Abstract
The feed additive Availa-ZMC was investigated for the ability to reduce lameness in broilers using 2 alternative models for inducing lameness. The mixture of organic trace minerals was effective in reducing lameness by 20% in the wire flooring model and 25% in the litter flooring model with the bacterial challenge. Lameness in both models is overwhelmingly attributable to bacterial chondronecrosis with osteomyelitis. The reduction in lameness was associated, at least in part, with enhanced intestinal barrier integrity mediated by elevated expression of tight junction proteins and stimulation of bactericidal killing of adherent peripheral blood monocytes obtained from the birds treated with Availa-ZMC. Lameness is a major animal welfare concern in broiler production. The wire flooring model and litter flooring model with the bacterial challenge are effective models for evaluation of management strategies for mitigating infectious causes of lameness.
Key words: broiler, lameness, chondronecrosis, Staphylococcus, organic trace mineral
Introduction
Lameness is one of the most significant animal welfare issues in the broiler industry, resulting in annual losses of millions of dollars (Wideman, 2016; Siegel et al., 2019). A wire flooring model has been shown to induce a high incidence of lameness in broilers (Wideman et al., 2012; Wideman and Prisby, 2013; Wideman et al., 2013; Wideman et al., 2014; Wideman, 2016). Lameness induced in this system is overwhelmingly bacterial chondronecrosis with osteomyelitis (BCO) of the proximal tibiae and femora (Wideman et al., 2012; Wideman and Prisby, 2013; Wideman et al., 2013; Wideman, 2016). The predominant isolates from BCO lesions of birds grown on wire flooring on our research farm are Staphylococcus agnetis, and the BCO lameness is sometimes associated with significant bacteremia (Al-Rubaye et al., 2015). The type strain, S. agnetis 908, when administered in drinking water can induce high levels of lameness in birds grown on wire or on litter (Al-Rubaye et al., 2015; Alrubaye et al., 2020). The BCO lameness model has demonstrated the following: 1) translocation of bacteria into blood in birds grown on litter, with higher translocation in birds grown on wire flooring (Al-Rubaye et al., 2017); 2) transmission of BCO-inducing pathogens within a flock (Al-Rubaye et al., 2017) or within a facility (Alrubaye et al., 2020); and 3) protection against BCO-inducing pathogens by probiotics and prebiotics (Wideman et al., 2012; Wideman et al., 2015; Wideman, 2016; Alrubaye et al., 2020). We now extend these studies to investigation of a commercial combination of zinc, manganese, and copper from metal amino acid complexes (Availa-ZMC) using the previously validated BCO lameness model. Organic zinc is reported to enhance epithelial integrity, gut health, and immune function (Hudson et al., 2004; Star et al., 2012; Zakaria et al., 2017). Adequate manganese nutrition and copper nutrition are reported to enhance immune function, connective tissue synthesis, and bone development (Hurley and Keen, 1987; Kidd, 2004). The manganese amino acid complex has shown an improved immune response (Burin et al., 2019). The same combination of zinc, manganese, and copper amino acid complexes used in this study has previously shown improvement in bone development in embryos (Favero et al., 2013). We therefore hypothesized that supplementation with Availa-ZMC–complexed trace minerals could be efficacious in reducing BCO lameness in our 2 models for inducing BCO lameness.
Materials and methods
Lameness Trials
All animal experiments were approved by the University of Arkansas Institutional Animal Care and Use Committee under protocols 18,010 and 18,075. One-day (d)-old chicks representing surplus males from a female broiler-breeder product were kindly provided by Cobb-Vantress (Siloam Springs, AR). Depending on the model in use, chicks were placed in 5 × 10 ft. pens on either suspended wire flooring (Wideman et al., 2012; Wideman, 2016) or on standard wood shaving litter, at 60 per pen. Nipple water lines were supplied with city tap water on one side of the pen, and 2 feeders were placed on the opposite side. Feed was standard starter through day 35 and finisher through day 56. Computer controllers regulated the temperature, photoperiod, and ventilation. Tunnel ventilation and evaporative cooling pads were automatically activated when needed. The photoperiod was set for 23-hour light:1-hour darkness for the duration of the experiment. Thermoneutral temperature targets were as follows: 90°F for day 1 to day 3, 88°F for day 4 to day 6, 85°F for day 7 to day 10, 80°F for day 11 to day 14, and 75°F thereafter. On day 19, all pens were culled to 50 birds.
In the litter flooring model with the bacterial challenge, source pens were challenged with S. agnetis in the drinking water. The tap water supplying the nipple waterer was replaced with a gravity flow from an elevated 20-L carboy of tap water. The bacteria (stationary overnight culture) were mixed in tap water in the carboy to a concentration of 104 colony-forming units per mL. After day 21, the nipple supply was returned to the tap water. All water lines were flushed with dilute bleach and fresh tap water before each experiment.
For both wire and litter flooring models, beginning on day 20, all birds were encouraged twice per day to move using standard kitchen brooms. Any bird that was reticent to move was marked with spray paint. Birds that continued to be unwilling or unable to walk were diagnosed as “clinically lame” and euthanized. All birds that died or were diagnosed as clinical lame were recorded by date and pen number. Necropsy for BCO lameness was as described by Wideman, 2016 and categorized as follows: N = normal proximal femur head or proximal tibia head; KB = Kinky Back (spondylolisthesis); FHS = proximal femoral head separation (epiphyseolysis); FHT = proximal femoral head transitional degeneration; FHN = proximal femoral head necrosis; THN = proximal tibial head necrosis; other = symptoms other than BCO, and total lame included all birds with any FHS, FHT, FHN, THN, or KB lesions.
For trace mineral dietary supplementation, both inorganic and complex organic sources were used. Inorganic sources consisted of zinc sulfate (ZnSO4·H2O), with 35.5% of Zn, manganese sulfate (MnSO4·H2O) with 32.0% of Mn, and copper sulfate (CuSO4 5H2O) with 25.2% of Cu. Availa-ZMC (Zinpro Corporation, Eden Prairie, MN) with 4% Zn, 4% Mn, and 0.7% Cu from amino acid complexes accounted for the organic portion. The control inorganic group included 100 ppm of Zn, 100 ppm of Mn, and 20.5 ppm of Cu from sulfate sources, with no added complexed organic portion. The Avila-ZMC normal treatment included 1,000 mg of the commercial product per kilogram of feed, providing 40 ppm of Zn, 40 ppm of Mn, and 7 ppm of Cu from the complexed organic mix, plus 40 ppm of Zn, 40 ppm of Mn, and 10 ppm of Cu from inorganic sulfate sources, giving a total supplementation of 80 ppm of Zn. 80 ppm of Mn, and 17 ppm of Cu. The Availa-ZMC high treatment included 1,500 mg of the commercial product per kilogram of feed, providing 60 ppm of Zn, 60 ppm of Mn, and 10.5 ppm of Cu from the complexed organic mix, plus 40 ppm of Zn, 40 ppm of Mn, and 10 ppm of Cu from sulfate sources, giving a total supplementation of 100 ppm of Zn, 100 ppm of Mn, and 20.5 ppm of Cu. Details on the supplementation levels are described in Table 1. This supplementation substituted for these minerals in the mineral premix, so no background levels other than those provided by feed ingredients were present. The commercial product was added to the feed before pelleting by the University of Arkansas Poultry Research feedmill. The mixer and lines were flushed and cleaned between preparations of feed formulations.
Table 1.
Supplemental levels and sources of minerals added to dietary treatments (ppm).
| Treatment > |
Control: inorganic |
Availa-ZMC normal2 |
Availa-ZMC high3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mineral | Sulfate1 | Complex | Total | Sulfate1 | Complex | Total | Sulfate1 | Complex | Total |
| Zn | 100 | 0 | 100 | 40 | 40 | 80 | 40 | 60 | 100 |
| Mn | 100 | 0 | 100 | 40 | 40 | 80 | 40 | 60 | 100 |
| Cu | 20.5 | 0 | 20.5 | 10 | 7 | 17 | 10 | 10.5 | 20.5 |
Availa-ZMC (4% Zn, 4% Mn, and 0.7% Cu from the metal amino acid complex) added on top of control diet. This supplementation substituted that for each one of these minerals in the mineral premix.
Zinc sulfate monohydrate (ZnSO4 H2O), 35.5% Zn; manganese sulfate monohydrate (MnSO4 H2O), 32.0% Mn; copper sulfate pentahydrate (CuSO4 5H2O), 25.2% Cu.
One thousand milligrams of the commercial product in 1 kg of feed provided additional 40 ppm of Zn, 40 ppm of Mn, and 7 ppm of Cu.
One thousand five hundred milligrams of the commercial product in 1 kg of feed provided additional 60 ppm of Zn, 60 ppm of Mn, and 10.5 ppm of Cu from the amino acid complex.
Histological Evaluation of Intestinal Villi
Intestinal samples (3-cm section) for histopathology were the distal jejunum (1 cm proximal to Meckel's diverticulum) and proximal ileum (1 cm distal to Meckel's diverticulum). The samples from freshly euthanized birds were flushed with 1× PBS and fixed in phosphate-buffered formalin. The fixed samples were processed at the histology laboratory in the Department of Poultry Science at the University of Arkansas. Hematoxylin and eosin–stained sections were imaged under an Olympus inverted scope (Shinjuku City, Tokyo, Japan) at a magnification of 400× using a CCD camper to display on an liquid crystal display monitor. Villus length was measured on a 21-inch diagonal liquid crystal display monitor using a flexible ruler. Calibration was based on a stage micrometer. For villus length and pathology analysis, at least 4 sections were examined from each tissue for each bird (8–10 birds per treatment). For each section, villus length was measured and gross pathology (villus tip integrity) was scored, on 4 sides (top, left, right, and bottom). For some sections, villus length and tip integrity could not be measured on all 4 sides owing to tissue damage in sectioning.
Assay of Intestinal Gene Expression
One microgram of total RNA was extracted from tissue samples by homogenization using Trizol Reagent (Thermo Fisher Scientific, Rockford, IL) in accordance with the manufacturer's recommendations. RNA concentration, quality, and integrity were assessed by the ratio of absorbance (260/280), and electrophoresis was carried out in 1% agarose gels using a Take 3 microvolume plate and the Synergy HT multimode microplate reader (BioTek, Winooski, VT). RNAs were treated with DNAseI and reverse transcribed using the qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). The cDNA was then amplified by real-time quantitative polymerase chain reaction (PCR) (7500 Real-Time PCR system; Applied Biosystems, Foster City, CA) with Power SYBR green Master Mix (Thermo Fisher Scientific, Rockford, IL) in triplicate, with 20 μL per reaction. Oligonucleotide primers specific for chicken were used: occludin (OCLN)—forward 5′-CGCAGATGTCCAGCGGTTA-3′ and reverse 5′-GTAGGCCTGGCTGCACATG-3′; claudin 1 (CLDN1)—forward 5′-CCCACGTTTTCCCCTGAAA-3′ and reverse 5′-GCCAGCCTCACCAGTGTTG-3′; gap junction protein alpha 1—forward 5′-TGGCAGCACCATCTCCAA-3′ and reverse 5′-GGTGCTCATCGGCGAAGT-3′; and catenin beta 1—forward 5′-TGCCCCACTGCGTGAAC-3′ and reverse 5′-TGCTCTAACCAGCAGCTGAACT-3′. Primers for the reference, housekeeping gene r18S, have been published previously (Lassiter et al., 2015; Piekarski-Welsher et al., 2018; Dhamad et al., 2019; Greene et al., 2019). The cycling conditions were as follows: 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s, and 58°C for 1 min with plate read. After PCR, melting curve analysis was applied using the dissociation protocol from the sequence detection system to exclude samples with nonspecific products. PCR products were also confirmed for one specific size band by agarose gel electrophoresis. Negative controls lacked cDNA input as the template for the PCR and were verified for the absence of gel bands. Relative expression of target genes were determined by the 2−ΔΔCt method using r18S as the reference and the control group as the calibrator (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
Phagocytosis Assay
Blood (1 mL) was collected from a wing vein using a vacutainer containing EDTA (Becton, Dickinson and Company, Franklin Lakes, NJ). Monocytes were enriched and cultured using published protocols (Drechsler et al., 2013; Dawes et al., 2014). The medium was RPMI (VWR) with 1× GlutaMax (Life Technologies, Carlsbad, CA) and 10% low-endotoxin fetal bovine serum. After 5 d in culture (37°C; 5% CO2), we challenged the adherent cells in triplicate with an approximate multiplicity of infection of 1:1 with S. agnetis 908 for 2 d following the published methods (Campbell et al., 1994; Drevets et al., 2015). Specifically, the bacteria were added to the medium for 2 h; then, the medium was replaced with media supplemented with gentamycin (50 μg/mL) for 6 h to kill noninternalized bacteria. The medium was replaced with an antibiotic-free medium. After 2 d in culture, the adherent cells are lysed by addition of pure water, and a dilution series of the lysate was plated on Luria Broth agar plates for viable bacterial cell counts.
Statistical Analyses
Data were compared using either the t-test function in Microsoft Excel or a generalized linear model (GLM) module in R 3.4.2 (https://cran.r-project.org) to produce P-values between treatments, as indicated. Gene expression data were analyzed by one-way ANOVA. If ANOVA revealed significant effects, the means were compared using Tukey's multiple range test using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, California). Significant difference was accepted at P ≤0.05. Data are expressed as mean ± SEM.
Results
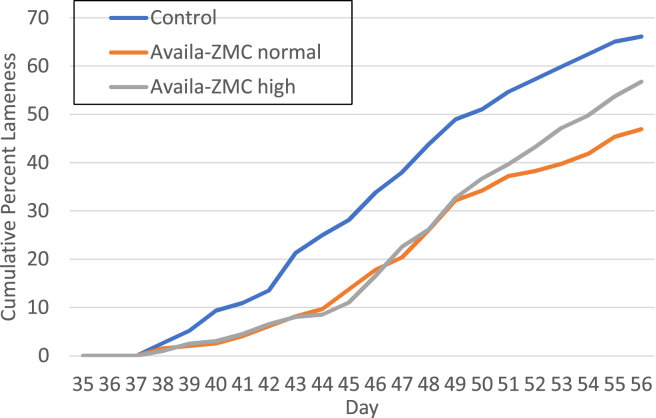
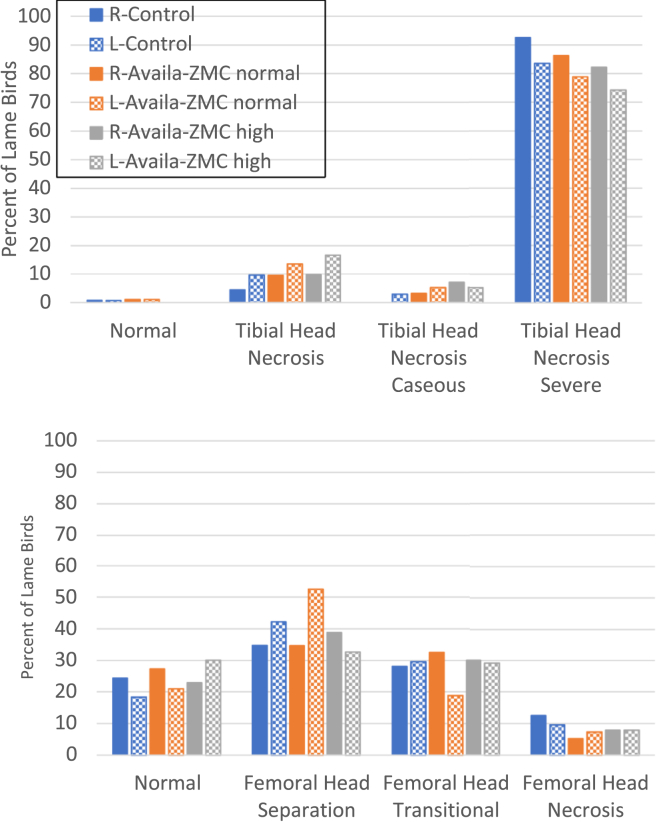
Experiment 1 evaluated whether Availa-ZMC could reduce lameness for birds raised on wire flooring to induce lameness. Chicks (1-day-old) were raised to day 56 on wire flooring with no direct administration of a bacterial challenge. There were 4 pens in each treatment group, and the control treatment received standard feed formulations (Table 1), whereas the Availa-ZMC normal treatment received the product at a concentration of 1,000 mg/kg of feed, and the Availa-ZMC high treatment group received the product at a concentration of 1,500 mg/kg of feed. Feed formulations were continuous through day 56, the end of the experiment. Lameness began to appear in all 3 treatments on day 37, but the trajectory of lameness accumulation was higher for the birds on standard feed (Figure 1). The final cumulative lameness for the control group was 66%, but the Availa-ZMC normal treatment group had 47% lameness, and the Availa-ZMC high treatment group had 57% lameness. Comparison of the lameness data using the GLM with the individual bird as the experimental unit showed that the Availa-ZMC normal treatment was statistically different from the control (P = 0.0003) and Availa-ZMC high treatments (P = 0.03). The control and Availa-ZMC high treatment were not statistically different (P = 0.15). Pen-to-pen variability for the 3 treatments in experiment 1 reveals a degree of variability in total lame per pen within a treatment (Table 2). Loss of birds to mortalities unrelated to lameness and final body weights were not different between treatments. Supplementation of feed with Availa-ZMC at either of the levels had no discernable effect on the severity of BCO lesions of proximal tibiae and femora from birds diagnosed as lame through the course of the experiment (Figure 2).
Figure 1.
Cumulative lameness for broilers raised on wire flooring treated with control feed or feed supplemented with Availa-ZMC. Cumulative percentage of lameness is plotted from day 35 to day 56. Details of the 3 treatments are shown in Table 1. Abbreviation: d, day.
Table 2.
Lame and mortality by pen and ending BW for 3 treatments in experiments 1 and 2.
| Experiment | Pen | Count, lame |
Count, mortality1 |
BW (kg)2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Avg3 | 1 | 2 | 3 | 4 | N | Avg3 | ||
| 1 | Control | 36 | 24 | 27 | 40 | 31.8 ± 3.2a | 2 | 2 | 4 | 0 | 10 | 4.27 ± 0.17 |
| 1 | Availa-ZMC normal | 28 | 25 | 23 | 16 | 23.0 ± 2.2b | 0 | 1 | 0 | 2 | 12 | 4.27 ± 0.07 |
| 1 | Availa-ZMC high | 26 | 25 | 43 | 19 | 28.3 ± 4.5a | 0 | 0 | 0 | 0 | 10 | 4.23 ± 0.09 |
| 2 | Control | 32 | 35 | 32 | 30 | 32.3 ± 0.9a | 0 | 1 | 0 | 0 | 5 | 4.37 ± 0.05 |
| 2 | Availa-ZMC normal | 22 | 23 | 31 | 26 | 25.5 ± 1.8b | 0 | 0 | 0 | 0 | 5 | 4.18 ± 0.05 |
| 2 | Availa-ZMC high | 26 | 26 | 21 | 28 | 25.3 ± 1.3b | 0 | 0 | 0 | 0 | 5 | 4.40 ± 0.07 |
a,bValues within an experiment that are significantly different (P < 0.05) have different superscripts.
Mortality from issues other than lameness.
BW for apparently healthy birds (N) at the end of the experiment (day 56).
Average (Avg) ± SEM.
Figure 2.
Tibial (upper) and femoral (lower) lesion diagnoses for all lame birds raised on wire flooring in experiment 1. Proximal heads were diagnosed for necropsy in right (R) and left (L) leg bones in each of the 3 treatment groups.
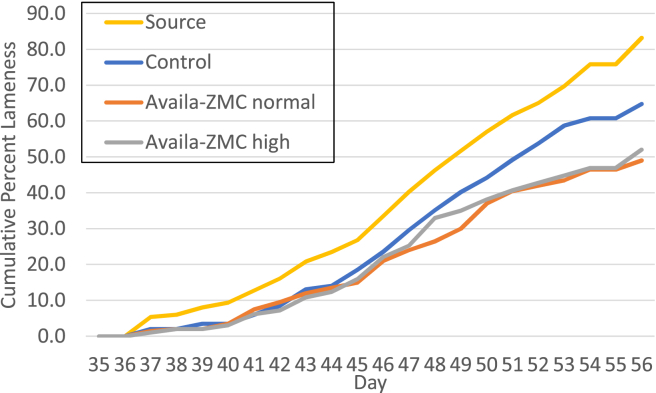
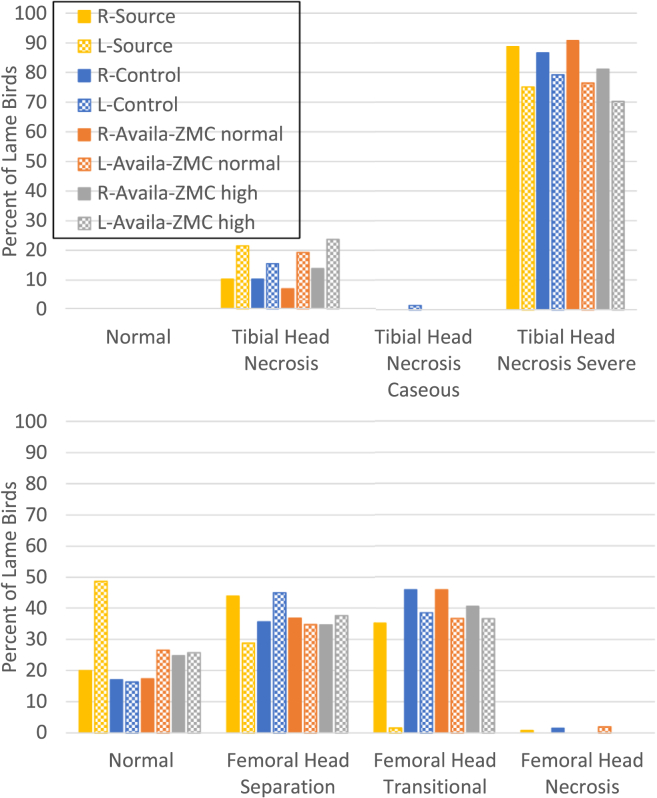
Experiment 2 evaluated whether Availa-ZMC could reduce lameness in birds raised on litter flooring when a bacterial challenge is imposed. The feed supplementation was the same, but lameness was induced by the transmission of the hypervirulent strain S. agnetis 908 from birds challenged with the bacterium in drinking water on day 20 and day 21. There were 3 pens of birds on litter flooring on standard feed that were the source population. These 3 pens were “upwind,” relative to the exhaust fans, of the treatment pens (Table 1). There were 4 pens for each of the 3 treatments: control, Availa-ZMC normal, and Availa-ZMC high, arrayed in a randomized block design and separated by at least 3 m from the source pens. Lameness began to appear on day 36, but lameness accumulation was accentuated in the source population (Figure 3). Accumulation of lameness in the 3 treatment groups lagged behind that for the source population by about 3 to 4 d through day 48, when the cumulative lameness in the control group continued to parallel that for the source population, but the lameness accumulation is reduced for both Availa-ZMC treatments. Final percentage of lameness was as follows: source, 83%; control, 65%; Availa-ZMC normal, 49%; and Availa-ZMC high, 52%. GLM-based comparisons of the lameness data with the individual bird as the experimental unit showed that the percentage of lameness was statistically higher in the control treatment than in the Availa-ZMC normal (P = 0.002) and Availa-ZMC high (P = 0.006) treatments. Pen-to-pen total lame was more uniform in this experiment compared with experiment 1, and losses due to mortalities unrelated to lameness were lower (Table 2). Final body weights were comparable between experiments 1 and 2, but the body weights of the Availa-ZMC normal treatment group were lower (t test, P = 0.02) than those of the other 2 treatment groups in experiment 2. There was no clear difference in the distribution of BCO lesions between any of the 4 treatment groups in experiment 2 (Figure 4). Figure 4 only shows BCO lesion severity for lame birds, which is consistent with previous experiments (Wideman et al., 2012; Wideman et al., 2013; Wideman et al., 2015; Wideman, 2016), namely, when birds exhibit severe lameness, it is because they have reached a particular level of BCO severity regardless of the treatment group. We did notice that a rather high percentage of normal left femoral head diagnoses in the source population, which we cannot explain.
Figure 3.
Cumulative lameness for broilers with a bacterial challenge, raised on litter flooring, treated with control feed or feed supplemented with Availa-ZMC in experiment 2. Cumulative percentage of lameness is plotted from day 35 to day 56. Details of the control and Availa treatments are shown in Table 1. The source population was the same as the control population but was challenged with S. agnetis 908 at a concentration of 104 cfu/mL in drinking water at day 20 and day 21. Abbreviation: d, day.
Figure 4.
Tibial (upper panel) and femoral (lower panel) lesion diagnoses for all lame birds raised on litter flooring in experiment 2. Proximal heads were diagnosed for necropsy in right (R) and left (L) leg bones in each of the 4 treatment groups (see the legend of Figure 3 and Table 1).
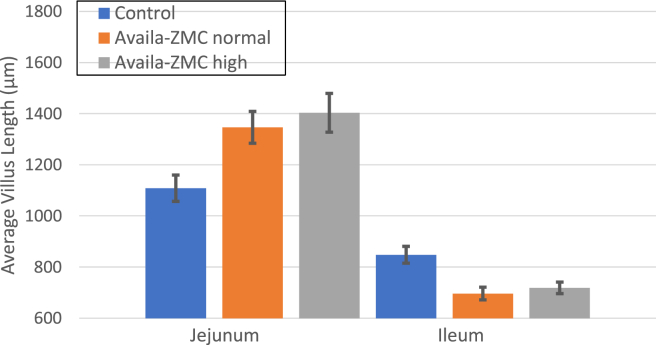
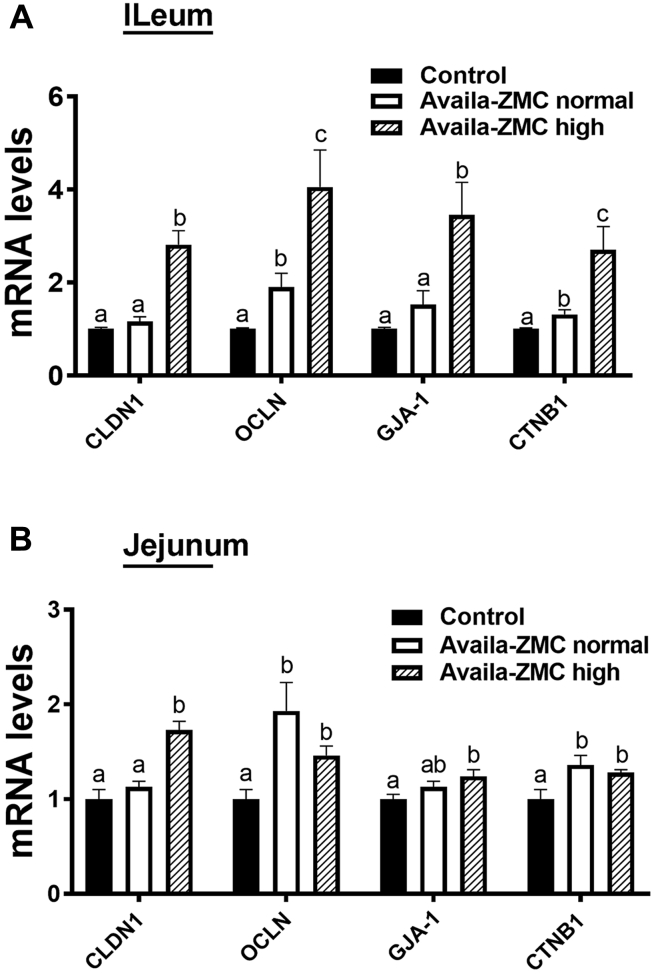
Sections of the distal jejunum and proximal ileum were collected from 5 apparently healthy birds on day 57 from the control and Availa-ZMC treatment groups. Villus length was assessed for multiple sections from each bird, and the average villus lengths were computed (Figure 5). The Student t test analysis of the villus length data indicates that villus length in the ileum was reduced in the Availa-ZMC treatment groups (P < 0.001), but villus length in the jejunum increased (P < 0.001). There was no difference in villus length between the 2 different levels of Availa-ZMC. In terms of important determinants of villus integrity, we examined expression of critical tight junction genes by reverse transcription quantitative PCR. The ileum and jejunum from the samples of the Availa-ZMC high treatment group showed significantly upregulated expression for CLDN1, OCLN, GJA-1, and CTNB1, compared with the control group (Figure 6). Complimentary data for histology and expression from experiment 1 are not provided as the stress imposed by the wire flooring model appears to significantly impact intestinal development and villus formation (A. Hasan, unpublished).
Figure 5.
Villus length at day 57 for apparently healthy birds from 3 treatment groups in experiment 2. Plot is for average villus length for the distal jejunum and proximal ileum for 5 birds from each treatment. Error bars indicate SEM. Abbreviation: d, day.
Figure 6.
Expression of intestinal barrier integrity–related genes from 3 treatment groups in experiment 2. The relative expression of ileal and jejunal OCLN, CLDN1, GJA-1, and CTNB1 was determined by quantitative PCR and analyzed by the 2–ΔΔCt method using the control group as the calibrator. Data are presented as mean ± SEM (n = 6/group). Different letters indicate significant difference at P <0.05. Abbreviation: PCR, polymerase chain reaction.
Wing vein blood was collected from the same birds examined for intestinal histopathology. Monocytes were enriched, and adherent cells were cultured for 5 d. The cells were then used in phagocytosis assays against S. agnetis 908 at an approximate multiplicity of infection of 1:1. Bacterial survival was assessed after 2 d (Table 3). The bactericidal activity was variable between birds within each treatment. The most variation was observed in the birds of the Availa-ZMC normal treatment group, in which the adherent cells of bird 3 were highly active in killing S. agnetis 908. For the lowest dilution plated, 10−2, there were only 5 colonies from one of the 3 triplicate wells. Therefore, verifying that, bacteria were added, but the bacterial survival was very low within the monocytes from this broiler. The high variability for the 5 birds from the Availa-ZMC normal treatment group meant that this treatment group was not statistically different from either the control or Availa-ZMC high treatment groups. Bactericidal activity of cells from the birds of the Availa-ZMC high treatment group was higher than the activity of cells from the birds of the control treatment group (t test, P = 0.00085).
Table 3.
Bacterial survival in adherent peripheral blood monocytes from 5 birds from the 3 treatments in experiment 2.
| Bird | Treatment |
||
|---|---|---|---|
| Control1 | Availa-ZMC normal1 | Availa-ZMC high1 | |
| 1 | 1.3 × 107 | 1.6 × 107 | 5.8 × 106 |
| 2 | 1.7 × 107 | 1.2 × 107 | 1.1 × 107 |
| 3 | 1.7 × 107 | 1.7 × 102 | 9.9 × 106 |
| 4 | 1.6 × 107 | 1.1 × 107 | 7.6 × 106 |
| 5 | 1.2 × 107 | 2.0 × 107 | 8.6 × 106 |
| Average2 | 1.5 × 107,a | 1.2 × 107,a | 8.6 × 106,b |
| SEM | 9.2 × 105 | 3.0 × 106 | 8.2 × 105 |
cfu average from triplicate wells—details of the assay mentioned in Materials and methods.
Values which are significantly different (P < 0.05) have different superscripts.
Discussion
In our previous research publications, we had reported that S. agnetis 908 can induce 80 to 90% lameness when administered to birds raised on wire flooring (Al-Rubaye et al., 2015; Al-Rubaye et al., 2017). We also demonstrated that S. agnetis 908 can induce 50 to 80% lameness when administered in drinking water to birds raised on litter flooring (Alrubaye et al., 2020). Furthermore, the lameness can be transmitted from the birds challenged with bacteria to unchallenged birds within the same broiler house. We also demonstrated that specific probiotics in the feed can protect broilers when raised on wire flooring (Wideman et al., 2012; Al-Rubaye et al., 2017), but different probiotics in the feed can protect the unchallenged birds in the litter flooring model with the bacterial challenge (Alrubaye et al., 2020). The combination of organic zinc, manganese, and copper has been reported to improve poultry health, reduce bacterial pathogen colonization, and reduce femoral head necrosis (Sirri et al., 2016; McKnight et al., 2020). The results from experiments 1 and 2 confirm that Availa-ZMC is effective in reducing lameness in both models for inducing lameness. When birds were raised on wire flooring with no direct bacterial challenge, the mineral supplement reduced lameness by 14 to 29% (Figure 1) and appears to have reduced mortality owing to causes not attributable to lameness (Table 2). In the litter flooring model with the bacterial challenge, the Availa-ZMC treatment similarly reduced lameness by 20 to 25% relative to the control treatment (Figure 3). Most importantly, this latter model uses the contagious spread of the infection observed in some broiler operations, whereas our model uses a hypervirulent bacterial strain; the reduction could be greater against less virulent species/strains. Although the Availa-ZMC normal treatment had lower levels of zinc, manganese, and copper than the other 2 treatments, lameness was also reduced compared with the control. This could result if organic trace minerals have higher bioavailability than inorganic sources (Hudson et al., 2004; Favero et al., 2013; Burin et al., 2019). Substituting complexed organic trace minerals for inorganic minerals appears to have resulted in reduced villus length in the ileum and increased villus length in the jejunum (Table 2). Further investigations are warranted to determine whether these changes arising from the difference in the source of trace minerals alter any aspect of assimilation or feed conversion rates in infectious models. In these studies, the final body weights did not seem to have been impacted (Table 2). However, with differences in lameness and mortality between treatments, differences in stocking density could have influenced body weights. Interestingly, the organic trace mineral upregulated the expression of the genes for tight junction (CLDN1 and OCLN), gap junction (GJA-1), and desmosome (CTNB1) proteins, consistent with improved gut barrier integrity. Although the exact functions of the individual tight junction proteins remain elusive, in avian species, occludin has been reported to be an integral component in tight junction barrier function (Balda et al., 2000). Studies conducted in occludin-deficient mice showed gut inflammation and defective epithelial barrier function (Schulzke et al., 2005). Similarly, it has been reported that downregulation of CLDN1 can drastically reduce barrier integrity (Zeissig et al., 2007). Upregulation of these genes is consistent with Availa-ZMC enhancing barrier functions and reducing translocation of bacteria into the blood, a critical first step in the progression of BCO lameness (Wideman and Prisby, 2013; Wideman, 2016; Al-Rubaye et al., 2017). Furthermore, the organic trace minerals appear to enhance the bacterial killing activity of adherent peripheral blood monocytes (Table 3). Availa-ZMC has been reported to improve intestinal health, epithelial integrity, and immune function (Hudson et al., 2004; Star et al., 2012; Zakaria et al., 2017). The reduction in bacterial lameness in both models could result from either enhanced barrier function or enhanced bactericidal activity of phagocytes, or both. Growth on wire flooring increases translocation of bacteria into the blood relative to growth on litter flooring (Al-Rubaye et al., 2017). The litter flooring model with the bacterial challenge involves noncontact spread of the infection from the source population, and we have speculated on whether the infection is through the pulmonary or gastrointestinal path (Alrubaye et al., 2020). The adherent peripheral blood monocyte phagocytosis results suggest that the Availa-ZMC reduces lameness in part by enhanced killing of bacteria that translocate into the blood on either type of flooring (Al-Rubaye et al., 2017). The enhanced gene expression data for gut integrity markers argue that both immunity and barrier functions have been enhanced for the Availa-ZMC–treated birds raised on litter flooring. Most intriguing is the high bactericidal activity of the monocytes from bird 3 of the Availa-ZMC normal treatment group. Only one of the 15 birds displayed such superior activity. However, we cannot discern whether the activity of cells from this bird was inherent to that bird or resulted from stimulation by the Availa-ZMC supplementation. There could be a small percentage of birds with superior innate immunity or a small percentage of birds with immune systems that are highly activated by organic trace minerals. Regardless, identification of these birds would provide a major new tool for improving animal welfare.
The data reported herein demonstrate that Availa-ZMC can reduce lameness in both the wire flooring model and the litter flooring model with the bacterial challenge. In addition, we observed that Availa-ZMC shows a dose-dependent enhancement of bacterial killing activity by adherent peripheral blood monocytes cultured from treated birds. These data extend the range of products that can be used to reduce BCO lameness, highlight the importance of organic trace minerals in improving animal well-being, and provide further validation of the 2 models we have developed to investigate treatments and management strategies for reducing BCO lameness in broiler operations. Our recent work demonstrating that certain probiotics can also reduce BCO lameness (Alrubaye et al., 2020) strongly supports investigations pairing probiotics with Availa-ZMC to determine whether the protective effects are overlapping, additive, or synergistic. The litter flooring model with the bacterial challenge provides an excellent system for evaluation of these interactions. Development of effective management strategies that can be used in the broiler industry will improve productivity and reduce animal welfare concerns.
Acknowledgments
Funding for this work was provided by Zinpro Corporation (Eden Prairie, MN), which also contributed to the design of these experiments.
Conflict of Interest Statement: The authors declare that they have no competing interests.
References
- Al-Rubaye A.A.K., Couger M.B., Ojha S., Pummill J.F., Koon J.A., II, Wideman R.F., Jr., Rhoads D.D. Genome analysis of Staphylococcus agnetis, an agent of lameness in broiler chickens. PLoS One. 2015;10:e0143336. doi: 10.1371/journal.pone.0143336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rubaye A.A.K., Ekesi N.S., Zaki S., Emami N.K., Wideman R.F., Rhoads D.D. Chondronecrosis with osteomyelitis in broilers: further defining a bacterial challenge model using the wire flooring model. Poult. Sci. 2017;96:332–340. doi: 10.3382/ps/pew299. [DOI] [PubMed] [Google Scholar]
- Alrubaye A., Ekesi N.S., Hasan A., Koltes D.A., Wideman R., Jr., Rhoads D. Chondronecrosis with osteomyelitis in broilers: further defining a bacterial challenge model using standard litter flooring and protection with probiotics. Poult. Sci. 2020;96:332–340. doi: 10.1016/j.psj.2020.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M.S., Flores-Maldonado C., Cereijido M., Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J. Cell. Biochem. 2000;78:85–96. [PubMed] [Google Scholar]
- Burin A.M., Jr., Fernandes N.L.M., Snak A., Fireman A., Horn D., Fernandes J.I.M. Arginine and manganese supplementation on the immune competence of broilers immune stimulated with vaccine against Salmonella Enteritidis. Poult. Sci. 2019;98:2160–2168. doi: 10.3382/ps/pey570. [DOI] [PubMed] [Google Scholar]
- Campbell P.A., Canono B.P., Drevets D.A. Measurement of bacterial Ingestion and killing by macrophages. Curr. Protoc. Immunol. 1994;12:14.16.11–14.16.13. doi: 10.1002/0471142735.im1406s12. [DOI] [PubMed] [Google Scholar]
- Dawes M.E., Griggs L.M., Collisson E.W., Briles W.E., Drechsler Y. Dramatic differences in the response of macrophages from B2 and B19 MHC-defined haplotypes to interferon gamma and polyinosinic:polycytidylic acid stimulation. Poult. Sci. 2014;93:830–838. doi: 10.3382/ps.2013-03511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamad A.E., Greene E., Sales M., Nguyen P., Beer L., Liyanage R., Dridi S. 75-kDa glucose-regulated protein (GRP75) is a novel molecular signature for heat stress response in avian species. Am. J. Physiol. Cell Physiol. 2019;318:C289–C303. doi: 10.1152/ajpcell.00334.2019. [DOI] [PubMed] [Google Scholar]
- Drechsler Y., Tkalcic S., Saggese M.D., Shivaprasad H.L., Ajithdoss D.K., Collisson E.W. A DNA vaccine expressing ENV and GAG offers partial protection against Reticuloendotheliosis Virus in the Prairie chicken (Tympanicus cupido) J. Zoo Wildl. Med. 2013;44:251–261. doi: 10.1638/2011-0229R1.1. [DOI] [PubMed] [Google Scholar]
- Drevets D.A., Canono B.P., Campbell P.A. Measurement of bacterial ingestion and killing by macrophages. Curr. Protoc. Immunol. 2015;109:14.16.11–14.16-17. doi: 10.1002/0471142735.im1406s109. [DOI] [PubMed] [Google Scholar]
- Favero A., Vieira S., Angel C., Bos-Mikich A., Lothhammer N., Taschetto D., Cruz R., Ward T. Development of bone in chick embryos from Cobb 500 breeder hens fed diets supplemented with zinc, manganese, and copper from inorganic and amino acid-complexed sources. Poult. Sci. 2013;92:402–411. doi: 10.3382/ps.2012-02670. [DOI] [PubMed] [Google Scholar]
- Greene E., Flees J., Dhamad A., Alrubaye A., Hennigan S., Pleimann J., Smeltzer M., Murray S., Kugel J., Goodrich J., Robertson A., Wideman R., Rhoads D., Dridi S. Double-stranded RNA Is a novel molecular target in osteomyelitis pathogenesis: a translational avian model for human bacterial chondronecrosis with osteomyelitis. Am. J. Pathol. 2019;189:1897. doi: 10.1016/j.ajpath.2019.06.013. [DOI] [PubMed] [Google Scholar]
- Hudson B.P., Dozier W.A., Wilson J.L., Sander J.E., Ward T.L. Reproductive performance and immune status of cage broiler breeder hens provided diets supplemented with either inorganic or organic sources of zinc from Hatching to 65 wk of Age. J. Appl. Poult. Res. 2004;13:349–359. [Google Scholar]
- Hurley L.S., Keen C.L. Manganese. In: Mertz W., editor. Trace Elements in Human and Animal Nutrition. Academic Press; Orlando, Fla: 1987. pp. 185–223. [Google Scholar]
- Kidd M.T. Nutritional modulation of immune function in broilers. Poult. Sci. 2004;83:650–657. doi: 10.1093/ps/83.4.650. [DOI] [PubMed] [Google Scholar]
- Lassiter K., Greene E., Piekarski A., Faulkner O.B., Hargis B.M., Bottje W., Dridi S. Orexin system is expressed in avian muscle cells and regulates mitochondrial dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R173–R187. doi: 10.1152/ajpregu.00394.2014. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McKnight L., Page G., Han Y. Effect of replacing in-feed antibiotics with synergistic organic acids, with or without trace minerals and/or water acidification, on growth performance and health of broiler chickens under a Clostridium perfringens type A challenge [e-pub ahead of print] Avian Dis. 2020 doi: 10.1637/aviandiseases-D-19-00115. [DOI] [PubMed] [Google Scholar]
- Piekarski-Welsher A., Greene E., Lassiter K., Kong B.C., Dridi S., Bottje W. Enrichment of autophagy and proteosome pathways in breast muscle of feed efficient pedigree male broilers. Front. Physiol. 2018;9:1342. doi: 10.3389/fphys.2018.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schulzke J.D., Gitter A.H., Mankertz J., Spiegel S., Seidler U., Amasheh S., Saitou M., Tsukita S., Fromm M. Epithelial transport and barrier function in occludin-deficient mice. BBA. Biomembranes. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Siegel P.B., Barger K., Siewerdt F. Limb health in broiler breeding: History using genetics to improve welfare. J. Appl. Poult. Res. 2019;28:785–790. [Google Scholar]
- Sirri F., Maiorano G., Tavaniello S., Chen J., Petracci M., Meluzzi A. Effect of different levels of dietary zinc, manganese, and copper from organic or inorganic sources on performance, bacterial chondronecrosis, intramuscular collagen characteristics, and occurrence of meat quality defects of broiler chickens. Poult. Sci. 2016;95:1813–1824. doi: 10.3382/ps/pew064. [DOI] [PubMed] [Google Scholar]
- Star L., Van der Klis J., Rapp C., Ward T. Bioavailability of organic and inorganic zinc sources in male broilers. Poult. Sci. 2012;91:3115–3120. doi: 10.3382/ps.2012-02314. [DOI] [PubMed] [Google Scholar]
- Wideman R.F. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult. Sci. 2016;95:325–344. doi: 10.3382/ps/pev320. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Al-Rubaye A., Gilley A., Reynolds D., Lester H., Yoho D., Hughes J.M., Pevzner I. Susceptibility of 4 commercial broiler crosses to lameness attributable to bacterial chondronecrosis with osteomyelitis. Poult. Sci. 2013;92:2311–2325. doi: 10.3382/ps.2013-03150. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Al-Rubaye A., Kwon Y.M., Blankenship J., Lester H., Mitchell K.N., Pevzner I.Y., Lohrmann T., Schleifer J. Prophylactic administration of a combined prebiotic and probiotic, or therapeutic administration of enrofloxacin, to reduce the incidence of bacterial chondronecrosis with osteomyelitis in broilers. Poult. Sci. 2015;94:25–36. doi: 10.3382/ps/peu025. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Al-Rubaye A., Reynolds D., Yoho D., Lester H., Spencer C., Hughes J.D., Pevzner I.Y. Bacterial chondronecrosis with osteomyelitis in broilers: Influence of sires and straight-run versus sex-separate rearing. Poult. Sci. 2014;93:1675–1687. doi: 10.3382/ps.2014-03912. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Hamal K.R., Stark J.M., Blankenship J., Lester H., Mitchell K.N., Lorenzoni G., Pevzner I. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult. Sci. 2012;91:870–883. doi: 10.3382/ps.2011-01907. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Prisby R.D. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front. Endocrinol. (Lausanne) 2013;3:183. doi: 10.3389/fendo.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria H., Jalal M., AL-Titi H., Souad A. Effect of sources and levels of dietary zinc on the performance, carcass traits and blood parameters of broilers. Braz. J. Poult. Sci. 2017;19:519–526. [Google Scholar]
- Zeissig S., Bürgel N., Günzel D., Richter J., Mankertz J., Wahnschaffe U., Kroesen A.J., Zeitz M., Fromm M., Schulzke J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]