Gondar.
Serology
About 2–3 mL of whole blood was collected, and sera were harvested into cryotubes and preserved at −20°C until being used for testing of specific antibodies to NDVs. Serum samples were inactivated by heating overnight at 56°C, and the presence of ND antibodies were determined using the HI test, with two fold serum dilutions, 4 units of hemagglutinin as recommended by the World Organization for Animal Health (OIE, 2009). In brief, 0.025 mL of PBS was dispensed into each well of a plastic V-bottomed microliter plate; then, 0.025 mL of serum was placed into the first well of the plate. Two fold dilutions of 0.025 mL of serum were then prepared across the plate. Newcastle diesease viral antigen (0.025 mL) was added to each well, and the plate was left for 30 min at room temperature. Chicken RBC (0.025 mL of 1% [v/v]) were then added to each well, and after gentle mixing, the RBC were allowed to settle for about 30 min at room temperature. Agglutination is assessed by tilting the plates with those wells in which the RBC streaming was observed at the same rate as the control wells (positive serum, virus and antigen, and PBS controls) considered to show inhibition. The antibody titers were expressed in log2 units, and the HI titer of ≥4 log2 is generally accepted as positive for specific immunity (OIE, 2009).
Pathogenicity Test of Isolates
The virulence of isolates against NDV was tested by MDT and ICPI as per the OIE (2008) standard manual. Accordingly, isolates with MDT values of 40–60 h, of 60–90 h, or higher than 90 h were designated as velogenic, mesogenic, and lentogenic respectively. The most widespread NDV pathotyping tool ICPI was used on 1-day-old pathogen-free chicks, and the virus isolates scored an ICPI of 1.3 to 2.0 virulent isolates, mesogenic isolates ranged from 0.7 to 1.3, and lentogenic isolates values ranged from 0.0 to 0.7 (OIE, 2004, Alexander et al., 2004).
Data Analysis
Stata software version 12 (Stata Corp, College Station, TX) was used for computing both descriptive and analytic statistics. The prevalence between the districts or markets was compared using Fisher's exact test. Analysis of variance was used to determine whether the log-transformed titers from the 4 markets were significantly different.
Results
Questionnaire Survey
Socioeconomic Status and Live Chicken Marketing
The socioeconomic status of traders and live chicken marketing and constraints is summarized in Table 1. Of the 27 traders interviewed, majority (55.6%) were financially dependent on chicken trade to support their households, and many traders (77.8%) purchase chickens from farmers either directly at the farm gate or at primary and secondary markets and sell mainly directly to customers and restaurants (77.8%).
Table 1.
Socioeconomic status of the chicken traders in northwest Ethiopia.
| Description | Category | Frequency | Percentage (%) |
|---|---|---|---|
| Sex of the respondents | Male | 15 | 55.6 |
| Female | 12 | 44.4 | |
| Age (average age of the respondents) | <30 | 8 | 29.6 |
| 30–40 | 14 | 51.9 | |
| >40 | 5 | 18.5 | |
| Educational status | Illiterate | 11 | 40.5 |
| Writing and reading | 3 | 11.1 | |
| Primary education | 8 | 29.9 | |
| Secondary education and higher | 5 | 18.5 | |
| Marital status | Single and divorced | 5 | 18.5 |
| Married | 22 | 81.5 | |
| Family size | 1–5 | 21 | 77.8 |
| 6–10 | 6 | 22.2 | |
| Sources of income | Poultry trade only | 15 | 55.6 |
| Poultry trade and others | 12 | 44.4 | |
| Trading experience (year) | 1–5 | 15 | 55.6 |
| 6–10 | 8 | 29.6 | |
| >10 | 4 | 14.8 | |
| Sources of chickens purchased | Producers and farmers | 21 | 77.8 |
| Farmers and traders | 6 | 22.2 | |
| Selling for | Customers | 21 | 77.8 |
| Customers and traders | 6 | 22.2 |
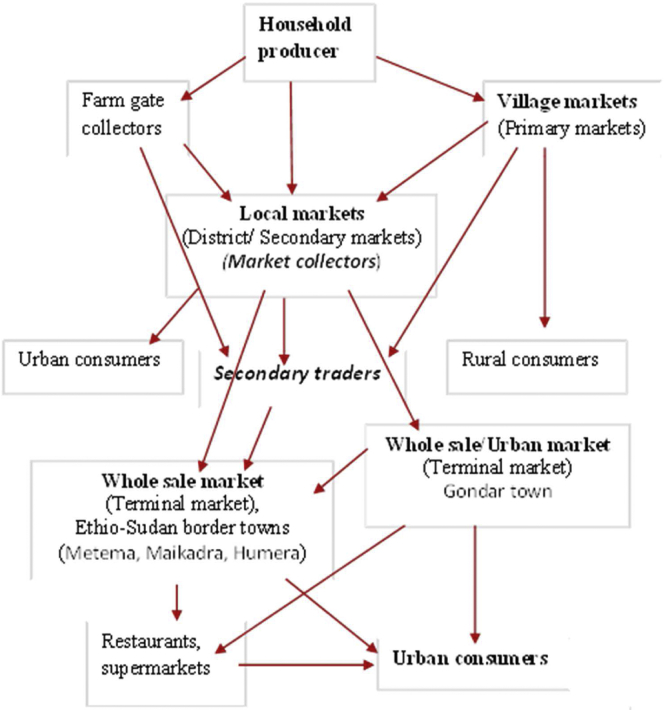
According to our observation and most of the respondents, the live chicken marketing chain was dominantly informal and poorly developed. Village chicken and eggs are sold off the farm directly to consumers or to middle traders through a local market in both rural and urban areas. About 42% of live sales were made between farmers and consumers, and 39% of live sales were made from farmers to traders. The detailed market channel is presented in Figure 1.
Figure 1.
Market channel for indigenous chickens in the study area.
Knowledge Assessment
Knowledge of chicken producers about ND and the reasons for the occurrence of the ND outbreak in the village chickens were assessed. Most chicken owners recognize ND by clinical signs such as depression, loss of appetite, unable to walk, green to yellow diarrhea, and sudden death. According to the respondents, the ND outbreak occurs every year mainly during the dry season, at the beginning of rainy seasons, and at peak marketing times especially during Easter. The most important reasons for occurrence of the disease in the villages was the presence of outbreaks in the neighboring village (40%), followed by introduction of newly purchased chickens (25%) and chickens returned from markets (15%) (Table 2).
Table 2.
Knowledge of village chicken keepers on the occurrence of the ND outbreak in northwest Ethiopia.
| Reasons | No. of respondents | Percentage (%) |
|---|---|---|
| Outbreak in the neighboring village | 48 | 40 |
| Introduction of newly purchased birds | 30 | 25 |
| Chickens returned from markets | 18 | 15 |
| Presence of scavenging dogs | 10 | 8.3 |
| Small rain shower and moisture | 8 | 6.7 |
| Unknown reasons | 6 | 5 |
| Total | 120 | 100 |
Abbreviation: ND, Newcastle disease.
Newcastle Disease Virus and Antibody Detection
Sera and swab (tracheal and cloacal) samples were collected from 480 chickens in the chicken markets to detect the NDVs and antibodies. The overall prevalence of NDV infection and antibodies in the study area was 39.2 (95% CI: 34.8–43.5%) and 34.6% (95% CI: 30.3–38.9%), respectively (Table 3). Prevalence difference was significant between districts (P < 0.05). High prevalence rates of NDVs and antibodies were reported in Gondar Zuria (46.7 and 42.5%), followed by Gondar town district (40 and 37.5%), respectively. The mean HA titer for the NDV was reported as 6.0 log2, whereas the mean antibody titer was reported to be 6.2 log2, with no variation among the markets.
Table 3.
Prevalence of Newcastle disease viruses and antibodies in live chicken markets.
| District | Study market | No. of tested birds | NDV isolation |
ND antibodies |
||||
|---|---|---|---|---|---|---|---|---|
| No. of positive | Percentage (%) | Mean HA titer log2 (±SD) | No. of positive | Percentage (%) | Mean HI titer log2 (±SD) | |||
| Dembia | Aymba | 120 | 38 | 31.7a | 5.6 (1.0) | 32 | 26.7a | 6.2 (1.0) |
| Gondar Zuria | Maksegnit | 120 | 56 | 46.7b | 5.8 (1.2) | 51 | 42.5b | 6.1 (1.2) |
| Wogera | Ambagiorgis | 120 | 46 | 38.3b | 6.1 (1.0) | 38 | 31.7b | 6.1 (1.2) |
| Gondar town | Gondar | 120 | 48 | 40.0b | 6.3 (1.1) | 45 | 37.5b | 6.3 (0.9) |
| Total | 480 | 188 | 39.2 | 6.0 (1.2) | 166 | 34.6 | 6.2 (1.1) | |
Percentages within a column with different superscripts differ significantly (P < 0.05).
Abbreviations: HA, hemagglutination; ND, Newcastle disease; NDV, Newcastle disease virus.
The distribution of NDV titers in different seasons is presented in Table 4. The outbreak of NDV was recorded in all seasons of the year, and variation was significantly seen among seasons (P < 0.05) in the chicken markets. However, the highest prevalence (55.8%) of ND was observed in prerainy season (April to May), followed by the dry season (45%) (November to March).
Table 4.
Seasonal distribution of NDV infection in live chicken markets of northwest Ethiopia.
| Season | District |
||||
|---|---|---|---|---|---|
| Dembia, n (%) | Gondar Zuria, n (%) | Wogera, n (%) | Gondar town, n (%) | Total, n (%) | |
| Predry season (September–October) | 9 (30) | 11 (33.3%) | 8 (26.7) | 10 (36.7) | 38 (31.8) |
| Dry season (November–March) | 10 (33.3) | 17 (56.7) | 15 (50.0) | 12 (40.0) | 54 (45) |
| Prerainy season (April–May) | 14 (46.7) | 20 (66.7) | 16 (53.3) | 17 (56.7) | 67 (55.8) |
| Rainy season (June–August) | 5 (16.7) | 8 (26.7) | 7 (23.3) | 9 (30.0) | 29 (24.2) |
| Total (n) | 38 | 55 | 46 | 48 | 188 |
n = number of positive chickens.
Abbreviation: NDV, Newcastle disease virus.
Isolation and Characterization of NDV
The virulence of NDV was determined by MDT of the embryo and ICPI, and the test result is summarized in Table 5. As per the MDT test, 21.1% of NDV isolates were velogenic, 20% were mesogenic, and 58.9% were lentogenic. In the pathogenicity characterization of NDV isolates, the ICPI of 8 isolates ranged between 0.7 and 1.87 (velogenic), and in 17 isolates, the ICPI varied from 0.0 to 0.61 (lentogenic).
Table 5.
Virulence of Newcastle disease virus isolates based on mean embryonic death time and intracerebral pathogenicity index tests.
| Districts | No. of NDV isolates | Mean death time (MDT) of the embryo |
Intracerebral pathogenicity index |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 48–60 h | Percentage (%) | 60–90 h | Percentage (%) | >90 h | Percentage (%) | No. of isolates | ICPI range | ||
| Dembia | 15 | 4 | 26.7 | 3 | 20.0 | 8 | 53.3 | 5 | 0.0–1.61 |
| Gondar Zuria | 30 | 7 | 23.3 | 6 | 20.0 | 17 | 56.7 | 8 | 0.31–1.87 |
| Wogera | 24 | 5 | 20.8 | 4 | 16.7 | 15 | 62.5 | 6 | 0.0–1.70 |
| Gondar town | 26 | 4 | 15.4 | 6 | 23.1 | 16 | 61.5 | 6 | 0.51–1.75 |
| Total | 95 | 20 | 21.1 | 19 | 20.0 | 56 | 58.9 | 25 | 0.0–1.87 |
MDT: <60 h = velogenic ND strain; 60–90 h = mesogenic ND strain; >90 h = lentogenic ND strain.
Abbreviations: ICPI, intracerebral pathogenicity index; ND, Newcastle disease; NDV, Newcastle disease virus.
Discussion
The present study revealed that live chicken markets are highly productive sources of ND and an ideal environment for virus amplification. A similar observation was reported by Jibril et al. (2014) in Nigeria and Serkalem et al. (2005) in central Ethiopia that live bird markets contribute to the persistence and spread of NDVs and serve as a source of infection to village chickens when they are newly introduced.
Very often in the study area, chickens in the terminal market shed usually get insufficient feed and water and are kept confined together in unhygienic shelters, which facilitate feed and water contamination with droppings, thus creating a conducive environment for feco-oral route transmission of NDV. Whenever there is price fluctuation or in the absence of marketing, most of the traders keep their chickens, both the newly introduced and existing birds, mixed together for a week or more, which would allow for perpetuation and persistence of NDV or an uninterrupted cycle of infection as the virus passes from infected or incubated chickens to other susceptible chicken. Similar to these study findings, Zeleke et al. (2005) also suggested that local open markets where huge numbers of chickens are gathered might serve as continuous foci of the NDV infections. Therefore, live chicken market would be the most common sources for the persistence and dissemination of NDV.
An occurrence of the ND outbreak in village chickens was more frequent during and after a peak marketing season, typically in holidays and festivals. The majority of farmer respondents recognized that ND occurrence in village poultry is associated with the introduction of purchased or returned birds from markets. This can be due to chickens acquiring infection from the markets. This is in agreement with Serkalem et al. (2005) who reported that ease of contact of chickens from different areas at local markets, which are then taken back to various localities, can undoubtedly facilitate the rapid spread and persistence of ND among village chickens. In addition, Alexander (2003) also stated the occurrence of the ND outbreak in villages is due to either newly introduced or recovered birds.
According to the farmer respondents, the occurrence of ND in the village chicken is associated with the presence of the outbreak in the neighboring villages and the presence of dogs in the household that scavenge dead birds' cadavers. This agrees with a Kenyan study conducted by Ipara et al. (2019) that showed the lack of uniformity in hygienic practices used in live chicken markets, leading to increased disease outbreaks. Similarly, Demeke (2007) also reported no practices of isolating sick birds from the household flocks and dead birds could sometimes be left for either domestic or wild predators, which favors ND outbreaks. The occurrence of ND is very frequent once or twice a year as the virus may enter from outside or circulate in village chickens for there is no ND vaccination practice in village chickens in the study area.
The present study indicated that NDV infection is widespread in the study area. The distribution of NDV varied significantly among the study districts, with higher prevalence in Gondar Zuria (46.7%) and Gondar town (40%). This finding was consistent with the previous study reports in different parts of Ethiopia: 12.9 to 47.6% seroprevalence in Southern and Rift valleys (Zeleke et al., 2005) and 32.22% in the central part of Ethiopia (Serkalem et al., 2005). Both the present and previous study reports are indicative of the endemicity of ND and presence of continuous infection pressure in village chickens.
In the present study, a significant rate of ND prevalence has been observed in district markets. This may be attributed to the diversity in climate, management practices including confinement, mode of waste disposal, and recovery rates of chickens from outbreaks, which further enhance the differences in disease outbreaks for the different localities both at villages and markets (Musa et al., 2009; Njagi et al., 2010). It is likely that the increasing population and mix of chickens of different origin in study market sheds also provided a sustainable reservoir for the maintenance of ND strains, which could have allowed the infection to persist or facilitated the introduction of viruses more frequently to villages.
Although ND occurs year-round in most rural poultry populations, it is most common and severe at times of climatic stress. Previous studies associated the outbreak of ND with the change of the season, and both cold and hot dry weathers have been cited as contributory factors for ND outbreaks (Musa et al., 2009). In the present study, relatively high prevalence was reported during the prerainy dry season (55.8%) (April and May), followed by the dry season (45%) (November to March). The same report has demonstrated a high seroprevalence rate in dry seasons in Nigeria and other parts of Ethiopia from November to March (Chaka et al., 2013; Abraham-Oyiguh et al., 2014). This may be attributed to seasonal and socioeconomic reasons. In the study region, northwest Ethiopia, the period from April to May is a hot humid season with less rainfall, which may favor the persistence and transmission of ND. Moreover, the dry seasons are particularly harsh and put free-ranging village chickens under severe stress. These environmental conditions might weaken immunity and increase susceptibility of chickens to ND infection (Miguel et al., 2013).
The socioeconomic factors such as cultural practices, trading modalities, as well as chicken rearing and disease management practices are also important factors in the transmission and maintenance of NDV in rural poultry production systems (Miguel et al., 2013). In our observation, occurrence of the ND outbreak in village chickens was more frequent during and after a peak marketing season, typically in holidays and festivals. This may be associated with massive movement of live chickens from rural areas to urban areas during festive seasons and increased trading at markets in these periods to generate instant income for purchasing home items. Besides, most of the religious festivals in Ethiopia are celebrated during the dry seasons. Thus, such a seasonal bird movement for trade and the free-range management system may be responsible in creating uninterrupted cycles of infections throughout the year (Musako and Abolnik, 2012; Chaka et al., 2013).
None of the chickens sampled had a history of previous vaccination against ND. It is therefore deduced that viruses and antibodies detected in the chickens in this study may be a result of natural infection by NDV. Therefore, the 39.2% prevalence rate of ND in the study markets could be attributed to factors such as the management system in traditional production and marketing, which may serve as a stress factor and favors infection.
The study detected more than half (58.9%) strains as lentogenic strains by using the MDT standard pathogenicity index. This higher proportion of lentogenic strains may be a potential risk factor for easy emergence of the virulent NDV (Bello et al., 2018). Moreover, ICPI assessment indicated that 8 NDV isolates had virulent strain characteristics ranging between 0.7 and 1.87.
Conclusions
The increasing prevalence of ND in chicken markets in the present study highlights the ease with which the infection can spread. The findings revealed the importance of live chicken markets as a focal source of virus replication and maintenance and spreading of the disease to the susceptible chicken population. Mixing of birds of different origin and overcrowding at markets, free disposal of dead birds both at market places and during transportation, and the absence of movement restriction during the onset of disease outbreaks favored the spread of the disease-causing uninterrupted cycle of infection. Although highly virulent NDV strains are apparently widespread in the study area, little attention has been given to the control of the disease in village chickens. Incursion of a highly virulent NDV strain to which the chicken population has insufficient immunity could have continued devastating consequences. However, these could be mitigated by a good vaccination strategy. The vaccination program among village chickens would limit mortalities and improve the flock size.
Acknowledgments
The authors would like to thank University of Gondar for their financial support throughout the study period. The authors gratefully extend their thanks to Aklilu Lemma Institute of Pathobiology for reagent support and Veterinary Microbiology Laboratory, University of Gondar, for the provision of laboratory facilities and reagents and its laboratory personnel for their cooperation during the laboratory work.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Abraham-Oyiguh J., Sulaiman L.K., Meseko C.A., Ismail S., Suleiman I., Ahmed S.J., Onate E.C. Prevalence of newcastle disease antibodies in local chicken in Federal Capital Territory, Abuja, Nigeria. Int. Sch. Res. Notices. 2014;2014:796148. doi: 10.1155/2014/796148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D.J. Disease of Poultry. 11th ed. Iowa State University Press; Ames, IA: 2003. Newcastle disease virus, other avian paramyxoviruses, and pneumovirus infections. [Google Scholar]
- Alexander D.J., Bell J.G., Alders R.G. Iowa State University Press; Ames, IA: 2004. A Technology Review: Newcastle Disease. With Special Emphasis on its Effect on Village Chickens. FAO Animal Production and health Paper (FAO) 0254-6019, no 161. [Google Scholar]
- Bello M.B., Yusoff K., Ideris A., Hair-Bejo M., Peeters B.P.H., Omar A.R. Diagnostic and vaccination Approaches for newcastle disease virus in poultry: the Current and emerging Perspectives. Biomed. Res. Int. 2018;2018:7278459. doi: 10.1155/2018/7278459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V.R., Bevins S.N. A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Vet. Res. 2017;48:68. doi: 10.1186/s13567-017-0475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaka H., Goutard F., Bisschop S.P.R., Thompson P.N. Seroprevalence of Newcastle disease and other infectious diseases in backyard chickens at markets in Eastern Shewa zone, Ethiopia. Poult. Sci. 2012;91:862–869. doi: 10.3382/ps.2011-01906. [DOI] [PubMed] [Google Scholar]
- Chaka H., Goutard F., Gil P., Abolnik C., Servan de Almeida R., Bisschop S., Thompson P.N. Serological and molecular investigation of Newcastle disease in household chicken flocks and associated markets in Eastern Shewa zone, Ethiopia. Trop. Anim. Health Prod. 2013;45:705–714. doi: 10.1007/s11250-012-0278-y. [DOI] [PubMed] [Google Scholar]
- CSA . Amhara region; Ethiopia: 2009. Census Central Statics Authority, Ethiopian Agricultural Enumerations, Result for Amhara Region. [Google Scholar]
- Damena D., Fusaro A., Sombo M., Belaineh R., Heidari A., Kebede A., Kidane M., Chaka H. Characterization of Newcastle disease virus isolates obtained from outbreak cases in commercial chickens and wild pigeons in Ethiopia. Springerplus. 2016;5:476. doi: 10.1186/s40064-016-2114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeke S. Suitability of hay-box brooding technology to rural household poultry production system. Livest. Res. Rural Dev. 2007;19:1. [Google Scholar]
- Fentie T., Heidari A., Aiello R., Kassa T., Capua I., Cattoli G., Sahle M. Molecular characterization of Newcastle disease viruses isolated from rural chicken in northwest Ethiopia reveals the circulation of three distinct genotypes in the country. Trop. Anim. Health Prod. 2014;46:299–304. doi: 10.1007/s11250-013-0487-z. [DOI] [PubMed] [Google Scholar]
- Ipara B.O., Otieno D.O., Nyikal R.A., Makokha S.N. The role of unregulated chicken marketing practices on the frequency of Newcastle disease outbreaks in Kenya. Poult. Sci. 2019;98:6356–6366. doi: 10.3382/ps/pez463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibril A.H., Umoh J.U., Kabir J., Saidu L., Magaji A.A., Bello M.B., Raji A.A. Newcastle disease in local chickens of live bird markets and households in Zamfara state, Nigeria. ISRN Epidemiol. 2014;2014:1–4. [Google Scholar]
- Mazengia H., Gelaye E., Nega M. Evaluation of newcastle disease antibody level after different vaccination regimes in three districts of Amhara Region, Northwestern Ethiopia. J. Infect. Dis. Immun. 2010;1:16–19. [Google Scholar]
- Miguel E., Grosbois V., Berthouly-Salazar C., Caron A., Cappelle J., Roger F. A meta-analysis of observational epidemiological studies of Newcastle disease in African agro-systems, 1980-2009. Epidemiol. Infect. 2013;141:1117–1133. doi: 10.1017/S0950268812002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa U., Abdu P.A., Dafwang I.I., Umoh J.U., Sa`idu L., Mera U.M., Edache J.A. Seroprevalence, seasonal occurrence and clinical Manifestation of newcastle disease in rural household chickens in plateau state, Nigeria. Int. J. Poult. Sci. 2009;8:200–204. [Google Scholar]
- Musako C., Abolnik C. Determination of the seroprevalence of Newcastle disease virus (avian paramyxovirus type 1) in Zambian backyard chicken flocks. Onderstepoort J. Vet. Res. 2012;79:1–4. doi: 10.4102/ojvr.v79i1.502. [DOI] [PubMed] [Google Scholar]
- Njagi L.W., Nyaga P.N., Mbuthia P.G., Bebora L.C., Michieka J.N., Kibe J.K., Minga U.M. Prevalence of Newcastle disease virus in village indigenous chickens in varied agro-ecological zones in Kenya. Livest. Res. Rural Dev. 2010;22:95. [Google Scholar]
- OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Mammals, Birds and Bees. Office International des Epizooties; Paris, France: 2004. Newcastle disease. Chapter 2.1.15. OIE manual of standards for Diagnostic tests and vaccines; pp. 270–282. [Google Scholar]
- OIE . vol. 1. 2008. pp. 576–589. (“Newcastle disease,” manual of Diagnostic tests and vaccines for Terrestrial animals (Mammals, Birds and Bees)). [Google Scholar]
- OIE . 2009. World Organization for Animal Health, Newcastle Disease, Etiology, Epidemiology, Diagnosis, Prevention and Control References.http://www.oie.int/en/animal-health-in-the-world/animal-diseases/newcastle-disease/ Accessed Dec. 2019. [Google Scholar]
- Putri D.D., Handharyani E., Soejoedono R.D., Setiyono A., Mayasari N., Poetri O.N. Pathotypic characterization of Newcastle disease virus isolated from vaccinated chicken in West Java, Indonesia. Vet. World. 2017;10:438–444. doi: 10.14202/vetworld.2017.438-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reta D. Understanding the role of indigenous chickens during the long walk to food security in Ethiopia. Livest. Res. Rural Dev. 2009;21:8. http://www.lrrd.org/lrrd21/8/dugu21116.htm 21 [Google Scholar]
- Schirrmacher V. Immunobiology of newcastle disease virus and its Use for Prophylactic vaccination in poultry and as Adjuvant for Therapeutic vaccination in Cancer Patients. Int. J. Mol. Sci. 2017;18:1103. doi: 10.3390/ijms18051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serkalem T., Hagos A., Zeleke A. Seroprevalence study of newcastle disease in local chickens in Central Ethiopia. Intern. J. Appl. Res. Vet. Med. 2005;3:1. [Google Scholar]
- Tadelle, Jobre A review of the importance and control of Newcastle disease in Ethiopia. Ethiop Vet. J. 2004;1:71–81. [Google Scholar]
- Wambura P.N. Oral vaccination of chickens against Newcastle disease with I-2 vaccine coated on oiled rice. Trop. Anim. Health Prod. 2009;41:205–208. doi: 10.1007/s11250-008-9176-8. [DOI] [PubMed] [Google Scholar]
- Zeleke A., Sori T., Gelaye E., Ayelet G. Newcastle disease in village chickens in the Southern and Rift valley districts in Ethiopia. Int. J. Poult. Sci. 2005;4:507–510. [Google Scholar]