Abstract
This study was aimed to investigate whether 1-deoxynojirimycin (DNJ) affects the digestion system of young geese and assess whether mulberry leaf, which contains this substance, has disadvantages that compromise its value as poultry feed. One hundred and twenty-eight 12-day-old male Wanxi white geese were randomly assigned into 4 treatment groups. The control group was fed an ordinary diet without DNJ. The other groups namely L-DNJ, M-DNJ, and H-DNJ had their basic diets supplemented with 0.05 mg/g, 0.1 mg/g, and 0.15 mg/g DNJ, respectively. The geese were fed for 6 wk, and the apparent digestibility test was conducted in the last week. Intestinal parameters, digestive organs, and enzymes were determined. 16S rRNA gene sequencing was conducted for cecal flora composition. The results revealed that DNJ decreased body and liver weight and increased feed conversion ratio in comparison with the control (P < 0.05); however, it did not influence the weight and length of the intestine or the pancreas weight. The utilization of organic matter, metabolizable energy, ether extract, acid detergent fiber, and calcium in feed were reduced in the M-DNJ and L-DNJ groups compared with those in the control (P < 0.05); however, the utilization of crude protein was increased in all DNJ-treated groups (P < 0.01). In the H-DNJ group, the usage of soluble phosphorus was also increased (P < 0.05). High-dose DNJ increased the activity of trypsin in the pancreas but reduced those of amylase (P < 0.05) and lipase (P > 0.05) in the pancreas and duodenum. The intestinal villi were short, even impaired, in DNJ-treated groups. High-throughput sequencing data revealed that DNJ supplement reduced the α-diversity indices of the cecal microbiota. The principal component analysis further suggested a difference in community structure between the DNJ treatment groups and control. High-dose DNJ increased the relative abundance of Bacteroides, Escherichia-Shigella, and Butyricicoccus but reduced that of unclassified Ruminococcaceae compared with the control (P < 0.05). In conclusion, changes in the digestive system caused by DNJ seriously affected the metabolism of nutrients in geese and reduced their growth performance. Attention should be paid to the adverse effects of DNJ when using mulberry leaves as poultry feed.
Key words: Morus alba, 1-deoxynojirimycin, digestion, intestine, 16S rRNA gene sequencing
Introduction
Mulberry (Morus alba) is a widely distributed multipurpose agro-forestry plant in tropical, subtropical, and temperate areas, that has a large biological yield (Zhang et al., 2001). Mulberry leaves are rich in organic substances, vitamins, and minerals. The content of crude protein and carbohydrate in mulberry leaves is significantly higher than those in other green forage and is comparable to that of high-quality legume forage such as alfalfa (Doran et al., 2007). At present, as a feed or feed additive, mulberry leaf has been widely used in the breeding of ruminants, rabbits, and pigs and has been reported to significantly improve the yield and quality of meat and milk (Chhay et al., 2010; Venkatesh Kumar et al., 2015; Ma et al., 2017; Hou et al., 2020b).
However, the nutritional value of mulberry leaf and its effect in promoting production are not obvious in poultry breeding. Mulberry leaves do not significantly increase the production of poultry meat and eggs, nor do they accelerate the growth rate of poultry (Saenthaweesuk, 2009; Olteanu et al., 2015). Researchers have suggested that diets containing ≥4% mulberry leaf powder would inhibit feed intake and body-weight gain of poultry (ItzáOrtiz et al., 2010; Wang et al., 2016). However, it is not known why nutrient-rich mulberry leaves are unsuitable for poultry.
Mulberry leaves contain flavonoids, polysaccharides, superoxide dismutase, sitosterol, isoquercitrin, r-amino acids, 1-deoxynojirimycin, and other bioactive substances. As a result, they can have various biological effects, including antioxidant, antimicrobial, glucosidase inhibition, anti-hyperlipidemic, and anti-atherosclerotic (Chan et al., 2016; Sánchez-Salcedo et al., 2017; Hou et al., 2020a). These bioactive components in mulberry leaves affect the digestive function of the gastrointestinal tract and the absorption capacity of nutrients (Liang et al., 2012; Hu et al., 2018). The compound 1-deoxynojirimycin (DNJ), an important piperidine alkaloid, is a potent α-glucosidase inhibitor that affects the digestion of disaccharides and the absorption of glucose in animals (Yatsunami et al., 2008). Carbohydrate/sugar is an important energy source, and insufficient energy supply leads to weight loss in animals, as well as reduces production performance (Mikkelsen et al., 2000). It has been found that DNJ improves the metabolism of glucose, lipid, and amino acid in mice with type-2 diabetes (Liu et al., 2011b; Hu et al., 2017), indicating that it can affect the metabolic processes of various nutrients in animals. However, most studies on DNJ have focused on its role in the treatment and prevention of obesity and diabetes (Tsuduki et al., 2013; Hu et al., 2017), while few have focused on its effect on dietary nutrients digestion and the gastrointestinal function of animals.
Wanxi white goose is a superior local goose breed, which is raised in mulberry gardens in many parts of China. It is famous for its large size, fast growth, strong disease resistance, and good meat quality. In this study, we investigated the effect of mulberry-derived DNJ on digestive enzyme activity, intestinal flora, and apparent digestibility of nutrients in young Wanxi white goose, to reveal whether DNJ contributes to effect exerted by mulberry leaves on the goose digestive system, compromising the value of mulberry leaf in feed.
Materials and methods
Preparation of DNJ
DNJ was extracted from mulberry (M. alba L.) leaves and purified by an LC-MS system (Waters, St. Milford, MA) as described by Liu et al. (2015). The purity of DNJ was confirmed to be more than 96.5% by HPLC analysis. The yield of DNJ was about 0.24% wet weight of mulberry leaves and 0.04% of the dry matter.
Animal and Experimental Design
Animal experiments were performed according to the national guidelines for the care and use of experimental animals with approval from the Institutional Animal Care and Use Committees of Jiangsu University of Science and Technology.
The experiment was conducted in the fall of 2019. A total of 128 twelve-day-old male Wanxi white geese (376 ± 11.35 g) were obtained from the National Waterfowl Gene Pool in Taizhou, Jiangsu, and randomly assigned into 4 treatment groups of 4 replicates with 8 birds per replicate. The Con group (control) was fed a basic diet without DNJ, while the L-DNJ, M-DNJ, and H-DNJ groups were fed basal diet supplemented with 0.05 mg/g, 0.1 mg/g, and 0.15 mg/g DNJ, respectively. The basic diet was prepared with reference to NRC (1994) goose standard and Chinese feed nutrient composition list. Compositions and chemical analyses of the basic diet are shown in Table 1. During the pretest period, all the geese were fed the basic diet for 1 wk. In the formal feeding trial, the geese were fed the allocated group diets and clean water for 6 wk, and maintained at 18°C∼25°C, with average relative humidity of 65%. The geese were vaccinated according to the routine procedure, and the health status of each goose was observed every day. Body-weight and feed intake were recorded weekly.
Table1.
The composition and nutrient level of basic diet (air-dry basis).
| Ingredients | Nutrient levels | % | |
|---|---|---|---|
| Corn | 57.78 | Metabolizable energy3/(MJ/kg) | 11.63 |
| Soybean meal | 28.72 | Crude protein | 19.15 |
| Corn stalk | 7.65 | Crude fiber | 6.18 |
| Fish meal | 2.49 | Neutral detergent fiber | 17.15 |
| Soybean oil | 0.48 | Acid detergent fiber | 6.35 |
| Calcium hydrogen phosphate | 1.15 | Soluble phosphorus | 0.40 |
| Calcium carbonate | 0.91 | Calcium | 0.84 |
| Sodium chloride | 0.3 | Lysine | 1.06 |
| Trace elements1 | 0.5 | Methionine + Cysteine | 0.62 |
| Multivitamin2 | 0.02 |
The trace elements provided the following per kg of diets: Fe 90 mg, Cu 6 mg, Mn 85 mg, Zn 85 mg, I 0.42 mg, Se 0.3 mg, Co 2.5 mg.
The multivitamin provided the following per kg of diets: VA 1 500 IU, VD3 200 IU, VE 12.5 mg, VK3 1.5 mg, VB1 2.2 mg, VB2 5.0 mg, nicotinic acid 65 mg, pantothenate 15 mg, VB6 2 mg, biotin 0.2 mg, folic acid 0.5 mg, choline 1,000 mg.
Calculated value.
Apparent Nutrient Utilization
In the last week of the feeding tria, 3 geese were selected according to the average body-weight from each replicate (n = 12 each treatment) and raised in metabolic cages. The animals fasted for 6 h before the start of the metabolic test, and then each goose was fed 160 g of diet per day with unlimited drinking water. The test was divided into 2 stages: the pretest period lasted 4 d and the formal test period lasted 3 d. In the formal test period, excreta were collected continuously for 3 d by the method of full fecal collection. Dander and feathers were taken out with tweezers.
The feed and excreta samples were dried at 65°C and ground to pass through a 0.5-mm screen, and then stored in wide-mouth bottles for analysis of dry matter, organic matter, crude protein, ether extract, and gross energy based on the Association of Official Analytical Chemists' procedures (AOAC, 2000). Neutral detergent fiber and acid detergent fiber were determined as described by Goering and Van Soest (1970). Calcium content was determined by potassium permanganate titration; soluble phosphorus content was extracted using sodium bicarbonate solution, and then examined by the molybdenum-antimony colorimetric method.
The apparent availability of dietary nutrients was calculated by the following formula:
Determination of Digestive Organ and Enzyme Activity
At the end of the experiment, 3 experimental geese were taken from each replicate (n = 12 each treatment) and sacrificed humanely. Organs, including large intestine, small intestine, liver, and pancreas, were individually dissected and weighed. The abdominal cavity was opened, and the pancreas and duodenum were separated. Samples were collected from the pancreatic head, body, and tail. The duodenum was cut longitudinally to collect chyme and intestinal mucosa. Ten times the volume of 4°C deionized water was added to the sample and homogenized at a low temperature. The homogenate was centrifuged at 4°C for 20 min (1,200 × g), and the supernatant was collected. Trypsin, amylase, and lipase activity in the pancreas and duodenum were measured according to the respective kit instructions. All kits were purchased from Nanjing Jiancheng Institute of Biological Engineering.
Jejunum tissues were separated and paraffin embedded for hematoxylin and eosin (HE) staining. Serial sections (5-μm) were cut onto slides using a rotary microtome (Leica, Wetzlar, Germany) and deparaffinized before staining with Gill-2 hematoxylin and eosin Y (HE staining, Sigma-Aldrich St. Louis, MO), according to the manufacturer's instructions. A light microscope (BX51, Olympus, Tokyo, Japan) was used for capturing images.
DNA Extraction and 16S rRNA Gene Sequencing
Caecum contents were collected from geese in each treatment (n = 8). A total of 500 mg of content was used for DNA extraction with the Power Soil-htp 96 Well Soil DNA Isolation Kit (Mobio Laboratories Inc., Carlsbad, CA). The quality of DNA was determined using Onedrop OD-1000+ spectrophotometer (Onedrop, Shanghai, China) and 1% agarose gel electrophoresis. Subsequently, the V4 region of the 16S rRNA gene was amplified using the universal barcode PCR primer pair (515 F/806R) by the GoTaq Hot Start Colorless Master Mix and DNA polymerase (Promega, Madison). The PCR products were evaluated using PicoGreen fluorescent real-time DNase assay (Invitrogen, Carlsbad, CA). Samples were purified using 2% agarose gel and QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). Final quality was evaluated by PicoGreen fluorescent real-time DNase assay and Agilent 2,200 Tapestation System (Agilent Technologies, Santa Clara, CA). Illumina Miseq pair-end 300 sequencing platform was used for16S rRNA gene sequencing.
Data Processing and Analysis
Raw reads were processed using Trimmomatic software (version 0.32, http://www.usadellab.org/cms/?page=trimmomatic). Data splicing was performed using FLASH (v1.2.7; http://ccb.jhu.edu/software/FLASH/), while data filtering was conducted using Qiime (v1.9.1; http://qiime.org/scripts/split_libraries_fastq.html) and UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html). Operational taxonomic units (OTUs) were analyzed using Mothur software (http://www.mothur.org/) with 97% identity. The OTU annotation was performed by SILVA rRNA database (http://www.arb-silva.de/), and alignment was carried out using the Ribosomal Database Project (RDP) classifier. The relative abundance of OTUs at each taxonomic level (confidence threshold of 70%) was analyzed using the RDP classifier. The alpha and beta diversity index (Unweighted UniFrac distance) indicators were calculated. Principal component analysis (PCA) and heatmap analysis were performed based on the beta diversity index Unweighted UniFrac distance.
Statistical Analyses
The results were subjected to analysis of variance (ANOVA), using PROC GLM in SAS software (SAS 9.2), and comparison of means through the Least Significant Difference (LSD) test with a significance level of P < 0.05. All data were expressed as mean ± SD. Differences in the relative abundances of OTUs between groups were analyzed using Mann-Whitney U test.
Results
Effect of DNJ on Growth Performance
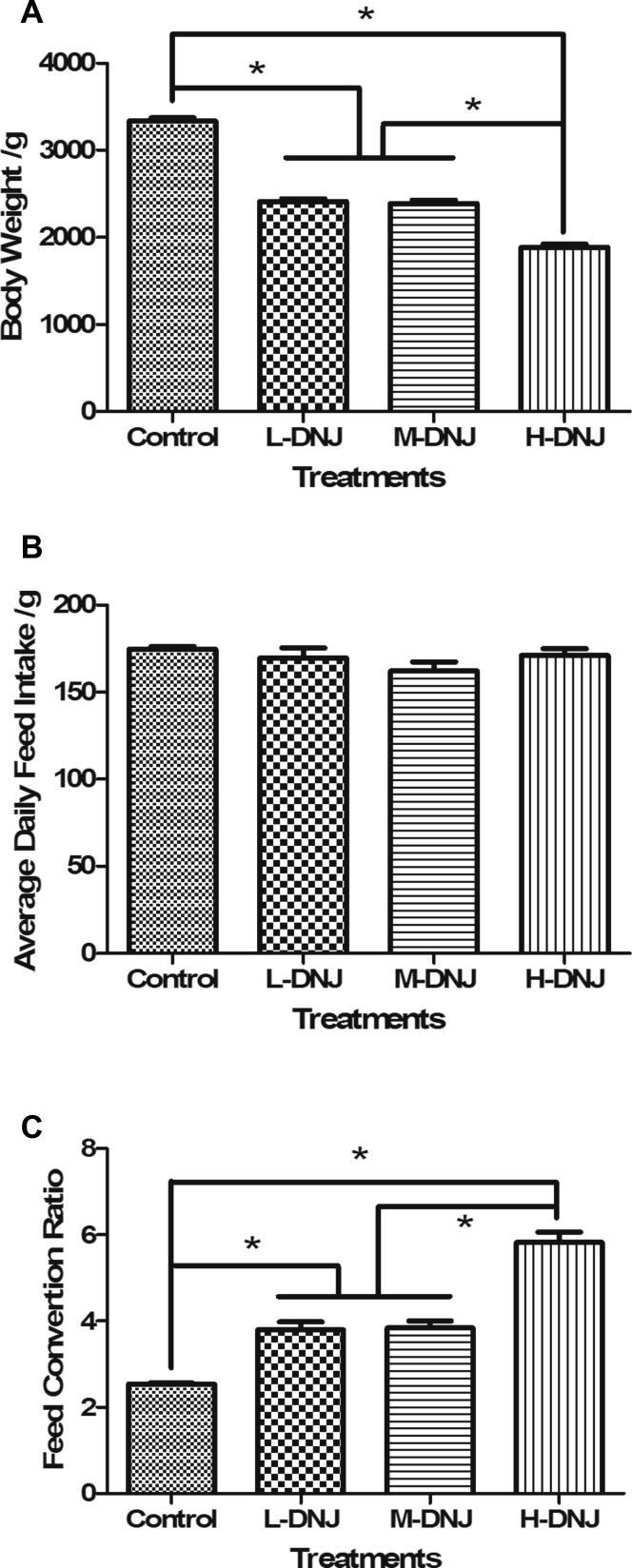
At the end of the experiment, the weight of geese in the DNJ treated groups was lower than that in the control group (P < 0.05, Figure 1A). The body-weight of the geese in the L-DNJ and M-DNJ treatments were similar, and both were higher than that in the H-DNJ treatment. The DNJ supplement did not significantly affect the feed intake of the geese (P > 0.05, Figure 1B). The feed conversion ratio was calculated according to the body-weight gain and feed intake and increased in DNJ-treated groups (P < 0.05, Figure 1C). High-dose DNJ in the H-DNJ treatment increased the feed conversion ratio by almost half.
Figure 1.
Effects of mulberry-derived 1-deoxynojirimycin on body weight (A), average daily feed intake (B), and feed conversion ratio (C). ∗P < 0.05 vs. control.
Effect of DNJ on Nutrient Utilization
As showed in Table 2, the apparent utilization of organic matter, metabolizable energy, ether extract, acid detergent fiber, and calcium were lower in the M-DNJ and L-DNJ groups than those in the control (P < 0.05), while that of crude protein was enhanced in all the DNJ treated groups (P < 0.01). A high dose of DNJ in the H-DNJ group improved the utilization of soluble phosphorus (P < 0.05). The DNJ supplement did not significantly affect the utilization of dry matter and neutral detergent fiber (P > 0.05).
Table 2.
Effects of mulberry-derived 1-deoxynojirimycin on nutrient apparent utilization of Wanxi white geese1.
| Items | Treatments |
SEM | % |
|||
|---|---|---|---|---|---|---|
| Con | L-DNJ | M-DNJ | H-DNJ | P value | ||
| Dry matter | 76.49 | 76.81 | 76.90 | 77.10 | 3.24 | 0.267 |
| Organic matter | 78.70a | 77.52a,b | 75.30b | 70.47c | 3.37 | 0.007 |
| Metabolizable energy | 69.07a | 68.05a | 64.76b | 62.49b | 1.21 | 0.004 |
| Crude protein | 66.62c | 69.40b | 71.84a,b | 73.74a | 2.96 | 0.006 |
| Ether extract | 80.89a | 79.79a,b | 76.93b | 69.46c | 2.38 | 0.002 |
| Neutral detergent fiber | 39.37 | 39.75 | 39.73 | 39.55 | 1.33 | 1.245 |
| Acid detergent fiber | 24.58a | 23.21a,b | 22.35b | 20.94c | 1.67 | 0.022 |
| Calcium | 36.35a | 35.31a | 31.87b | 30.58b | 2.48 | 0.005 |
| Soluble phosphorus | 36.53b | 36.65b | 37.07a,b | 38.16a | 2.30 | 0.013 |
a-cValues in the same row with different letter superscripts were significantly different (P < 0.05).
Data represent the means of 12 replicates.
Effect of DNJ on Digestive Organ and Enzymes Activity
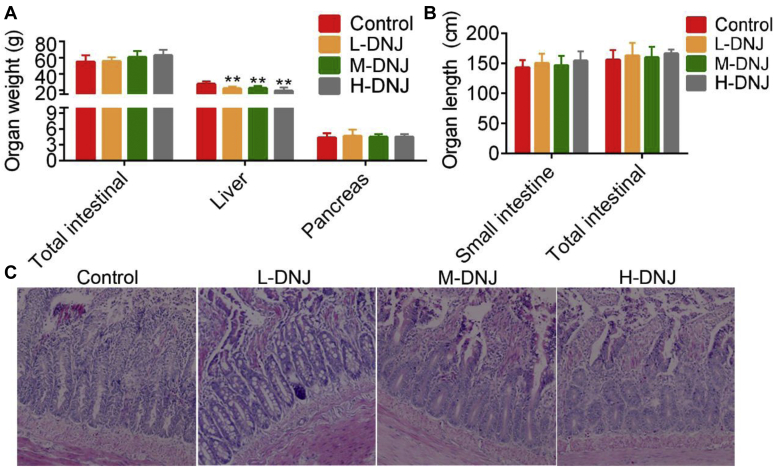
DNJ treatments reduced liver weight in the geese (P < 0.01, Figure 2A), but did not influence the weight of the total intestine and pancreas (Figure 2A) or the length of the small intestine and total intestinal (Figure 2B). Histopathology analysis using HE staining showed that the intestinal villi in the control group were normal and the finger-like structures were clearly observed. With increased of DNJ in the diets, the epithelial cells of the small intestinal mucosa vacuolated; also, the villi length was significantly shortened, became sparse and partially fractured, and was shed. In the M-DNJ and H-DNJ groups, the infiltration of inflammatory cells into the submucosa of the intestinal wall increased, and a greater secretion of mucus caused a light staining color. Dotted hemorrhagic foci were also seen in the lamina propria of the intestinal wall in M-DNJ and H-DNJ groups (Figure 2C).
Figure 2.
Effects of mulberry-derived 1-deoxynojirimycin on organ weight and length. (A) The weight of total intestinal, liver, and pancreas. (B) The length of small intestine and total intestinal. (C) Representative pictures of HE histopathology analysis for small intestine (200 × microscope). ∗∗P < 0.01 vs. control.
As can be seen from Table 3, a high dose of DNJ in the H-DNJ group increased the activity of trypsin in the pancreas (P < 0.01) but lowered that of amylase in the duodenum (P < 0.05). The activity of amylase in the pancreas was inhibited in all the DNJ treated groups (P < 0.05). Lipase activity in the pancreas and duodenum was reduced slightly (P > 0.05).
Table 3.
Effects of mulberry-derived 1-deoxynojirimycin on digestive enzyme activities of Wanxi white geese1.
| Organs | Items | Treatments |
SEM | U/g |
|||
|---|---|---|---|---|---|---|---|
| Con | L-DNJ | M-DNJ | H-DNJ | P value | |||
| Pancreas | Trypsin | 192.35b | 196.14b | 200.27a,b | 206.25a | 16.31 | 0.008 |
| Amylase | 45975a | 44720b | 44539b | 43702b | 2,311.21 | 0.010 | |
| Lipase | 200.98 | 199.18 | 199.04 | 196.24 | 8.65 | 1.561 | |
| Duodenum | Trypsin | 29.41 | 29.53 | 29.73 | 29.90 | 1.21 | 1.385 |
| Amylase | 74513a | 74438a | 73872a,b | 72673b | 2,696.14 | 0.041 | |
| Lipase | 18.08 | 18.04 | 17.94 | 17.72 | 1.05 | 0.260 | |
a,bValues in the same row with different letter superscripts were significantly different (P < 0.05).
Data represent the means of 12 replicates.
Effect of DNJ on Intestinal Flora
Mulberry-derived DNJ reduced the ACE, Chao 1, and Shannon indices, and the OTU number of the intestinal microorganisms in the geese (Table 4, P < 0.05 or P < 0.01), indicating that the abundance and diversity of the bacteria decreased. The coverage values of the samples were all greater than 0.999, indicating that the species in the samples had a high probability of being detected. The rarefaction curves are shown in Supplementary Figure 1. The OTU clustering analysis was conducted after the sequence declutter optimization, and the rarefaction curves indicated that the data volume of the sequencing was reasonable.
Table 4.
Effects of mulberry-derived 1-deoxynojirimycin on gut microbiotaαdiversity indexes of Wanxi white geese1.
| Indexes | Treatments |
SEM | P value | |||
|---|---|---|---|---|---|---|
| Con | L-DNJ | M-DNJ | H-DNJ | |||
| ACE | 176.35a | 175.28a | 154.76b | 157.10b | 7.23 | 0.006 |
| Chao 1 | 177.45a | 175.40a | 156.41b | 161.35b | 9.69 | 0.006 |
| Shannon | 2.49a | 1.83b | 2.06a,b | 2.04b | 0.21 | 0.005 |
| Simpson | 0.25 | 0.38 | 0.32 | 0.27 | 0.08 | 0.253 |
| OTU number | 169.25a | 151.86b | 145.12b | 139.12b | 7.11 | 0.005 |
| Coverage | 0.9997a | 0.9994b | 0.9997a | 0.9996a,b | 0.0001 | 0.043 |
a,bValues in the same row with different letter superscripts were significantly different (P < 0.05).
Data represent the means of 8 replicates.
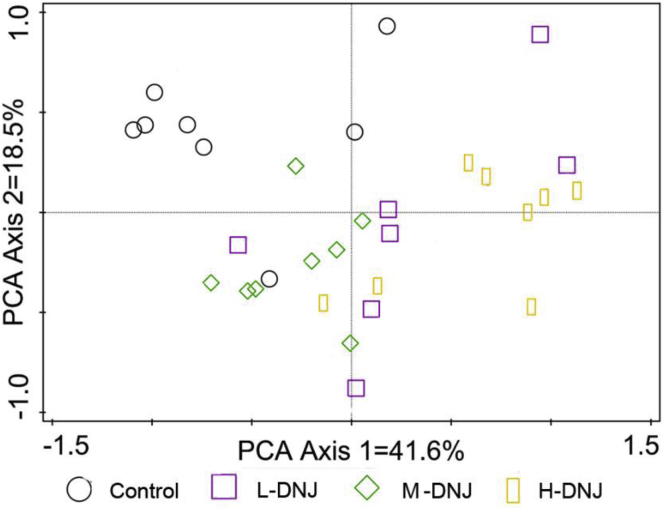
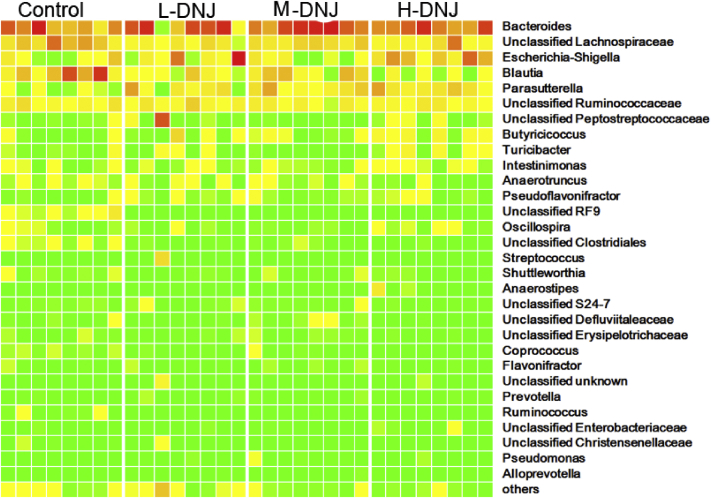
Principal component analysis based on the Unweighted UniFrac distance showed mulberry-derived DNJ treated samples were distinct from the control (Figure 3). Heatmap analysis based on OTU abundance at the genera level is shown in Supplementary Figure 2.
Figure 3.
Principal component analysis (PCA) of intestinal bacterial OTUs of geese in the 4 groups.
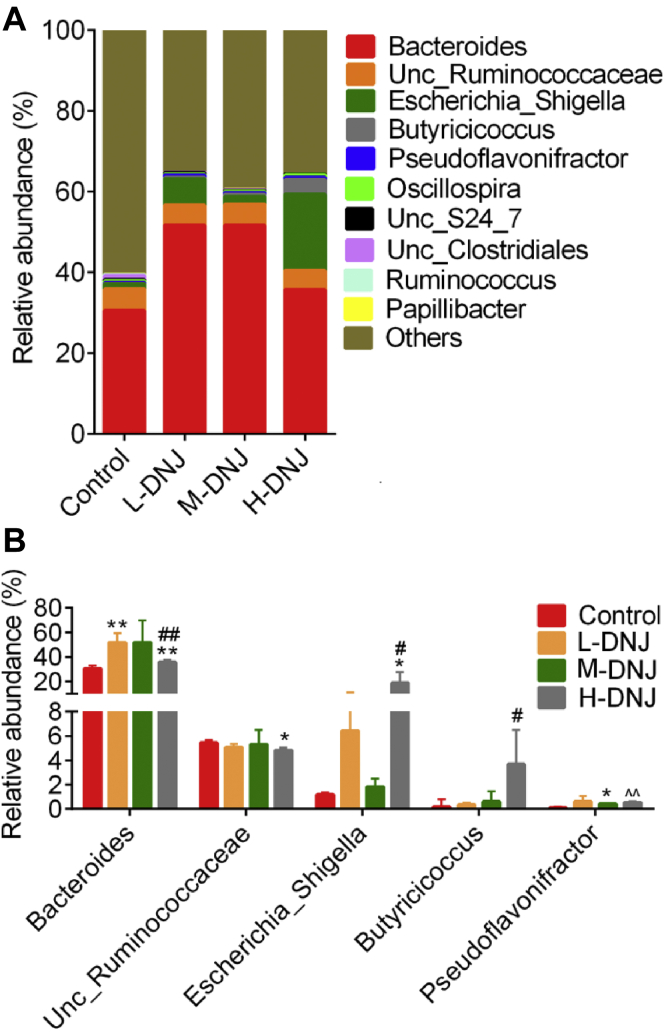
Figure 4A showed the Bacteroides, unclassified Ruminococcaceae, and Escherichia-Shigella were the dominant genera in the intestinal flora of the geese. The DNJ increased the relative abundance of Bacteroides (35.73 ∼ 51.66%) in geese compared with the control (30.52%, P < 0.05, Figure 4B). High-dose DNJ in H-DNJ group increased the abundance of Escherichia-Shigella (18.76 vs. 1.19%) and Butyricicoccus (3.68 vs. 0.17%, P < 0.05) and reduced the unclassified Ruminococcaceae (4.82 vs. 5.43%, P < 0.05) compared to the control. The abundances of Pseudoflavonifractor (0.12 ∼ 0.63%), Oscillospira (0.20 ∼ 0.67%), unclassified_S24_7 (0.18 ∼ 0.34%), and unclassified Clostridiales (0.01 ∼ 1.03%) were low in the intestinal bacteria of the geese, the proportions of these bacteria were also varied between groups (Figure 4 and Supplementary Table 1).
Figure 4.
The relative abundance of intestinal bacteria at genus level of geese in the 4 groups. (A) The relative abundance of several genera with more than 0.1% relative abundance. (B) The relative abundance of genera with >1% relative abundance. ∗P < 0.05 vs. control. ∗∗P < 0.01 vs. control. #P < 0.05 vs. L-DNJ. ##P < 0.01 vs. L-DNJ. ˆˆP < 0.01 vs. M-DNJ.
Discussion
ItzáOrtiz et al. (2010) reported that, with an increase of mulberry flour in diet, a linear decrease in the body-weight of broilers was observed, together with a quadratic effect in feed intake and conversion. In the study by Wang et al. (2016), geese fed mulberry leaf meal exhibited low weight gains, elevated feed consumption, and increased feed to gain ratio compared with geese without mulberry in their diet. However, it remained unclear why nutrient-rich mulberry leaves could not be used in poultry feed or widely used. Our study showed that DNJ from mulberry leaves inhibited the body-weight gain and increased the feed conversion ratio in geese. Indeed, DNJ was one factor in mulberry leaf that affected the digestion system of the geese.
Previous studies have shown that mulberry leaves affect feed intake and intestinal development in animals, prolong the retention of chyme in the intestinal tract, and increase intestinal weight (Prasad et al., 2003; Simol et al., 2012; Hou et al., 2020b). In our study, DNJ did not greatly affect feed intake, neither did it affected intestinal development or cause chyme accumulation. In these respects, it was different from the effect of mulberry leaves, indicating that DNJ was not the component in mulberry leaf that affected feed intake and intestinal development in healthy animals. However, DNJ does reduce food intake and so can be used to prevent and treat obesity. As a study on high-fat-induced obesity mice reported, DNJ regulates body-weight by increasing adiponectin levels in the hypothalamus, which affects food intake (Kim et al., 2017).
Metabolome analysis showed that mulberry-derived DNJ administration had positive effects on glucose, lipid, and amino acid metabolism in mice with type-2 diabetes (T2DM) (Liu et al., 2011b; Hu et al., 2017). In this study, the apparent digestibility data showed that the metabolism of most nutrients in feed was affected by the DNJ supplement. Many enzymes are involved in the metabolism of nutrients, and the activity of enzymes determines the rate of nutrients metabolism. DNJ and its derivatives strongly inhibit the activities of many carbohydrases, including glucoamylase, α-amylase, and sucrose (Samulitis et al., 1987). The regulation of glucose and lipid metabolism by DNJ has been demonstrated in many animal models (Kim et al., 2011; Li et al., 2019). Carbohydrates and lipids are important energy sources for animal growth (Singh et al., 2013), but DNJ slows down their metabolism. To compensate for the lack of energy needed by the body, the geese improved their ability to digest proteins and amino acids by stimulating the activity of trypsin; this was consistent with the findings of a previous research on mice (Hu et al., 2017). Another evidence for reduced nutrient digestibility comes from the reduced liver weight and damaged villi of the small intestine. The liver is an important digestive gland in poultry. It secretes bile, participates in the metabolism of sugars, proteins, and fat and has important functions such as detoxification and provision of immunity (Hu et al., 2019). The liver weight of the geese in DNJ-treated groups was lower (P < 0.01) than that in the control group. A goose liver that is significantly less than the normal weight may have suffered injury and become incapacitated (van Zutphen et al., 2016). The villi of the small intestine enlarge the surface area of intestinal epithelial cells in contact with nutrients; however, the shortened or damaged villi caused by exposure to DNJ were not conducive to the digestion and absorption of nutrients in the intestines of the geese (Kumar and Patra, 2017). Most of the liver weight loss is due to the reduced liver fat content, which is consistent with DNJ's hypoglycemic and lipid reduction effect (Tsuduki et al., 2013). Nutritional deficiencies, inflammation, and stress can all lead to villi damage (Liu et al., 2017; Murugaiah et al., 2017; Ilić et al., 2018). At present, it has not been reported that DNJ can directly damage intestinal villi, but the effect of DNJ on animal digestive metabolism may indirectly cause structural changes in intestinal villi.
Diet has a dominant role in shaping intestinal microbiome (Zhang et al., 2010). Numerous bacteria in the intestinal tract are capable of digesting and supplying nutrients (Yin and Huang, 2016). With sufficient OTU coverage to accurately describe the bacterial composition of each sample, our sequencing results showed that both the richness indices (ACE, Chao 1, and OTU number) and diversity indices (Shannon) were decreased by DNJ supplementation. The principal component analysis further indicated differences in cecum microbiota community structure between DNJ treatments and the control. Thus, we concluded that feeding mulberry leaves containing DNJ could change the intestinal bacterial community of geese, which agreed with the results of a previous study in which DNJ improved glycolipid metabolism by restoring the structure of intestinal flora (Zheng et al., 2019b).
In this study, Bacteroides, unclassified Ruminococcaceae, and Escherichia-Shigella were considered the dominant genera of intestinal bacteria in geese, which was consistent with the report of Liu et al. (2011a). Bacteroides was the most dominant genus in the geese cecum contents and was increased by DNJ supplementation. Gut Bacteroide are beneficial in preventing obesity and insulin resistance, and it has been reported that DNJ can produce therapeutic effects by increasing the abundance of Bacteroide (Yan et al., 2016; Yang et al., 2016; Sheng et al., 2017). It has also been reported that the relative abundance of Bacteroides in lean pigs is higher than that in obese pigs (Yan et al., 2016), and an increased abundance of Bacteroides in the cecum indicates improved meat quality in chicken (Stanley et al., 2014; Zheng et al., 2019a).
Members of the genus Shigella are phenotypically similar to Escherichia coli and, with the exception of Shigella boydii serotype 13, would be considered the same species by DNA-DNA hybridization analysis and whole-genome sequence analysis (Strockbine et al., 2015). Reduced diversity of bacterial species and greater abundance of Escherichia-Shigella are considered harmful and can cause irritable bowel syndrome (IBS), which is a common disorder that affects the large intestine. Signs and symptoms include cramping, abdominal pain, bloating, gas, and diarrhea or constipation that causes changes in defecation habits (Distrutti et al., 2016). Many species within the phylum Firmicutes are thought to exert anti-inflammatory effects. Butyrate-producing bacteria are promising probiotic candidates to target microbial dysbiosis in gastrointestinal disorders such as inflammatory bowel diseases (Geirnaert et al., 2015). DNJ increased the abundance of Escherichia-Shigella and Butyricicoccus in the goose gut, indicating that the defecation habit of the geese had changed, and the cause of the change was probably enteritis.
Ruminococcaceae is a big family from the order Clostridiales that specialize in the degradation of complex plant material and has been associated with the maintenance of gut health (Biddle et al., 2013). A high-dose of NDJ in the H-DNJ group reduced the abundance of unclassified Ruminococcaceae in the goose gut. This decrease in the abundance of Ruminococcaceae was not conducive to the digestion of the coarse fiber in the feed, which may be one reason for the low apparent digestibility of fiber.
The relative abundances of many other intestinal bacteria were also changed by the addition of mulberry DNJ in feed. Changes in the structure of the intestinal flora are often linked to nutrient metabolism and energy.
In brief, DNJ inhibited the activities of amylase and lipase, reduced liver weight, induced shorter or impaired intestinal villi, reduced the abundance and diversity of the intestinal flora, and changed its composition. All these changes in the digestive system caused by DNJ seriously affected the metabolism of nutrients in the feed and were related to the decrease in the growth performance of the geese. Thus, it can be concluded that DNJ may be one factor influencing the utilization value of mulberry leaf in poultry feed. Attention should be paid to the role of DNJ when using mulberry leaves as feed for poultry.
Acknowledgments
This research was supported by the Construction of Modern Agricultural Industry Technology System of China (Sericulture Industry Technology in China Agriculture Research System [CARS-18-ZJ0207]), the Overseas Study Program 2016 of Colleges and Universities in Jiangsu Province for Outstanding Young and Middle-aged Teachers, Jiangsu Science and Technology Project (BZ2016055).
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.07.048.
Contributor Information
Qirui Hou, Email: houqr@just.edu.cn.
Weiguo Zhao, Email: wgzsri@126.com.
Supplementary data
Supplementary Figure 1.
The rarefaction curves of intestinal bacterial OTUs of the geese.
Supplemenary Figure 2.
Heatmap of intestinal bacteria of geese in the 4 groups based on OTU abundance at the genera level.
References
- AOAC . 18th ed. Association of Official Analytical Chemists Int.; Washington, DC: 2000. Official Methods of Analysis. [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S.J.D. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Chan W.C., Lye P.Y., Wong S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Medicines. 2016;14:17–30. doi: 10.3724/SP.J.1009.2016.00017. [DOI] [PubMed] [Google Scholar]
- Chhay T., Borin K., Preston T. Effect of Taro (Colocasia esculenta) leaf + stem silage and mulberry leaf silage on digestibility and N retention of growing pigs fed a basal diet of rice bran. Livest. Res. Rural Dev. 2010;22:109. [Google Scholar]
- Distrutti E., Monaldi L., Ricci P., Fiorucci S. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J. Gastroentero. 2016;22:2219. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran M., Laca E., Sainz R. Total tract and rumen digestibility of mulberry foliage (Morus alba), alfalfa hay and oat hay in sheep. Anim. Feed Sci. Tech. 2007;138:239–253. [Google Scholar]
- Geirnaert A., Wang J., Tinck M., Steyaert A., Van den Abbeele P., Eeckhaut V., Vilchez-Vargas R., Falony G., Laukens D., De Vos M., Van Immerseel F., Raes J., Boon N., Van de Wiele T. Interindividual differences in response to treatment with butyrate-producing Butyricicoccus pullicaecorum 25–3T studied in an in vitro gut model. FEMS Microbiol. Ecol. 2015;91:054. doi: 10.1093/femsec/fiv054. [DOI] [PubMed] [Google Scholar]
- Goering H.K., Van Soest P.J. US Department of Agriculture; 1970. Forage Fiber Analyses: Apparatus, Reagents, Procedures, and Some Applications (No. 379). Agricultural Research Service. Accessed Sep. 2020. https://www.researchgate.net/publication/275346632_Forage_Fiber_Analyses_Apparatus_Reagents_Procedures_and_Some_Applications. [Google Scholar]
- Hu R., He Y., Arowolo M.A., Wu S., He J. Polyphenols as potential attenuators of heat stress in poultry production. Antioxidants. 2019;8:67. doi: 10.3390/antiox8030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.Q., Thakur K., Chen G.H., Hu F., Zhang J.G., Zhang H.B., Wei Z.J. Metabolic effect of 1-Deoxynojirimycin from mulberry leaves on db/db diabetic mice using liquid chromatography–mass spectrometry based metabolomics. J. Agr. Food Chem. 2017;65:4658–4667. doi: 10.1021/acs.jafc.7b01766. [DOI] [PubMed] [Google Scholar]
- Hu D., Xu Y., Xie J., Sun C., Zheng X., Chen W. Systematic evaluation of phenolic compounds and protective capacity of a new mulberry cultivar J33 against palmitic acid-induced lipotoxicity using a simulated digestion method. Food Chem. 2018;258:43–50. doi: 10.1016/j.foodchem.2018.03.049. [DOI] [PubMed] [Google Scholar]
- Hou Q., Thidza I.Z., Miao L., Dong Z., Pan W., Zhu W., Wang L., Fu J., Zhao W., Li L. Lipid metabolism responses of common carp (Cyprinus carpio L.) to mulberry leaf meal in diet. Aquac. Res. 2020;51:719–727. [Google Scholar]
- Hou Q.R., Zhang J., Chen T., Zhao W.G., Li L. Effects of dietary supplement of mulberry leaf (Morus alba) on growth and meat quality in rabbits. Indian J. Anim. Res. 2020;54:317–321. [Google Scholar]
- Ilić I.R., Stojanović N.M., Randjelović P., Mitić K., Sokolović D., Stevanović M., Radulović N., Živković V. Oregano (Origanum vulgare) essential oil prevents L-arginine-induced rat ileum villi damage. FU Phys. Chem. Tech. 2018;16:90. [Google Scholar]
- ItzáOrtiz M., Lara y Lara P., Magaña M., Sanginés G. Evaluation of mulberry (Morus alba) leaf flour in broiler feeding. Zootecn. Trop. 2010;28:477–488. [Google Scholar]
- Kim G.N., Kwon Y.I., Jang H.D. Mulberry leaf extract reduces postprandial hyperglycemia with few side effects by inhibiting α-glucosidase in normal rats. J. Med. Food. 2011;14:712–717. doi: 10.1089/jmf.2010.1368. [DOI] [PubMed] [Google Scholar]
- Kim J., Yun E.Y., Quan F.S., Park S.W., Goo T.W. Central administration of 1-deoxynojirimycin attenuates hypothalamic endoplasmic reticulum stress and regulates food intake and body weight in mice with high-fat diet-induced obesity. Evid-Based Complement. Altern. Med. 2017;2017:1–11. doi: 10.1155/2017/3607089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Patra A. Beneficial uses of black cumin (Nigella sativa L.) seeds as a feed additive in poultry nutrition. World. Poult. Sci. J. 2017;73:872–885. [Google Scholar]
- Li Y., Zhong S., Yu J., Sun Y., Zhu J., Ji D., Wu C. The mulberry-derived 1-deoxynojirimycin (DNJ) inhibits high-fat diet (HFD)-induced hypercholesteremia and modulates the gut microbiota in a gender-specific manner. J. Func. Foods. 2019;52:63–72. [Google Scholar]
- Liang L., Wu X., Zhao T., Zhao J., Li F., Zou Y., Mao G., Yang L. In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 2012;46:76–82. [Google Scholar]
- Liu S.X., Hua J.L., Wang B. Hypoglycemic function of mulberry leaf polysaccharide and 1-deoxynojirimycin (DNJ) Adv. Mater. Res. 2011;361:808–812. [Google Scholar]
- Liu Y., Luo S., Kou L., Tang C., Huang R., Pei Z., Li Z. Ischemic stroke damages the intestinal mucosa and induces alteration of the intestinal lymphocytes and CCL19 mRNA in rats. Neurosci. Lett. 2017;658:165–170. doi: 10.1016/j.neulet.2017.08.061. [DOI] [PubMed] [Google Scholar]
- Liu B., Wang Z., Wang H., Hu P., Xu D., Wang Q. Molecular profiling of bacterial species in the caecum of geese. Czech J. Anim. Sci. 2011;56:192–203. [Google Scholar]
- Liu C., Xiang W., Yu Y., Shi Z.Q., Huang X.Z., Xu L. Comparative analysis of 1-deoxynojirimycin contribution degree to α-glucosidase inhibitory activity and physiological distribution in Morus alba L. Ind. Crop Prod. 2015;70:309–315. [Google Scholar]
- Ma T., Chen D.D., Tu Y., Zhang N.F., Si B.W., Diao Q.Y. Dietary supplementation with mulberry leaf flavonoids inhibits methanogenesis in sheep. Anim. Sci. J. 2017;88:72–78. doi: 10.1111/asj.12556. [DOI] [PubMed] [Google Scholar]
- Mikkelsen P.B., Toubro S., Astrup A. Effect of fat-reduced diets on 24-h energy expenditure: comparisons between animal protein, vegetable protein, and carbohydrate. Am. J. Clin. Nutr. 2000;72:1135–1141. doi: 10.1093/ajcn/72.5.1135. [DOI] [PubMed] [Google Scholar]
- Murugaiah C., Noor N.Z.N.M., Mustafa S., Manickam R., Pattabhiraman L. Evaluation of intestinal damage caused by V. ácholerae O139, an inávivo study. Microb. Pathogenesis. 2017;105:25–29. doi: 10.1016/j.micpath.2017.02.002. [DOI] [PubMed] [Google Scholar]
- NRC . National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry: National Research Council. [Google Scholar]
- Olteanu M., Criste Rodica D., Cornescu Gabriela M., Ropota M., Panaite T., Varzaru I. Effect of dietary mulberry (Morus alba) leaves on performance parameters and quality of breast meat of broilers. Indian J. Anim. Sci. 2015;85:291–295. [Google Scholar]
- Prasad R., Misra A., Sankhyan S., Mishra A., Tripathi M., Karim S., Jakhmola R. Growth performance and caecal fermentation in growing rabbits fed on diets containing graded levels of mulberry (Morus alba) leaves. Asian Austral. J. Anim. Sci. 2003;16:1309–1314. [Google Scholar]
- Saenthaweesuk S. Effect of dietary supplementation of mulberry (Morus alba L.) leaf powder in place of antibiotic in feed ration to improve growth performance, meat quality and production of broiler chicken. J. Sci. Technol. Mahasarakham Univ. 2009;28:285–290. [Google Scholar]
- Samulitis B., Goda T., Lee S., Koldovský O. Inhibitory mechanism of acarbose and 1-deoxynojirimycin derivatives on carbohydrases in rat small intestine. Drugs Exp. Clin. Res. 1987;13:517–524. [PubMed] [Google Scholar]
- Sánchez-Salcedo E., Amorós A., Hernández F., Martínez J. Physicochemical properties of white (Morus alba) and black (Morus nigra) mulberry leaves, a new food supplement. J. Food Nutr. Res. 2017;5:253–261. [Google Scholar]
- Sheng Y., Zheng S., Ma T., Zhang C., Ou X., He X., Xu W., Huang K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-12245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simol C.F., Tuen A.A., Khan H.H.A., Chubo J.K., King P.J.H., Ong K.H. Performance of chicken broilers fed with diets substituted with mulberry leaf powder. Afr. J. Biotechnol. 2012;11:16106–16111. [Google Scholar]
- Singh V.K., Pattanaik A.K., Goswami T.K., Sharma K. Effect of varying the energy density of protein-adequate diets on nutrient metabolism, clinical chemistry, immune response and growth of muzaffarnagari lambs. Asian Austral. J. Anim. Sci. 2013;26:1089–1101. doi: 10.5713/ajas.2012.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Strockbine N.A., Bopp C.A., Fields P.I., Kaper J.B., Nataro J.P. Escherichia, Shigella, and Salmonella. In: Jorgensen J., Pfaller M., Carroll K., Funke G., Landry M., Richter S., editors. Manual of Clinical Microbiology. D. Warnock.; Washington, DC: 2015. pp. 685–713. [Google Scholar]
- Tsuduki T., Kikuchi I., Kimura T., Nakagawa K., Miyazawa T. Intake of mulberry 1-deoxynojirimycin prevents diet-induced obesity through increases in adiponectin in mice. Food Chem. 2013;139:16–23. doi: 10.1016/j.foodchem.2013.02.025. [DOI] [PubMed] [Google Scholar]
- van Zutphen T., Ciapaite J., Bloks V.W., Ackereley C., Gerding A., Jurdzinski A., de Moraes R.A., Zhang L., Wolters J.C., Bischoff R. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J. Hepatol. 2016;65:1198–1208. doi: 10.1016/j.jhep.2016.05.046. [DOI] [PubMed] [Google Scholar]
- Venkatesh Kumar R., Gautam C., Shobha N., Lingappa R.S. Use of mulberry leaves as supplementary food in cow and goat to improve milk production. Int. J. Appl. Res. 2015;1:81–84. [Google Scholar]
- Wang C., Yang F., Wang Q., Zhou X., Xie M., Kang P., Wang Y., Peng X. Nutritive value of mulberry leaf meal and its effect on the performance of 35-70-day-old geese. J. Poult. Sci. 2016;54:0160070. doi: 10.2141/jpsa.0160070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Diao H., Xiao Y., Li W., Yu B., He J., Yu J., Zheng P., Mao X., Luo Y. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci. Rep. 2016;6:31786. doi: 10.1038/srep31786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.Y., Lee Y.S., Kim Y., Lee S.H., Ryu S., Fukuda S., Hase K., Yang C.S., Lim H.S., Kim M.S. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2016;10:104–116. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- Yatsunami K., Ichida M., Onodera S. The relationship between 1-deoxynojirimycin content and α-glucosidase inhibitory activity in leaves of 276 mulberry cultivars (Morus spp.) in Kyoto, Japan. J. Nat. Medicines. 2008;62:63–66. doi: 10.1007/s11418-007-0185-0. [DOI] [PubMed] [Google Scholar]
- Yin H., Huang J. Effects of soybean meal replacement with fermented alfalfa meal on the growth performance, serum antioxidant functions, digestive enzyme activities, and cecal microflora of geese. J. Integr. Agr. 2016;15:2077–2086. [Google Scholar]
- Zhang C., Zhang M., Wang S., Han R., Cao Y., Hua W., Mao Y., Zhang X., Pang X., Wei C., Zhao G., Chen Y., Zhao L. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tang Y., Chen K.A., Sun H. Leaf yield and biological production of mulberry planted in contour hedgerows of nitrogen-fixing plants. Chin. J. Appl. Environ. Biol. 2001;7:303–307. [Google Scholar]
- Zheng M., Mao P., Tian X., Qiang G., Lin M. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult. Sci. 2019;98:2250–2259. doi: 10.3382/ps/pey550. [DOI] [PubMed] [Google Scholar]
- Zheng J., Zhu L., Hu B., Zou X., Hu H., Zhang Z., Jiang N., Ma J., Yang H., Liu H. 1-Deoxynojirimycin improves high fat diet-induced nonalcoholic steatohepatitis by restoring gut dysbiosis. J. Nutr. Biochem. 2019;71:16–26. doi: 10.1016/j.jnutbio.2019.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.